Chagas disease remains neglected, and current chemotherapeutics present severe limitations. Lychnopholide (LYC) at low doses loaded in polymeric poly(d,l-lactide)-block-polyethylene glycol (PLA-PEG) nanocapsules (LYC-PLA-PEG-NC) exhibits anti-Trypanosoma cruzi efficacy in mice infected with a partially drug-resistant strain. This study reports the efficacy of LYC-PLA-PEG-NC at higher doses in mice infected with a T. cruzi strain resistant to benznidazole (BZ) and nifurtimox (NF) treated at both the acute phase (AP) and the chronic phase (CP) of infection by the oral route.
KEYWORDS: lychnopholide, nanocapsules, Trypanosoma cruzi, oral treatment, acute phase, chronic phase
ABSTRACT
Chagas disease remains neglected, and current chemotherapeutics present severe limitations. Lychnopholide (LYC) at low doses loaded in polymeric poly(d,l-lactide)-block-polyethylene glycol (PLA-PEG) nanocapsules (LYC-PLA-PEG-NC) exhibits anti-Trypanosoma cruzi efficacy in mice infected with a partially drug-resistant strain. This study reports the efficacy of LYC-PLA-PEG-NC at higher doses in mice infected with a T. cruzi strain resistant to benznidazole (BZ) and nifurtimox (NF) treated at both the acute phase (AP) and the chronic phase (CP) of infection by the oral route. Mice infected with the T. cruzi VL-10 strain were treated by the oral route with free LYC (12 mg/kg of body weight/day), LYC-PLA-PEG-NC (8 or 12 mg/kg/day), or BZ at 100 mg/kg/day or were not treated (controls). Treatment efficacy was assessed by hemoculture (HC), PCR, enzyme-linked immunosorbent assay (ELISA), heart tissue quantitative PCR (qPCR), and histopathology. According to classical cure criteria, treatment with LYC-PLA-PEG-NC at 12 mg/kg/day cured 75% (AP) and 88% (CP) of the animals, while at a dose of 8 mg/kg/day, 43% (AP) and 43% (CP) were cured, showing dose-dependent efficacy. The negative qPCR results for heart tissue and the absence of inflammation/fibrosis agreed with the negative results obtained by HC and PCR. Thus, the mice treated with the highest dose could be considered 100% cured, in spite of a low ELISA reactivity in some animals. No cure was observed in animals treated with free LYC or BZ or the controls. These results are exceptional in terms of experimental Chagas disease chemotherapy and provide evidence of the outstanding contribution of nanotechnology in mice infected with a T. cruzi strain totally resistant to BZ and NF at both phases of infection. Therefore, LYC-PLA-PEG-NC has great potential as a new treatment for Chagas disease and deserves further investigations in clinical trials.
INTRODUCTION
Chagas disease (CD) is an important neglected disease caused by Trypanosoma cruzi, an intracellular hemoflagellate protozoan parasite. WHO estimated that 8 million people worldwide are infected, leading to 12,500 deaths per year, mostly in Latin America (1). CD is mainly transmitted to humans by triatomine insect vectors, blood transfusion, transplantation of infected organs, congenital transmission, or ingestion of contaminated food (2). CD chemotherapy is based on two drugs: nifurtimox (NF) and benznidazole (BZ). However, their efficacies vary depending on the genetic variability of the parasite, treatment schedules, time of infection (acute and recent infection versus later chronic infection), and host immune status (3–5). Unfortunately, both drugs induce serious adverse effects (6). Nowadays, CD occurs in other countries because human migration has led to new cases in countries where the disease is not endemic via transmission mechanisms independent of the triatomine insect vectors. Consequently, this infection is no longer considered exclusive to the Americas (1).
The search for new chemical entities as lead compounds or drug candidates in the short- and medium-term attempts to treat CD is absolutely necessary. Besides, new technological strategies, such as the use of new formulations, are of increasing importance in lead optimization, particularly to improve in vivo pharmacokinetics (7, 8). The recently published results of clinical trials demonstrated that the most promising synthetic azole derivative (posaconazole) and E1224 (a prodrug of ravuconazole) were less active as chemotherapeutic agents in humans than in preclinical studies in animal models (9, 10).
In addition, 50 to 70% of all chemotherapeutic agents currently in clinical use were originally from natural sources (11). Thus, the use of natural products in Chagas disease chemotherapy has received increased attention. Studies using substances isolated from the Asteraceae family, which demonstrated anti-T. cruzi activity, were previously performed in vitro by de Oliveira et al. (12) and Chiari et al. (13). Lychnopholide (LYC) is a lipophilic sesquiterpene lactone (SL) isolated from Lychnophora trichocarpha (Spreng.) of the Asteraceae family and was extensively investigated in vivo by our team in the context of experimental CD chemotherapy. Robust data show that the efficacy of LYC against murine T. cruzi infection was improved by encapsulation in biodegradable poly(d,l-lactide) (PLA)-block-polyethylene glycol (PEG) and poly(ε-caprolactone) (PCL) nanocapsules (NC) in the acute and chronic phases, when administered by the oral and intravenous routes (14, 15). The higher cure rates obtained with LYC loaded in polymeric NC stabilized by PLA-PEG (LYC-PLA-PEG-NC) than with LYC loaded in polymeric NC stabilized by PCL (LYC-PCL-NC) may have been due to the smaller particle size and the better steric stabilization of the nanoparticle surface provided by the hydrophilic PEG segment of the PLA-PEG diblock copolymer than those provided by the more hydrophobic PCL polymer. Furthermore, LYC in PLA-PEG NC led to LYC plasma concentration-time profiles higher than those obtained with free LYC and PCL NC. The NC improved dramatically the body exposure to LYC (they produced a higher area under the curve [AUC]), and LYC clearance (12.4-fold) was drastically reduced when it was loaded in PLA-PEG NC (16). As body exposure to LYC increases when LYC is administered in the NC dosage form, a detailed investigation of the potential side effects of LYC on the cardiovascular system during long-term (120 days) administration of free LYC was performed (17). While free LYC showed cardiotoxicity in mice, the association of LYC with biodegradable NC abolished these adverse cardiac effects. Altogether, these results indicate not only that LYC in NC is able to induce a cure in animals (14, 15) but also that it is safer for the cardiovascular system (17).
Following up on these excellent results, this study reports the efficacy of oral LYC (free LYC) and LYC loaded in PLA-PEG NC (LYC-PLA-PEG-NC) at doses higher than those previously studied for the treatment of mice experimentally infected with a T. cruzi strain totally resistant to BZ and NF in the acute and chronic phases of the infection.
RESULTS
LYC was prepared, characterized, and loaded in PLA-PEG NC with a 99% entrapment efficiency, as described by Branquinho et al. (16). The mean hydrodynamic diameter of LYC-PLA-PEG-NC was 107 ± 8 nm, with a polydispersity index (PdI) lower than 0.3 and a zeta potential of −31 ± 8 mV. No signs of general toxicity were observed upon administration by oral gavage, according to the behavior and clinical or physical aspects of the animals treated with the formulations containing LYC (free LYC, LYC-PLA-PEG-NC) or the mice receiving the control formulations, i.e., mice that were infected and not treated (INT), mice that were infected and treated with the excipients of the free LYC dispersion, and mice that received blank NC.
Animals treated during the acute phase.
