In this study, we investigated VIM-1-producing Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Citrobacter freundii, and Enterobacter cloacae strains, isolated in 2019 during a period of active surveillance of carbapenem-resistant Enterobacterales in a large university hospital in Italy. VIM-1-producing strains colonized the gut of patients, with up to three different VIM-1-positive bacterial species isolated from a single rectal swab, but also caused bloodstream infection in one colonized patient.
KEYWORDS: antibiotic resistance, carbapenems, hospital infections, metallo-beta-lactamases, plasmid-mediated resistance
ABSTRACT
In this study, we investigated VIM-1-producing Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Citrobacter freundii, and Enterobacter cloacae strains, isolated in 2019 during a period of active surveillance of carbapenem-resistant Enterobacterales in a large university hospital in Italy. VIM-1-producing strains colonized the gut of patients, with up to three different VIM-1-positive bacterial species isolated from a single rectal swab, but also caused bloodstream infection in one colonized patient. In the multispecies cluster, blaVIM-1 was identified in a 5-gene cassette class 1 integron, associated with several genetic determinants, including the blaSHV-12, qnrS1, and mph(A) genes, located on a highly conjugative and broad-host-range IncA plasmid. The characteristics and origin of this IncA plasmid were studied.
INTRODUCTION
The rapid spread of carbapenemase-producing Enterobacteriaceae (CPE) is a growing worldwide public health threat. Plasmid-mediated horizontal transfer of carbapenemase genes accounts for the dissemination of carbapenem resistance (1–3).
In Italy, the national surveillance performed in 2014 to 2017 on bloodstream infections (BSI) showed that of 7,632 CPE-BSI cases reported from Italian hospitals, almost all (98.1%) were due to K. pneumoniae carbapenemase (KPC) enzyme (95.2%), and two species were prevalent, Klebsiella pneumoniae and Escherichia coli. Metallo-β-lactamases (MBLs) were detected in only 1% of CPE cases, and 1.2% were positive for OXA-48 enzymes. Among the MBLs, the most frequent was VIM (0.67%), accounting for 75.6% of all MBL-producing Enterobacterales (4). The blaVIM- and blaIMP-MBL genes typically are gene cassettes inserted into integron platforms (5). In Enterobacterales, MBL-encoding integrons are mostly associated with plasmid lineages of IncN, IncC (previously termed IncA/C2 [6]), IncF, and IncHI type (7, 8).
The high incidence of KPC-CPE in Italy favors the use of ceftazidime-avibactam (CAZ-AVI), a novel beta-lactam/β-lactamase inhibitor combination, as the first drug of choice for treatment of KPC-CPE and OXA-48–CPE infections. The possible emergence of resistance to CAZ-AVI is of great concern, and since June 2018, the ECDC has launched actions, including active and passive surveillance, to prevent this threat and control the transmission of CAZ-AVI-resistant strains in hospitals and other health care settings (https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Emergence-of-resistance-to%20CAZ-AVI-in-CRE-Enterobacteriaceae.pdf). MBL enzymes are not targeted by the avibactam inhibitor and confer resistance to CAZ-AVI (9). It is tempting to speculate that the increased consumption of CAZ-AVI for KPC-producing bacteria will lead MBL-producing organisms to increase their prevalence in Italian hospitals.
A multispecies cluster of VIM-1-producing strains was identified in 2019 at the Sapienza University Hospital “Policlinico Umberto I” in Rome, Italy. CPE were identified as colonizers of the gut of patients recovered in different wards. Horizontal transmission of a highly conjugative and broad-host-range plasmid of the IncA type (previously termed IncA/C1) (6), carrying the blaVIM-1 gene, was detected in Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Citrobacter freundii, and Enterobacter cloacae strains.
RESULTS
A multispecies cluster of VIM-1-producing Enterobacterales.
In January 2019, during routine surveillance for CPE colonization in critical patients, Escherichia coli, Klebsiella oxytoca, and Citrobacter freundii blaVIM-positive strains were isolated at the Microbiology Unit of the University Hospital from a single rectal swab of a patient (patient 1).
In May 2019, the Italian Ministry of Health, in the framework of a plan aimed to extend and consolidate the prevention/control of CPE transmission launched a few years ago, reinforced the recommendations for active surveillance for CPE colonization. In our hospital, such activity has been intensified, and, from July to October 2019, a total of 1,066 rectal swabs from patients recovered in 58 different wards were screened for carbapenem-resistant Enterobacterales (CRE). Among them, 272 samples (1 per patient) were suspected positive for CRE, showing growth on Brilliance CRE medium plates (Oxoid Ltd., Basingstoke, UK). Carbapenemase gene identification was confirmed by Xpert Carba-R (Cepheid, Sunnyvale, CA) on colonies isolated from 196 samples. CPE with the blaKPC gene were identified in most of the samples (185/196, 94.4%), but 5 NDM-, 2 OXA-48-, and 4 VIM-positive CPE were also identified as colonizers of patients. The blaVIM-1 gene was identified in E. coli (patients 2 and 4), K. pneumoniae (patient 3), and E. cloacae (patient 5).
After colonization, patient 2 developed a BSI sustained by VIM-1-producing E. cloacae, which was not detected during the rectal swab screening. The patients were from several different wards and had no identifiable common epidemiological links. Cluster analysis of E. cloacae from patients 2 and 5 and E. coli from patients 1, 2, and 4 was performed by maximum likelihood (ML) parsimony single-nucleotide polymorphism (SNP) analysis by the kSNP (Galaxy version 3.1) software, demonstrating that the strains were not of clonal origin (see Fig. S1 in the supplemental material).
Overall, a total of 8 multispecies VIM-1-producing strains identified in the hospital in 2019 were studied.
Complete sequences of the VIM-1–IncA plasmids.
Complete whole-genome sequencing (WGS) of the three multispecies VIM-1-positive strains from patient 1 was obtained by short-read/Illumina and long-read/Nanopore methods (BioProject no. PRJNA592166). In E. coli serotype O86:H2 (ST349), K. oxytoca (nearest match to ST165), and C. freundii ST18, several plasmids were identified in the sequenced strains (Table 1) and the blaVIM-1 gene was located on IncA plasmids (PlasmidFinder detected IncA/C_1 at 98.32% and IncA/C_2 at 94% nucleotide identity) and assigned to ST12 by plasmid multilocus sequence typing (pMLST).
TABLE 1.
Features of the VIM-1-IncA-positive strains described in this study
| Species and STa | Patient-wardb and infection status | β-Lactamase(s) | Other acquired resistance genesc | Replicon contentd/IncA plasmid name |
|---|---|---|---|---|
| E. coli ST349 | Patient 1-SUR, colonizer | VIM-1, OXA-10, SHV-12, TEM-1C | aadA1, aac(6')-Ib3, aph(3″)-Ib, aph(3′)-XV, aph(6)-Id, arr-2, catB2, cmlA, dfrA14, mph(A), qnrS1, sul1, sul2, tetA(A) | A, FII, FIB, X1/pGA_VIM |
| K. oxytoca ST165* | Patient 1-SUR, colonizer | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3″)-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | A, ND/pFDL_VIM |
| C. freundii ST18 | Patient 1-SUR, colonizer | VIM-1, OXA-1, SHV-12, CTX-M-15, TEM-1B | aadA1, aac(3)-IIa, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, catB3, catA1, dfrA14, mph(A), qnrB1, qnrS1, sul1, sul2, tet(A) | A/pGA_VIM |
| E. coli ST131 | Patient 2-ICU, colonizer | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2, tet(B) | A, FIB, FII, I1, Col8282/pGB_VIM |
| E. cloacae ST574 | Patient 2-ICU, bloodstream infection | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, fosA, mph(A), qnrS1, sul1, sul2 | A, ColpVC, Col8282/pGB_VIM |
| K. pneumoniae ST3155* | Patient 3-NICU, colonizer | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3′)-XV, catB2, dfrA14, mph(A), qnrS1, sul1 | A, FIB(K), Col440/pAC_VIM |
| E. coli ST6496 | Patient 4-CICU, colonizer | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3″)-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul2 | A, X1, Col440/pLF_VIM |
| E. cloacae ST106 | Patient 5-MED, colonizer | VIM-1, SHV-12 | aadA1, aac(6')-Ib3, aph(3″)-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | A, FIB(K)/pMB_VIM |
ST, sequence type determined at the https://pubmlst.org/ website. An asterisk indicates that the ST was not determined for differences, so the nearest match with STs in the database is reported.
SUR, surgical ward; ICU, intensive care unit; NICU, neonatal intensive care unit; CICU, cardiological intensive care unit; MED, internal medicine ward.
Resistance gene content was determined by ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/).
Plasmid replicons were determined by PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/). ND, not determined.
IncA plasmids showed a scaffold coherent with that described for the pRA1 IncA reference plasmid from Aeromonas hydrophila, including the replicon with the repA replicase gene, two transfer loci (tra1 and tra2), and a resistance region (10). In pRA1, the resistance region was localized between the DNA replication terminator tus gene and a gene (hyp) encoding a conserved hypothetical protein (nucleotide [nt] positions 127046 to 142813; FJ705807). The resistance region of VIM-1-IncA plasmids was integrated at the same position as pRA1 (Fig. 1).
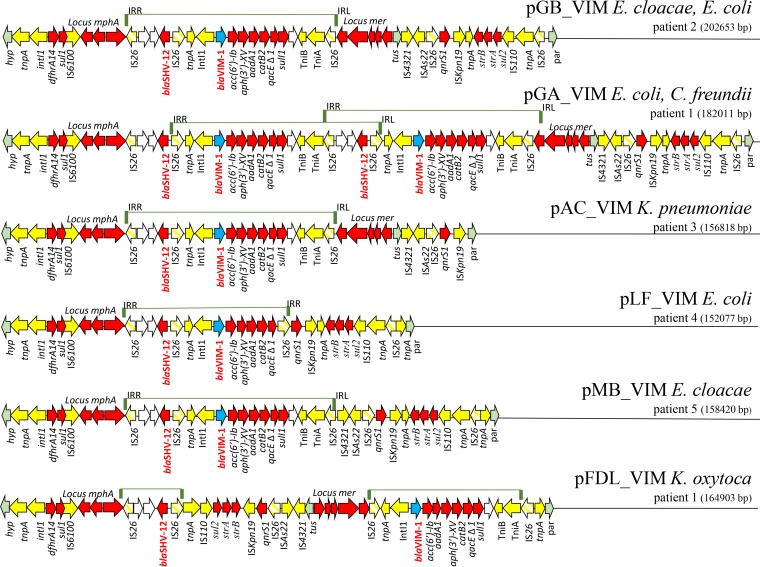
FIG 1.
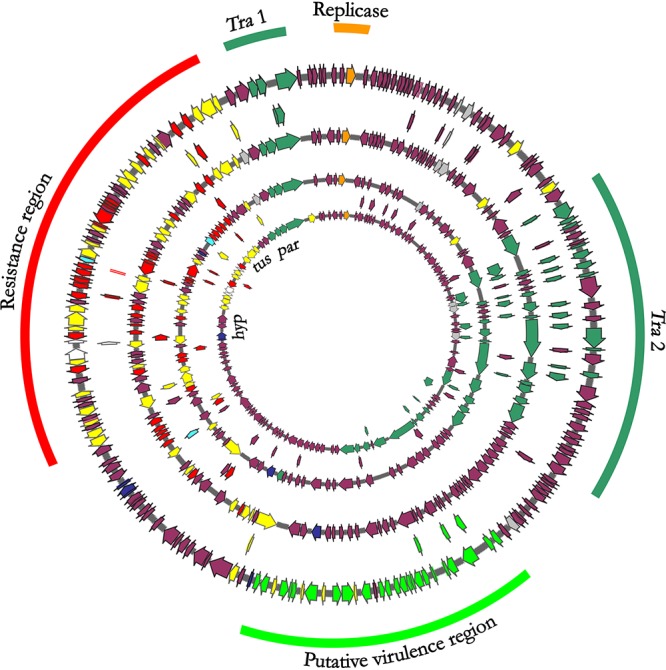
Circular maps of IncA plasmids. Circular maps of (starting from the inner circle) IncA reference plasmid pRA1 from Aeromonas hydrophila (NC_012885.1), plasmid pFDL_VIM from Klebsiella oxytoca of patient 1, pGA_VIM from Escherichia coli of patient 1, and pGB_VIM Enterobacter cloacae from patient 2. Colored arrows represent the blaVIM-1 gene (turquoise), conjugal transfer system (green), resistance genes (red), mobile elements (yellow), hypothetical proteins (plum), replication protein repA (orange), and virulence region identified only in pGB-VIM plasmids from patient 2 (bright green).