All treatments were well tolerated during the 20 days of treatment. Animals infected and treated with oral free LYC at 12 mg/kg of body weight/day and LYC-PLA-PEG-NC at 8 mg/kg/day showed similar levels of parasitemia (P > 0.05), evaluated from the area under the curve (AUC) (Fig. 1A and B), with a significant reduction in the level of parasitemia being seen in the treated mice compared to that seen in the control groups (Fig. 1A and B). The group treated with LYC-PLA-PEG-NC at 12 mg/kg/day presented an even more significant reduction in parasitemia (AUC) than all other infected groups (the groups treated with free LYC and LYC-PLA-PEG-NC at 8 mg/kg/day and the INT and blank NC groups) and showed levels of parasitemia similar to those in mice treated with BZ (Fig. 1A and B). No significant differences between treatment with BZ at 100 mg/kg/day and treatment with LYC-PLA-PEG-NC at 12 mg/kg/day were observed in terms of parasitemia (Fig. 1A and B). Animals treated with LYC-PLA-PEG-NC at 12 mg/kg/day and 8 mg/kg/day showed 100% and 50% suppression of parasitemia, respectively, which became subpatent during and after treatment, which is different from the findings for the animals treated with free LYC and BZ, which maintained patent parasitemia (Fig. 1B). Free LYC at 12 mg/kg/day reduced the level of parasitemia and increased the rate of survival compared to those seen for the other control groups.
FIG 1.
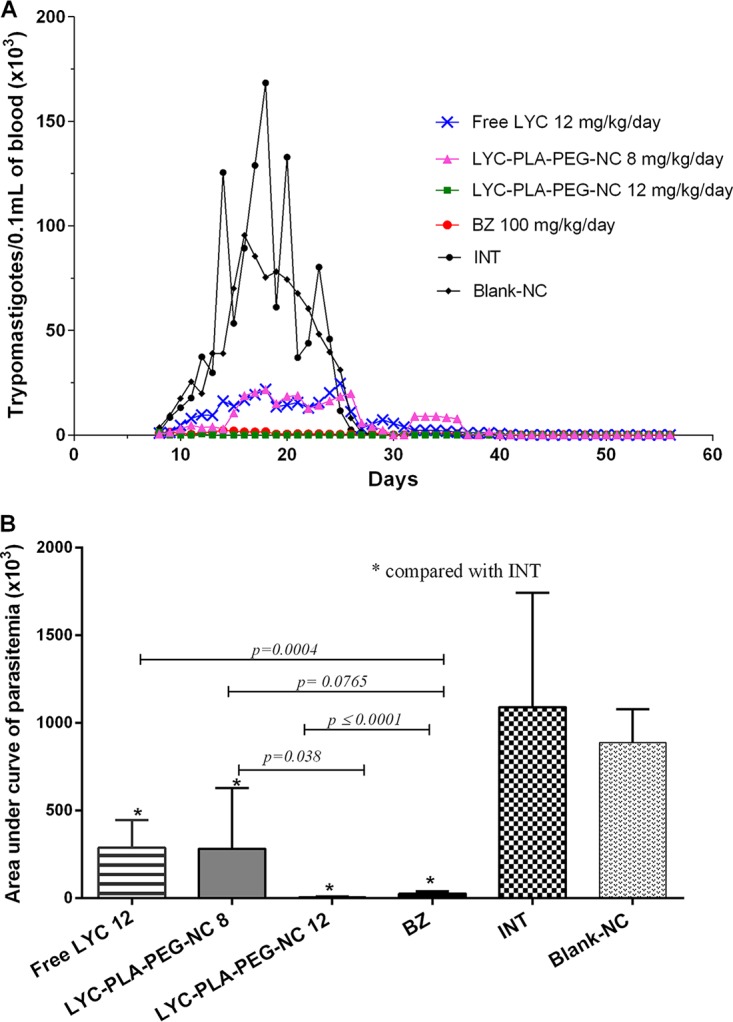
Parasitemia curve (A) and area under the curve of parasitemia (B) for mice infected with the Trypanosoma cruzi VL-10 strain and treated with lychnopholide in nanocapsules during the acute phase. Free LYC was administered at 12 mg/kg/day, LYC-PLA-PEG-NC was administered at 8 and 12 mg/kg/day, and benznidazole (BZ) was administered at 100 mg/kg/day. The control groups consisted of mice that were infected and not treated (INT), mice that received DMA-PEG solution, and mice that received blank NC. All animals were treated by oral gavage daily for 20 consecutive days, which started in the patent period, which was on the 9th day after infection.
Posttreatment evaluations and therapeutic efficacy. (i) Animals treated during the acute phase.
Treatment efficacy was evaluated by fresh blood examination (FBE), hemoculture (HC), PCR of blood eluate, and enzyme-linked immunosorbent assay (ELISA) and interpreted according to the combined results of all laboratory evaluations or the classic cure criterion (18) in animals treated with the different formulations (Table 1).
TABLE 1.
Posttreatment evaluations and efficacy of oral treatment with LYC formulations in mice infected with Trypanosoma cruzi VL-10 in acute phase of infection
| Experimental group | Dose (mg/kg/day) | % of animals (no. of animals with the indicated outcome or result/total no. tested) |
||||||
|---|---|---|---|---|---|---|---|---|
| Survival | Negative laboratory tests |
Cured by classic criterion | Negative heart tissue qPCRe | |||||
| FBEa | HCb | PCRc | ELISAd | |||||
| Free LYC | 12 | 87.5 (7/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | |
| LYC-PLA-PEG-NC | 8 | 87.5 (7/8) | 62.5 (5/8) | 100 (3/3) | 100 (3/3) | 42.8 (3/7) | 42.8 (3/7) | 57.1 (4/7) |
| LYC-PLA-PEG-NC | 12 | 100 (8/8) | 100 (8/8) | 100 (8/8) | 100 (8/8) | 75 (6/8) | 75 (6/8) | 100 (8/8) |
| BZ | 100 | 87.5 (7/8) | 0 (8/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) |
| INTf | 87.5 (6/7) | 0 (0/7) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/7) | 0 (0/8) | |
| Blank NC | —g | 87.5 (7/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) |
FBE was performed during treatment and at 30 d.p.t.
HC was performed at 30, 60, 90, and 120 d.p.t.
PCR was performed at 60 and 90 d.p.t.
ELISA was performed at 90 and 180 d.p.t.
qPCR was performed at 180 d.p.t.
INT, infected and not treated.
—, the amounts of excipients used in blank NC were the same as the amounts used in the LYC-loaded NC formulation.
Animals in the groups treated with LYC-PLA-PEG-NC at 8 mg/kg/day and 12 mg/kg/day during the acute phase showed cure rates of 42.8% (3/7) and 75.0% (6/8), respectively, revealing dose-dependent efficacy. No animals (0%) treated with BZ or free LYC and none of the animals in the control groups (the INT and blank NC groups) were cured. The animal survival rates were 87.5% until necropsy at 180 days posttreatment (d.p.t.) for all groups. On the other hand, 100% (8/8) of the mice treated with LYC-PLA-PEG-NC at 12 mg/kg/day survived (Table 1). Negative FBE, HC, and PCR test results during and after treatment (Table 1) for the group treated with LYC-PLA-PEG-NC at 12 mg/kg/day already indicated the cure of these animals according to the criteria established by Filardi and Brener (3) and Toledo et al. (19).
Evaluation of tissue parasitism by quantitative PCR (qPCR) of heart tissue samples showed that all animals infected and treated with LYC-PLA-PEG-NC at 12 mg/kg/day were negative (8/8), while 57.1% (4/7) of the mice treated with LYC-PLA-PEG-NC at 8 mg/kg/day were negative (Table 1). The two animals in the group treated with LYC-PLA-PEG-NC at 12 mg/kg/day and the one animal in the group treated with LYC-PLA-PEG-NC at 8.0 mg/kg/day that consistently showed negative results by FBE, HC, and PCR and a positive ELISA result (Table 1) were also negative by the heart tissue qPCR and were considered cured, according to the results of parasitological tests.