Conjugation experiments were performed using the VIM-1-IncA K. oxytoca strain as the donor and the susceptible E. coli JM83 laboratory strain as the recipient. Conjugation frequency varied in the experiments repeated in triplicate in the range of 1 × 10−2 to 0.5 × 10−3 conjugants per recipient cell. The frequency of conjugation was reproducible with that described for the pRA1 reference plasmid (10).
The repA replicase gene of the VIM-1-IncA plasmid showed 11 and 71 mismatch mutations with the repA genes of IncA pRA1 and IncC R16a (KX156773.1) reference plasmids, respectively. An IncA repA PCR was devised (A-RV, 5′-CCCATCTTCGAGAGCTCCTTCTG-3′; A/C FW, 5′-GAGAACCAAAGACAAAGACCTGGA-3′), producing a 417-bp amplicon.
The five isolates identified in the July to October 2019 period, including E. coli ST131 (colonizer of patient 2), E. cloacae ST574 (BSI of patient 2), K. pneumoniae ST3155 (colonizer of patient 3), E. coli ST6496 (colonizer of patient 4), and E. cloacae ST106 (colonizer of patient 5), were positive for the IncA repA PCR and for the blaVIM-1 gene, respectively. The presence of the VIM-IncA plasmid in these strains was confirmed by Nanopore technology complete genome sequencing (BioProject no. PRJNA592166) (Table 1).
IncA plasmids in the 8 VIM-1 producers were highly related to each other but showed variable length, ranging from 152,077 bp to 202,653 bp. The largest plasmid was pGB_VIM, identified in E. coli (colonizer) and E. cloacae (causing BSI) from patient 2. This plasmid showed a 33-kb fragment, which was not detected in the other IncA plasmids, integrated in the region between the tra2 locus and the resistance region (Fig. 1). This region encoded several hypothetical proteins and had genes predicted to encode lactoylglutathione lyase, S-(hydroxymethyl)glutathione dehydrogenase, and other putative virulence factors of unknown origin and function. Other differences in plasmid length were due to different assortments of genetic determinants identified in the resistance region (Fig. 2).
FIG 2.
VIM-1 resistance regions in IncA plasmids identified in this study. A linear map of resistance regions of the 8 IncA plasmids sequenced in this study is shown. Resistance genes are indicated by red-colored arrows, except the blaVIM-1 gene, which is indicated by a turquoise arrow; transposon-related genes (tnpA, tnpR, and tnpM) and class 1 integrase and insertion sequences are indicated by yellow arrows, except the IS26 insertion sequences, which are represented by striped yellow arrows. Other genes are indicated by arrows in the following colors: light green, integration genes identified with respect to pRA1 reference sequence; white, genes trapped within the IS26-blaSHV-12-IS26 element. A bar above the map indicates the IS26-blaSHV-12-blaVIM-1-IS26 module amplified in pGA-VIM, flanked by the two inverted right (IRR) and left (IRL) repeats of IS26.
IncA plasmid resistance region.
Five regions of resistance determinants were identified in VIM-1-IncA: (i) a region including the Tn3-tnpA and IS6100 elements, a class 1 integron with the dfrA14 gene cassette, the ΔqacE-sul1 genes of the 3′ conserved region (3′-CS), and the macrolide resistance mrx-mph(A) locus; (ii) the blaSHV-12 extended-spectrum β-lactamase (ESBL) gene flanked by two inverted repeated IS26 elements; (iii) a Tn21 derivative transposon, carrying tniA, tniB, a class 1 integron with blaVIM-1, aac(6′)-Ib3, aph(3′)-XV, aadA1, and catB2 gene cassettes, the ΔqacE-sul1 genes in the 3′-CS, and mercuric ion resistance encoded by the locus mer; (iv) a Tn6292 derivative carrying the qnrS1 gene and the ISKpn19 insertion sequence; and (v) a region comprised of the Tn4380 transposase, aph(6)-Id (strB), aph(3″)-Ib (strA), sul2 genes, and IS110 insertion sequence (Fig. 2 and Table 1).
The first three resistance determinants were located between tus and a gene (hyp) encoding a hypothetical protein, namely, in the same position of the resistance region identified in the reference pRA1 plasmid. The Tn6292 and strB-strA-sul2 regions were located between tus and par genes (in pRA1, nt positions 127921 to 128077; FJ705807) (Fig. 1 and 2).
Comparing the resistance regions in the 8 IncA plasmids identified in our study, several rearrangements were observed. The pGB_VIM plasmid, identified in E. coli and E. cloacae from patient 2, carried all five resistance determinants, and pGA_VIM, identified in E. coli and C. freundii colonizing patient 1, carried two repeated copies in a head-to-tail configuration of the blaSHV-12 and blaVIM-1-resistance determinants. The duplication was likely mediated by amplification of the region between two directly repeated IS26 elements (Fig. 2).