(ii) Animals treated during the chronic phase.
Treatment efficacy was evaluated on the basis of HC, PCR, and ELISA results and was also interpreted according to the classic cure criterion. The cure rates were 87.5% (7/8) and 42.8% (3/7) for the groups of animals infected and treated with LYC-PLA-PEG-NC at 12 mg/kg/day and 8 mg/kg/day, respectively (Table 2). No animals (0%) treated with BZ (100 mg/kg/day) or free LYC (12 mg/kg/day) and none of the control animals (those in the INT and blank NC groups) were cured (Table 2). The rate of survival until necropsy (180 d.p.t.) of animals treated with LYC-PLA-PEG-NC at 12 mg/kg/day and BZ at 100 mg/kg/day was 80%, that of animals treated with free LYC at 12 mg/kg/day and LYC-PLA-PEG-NC at 8 mg/kg/day was 70%, and that of animals in the other control groups was 60%.
TABLE 2.
Posttreatment evaluations and efficacy of oral treatment with LYC formulations and BZ in mice infected with Trypanosoma cruzi VL-10 in chronic phase of infection
| Experimental group | Dose (mg/kg/day) | % of animals (no. of animals with the indicated outcome or result/total no. tested) |
|||||
|---|---|---|---|---|---|---|---|
| Survival | Negative laboratory tests |
Cured by classic criterion | Negative heart tissue qPCRd | ||||
| HCa | PCRb | ELISAc | |||||
| Free LYC | 12 | 70 (7/10) | 14.3 (1/7) | 57.1 (4/7) | 0 (0/7) | 0 (0/7) | 16.7 (1/6) |
| LYC-PLA-PEG-NC | 8 | 70 (7/10) | 86 (6/7) | 71 (5/7) | 42.8 (3/7) | 42.8 (3/7) | 42.8 (3/7) |
| LYC-PLA-PEG-NC | 12 | 80 (8/10) | 100 (8/8) | 100 (8/8) | 87.5 (7/8) | 87.5 (7/8) | 100 (8/8) |
| BZ | 100 | 80 (8/10) | 100 (8/8) | 75 (6/8) | 0 (0/8) | 0 (0/8) | 0 (0/8) |
| INTe | 60 (6/10) | 100 (6/6) | 50 (3/6) | 0 (0/6) | 0 (0/6) | 0 (0/8) | |
| Blank NC | —f | 60 (6/10) | 100 (7/7) | 71 (5/7) | 0 (0/7) | 0 (0/8) | 0 (0/8) |
HC was performed at 30, 60, 90, and 120 d.p.t.
PCR was performed at 60 and 90 d.p.t.
ELISA was performed at 90 and 180 d.p.t.
qPCR was performed at 180 d.p.t.
INT, infected and not treated.
—, the amounts of excipients used in blank NC were the same as the amounts used in the LYC-loaded NC formulation.
The results of qPCR analyses of heart tissue samples from the animals infected and treated during the chronic phase are shown in Table 2. All (100%) of the animals in the group treated with LYC-PLA-PEG-NC at 12 mg/kg/day were negative, while 42.8% of the animals treated with LYC-PLA-PEG-NC at 8 mg/kg/day were negative. Only one animal (16.7%) treated with free LYC at 12 mg/kg/day was qPCR negative. No animals in the BZ and INT groups were qPCR negative. Again, only two animals in the group treated with LYC-PLA-PEG-NC at 12 mg/kg/day and one animal in the group treated with free LYC at 12 mg/kg/day showed negative results by HC and PCR but positive results by ELISA (Table 2) and were also negative by the heart tissue qPCR. These animals were considered cured according to the results of parasitological tests.
Histopathological evaluation.
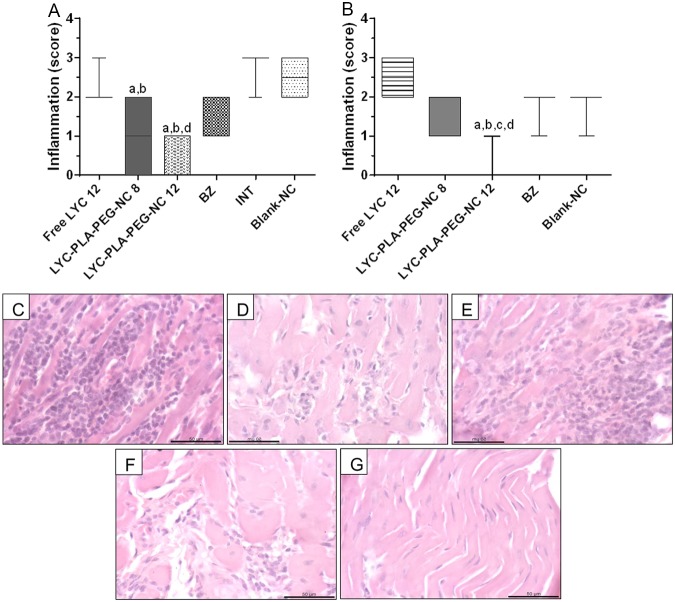
The animals treated during the acute phase of infection with LYC-PLA-PEG-NC at a dose of either 8 or 12 mg/kg/day showed a significant reduction of the inflammatory process compared to that seen in the animals in the INT and blank NC groups. The inflammatory process was absent in animals treated with LYC-PLA-PEG-NC at 12 mg/kg/day and was moderate in those treated with free LYC at the same dose (Fig. 2). Histopathology examination during the chronic phase of the animals treated with LYC-PLA-PEG-NC at 12 mg/kg/day showed no inflammatory process in the heart, in contrast to the findings for all the other groups (Fig. 2).
FIG 2.
Evaluation of the cardiac inflammatory process in mice infected with the Trypanosoma cruzi VL-10 strain and treated with lychnopholide in nanocapsules during the acute and chronic phases. (A and B) Inflammation scores obtained by histological analysis during treatment in the acute phase (A) and the chronic phase (B) of infection. Data are presented as the mean value ± SD. (C to H) Photomicrographs obtained during the acute phase analyzed with H&E staining, showing an intense inflammatory process in the group that was infected and not treated (INT) and the group that received blank NC (C), a discrete inflammatory process in the majority of animals treated with BZ (D), a moderate inflammatory process in most animals treated with free LYC (E), a discrete inflammatory process in the group treated with LYC-PLA-PEG-NC at 8 mg/kg/day (F), and the absence of an inflammatory process in animals treated with LYC-PLA-PEG-NC at 12 mg/kg/day (G). Bar = 50 μm. The letters a, b, c, and d in panel A represent significant differences between the INT, blank NC, BZ, and free LYC groups, respectively, in the acute phase. The letters a, b, c, and d in panel B represent significant differences between the groups receiving blank NC, BZ, free LYC, and LYC-PLA-PEG-NC at 8 mg/kg/day, respectively, in the chronic phase.