Plasmid pAC_VIM, identified in the K. pneumoniae colonizer of patient 3, lacked the Tn4380 transposase, strB-strA-sul2 genes, and the IS110 insertion sequence, while both pLF_VIM of the E. coli colonizer of patient 4 and pMB_VIM, identified in the E. cloacae from patient 5, lacked the mer locus (Fig. 2). In pLF_VIM, the IS26-qnrS1-strB-strA-sul2-IS110-IS26 element was juxtaposed to the 3′ end of the blaVIM-1 integron, causing the sul1 gene deletion in the 3′-CS. In plasmid pFDL_VIM from the K. oxytoca colonizer of patient 1, an inversion occurred involving qnrS1, strB-strA-sul2, and the locus mer region (Fig. 2).
VIM-1-IncA plasmid evolution.
A BLASTN analysis of the NCBI nucleotide databank was performed using the repA gene of pRA1 (FJ705807). Twenty sequences showing 98 to 100% nucleotide identity with the repA gene of pRA1 were identified. Further, 367 sequences were detected by BLASTN, but nucleotide identity dropped to 93% to 95%. Plasmids showing ≤95% nucleotide identity were IncC (confirmed by BLASTN using transfer loci and other peculiar IncC regions [11, 12, and data not shown]) and were not included in this study. The 20 sequences with >98% nucleotide identity were considered IncA plasmids. Among them, pRAx (FJ705806), representing a pRA1 (FJ705807)-deleted version (10), and pIBAC_IncX3_A/C (MH594478), identical to pIBAC_IncA/C (MH594477) but fused with an IncX3 plasmid, were excluded from the comparison.
The 18 IncA plasmid sequences identified were downloaded from GenBank (Table 1) and compared with the pGA-VIM and pFDL-VIM plasmids sequenced in this study to generate an SNP-based ML phylogenetic tree.
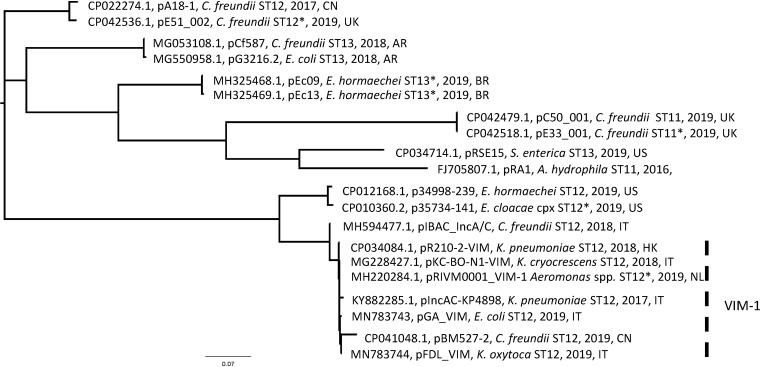
The ML phylogenetic tree showed multiple branches (Fig. 3). The IncA ancestor pRA1 (ST11 by pMLST) was distant from the VIM-1-IncA plasmids and clustered with C. freundii and Salmonella enterica plasmids, assigned to ST11 or ST13, respectively (Table 2). All VIM-1-positive plasmids clustered together, differing from 6 to 45 SNPs. These were with pGA-VIM and pFDL-VIM from this study, pBM527-2, identified in C. freundii in China (CP041048.1), pKC-BO-N1-VIM, from Kluyvera cryocrescens isolated in Bologne (13), Italy (MG228427.1), pIncAC-KP4898, from K. pneumoniae causing an outbreak in 2018 in Naples (14), Italy (KY882285.1), pR210-2-VIM, from K. pneumoniae isolated in Hong Kong (CP034084.1), and pRIVM0001_VIM-1, from an Aeromonas sp. from The Netherlands (MH220284.1) (Fig. 3 and Table 2). An average of 1,220 SNPs separated the pRA1 from the group of VIM-1-IncA plasmids.
FIG 3.
Maximum likelihood (ML) phylogenetic tree of IncA plasmids. The IncA phylogenetic tree was generated by comparing 20 complete plasmid sequences, including 18 sequences downloaded from GenBank and the pGA_VIM and pFDL_VIM sequences, which were determined in this study (Table 2). Parsimony SNP analysis was performed by kSNP3 (Galaxy version 3.1) software at the ARIES public Galaxy server (https://w3.iss.it/site/aries/). The phylogenetic tree was visualized using FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). The group of the VIM-1-IncA plasmid in the tree is indicated with a dashed vertical bar.
TABLE 2.