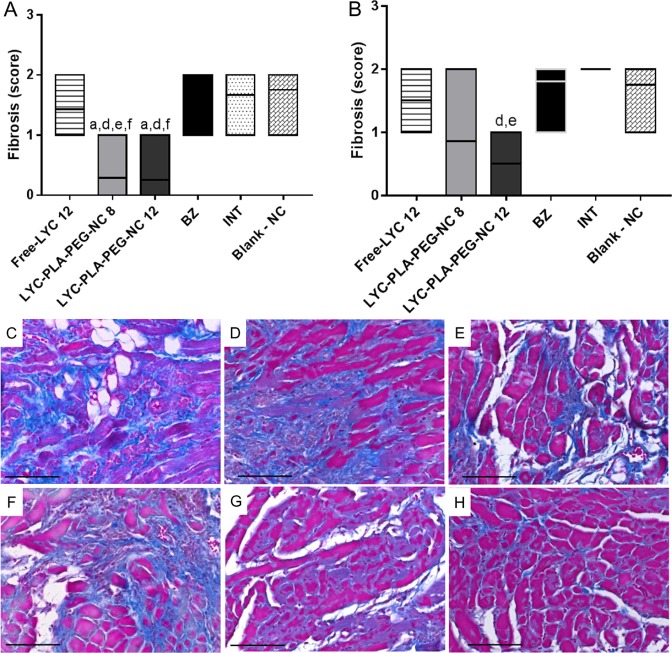
Fibrosis of animals treated in the acute phase with LYC-PLA-PEG-NC at 8 and 12 mg/kg/day presented lower scores (Fig. 3) compared to the other groups. In the chronic phase, reduction of cardiac fibrosis (Fig. 3) was observed only in the animals of the group treated with LYC-PLA-PEG-NC at 12 mg/kg/day when compared to the animals treated with BZ and blank NC.
FIG 3.
Evaluation of cardiac inflammatory fibrosis in mice infected with the Trypanosoma cruzi VL-10 strain and treated orally with lychnopholide in nanocapsules during the acute and chronic phases. (A and B) Fibrosis scores obtained by histological analysis during treatment in the acute phase (A) and the chronic phase (B) of infection. Data are presented as the mean value ± SD. (C to H) Photomicrographs obtained during the acute phase analyzed by Masson trichrome staining, showing moderate fibrosis in the group infected and not treated (INT) (C) and the groups receiving blank NC (D), BZ (E), and free LYC (F) and a discrete fibrosis process in the majority of animals treated with LYC-PLA-PEG-NC at 8 mg/kg/day (G) and with LYC-PLA-PEG-NC at 12 mg/kg/day (H). Bar = 50 μm. The letters a, d, e, and f in panel A represent significant differences between the free LYC-treated, BZ-treated, INT, and blank NC groups, respectively, in the acute phase. The letters d and e in panel B represent significant differences compared with the results for the BZ-treated and INT groups, respectively, in the chronic phase.
DISCUSSION
The search for new therapeutic alternatives against Chagas disease is strongly justified, considering the difficulties and complexity found in the treatment of Chagas disease; the low therapeutic efficacy of the existing drugs against intracellular parasites, mainly during the chronic phase (20); the natural resistance of T. cruzi strains to treatment (3); the toxicity of the current drugs active against T. cruzi (21); the beneficial action of the treatment in the lesions (22, 23); and the clinical evolution of the disease, even in treated patients (24).
In this critical scenario, natural substances represent a rich source of new compounds to be explored. Regarding trypanosomiases (25) and, specifically, Chagas disease, several sesquiterpene lactones have been demonstrated to be active in vitro and in vivo (26–30).
In this context, our team has brought significant advances with LYC, achieving high therapeutic efficacy against T. cruzi in experimental infections, particularly when LYC is loaded in biodegradable polymeric PLA-PEG NC (15). These NC improved the pharmacokinetics of LYC and increased its therapeutic efficacy in a murine model of Chagas disease (16), even when it was given by the oral route, protecting the host against the cardiotoxicity of LYC (17).
Therefore, the present study evaluated the therapeutic efficacy of LYC-PLA-PEG-NC administered by the oral route in animals infected with a T. cruzi strain 100% resistant to conventional treatments (the VL-10 strain), using doses of LYC higher than those used in previous work in the treatment of the acute and chronic phases of the infection.
Oral administration of LYC in PLA-PEG NC during the acute phase in mice infected with the VL-10 strain had a significant impact on the level of parasitemia compared to that seen in all other experimental groups. Animals that received the highest dose (12 mg/kg/day) presented the largest reduction of parasitemia, with the administration of only two doses being required for its suppression in 100% of the animals during and after treatment. Besides, following treatment, the results of HC and PCR performed at 90 days postinfection were also negative for these animals, showing cure (3, 19). Treatment with LYC-PLA-PEG-NC at 8 mg/kg/day and BZ also led to a significant reduction in the level of parasitemia compared to that seen in mice treated with free LYC and mice in the control groups. Free LYC also reduced the level of parasitemia compared to that in the other control groups.
HC, blood PCR, and ELISAs indicated that the animals in the group treated with the highest dose of LYC-PLA-PEG-NC had a higher cure rate than animals treated with the lowest dose, which indicated an LYC dose-dependent effect in vivo during the acute phase of infection with a T. cruzi strain resistant to conventional drugs. Animals treated with free LYC and BZ and the control animals were not cured. Furthermore, there was a clear gain in efficacy when LYC was associated with NC compared with the efficacy of free LYC at the same dose. The 100% tissue qPCR negativity for animals treated with the highest dose of LYC, including mice that remained reactive by ELISA, indicates that the parasitological healing of these animals was 100% during the acute phase of infection. This parasitological cure was reinforced by the absence of tissue parasitism, inflammation, and, consequently, cardiac fibrosis. It is well-known that conventional serology tests remain positive for long periods following treatment. The residual reactivity by ELISA in some animals may have mainly been due to memory antibodies, which have been found to persist for many years posttreatment in humans (31, 32) and at least for 6 to 10 months in treated mice (33), due to the existence of antigens in the spleen representing a continuous stimulation of long-lasting memory antibodies and several other reasons, as reviewed by Krettli (34).
All animals in the groups treated with free LYC at 12 mg/kg/day and BZ at 100 mg/kg/day presented positive results by qPCR of cardiac tissues, as did the animals from the control groups, and the overall results indicate the persistence of the infection in all animals. Caldas et al. (35) also evaluated by qPCR cardiac tissue from mice infected with the T. cruzi VL-10 strain during the acute phase and treated with BZ, itraconazole, posaconazole, and fexinidazole. Fexinidazole at a dose of 300 mg/kg/day led to negative qPCR results in 70% of the animals, which was not observed with a lower dose or with the other compounds cited.
The total cure (100%) obtained with LYC at 12 mg/kg/day in NC in the acute phase was probably achieved because the parasitemia was suppressed in all animals from the 2nd day of treatment, accompanied by 100% negative results by successive HC and PCR of blood eluates. This finding is not surprising, considering that negative results by evaluation by HC can be considered a cure criterion in the mouse model (3) because this technique is highly sensitive in mice evaluated during the acute phase, while during the chronic phase, more sensitive evaluations, such as PCR, are required (19). In this work, only animals treated with LYC-PLA-PEG-NC at the highest dose during the acute phase were 100% negative or cured, based on the HC results. Negative HC results in 100% of the animals treated with LYC-PLA-PEG-NC at the lower dose were also observed, but qPCR of cardiac tissue was negative for only 57.1% of the mice. The higher sensitivity of qPCR than of HC to identify the DNA of the parasite during the chronic phase has been well documented by several authors.
Our results, showing the cure of acute infection caused by T. cruzi parasites resistant to the conventional drugs, are original in the literature and are in contrast to the results obtained with all available drugs (BZ and NF) and other drugs in investigational phases, such as fexinidazole and E1224 (3, 7, 52).
Publications reporting the use of nanotechnology for the treatment of Chagas disease have been rare to date. Abriata et al. (36) carried out an in vivo study in mice infected with the Y strain, which is partially resistant to treatment, and treated with ursolic acid loaded in nanoparticles by the oral route. They found that the treatment had efficacy similar to that of BZ in controlling parasitemia and that the ursolic acid loaded in nanoparticles produced a better reduction in parasitemia than free ursolic acid. They attributed this effect to an improvement of the bioavailability of the encapsulated drug. Molina et al. (37) showed that DO870 used at a dose of 3 mg/kg/day loaded in PLA-PEG nanospheres and administered by the intravenous route produced a cure rate of 60% in animals infected with the Y strain. The nanospheres used may also have played a role in the increased efficacy of D0870.
The fast degradation of LYC was reported in mouse plasma, particularly after 3 h of incubation, but this fast degradation was prevented and retarded by its encapsulation in polymeric NC (16). In fact, LYC-PLA-PEG-NC formulations significantly decreased the rate of LYC degradation for up to 24 h. Binding of LYC to plasma proteins of mice probably occurs, and this plays a role in LYC efficacy in vivo, as recently reported for rat plasma (38). The protection of LYC by encapsulation is likely to occur even by oral administration, as suggested by the efficacy of this formulation in vivo, and this protection contributes to considerable improvements to the absorption, distribution, metabolism, and excretion (ADME) properties of this molecule. Our previous pharmacokinetic study showed that following intravenous administration, free LYC was rapidly cleared from the blood and had a short half-life (21 min) compared to that of LYC-PLA-PEG-NC, which showed a half-life of 538 min in mice when the same LYC doses were used (16).
The data presented here corroborate the findings of our earlier studies of the increased efficacy of LYC-PLA-PEG-NC compared to that of free LYC, which found rates of cure of 100% and 0% in mice infected with the CL strain (which is sensitive to treatment) and the Y strain (which is partially resistant to treatment), respectively, during the acute phase. The properties of the NC obtained from the diblock PLA-PEG polymer (39), showing its long-term circulation in the bloodstream, are convenient during the acute phase of Chagas disease, where T. cruzi trypomastigotes are present in the blood circulation.
The animals infected and treated with LYC-PLA-PEG-NC at 12 and 8 mg/kg/day during the chronic phase of infection showed rates of cure of 87.5% and 43.0%, respectively. Again, qPCR of heart tissue from mice treated with LYC-PLA-PEG-NC at the higher dose was negative for all (100%) of the mice, in agreement with previous FBE, HC, and blood PCR results, and these findings together indicate 100% parasitological cure.
The cardiac histopathological results for these animals also revealed the total absence of parasites and lesions after treatment with NC at the highest dose. Considering the previously reported anti-inflammatory activity of LYC (40), a combination of both effects may be involved in the absence of inflammatory processes in tissue.
To improve the precision in detecting parasites remaining in the mouse body (in blood and tissues) after treatment during the acute phase, we performed qPCR of blood and cardiac tissue and cardiac histopathology. The VL-10 strain, which is 100% resistant to BZ, is cardiomyotropic, and the infection naturally evolves to the chronic phase in mice (3, 41). Thus, our experimental design requires no immunosuppression in the protocol during the chronic phase but is more stringent in other aspects for the evaluation of cure.
It is known that during the acute phase of infection, T. cruzi amastigote nests are found in abundance in a wide variety of tissues. In contrast, during the chronic phase of infection, the level of parasitemia is very low and parasites are restricted to muscle tissue (8), mainly cardiac tissue. Then, during the chronic phase of infection, the strategy is to achieve the amastigote forms of the parasite inside the cell, using small, long-circulating nanocarriers (NC), and, additionally, in the interstitial matrix, where amastigotes or trypomastigotes derived from them are released. This approach takes advantage of the endothelial leakage of inflamed tissues, which results in nanoparticle perfusion to the extravascular region (8). Long-circulating NC may exhibit this property because the PLA-PEG polymer increases its ability to circulate longer and to extravasate to inflamed tissue, where the parasites are located after they are released from host cells. Furthermore, these NC have a small hydrodynamic diameter (approximately 100 nm), which could facilitate their passage through the fenestrate spaces of the endothelium of leaky vessels.
No study of the biodistribution of the lychnopholide molecule is available to date, so its special tropism or target tissue is unknown. T. cruzi parasites have a tropism for muscular cells, where they induce inflammation and fibrosis, which lead to irreversible damage. Targeting of muscular tissues is a challenge. However, when inflammation is established, as in chronic Chagas disease, nanoparticles that circulate longer in the bloodstream and that very slowly release their cargos have a chance to extravasate in particular sites with a leaky vasculature, such as infected, tumoral, and inflamed tissues.
LYC is a very lipophilic molecule and in its free form has a short half-life in mice (21 min when given via the intravenous route). Thus, the tissue biodistribution difference between free LYC and LYC loaded in NC is probably related to the presence of inflammation in tissues with a greater T. cruzi load, which cannot be distinguished by the free molecule but which passively affects the nanoparticle distribution in the body and, consequently, the efficacy of LYC-loaded NC against T. cruzi observed during the experimental chronic phase. A different and surprising finding is that after oral administration, the nanocapsules seems to be able to pass through gastrointestinal barriers while transiting through the enterocytes by surface adsorption of apoproteins and phospholipids via lymphatic absorption, as previously discussed and demonstrated by Attili-Qadri et al. (42). These lipoproteinated nanocapsules reduced the rate of drug release in plasma, and their distribution among the organs may be guided by the enhanced permeability (the enhanced permeability and retention [EPR] effect) of tissues with inflammation, where NC preferentially accumulate. The LYC-loaded NC were therefore more potent against T. cruzi than free LYC. LYC is a naturally occurring substance, and LYC-loaded NC are produced by a simple and scalable nanotechnological method (43). In particular, to the best of our knowledge, no other molecule reported in the literature has been as effective as LYC-loaded NC during the acute and chronic phases of infection with a T. cruzi strain 100% resistant to treatment.
In conclusion, this is the first study reporting a high level of oral efficacy of a new compound against infections caused by a resistant T. cruzi strain during the acute and chronic phases. These results are original in terms of the chemotherapy of CD and show the enormous potential of the natural substance lychnopholide (LYC) loaded in polymeric nanocapsules. It represents a potential innovative therapy for CD and offers a great prospective treatment to be explored by public or private pharmaceutical laboratories for the treatment of this endemic disease, which is today a global challenge.
MATERIALS AND METHODS
Preparation of LYC-loaded nanocapsules, free LYC solution, and benznidazole suspension.
LYC was extracted from Lychnophora trichocarpha as described by Branquinho et al. (44). LYC was loaded in sterically stabilized NC (LYC-PLA-PEG-NC) and characterized as described by Branquinho et al. (16). Free LYC was prepared as a dispersion (12 mg/ml) of LYC in a mixture of N,N-dimethylacetamide (DMA) and polyethylene glycol 300 (PEG) (4:6, vol/vol) and was further diluted in isotonic glucose solution to attain its final concentration, as described by de Mello et al. (15). BZ (N-benzyl-2-nitro-1-imidazole-acetamide) was obtained as benznidazole tablets from Roche and suspended in arabic gum for oral administration as described by Brener (45). The control group received the excipients of the oral solution (14, 15).
Parasite and animal ethics.
VL-10 T. cruzi strain DTU II (46), which is resistant to BZ and NF treatments (3), was used. This strain induces high levels of parasitemia and intense inflammation, mainly in the hearts of mice (47). Female Swiss mice (age, 28 to 30 days; body weight, 20 to 25 g) were used and maintained according to the guidelines established by the National Council on Animal Experimentation Control (CONCEA). The experiments were approved by the Ethical Committee on Animal Experimentation of the Universidade Federal de Ouro Preto, Brazil (CEUA-UFOP; approval no. 2014/25). Those responsible for the handling and euthanasia of the animals had technical and scientific knowledge, as well as an understanding of the reason for the animals’ death.