Characteristics of internationally identified IncA plasmids and those of pGA-VIM and pFDL-VIM sequenced in this study
| Accession no. | Name | Species | Date | Country | Resistance genes on the IncA plasmida | pMLSTb |
|---|---|---|---|---|---|---|
| FJ705807.1 | pRA1 | Aeromonas hydrophila | 2016 | United States | sul2, tetA(D) | ST11 |
| CP042479.1 | pC50_001 | Citrobacter freundii | 2019 | United Kingdom | No resistance gene detected | ST11 |
| CP042518.1 | pE33_001 | Citrobacter freundii | 2019 | United Kingdom | No resistance gene detected | ST11* |
| MH594477.1 | pIBAC_IncA/C | Citrobacter freundii | 2018 | Italy | blaOXA-1, aac(6')-Ib-cr, catB3, sul1 | ST12 |
| CP012168.1 | p34998-239 | Enterobacter hormaechei | 2019 | United States | blaKPC-4, blaOXA1, blaTEM-1, aadA1, aac(6')-Ib-cr, aph(3′)-Ia, aac(3)-Vla, catB3, mph(A), sul1 | ST12 |
| KY882285.1 | pIncAC-KP4898 | Klebsiella pneumoniae | 2017 | Italy | blaVIM-1, blaSHV-12, aadA1, aac(6')-Ib3, aph(3′)-XV, catB2, dfrA14, mph(A), qnrA1, sul1 | ST12 |
| CP034084.1 | pR210-2-VIM | Klebsiella pneumoniae | 2018 | Hong Kong | blaVIM-1, aadA1, aac(6')-Ib3, aph(3′)-XV, catB2, dfrA14, mph(A), sul1 | ST12 |
| MG228427.1 | pKC-BO-N1-VIM | Kluyvera cryocrescens | 2018 | Italy | blaVIM-1, blaSHV-12, aadA1, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | ST12 |
| CP041048.1 | pBM527-2 | Citrobacter freundii | 2019 | China | blaVIM-1, blaSHV-12, blaCTX-M-3, blaNDM-1, blaTEM-1B, aadA1, aac(6')-Ib3, aph(3′)-XV, catB2, sul1 | ST12 |
| MN783743 | pGA_VIM | Escherichia coli | 2019 | Italy | blaVIM-1, blaSHV-12, aadA1, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | ST12 |
| MN783744 | pFDL_VIM | Klebsiella oxytoca | 2019 | Italy | blaVIM-1, blaSHV-12, aadA1, aac(6')-Ib3, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | ST12 |
| CP022274.1 | pA18-1 | Citrobacter freundii | 2017 | China | No resistance gene detected | ST12 |
| MH220284.1 | pRIVM0001_VIM-1 | Aeromonas sp. | 2019 | The Netherlands | blaVIM-1, blaSHV-12, aadA1, aac(6')-Ib-cr, aph(3′')-Ib, aph(3′)-XV, aph(6)-Id, catB2, dfrA14, mph(A), qnrS1, sul1, sul2 | ST12* |
| CP010360.2 | p35734-141 | Enterobacter cloacae complex | 2019 | United States | No resistance gene detected | ST12* |
| CP042536.1 | pE51_002 | Citrobacter freundii | 2019 | United Kingdom | No resistance gene detected | ST12* |
| CP034714.1 | pRSE15 | Salmonella enterica | 2019 | United States | No resistance gene detected | ST13 |
| MG053108.1 | pCf587 | Citrobacter freundii | 2018 | Argentina | blaPER-2, blaTEM-1B, ant(2'')-Ia, aph(3′)-VIa, catA1, ereA, sul1 | ST13 |
| MG550958.1 | pG3216.2 | Escherichia coli | 2018 | Argentina | blaIMP-8, blaTEM-1b, aadA1, ant(2″)-Ia, aph(3′)-Vla, mph(A), sul1 | ST13 |
| MH325469.1 | pEc13 | Enterobacter hormaechei | 2019 | Brazil | blaCTX-M-2, blaTEM-1A, rmtG, sul1, sul2 | ST13* |
| MH325468.1 | pEc09 | Enterobacter hormaechei | 2019 | Brazil | blaCTX-M-2, blaOXA-9, aadA1, aac(6')-Ib3, blaTEM-1A, rmtG, sul1, sul2 | ST13* |
Resistance gene content was determined by submission of the FASTA file downloaded from GenBank to ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/).
Asterisks indicate that the closest sequence type (ST) is used, as provided by the in silico pMLST tool (https://cge.cbs.dtu.dk/services/pMLST/). This was due to some allele sequences not matching at 100% nucleotide identity with the IncA/C allelic profiles currently available in the pMLST database.
Interestingly, the VIM-IncA plasmid cluster was related (average of 81 SNPs) to the pIBAC_IncA plasmid (MH594477) of C. freundii, identified in Italy in 2017. This plasmid did not carry the blaVIM-1 integron but a class I integron with aac(6′)-Ib-cr5, blaOXA-1, and catB3 gene cassettes (15).
PlasmidFinder update for IncA/C plasmids.
The PlasmidFinder database currently includes 133 unique plasmid replicon sequences. Among them, two replicon sequences are available for detecting plasmids of the IncA/C family, dividing them into IncA/C1 and IncA/C2 groups (https://cge.cbs.dtu.dk/services/PlasmidFinder/).
Three modifications of the current PlasmidFinder database are proposed here to facilitate the detection of the former IncA/C group: a change of the IncA/C_1_FJ705807 and IncA/C2_1_JN157804 names, with IncA_FJ705807 and IncC_JN157804 nomenclature, respectively, and inclusion of a new probe, named IncA_pGA_VIM, detecting at 100% nucleotide identity the new replicon variant of the VIM-1-IncA plasmids described in this study (Table S1).
DISCUSSION
The study describes a multispecies cluster of VIM-1-producing Enterobacterales identified in 2019 at the University Hospital in Rome. Bacterial and plasmid typing demonstrated that there was no transmission of bacterial clones among patients; instead, VIM-1-producing strains shared a common IncA plasmid type.
IncA plasmids are not well-known and characterized, since they have only recently been recognized as a separate incompatibility group from the related IncC type, previously together termed the IncA/C group (12). The IncC group was assigned for the first time in 1972 to the R40a (also known as pIP40a) and R57b plasmids, which were compatible with plasmids of all other known groups at that time (16). The plasmid RA1, identified in 1971, was compatible with plasmids of all Inc groups, including IncC, and was assigned to the IncA group (17). The IncA/C group was defined in 1988 because of the high DNA sequence similarity shared by the two plasmid types (18). More recently, two distinct lineages, designated A/C1 (pRA1) and A/C2, were identified (11).
A/C2 plasmids were very frequently described as being associated with MBL genes, mostly of the NDM-1 type (19). When the complete sequence of pRA1 was available (10), it was clear that the backbone of pRA1 was different from that of the A/C2 plasmids (10). The group then was reseparated into IncA and IncC groups, again demonstrating each was compatible with the other by conjugation (6). However, the two groups had completely different epidemiological success levels: it is possible to find a vast collection of fully sequenced IncC plasmids in GenBank (367 complete sequences available as of January 2020; https://www.ncbi.nlm.nih.gov/), while IncA plasmids were only sporadically reported in association with relevant resistance genes. Currently, 20 complete sequences of plasmids of this type are available (13, 14, 20).