Acute infection model and treatment schedules.
For the experiments performed during the acute phase, the animals were intraperitoneally inoculated with 10,000 blood trypomastigotes, which were counted as described by Brener (45). The infection was confirmed in all animals at 9 days postinoculation by FBE (45). The infected animals were divided into groups of 8 animals each and received the LYC formulations (free LYC and LYC-PLA-PEG-NC) at doses of 8 or 12 mg/kg/day by oral gavage. The treatment was initiated on the first day of patent parasitemia (the 9th day after inoculation) and was given for 20 consecutive days. The control groups of animals were evaluated in parallel and consisted of animals that were infected and treated with BZ, employing the classical schedule for the murine model (45); animals that were infected and not treated (the INT group); animals that received solution excipients (DMA-PEG); and animals that received blank NC.
Chronic infection model and treatment schedules.
For the experiments performed during the chronic phase of the infection, the mice were intraperitoneally inoculated with 500 blood trypomastigotes (45) of the same T. cruzi strain mentioned above. This procedure allowed the natural evolution in mice of the chronic phase of the infection, as confirmed by FBE (45) at between 20 and 23 days postinoculation. The treatment in the chronic phase, consisting of the same therapeutic regime described above for the acute phase of infection, started at 90 days postinoculation. The same control groups used for the treatment during the acute phase, described above, were also evaluated in parallel.
Posttreatment evaluation.
The efficacy of all formulations in mice was evaluated according to the criteria described below.
(i) Parasitemia.
The level of parasitemia during the acute phase was determined by the FBE method (45). After the first week of inoculation, blood samples were collected daily from the caudal vein of the mice for the counting of the parasites in the peripheral blood until the mice were consistently negative.
(ii) HC.
The collection of blood for HC evaluation was performed 30, 60, 90, and 120 days after the culture of blood, as described by Filardi and Brener (3). The contents of the tubes were homogenized daily, and 1 drop of the blood culture was examined on a glass slide under an optical microscope to identify the presence of parasites.
(iii) PCR.
Blood samples (0.2 ml/mouse) were collected at 60 and 90 d.p.t. and added to guanidine solution (6 M guanidine-HCl, 0.2 M EDTA, pH 8.0 [48]), and the mixture was stored at room temperature. DNA was extracted with a DNA purification kit (Wizard Genomic; Promega). PCR was performed as described by Gomes et al. (49), with some modifications, using the primers S35 (5′-AAATAATGTACGGGKGAGATGCATGA-3′) and S36 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) (Invitrogen, São Paulo, SP, Brazil) in the presence of Platinum Taq DNA polymerase (Invitrogen, São Paulo, SP, Brazil) to amplify the kinetoplast DNA (k-DNA) minicircles. Positive- and negative-control blood samples were examined in parallel, as were the PCR reagents as a control. All samples were processed in triplicate.
(iv) ELISA.
Serum samples were collected at 90 and 180 d.p.t. for the detection of anti-T. cruzi IgG and analyzed as described by Voller et al. (50), with modifications (51). The antigen concentration was 4.5 μg/ml, the serum dilution was 1:80, and the peroxidase-anti-mouse IgG conjugate dilution was 1:2,000. The cutoff was calculated by considering the mean absorbance for 10 negative controls plus 2 standard deviations.
(v) Parasite load.
The parasite load in 25 to 30 mg of cardiac tissue was estimated by the quantification of T. cruzi DNA by qPCR. Extraction of genomic DNA from the heart was performed using a commercial kit (a genomic DNA purification kit; Promega) according to the manufacturer’s instructions. To quantify tissue parasitism, a standard curve was constructed with epimastigote forms of the T. cruzi Y strain successively diluted 1/10 (from 1.0 × 108 parasites down to 1 parasite) to determine the number of DNA copies of the parasite in each sample. The concentration of DNA obtained was determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific), and the quality of the samples was measured by an absorbance at 260 nm/absorbance at 280 nm ratio of between 1.8 and 2.0. PCR was performed under the conditions described by Caldas et al. (35). For amplification of the DNA, the PCR mixture contained 3 μl of genomic DNA (30 ng), 7 μl of SYBR green PCR Mastermix (Applied Biosystems, Carlsbad, CA, USA), and either 0.35 μM T. cruzi repeat DNA-specific primers or 0.50 μM mouse-specific tumor necrosis factor alpha (TNF-α) primers, used as an endogenous control. The primers for T. cruzi DNA (primers TCZ-F [5′-GCTCTTGCCCACAMGGGTGC-3′, where M is A or C] and TCZ-R [5′-CCAAGCAGCGGATAGTTCAGG-3′]) amplified a 182-bp fragment. The primers for murine TNF-α (primers TNF-5241 [5′-TCCCTCTCATCAGTTCTATGGCCCA-3′] and TNF [5′-CAGCAAGCATCTATGCACTTAGACCCC-3′]) amplified a 170-bp product. The PCR assay was performed under previously described analytical conditions (time, temperature, and number of cycles) (52). Each 96-well reaction plate contained a standard for preparation of a standard curve and two negative controls with T. cruzi-specific or murine-specific primers without DNA and also with tissue DNA from noninfected mice. The reactions were processed and analyzed in an ABI Prism 7500 sequence detection system (Applied Biosystems, USA).
(vi) Survival rates.
Animals were monitored daily, and survival was registered as the accumulated percentage and evaluated until necropsy at 180 d.p.t., the period established to be the end of the experiments.
(vii) Heart histopathology.
For histopathological analysis of myocardial tissues, mice were necropsied at 180 d.p.t. and heart tissues were collected. The cardiac tissue was chosen because the T. cruzi VL-10 strain has a special tropism for the heart in the mouse model and has a pronounced parasitism, according to a previous publication (41). Fragments of the heart were fixed in 4% phosphate-buffered formaldehyde for 24 to 48 h. The samples were washed in phosphate-buffered saline and dehydrated with different concentrations of ethanol (70, 80, 90, and 100%). After dehydration, the tissue was embedded in paraffin, and 4-μm-thick sections were cut and stained with hematoxylin and eosin (H&E). The slides were analyzed under an optical microscope (Leica model DM5000B). The H&E-stained sections were scored for inflammation, defined by the degree of infiltration of the heart tissue with mononuclear leukocytes, as follows: 0, absence of an inflammatory process; 1, mild inflammation; 2, moderate inflammation; and 3, a severe inflammatory process.
The histological analysis of the myocardium for fibrosis was also semiquantitative and used an optical microscope (model CH30; Olympus, Japan). The analyses were performed by the use of scores on a scale of from 0 to 3, in which 0 was the absence of myocardium fibrosis, 1 was the discrete presence of myocardium fibrosis, 2 was a moderate presence of myocardium fibrosis, and 3 was the intense presence of myocardium fibrosis. The scale was adapted from that of Iordanou et al. (53).
Cure criterion.
Animals were considered cured when they were simultaneously negative by all parasitological (HC, PCR, qPCR), serological (ELISA), and histopathological tests.
Animals negative by HC 30 d.p.t. at 90 d.p.t. were also considered cured (3, 19).
Statistical analysis.
Statistical analyses of the data were performed using GraphPad Prism software (v.6.0.1; GraphPad, San Diego, CA, USA). The Kolmogorov-Smirnov test was first performed to verify the data normality. One-way analysis of variance (ANOVA) with Tukey’s posttest was used to compare the area under the curve of parasitemia versus time between the experimental groups. For the histopathological analyses, the Kruskal-Wallis test, followed by Dunn’s posttest, was used. Differences between groups were considered significant when the P value was ≤0.05 (95% confidence intervals).
ACKNOWLEDGMENTS
We thank the Brazilian agencies CNPq (process 431413/2016-9), FAPEMIG (grants APQ-00766/16 and APQ-03514-18), and BRICS-STI/CNPq (grant 442351/2017) for financial support and UFOP and CAPES for undergraduate and postgraduate scholarships.
V. C. F. Mosqueira and M. de Lana are research fellows of CNPq.
There are no conflicts of interest between the authors and the institutions.
REFERENCES
- 1.WHO. 2018. Chagas disease—American trypanosomiasis. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
- 2.Dias JC. 2015. Chagas disease: still a challenge around the world. Rev Soc Bras Med Trop 48:367–369. doi: 10.1590/0037-8682-0269-2015. [DOI] [PubMed] [Google Scholar]
- 3.Filardi LS, Brener Z. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg 81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 4.Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez J, Davies C, Simonazzi A, Real JP, Palma S. 2016. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop 156:1–16. doi: 10.1016/j.actatropica.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Coura JR. 2009. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem Inst Oswaldo Cruz 104:549–554. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- 7.Bahia MT, Diniz LDF, Mosqueira VC. 2014. Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opin Investig Drugs 23:1225–1237. doi: 10.1517/13543784.2014.922952. [DOI] [PubMed] [Google Scholar]
- 8.Morilla MJ, Romero EL. 2015. Nanomedicines against Chagas disease: an update on therapeutics, prophylaxis and diagnosis. Nanomedicine (Lond) 10:465–481. doi: 10.2217/nnm.14.185. [DOI] [PubMed] [Google Scholar]
- 9.Molina I, Salvador F, Sánchez-Montalvá A. 2015. The use of posaconazole against Chagas disease. Curr Opin Infect Dis 28:397–407. doi: 10.1097/QCO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 10.Torrico F, Gascon J, Ortiz L, Alonso-Vega C, Pinazo MJ, Schijman A, Almeida IC, Alves F, Strub-Wourgaft N, Ribeiro I, E1224 Study Group . 2018. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis 18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman DJ, Cragg GM. 2016. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 12.de Oliveira AB, Saúde DA, Perry KSP, Duarte DS, Raslan DS, Boaventura MAD, Chiari E. 1996. Trypanocidal sesquiterpenes from Lychnophora species. Phytother Res 10:292–295. doi:. [DOI] [Google Scholar]
- 13.Chiari E, de Oliveira AB, Raslan DS, Mesquita AA, Tavares KG. 1991. Screening in vitro of natural products against blood forms of Trypanosoma cruzi. Trans R Soc Trop Med Hyg 85:372–374. doi: 10.1016/0035-9203(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 14.Branquinho RT, Mosqueira VC, de Oliveira-Silva JC, Simões-Silva MR, Saúde-Guimarães DA, de Lana M. 2014. Sesquiterpene lactone in nanostructured parenteral dosage form is efficacious in experimental Chagas disease. Antimicrob Agents Chemother 58:2067–2075. doi: 10.1128/AAC.00617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mello CG, Branquinho RT, Oliveira MT, Milagre MM, Saúde-Guimarães DA, Mosqueira VC, de Lana M. 2016. Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental Chagas disease. Antimicrob Agents Chemother 60:5215–5222. doi: 10.1128/AAC.00178-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branquinho RT, Pound-Lana G, Marques Milagre M, Saúde-Guimarães DA, Vilela JMC, Spangler Andrade M, de Lana M, Mosqueira V. 2017. Increased body exposure to new anti-trypanosomal through nanoencapsulation. Sci Rep 7:8429. doi: 10.1038/s41598-017-08469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branquinho RT, Roy J, Farah C, Garcia GM, Aimond F, Le Guennec JY, Saúde-Guimarães DA, Grabe-Guimarães A, Mosqueira VC, de Lana M, Richard S. 2017. Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti-trypanosomal lychnopholide. Sci Rep 7:44998. doi: 10.1038/srep44998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias JC, Ramos AN Jr, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, Torres RM, Melo JR, Almeida EA, Oliveira W Jr, Silveira AC, Rezende JM, Pinto FS, Ferreira AW, Rassi A, Fragata AAF, Sousa AS, Correia DF, Jansen AM, Andrade GM, Britto CF, Pinto AY, Rassi A Jr, Campos DE, Abad-Franch F, Santos SE, Chiari E, Hasslocher-Moreno AM, Moreira EF, Marques DS, Silva EL, Marin-Neto JA, Galvao LM, Xavier SS, Valente SA, Carvalho NB, Cardoso AV, Silva RA, Costa VM, Vivaldini SM, Oliveira SM, Valente VD, Lima MM, Alves RV. 2016. Brazilian consensus on Chagas disease, 2015. Epidemiol Serv Saude 25:7–86. doi: 10.5123/S1679-49742016002100002. [DOI] [PubMed] [Google Scholar]
- 19.Toledo M, Guilherme ALF, Silva JCD, Gasperi MVD, Mendes AP, Gomes ML, Araújo S. 1997. Trypanosoma cruzi: chemotherapy with benznidazole in mice inoculated with strains from Paraná State and from different endemic areas of Brazil. Rev Inst Med Trop Sao Paulo 39:283–290. doi: 10.1590/s0036-46651997000500007. [DOI] [PubMed] [Google Scholar]
- 20.Urbina JA. 2015. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol 62:149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- 21.Aldasoro E, Posada E, Requena-Mendez A, Calvo-Cano A, Serret N, Casellas A, Sanz S, Soy D, Pinazo MJ, Gascon J. 2018. What to expect and when: benznidazole toxicity in chronic Chagas’ disease treatment. J Antimicrob Chemother 73:1060–1067. doi: 10.1093/jac/dkx516. [DOI] [PubMed] [Google Scholar]
- 22.Andrade SG, Freitas LA, Peyrol S, Pimentel AR, Sadigursky M. 1991. Experimental chemotherapy of Trypanosoma cruzi infection: persistence of parasite antigens and positive serology in parasitologically cured mice. Bull World Health Organ 69:191–197. [PMC free article] [PubMed] [Google Scholar]
- 23.Bustamante JM, Tarleton RL. 2014. Potential new clinical therapies for Chagas disease. Expert Rev Clin Pharmacol 7:317–325. doi: 10.1586/17512433.2014.909282. [DOI] [PubMed] [Google Scholar]
- 24.Viotti R, Alarcon de Noya B, Araujo-Jorge T, Grijalva MJ, Guhl F, Lopez MC, Ramsey JM, Ribeiro I, Schijman AG, Sosa-Estani S, Torrico F, Gascon J, Latin American Network for Chagas Disease, NHEPACHA . 2014. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother 58:635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt TJ, Nour AM, Khalid SA, Kaiser M, Brun R. 2009. Quantitative structure–antiprotozoal activity relationships of sesquiterpene lactones. Molecules 14:2062–2076. doi: 10.3390/molecules14062062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulsen VP, Frank FM, Cazorla SI, Anesini CA, Malchiodi EL, Freixa B, Vila R, Muschietti LV, Martino VS. 2008. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob Agents Chemother 52:2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulsen VP, Frank FM, Cazorla SI, Barrera P, Freixa B, Vila R, Sosa MA, Malchiodi EL, Muschietti LV, Martino VS. 2011. Psilostachyin C: a natural compound with trypanocidal activity. Int J Antimicrob Agents 37:536–543. doi: 10.1016/j.ijantimicag.