In a recent study, IncA plasmids were demonstrated to be coresident within the same bacterial cell with plasmids carrying KPC in C. freundii isolates from Italy, sustaining the conjugation of an IncN plasmid carrying blaKPC-2 and an IncX3 plasmid carrying blaKPC-3. In the latter case, the IncA plasmid was in fusion with IncX3, which was defective in the conjugation loci. The IncA helper plasmid contributed to carbapenemase resistance, supporting conjugation of the coresident KPC-positive plasmids (15).
In the last 2 years, two highly related IncA plasmids, carrying the same resistance gene content as those described in this study, were identified in clinical strains from Italy. One was identified in K. cryocrescens (MG228427) from Bologne (13), and the other was in K. pneumoniae ST104, causing an outbreak in a neonatal intensive care unit in Naples (14). The presence of highly related plasmids in other Enterobacterales of Italian origin suggests that this plasmid is emerging in different hospitals of the country.
The blaVIM-1 gene was identified for the first time in Verona (21) and prevailed in the 2000s in Italy and Greece, being for a decade the most frequent carbapenemase in E. coli and K. pneumoniae strains (2, 22, 23), after which it was displaced by the emergence of KPC carbapenemases (4, 24, 25). The blaVIM-1 gene from Italy and Greece was mostly on In70-related integrons located on IncN-type plasmids (26).
The association of blaVIM-1, blaSHV-12, and qnrS1 genes was described for the first time in Italy in a K. oxytoca strain isolated in a tertiary-care hospital in Bolzano. All resistance determinants were located on an IncN plasmid (27). In 2016, a VIM-1 integron identical to that described in this study was identified in K. pneumoniae in river water in Switzerland, and it was located on an IncN plasmid as well (KF977034) (28). In the same year, the VIM-1 integron, associated with blaSHV-12, mph(A), and qnrS1 resistance determinants, was identified on IncY plasmids in E. coli strains isolated from retail seafood in Germany, but the contaminated Venus clam was harvested in Italy (29). The above-described reports suggest that the resistance determinants here found on the IncA plasmid were previously present in Italy, and more generally in Europe, but located on different plasmid scaffolds. We hypothesize that the acquisition of VIM-1 by the IncA plasmid opens new possibilities for a reemergence of this carbapenemase, thanks to the increased conjugation efficiency and wider host range abilities of the new plasmid. The previous localization on IncN plasmids conferred to the blaVIM-1 gene a conjugation frequency estimated by different studies to be at best around 1 × 10−4 conjugants per recipient cell (30), while IncA in this and previous studies can conjugate 100 times more frequently (10). Furthermore, IncN plasmids can conjugate in different Enterobacterales, but VIM-1-IncA plasmid was identified originally in Aeromonas spp. and spread in many different Enterobacterales, demonstrating a wider host range than IncN (13, 14).
MBLs confer resistance not only to carbapenems but also to the newest therapeutic drug, CAZ-AVI. Our hypothesis is that the increasing use of CAZ-AVI for treatment of infections sustained by KPC-producing carbapenem-resistant K. pneumoniae is favoring the emergence of the MBL producers. No VIM-1-clonal outbreak occurred in the hospital, and no secondary cases were linked to the five patients described in this study. However, the capacity of the VIM-1-IncA plasmid to spread within the gut, reaching three different bacterial species in a single patient, prompted us to suggest that, if not contained, it will cause larger diffusion of the VIM-1 carbapenemase, sustained by the increasing use of the CAZ-AVI combination drug. Thanks to this plasmid, VIM-1 can be expected to go beyond the Enterobacterales borders, causing multispecies plasmid outbreaks that are very difficult to trace and control.
MATERIALS AND METHODS
Bacterial strain isolation and susceptibility testing.
The isolation of the strains took place between January 2019 and October 2019 at the Microbiology Unit of the University Hospital in Rome. Bacteria investigated were isolated from samples processed during routine analysis, specifically rectal swabs and blood cultures. Rectal swabs were directly plated on Brilliance CRE medium plates (Oxoid Ltd.). Blood culture bottles were incubated in the automatic VirtuoBacT/Alert system (bioMérieux, Inc., Marcy l’Etoile, France). When positive for Gram-negative bacteria, aliquots of blood cultures were plated on BD Columbia agar with 5% sheep blood, MacConkey agar (Becton, Dickinson, Heidelberg, Germany), ESBL agar, and Brilliance CRE medium plates (Oxoid Ltd.). Isolated colonies from rectal swabs and blood cultures were identified by the matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) system (Bruker Daltonik GmbH, Bremen, Germany). Antimicrobial susceptibility was tested by the Vitek2 system (bioMérieux). The MICs of antibiotics were assessed by following EUCAST breakpoint tables for interpretation of MICs and zone diameters, version 9.0, valid from 2 January 2019 (http://www.eucast.org/clinical_breakpoints/; see Table S2 in the supplemental material). Etest strips (bioMérieux) were used to assess CAZ-AVI MICs.
Strains showing a carbapenem-resistant phenotype (according to EUCAST criteria; http://www.eucast.org/clinical_breakpoints/) were tested using the real-time PCR assay Xpert Carba-R kit for the GeneXpert system (Cepheid) to evaluate the presence of the blaVIM, blaIMP-1, blaKPC, blaOXA-48, and blaNDM carbapenemase genes.
Conjugation experiments.
Conjugation experiments were performed using the VIM-1-IncA K. oxytoca strain as the donor and the susceptible laboratory lactose-negative E. coli JM83 strain as the recipient. Conjugants were identified by plating 10-fold dilutions of the conjugation mixture on MacConkey agar plates with or without 100 μg/ml ampicillin. Approximately 2,000 colonies were screened for each experiment. Conjugants were recognized as lactose-negative, ampicillin-resistant colonies growing on MacConkey agar plates with 100 μg/ml ampicillin and confirmed by IncA-repA and blaVIM-1 PCRs. Mating experiments were repeated in triplicate, using 1:1, 1:1, and 1:2 donor/recipient ratios. Conjugation frequency was calculated as the ratio of the total number of lactose-negative, ampicillin-resistant colonies (CFU/ml) to the total number of lactose-negative colonies (CFU/ml) growing on MacConkey agar plates.
Whole-genome sequencing.
Genomic DNA was purified from bacterial strains using the Macherey-Nagel DNA extraction kit (Düren, Germany). Paired-end libraries were generated using the Nextera XT DNA sample preparation kit (Illumina, Inc., San Diego, CA, USA) and sequenced using the Illumina MiSeq instrument with the 2 × 300PE protocol (Illumina, Inc.). De novo assembly of Illumina reads was performed using Galaxy version 20150522 of the A5 pipeline through the ARIES public Galaxy server (https://w3.iss.it/site/aries/). Antimicrobial resistance and replicon genes were detected using the ResFinder and PlasmidFinder online tools (https://cge.cbs.dtu.dk/services/). Insertion sequences were identified by ISFinder (https://isfinder.biotoul.fr). Phage prediction was performed at the PHASTER website (https://phaster.ca/). Complete plasmid sequences were annotated using the RAST server (http://rast.nmpdr.org/).
Nanopore sequencing and assembly.
Nanopore sequencing was performed on DNA isolated from all strains described in this study using the Macherey-Nagel DNA extraction kit (Düren, Germany). About 400 ng of DNA was used for library preparation. The rapid DNA ligation kit (SQK-RBK004) from Nanopore was used to prepare the library, and the library was sequenced using R9.4.1 chemistry. Mini_assembler software from the package pomoxis (https://github.com/nanoporetech/pomoxis) was used to assemble bacterial genomes and plasmids.
Plasmid reconstruction.
Complete plasmid sequences were obtained by combining short-read/Illumina and long-read/Nanopore methods. Due to the abundance of repetitive elements, the assembly of Illumina contigs obtained by pair-end overlapping produced partial assemblies, including one large contig containing the entire plasmid scaffold, but did not resolve ambiguous repetitive element positioning. De novo assembly of long reads obtained by Nanopore technology yielded full-length, circular contigs, one for each plasmid in the genome, but with low nucleotide sequence accuracy. The combination of Illumina and Nanopore reads using hybrid assemblers did not solve all the problems of sequence accuracy and position ambiguity. The Nanopore assembly then was used as the skeleton by BLAST2N to order the Illumina partial assemblies.
IncA plasmid comparative analysis.
Cluster analysis of 20 complete IncA plasmid sequences was performed by maximum likelihood (ML) parsimony SNP analysis performed by the kSNP (Galaxy version 3.1) software at the ARIES public Galaxy server (https://w3.iss.it/site/aries/). Eighteen plasmids were selected by BLASTN with the repA gene of pGA_VIM plasmid and downloaded from GenBank. The characteristics of the plasmids listed in Table 2 were obtained by ResFinder and in silico pMLST online tools (https://cge.cbs.dtu.dk/services/). The phylogenetic tree was visualized using FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Escherichia coli and Enterobacter cloacae comparative analysis.
Cluster analysis of E. coli and E. cloacae genome sequences was performed by ML parsimony SNP analysis by the kSNP (Galaxy version 3.1) software at the ARIES public Galaxy server (https://w3.iss.it/site/aries/). E. coli strains from patients 1 (ST349), 2 (ST131), and 4 (ST6496) were compared with 3 ST349 and 3 ST131 genomes and the unique ST6496 genome available from the Enterobase database (https://enterobase.warwick.ac.uk/). E. cloacae strains from patients 2 (ST574) and 5 (ST106) were compared with the four E. cloacae ST78, ST106, ST133, and ST268 genomes, downloaded from GenBank (https://www.ncbi.nlm.nih.gov/nuccore). Since no complete genomes were available for ST574, the nearest sequence types, ST78 and ST268, were used in the comparative analysis.
Data availability.
The whole-genome project has been deposited at DDBJ/ENA/GenBank under the accession number PRJNA592166. Novel plasmid nucleotide sequences have been deposited in GenBank with the following accession numbers: pGA_VIM, MN783743; pFDL_VIM, MN783744; pFII-FIB, MN783745; pIncX1_p1, MN783746; pIron_OXY, MN783747.