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Sulsen VP, Lizarraga EF, Elso OG, Cerny N, Sanchez Alberti A, Bivona AE, Malchiodi EL, Cazorla SI, Catalan C. 2019. Activity of estafietin and analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 24:E1209. doi: 10.3390/molecules24071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurella LC, Cerny N, Bivona AE, Sánchez Alberti A, Giberti G, Malchiodi EL, Martino VS, Catalan CA, Alonso MR, Cazorla SI, Sülsen VP. 2017. Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. PLoS Negl Trop Dis 11:e0005929. doi: 10.1371/journal.pntd.0005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puente V, Laurella LC, Spina RM, Lozano E, Martino VS, Sosa MA, Sulsen VP, Lombardo E. 2019. Primary targets of the sesquiterpene lactone deoxymikanolide on Trypanosoma cruzi. Phytomedicine 56:27–34. doi: 10.1016/j.phymed.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Krettli AU, Cancado JR, Brener Z. 1982. Effect of specific chemotherapy on the levels of lytic antibodies in Chagas’s disease. Trans R Soc Trop Med Hyg 76:334–340. doi: 10.1016/0035-9203(82)90184-5. [DOI] [PubMed] [Google Scholar]
- 32.de Lana M, Martins-Filho OA. 2015. Revisiting the posttherapeutic cure criterion in Chagas disease: time for new methods, more questions, doubts, and polemics or time to change old concepts? Biomed Res Int 2015:652985. doi: 10.1155/2015/652985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira MT, Branquinho RT, Alessio GD, Mello CGC, Nogueira-de-Paiva NC, Carneiro CM, Toledo MJO, Reis AB, Martins-Filho OAM, de Lana M. 2017. TcI, TcII and TcVI Trypanosoma cruzi samples from Chagas disease patients with distinct clinical forms and critical analysis of in vitro and in vivo behavior, response to treatment and infection evolution in murine model. Acta Trop 167:108–120. doi: 10.1016/j.actatropica.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Krettli AU. 2009. The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem Inst Oswaldo Cruz 104(Suppl 1):142–151. doi: 10.1590/s0074-02762009000900020. [DOI] [PubMed] [Google Scholar]
- 35.Caldas S, Caldas IS, Diniz LDF, Lima WGD, Oliveira RDP, Cecílio AB, Ribeiro I, Talvani A, Bahia MT. 2012. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop 123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Abriata J, Eloy J, Riul T, Campos P, Baruffi M, Marchetti J. 2017. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mater Sci Eng C Mater Biol Appl 77:1196–1203. doi: 10.1016/j.msec.2017.03.266. [DOI] [PubMed] [Google Scholar]
- 37.Molina J, Urbina J, Gref R, Brener Z, Rodrigues Junior JM. 2001. Cure of experimental Chagas’ disease by the bis-triazole DO870 incorporated into ‘stealth’ polyethyleneglycol-polylactide nanospheres. J Antimicrob Chemother 47:101–104. doi: 10.1093/jac/47.1.101. [DOI] [PubMed] [Google Scholar]
- 38.Lachi-Silva L, Sy SK, Voelkner A, de Sousa JP, Lopes JL, Silva DB, Lopes NP, Kimura E, Derendorf H, Diniz A. 2015. Simultaneous characterization of intravenous and oral pharmacokinetics of lychnopholide in rats by transit compartment model. Planta Med 81:1121–1127. doi: 10.1055/s-0035-1546214. [DOI] [PubMed] [Google Scholar]
- 39.Mosqueira VC, Legrand P, Morgat JL, Vert M, Mysiakine E, Gref R, Devissaguet JP, Barratt G. 2001. Biodistribution of long-circulating PEG-grafted nanocapsules in mice: effects of PEG chain length and density. Pharm Res 18:1411–1419. doi: 10.1023/a:1012248721523. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari FC, Ferreira LC, Souza MR, Grabe-Guimarães A, Paula CA, Rezende SA, Saúde-Guimarães DA. 2013. Anti-inflammatory sesquiterpene lactones from Lychnophora trichocarpha Spreng. (Brazilian Arnica). Phytother Res 27:384–389. doi: 10.1002/ptr.4736. [DOI] [PubMed] [Google Scholar]
- 41.Caldas S, Caldas IS, Cecilio AB, Diniz LD, Talvani A, Ribeiro I, Bahia MT. 2014. Therapeutic responses to different anti-Trypanosoma cruzi drugs in experimental infection by benznidazole-resistant parasite stock. Parasitology 141:1628–1637. doi: 10.1017/S0031182014000882. [DOI] [PubMed] [Google Scholar]
- 42.Attili-Qadri S, Karra N, Nemirovski A, Schwob O, Talmon Y, Nassar T, Benita S. 2013. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci U S A 110:17498–17503. doi: 10.1073/pnas.1313839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. 1989. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharmaceutics 55:R1–R4. doi: 10.1016/0378-5173(89)90281-0. [DOI] [Google Scholar]
- 44.Branquinho RT, Mosqueira VCF, Kano EK, de Souza J, Dorim DDR, Saúde-Guimarães DA, de Lana M. 2014. HPLC-DAD and UV-spectrophotometry for the determination of lychnopholide in nanocapsule dosage form: validation and application to release kinetic study. J Chromatogr Sci 52:19–26. doi: 10.1093/chromsci/bms199. [DOI] [PubMed] [Google Scholar]
- 45.Brener Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–396. [PubMed] [Google Scholar]
- 46.Moreno M, D'ávila DA, Silva MN, Galvão LM, Macedo AM, Chiari E, Gontijo ED, Zingales B. 2010. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Mem Inst Oswaldo Cruz 105:918–924. doi: 10.1590/s0074-02762010000700014. [DOI] [PubMed] [Google Scholar]
- 47.Caldas IS, Talvani A, Caldas S, Carneiro CM, de Lana M, da Matta Guedes PM, Bahia MT. 2008. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res 103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 48.Avila HA, Sigman DS, Cohen LM, Millikan RC, Simpson L. 1991. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas’ disease. Mol Biochem Parasitol 48:211–221. doi: 10.1016/0166-6851(91)90116-n. [DOI] [PubMed] [Google Scholar]
- 49.Gomes ML, Macedo AM, Vago AR, Pena SD, Galvao LM, Chiari E. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol 88:28–33. doi: 10.1006/expr.1998.4191. [DOI] [PubMed] [Google Scholar]
- 50.Voller A, Bidwell DE, Bartlett A. 1976. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ 53:55–65. [PMC free article] [PubMed] [Google Scholar]
- 51.Santos LDS, Torres RM, Machado-de-Assis GF, Bahia MT, Martins HR, Teixeira-Carvalho A, Coelho-Dos-Reis JGA, Albajar-Viñas P, Martins-Filho OA, de Lana M. 2012. In-house ELISA method to analyze anti-Trypanosoma cruzi IgG reactivity for differential diagnosis and evaluation of Chagas disease morbidity. Rev Soc Bras Med Trop 45:35–44. doi: 10.1590/s0037-86822012000100008. [DOI] [PubMed] [Google Scholar]
- 52.Diniz LF, Mazzeti AL, Caldas IS, Ribeiro I, Bahia MT. 2018. Outcome of E1224-benznidazole combination treatment for infection with a multidrug-resistant Trypanosoma cruzi strain in mice. Antimicrob Agents Chemother 62:e00401-18. doi: 10.1128/AAC.00401-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iordanou P, Lykoudis EG, Athanasiou A, Koniaris E, Papaevangelou M, Fatsea T, Bellou P. 2009. Effect of visible and infrared polarized light on the healing process of full-thickness skin wounds: an experimental study. Photomed Laser Surg 27:261–267. doi: 10.1089/pho.2008.2237. [DOI] [PubMed] [Google Scholar]