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canton R, European Network on Carbapenemases, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Ø, Seifert H, Woodford N, Nordmann P. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Iacchini S, Sabbatucci M, Gagliotti C, Rossolini GM, Moro ML, Iannazzo S, D’Ancona F, Pezzotti P, Pantosti A. 2019. Bloodstream infections due to carbapenemaseproducing Enterobacteriaceae in Italy: results from nationwide surveillance, 2014 to 2017. Eurosurveillance 24:1800159. doi: 10.2807/1560-7917.ES.2019.24.5.1800159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge SR, Kwong SM, Firth N, Jensen SO. 2018. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:1–61. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrose SJ, Harmer CJ, Hall RM. 2018. Plasmid compatibility and entry exclusion of IncA and IncC plasmids revisited: IncA and IncC plasmids are compatible. Plasmid 96–97:7–12. doi: 10.1016/j.plasmid.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Carattoli A. 2013. Plasmids and the spread of resistance. Int J Med Microbiol 303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Peirano G, Bradford PA, Motyl MR, DeVinney R, Pitout J. 2018. Genomic characterization of IMP and VIM carbapenemase-encoding transferable plasmids of Enterobacteriaceae. J Antimicrob Chemother 73:3034–3038. doi: 10.1093/jac/dky303. [DOI] [PubMed] [Google Scholar]
- 9.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricke WF, Welch TJ, Mcdermott PF, Mammel MK, Leclerc JE, White DG, Cebula TA, Ravel J. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol 191:4750–4757. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carattoli A, Miriagou V, Bertini A, Loli A, Colinon C, Villa L, Whichard JM, Rossolini GM. 2006. Replicon typing of plasmids encoding resistance to newer β-lactams. Emerg Infect Dis 12:1145–1148. doi: 10.3201/eid1207.051555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmer CJ, Hall RM. 2015. The A to Z of A/C plasmids. Plasmid 80:63–82. doi: 10.1016/j.plasmid.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Gaibani P, Ambretti S, Scaltriti E, Cordovana M, Berlingeri A, Pongolini S, Landini MP, Re MC. 2018. A novel IncA plasmid carrying bla VIM-1 in a Kluyvera cryocrescens strain. J Antimicrob Chemother 73:9–11. doi: 10.1093/jac/dky304. [DOI] [PubMed] [Google Scholar]
- 14.Esposito EP, Gaiarsa S, Del Franco M, Crivaro V, Bernardo M, Cuccurullo S, Pennino F, Triassi M, Marone P, Sassera D, Zarrilli R. 2017. A novel IncA/C1 group conjugative plasmid, encoding VIM-1 metallo-beta-lactamase, mediates the acquisition of carbapenem resistance in ST104 Klebsiella pneumoniae isolates from neonates in the intensive care unit of V. Monaldi Hospital in Naples. Front Microbiol 8:1–12. doi: 10.3389/fmicb.2017.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bitar I, Caltagirone M, Villa L, Mattioni Marchetti V, Nucleo E, Sarti M, Migliavacca R, Carattoli A. 25 April 2019. Interplay among IncA and blaKPC-carrying plasmids in Citrobacter freundii. Antimicrob Agents Chemother doi: 10.1128/AAC.02609-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta N, Hedges RW. 1972. R factors identified in Paris, some conferring gentamicin resistance, constitute a new compatibility group. Ann Inst Pasteur 123:849–852. [PubMed] [Google Scholar]
- 17.Hedges RW, Datta N. 1973. R factors of compatibility group A. J Gen Microbiol 74:335–336. doi: 10.1099/00221287-74-2-335. [DOI] [PubMed] [Google Scholar]
- 18.Couturier M, Bex F, Bergquist PL, Maas WK. 1988. Identification and classification of bacterial plasmids. Microbiol Rev 52:375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zhong Z. 2019. NDM metallo-β -lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggiero M, Girlich D, Dabos L, Power P, Naas T, Gutkind G. 2018. Complete sequence of the IncA/C 1 plasmid pCf587 carrying blaPER-2 from Citrobacter freundii. Antimicrob Agents Chemother 62:1–6. doi: 10.1128/AAC.00006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. 1999. Cloning and characterization of bla(VIM), a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother 43:1584–1590. doi: 10.1128/AAC.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikonomidis A, Tokatlidou D, Kristo I, Sofianou D, Tsakris A, Mantzana P, Pournaras S, Maniatis AN. 2005. Outbreaks in distinct regions due to a single Klebsiella pneumoniae clone carrying a bla VIM-1 metallo-β-lactamase gene. J Clin Microbiol 43:5344–5347. doi: 10.1128/JCM.43.10.5344-5347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vatopoulos A. 2008. High rates of metallo-beta-lactamase-producing Klebsiella pneumoniae in Greece–a review of the current evidence. Euro Surveill 13:8023. doi: 10.2807/ese.13.04.08023-en. [DOI] [PubMed] [Google Scholar]
- 24.Giani T, D'Andrea MM, Pecile P, Borgianni L, Nicoletti P, Tonelli F, Bartoloni A, Rossolini GM. 2009. Emergence in Italy of Klebsiella pneumoniae sequence type 258. J Clin Microbiol 47:3793–3794. doi: 10.1128/JCM.01773-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conte V, AR-ISS Study Group on Carbapenemase-Producing K. pneumoniae, Monaco M, Giani T, D'Ancona F, Moro ML, Arena F, D'Andrea MM, Rossolini GM, Pantosti A. 2016. Molecular epidemiology of KPC-producing Klebsiella pneumoniae from invasive infections in Italy: increasing diversity with predominance of the ST512 clade II sublineage. J Antimicrob Chemother 71:3386–3391. doi: 10.1093/jac/dkw337. [DOI] [PubMed] [Google Scholar]
- 26.Miriagou V, Papagiannitsis CC, Kotsakis SD, Loli A, Tzelepi E, Legakis NJ, Tzouvelekis LS. 2010. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the bla VIM-1 metallo-β-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4497–4502. doi: 10.1128/AAC.00665-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carattoli A, Aschbacher R, March A, Larcher L, Livermore DM, Woodford N. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the USA. J Antimicrob Chemother 65:2070–2075. doi: 10.1093/jac/dkq269. [DOI] [PubMed] [Google Scholar]
- 28.Zurfluh K, Power KA, Klumpp J, Wang J, Fanning S, Stephan R. 2015. A novel Tn 3-like composite transposon harboring blaVIM-1 in Klebsiella pneumoniae spp. pneumoniae isolated from river water. Microb Drug Resist 21:43–49. doi: 10.1089/mdr.2014.0055. [DOI] [PubMed] [Google Scholar]
- 29.Roschanski N, Guenther S, Vu TTT, Fischer J, Semmler T, Huehn S, Alter T, Roesler U. 2017. VIM-1 carbapenemase-producing Escherichia coli isolated from retail seafood, Germany 2016. Eurosurveillance 22:1–7. doi: 10.2807/1560-7917.ES.2017.22.43.17-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert T, Gerbaud GUY, Courvalin P. 1994. Characterization of transposon Tn1528, which confers amikacin resistance by synthesis of aminoglycoside 3′-O-phosphotransferase type VI. Antimicrob Agents Chemother 38:702–706. doi: 10.1128/aac.38.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole-genome project has been deposited at DDBJ/ENA/GenBank under the accession number PRJNA592166. Novel plasmid nucleotide sequences have been deposited in GenBank with the following accession numbers: pGA_VIM, MN783743; pFDL_VIM, MN783744; pFII-FIB, MN783745; pIncX1_p1, MN783746; pIron_OXY, MN783747.