The intrinsic resistance of Pseudomonas aeruginosa to polymyxins in part relies on the addition of 4-amino-4-deoxy-l-arabinose (Ara4N) molecules to the lipid A of lipopolysaccharide (LPS), through induction of operon arnBCADTEF-ugd (arn) expression. As demonstrated previously, at least three two-component regulatory systems (PmrAB, ParRS, and CprRS) are able to upregulate this operon when bacteria are exposed to colistin. In the present study, gene deletion experiments with the bioluminescent strain PAO1::lux showed that ParRS is a key element in the tolerance of P. aeruginosa to this last-resort antibiotic (i.
KEYWORDS: Pseudomonas aeruginosa, colistin resistance, MexXY/OprM, antibiotic resistance
ABSTRACT
The intrinsic resistance of Pseudomonas aeruginosa to polymyxins in part relies on the addition of 4-amino-4-deoxy-l-arabinose (Ara4N) molecules to the lipid A of lipopolysaccharide (LPS), through induction of operon arnBCADTEF-ugd (arn) expression. As demonstrated previously, at least three two-component regulatory systems (PmrAB, ParRS, and CprRS) are able to upregulate this operon when bacteria are exposed to colistin. In the present study, gene deletion experiments with the bioluminescent strain PAO1::lux showed that ParRS is a key element in the tolerance of P. aeruginosa to this last-resort antibiotic (i.e., resistance to early drug killing). Other loci of the ParR regulon, such as those encoding the efflux proteins MexXY (mexXY), the polyamine biosynthetic pathway PA4773-PA4774-PA4775, and Ara4N LPS modification process (arnBCADTEF-ugd), also contribute to the bacterial tolerance in an intricate way with ParRS. Furthermore, we found that both stable upregulation of the arn operon and drug-induced ParRS-dependent overexpression of the mexXY genes accounted for the elevated resistance of pmrB mutants to colistin. Deletion of the mexXY genes in a constitutively activated ParR mutant of PAO1 was associated with significantly increased expression of the genes arnA, PA4773, and pmrA in the absence of colistin exposure, thereby highlighting a functional link between the MexXY/OprM pump, the PA4773-PA4774-PA4775 pathway, and Ara4N-based modification of LPS. The role played by MexXY/OprM in the adaptation of P. aeruginosa to polymyxins opens new perspectives for restoring the susceptibility of resistant mutants through the use of efflux inhibitors.
INTRODUCTION
Pseudomonas aeruginosa is a notorious cause of health care-associated infections and lung function deterioration in cystic fibrosis. Because of an increasing prevalence of extensively drug-resistant strains in hospitals (1, 2), polymyxin B and polymyxin E (colistin) have been reconsidered as potentially useful drugs to treat severely ill patients (3, 4).
The resistance of P. aeruginosa to these polycationic antimicrobial peptides (AMPs) is considered to rely essentially on the addition of 4-amino-4-l-deoxyarabinose (Ara4N) to the phosphate groups of lipopolysaccharide (LPS) (5). Such posttranslational modification of LPS mitigates the penetration of polymyxins across the outer membrane by reducing the net negative charge of the bacterial surface (5). Proteins for the whole process are encoded by a large operon named arnBCADTEF-ugd (here called arn), which governs the biosynthesis, transmembrane transport, and enzymatic attachment to one or two phosphate groups of lipid A of Ara4N (5, 6). Operon arn expression is upregulated in response to outer membrane damage or perturbations via at least four two-component systems (TCSs) (7–12). Whereas PhoPQ is sensitive to divalent cation depletion destabilizing the outer membrane lipid bilayer, PmrAB, ParRS, and CprRS are activated when bacteria are exposed to various AMPs (7–10). However, the respective contributions of the three latter systems to the natural resistance of P. aeruginosa to polymyxins have not been fully elucidated to date.
Over past years, it has become evident that higher levels of resistance to polymyxins can be reached by the pathogen when mutations in genes pmrB, phoQ, parS, parR, cprS, and/or colS stably activate the corresponding TCSs, thus causing constitutive overexpression of operon arn and subsequent LPS modification (9, 13–17). Recently, we found that, in addition to colistin, some pmrB mutants are more resistant to aminoglycosides than the wild-type parental strains (18). In such mutants, increased levels of resistance to aminoglycosides (but not polymyxins) result from activation of a gene cluster, PA4773-PA4774-PA4775, that codes for a pathway of norspermidine biosynthesis. The active efflux system MexXY/OprM, which is related to the resistance-nodulation-cell division (RND) family of bacterial transporters, also contributes to aminoglycoside resistance in pmrB mutants (18). This pump has a remarkably wide substrate specificity that encompasses diverse antibiotics such as aminoglycosides, cephalosporins (cefepime, cefpirome, and ceftobiprole), fluoroquinolones, tetracyclines, tigecycline, and macrolides (19). Its role in the wild-type resistance phenotype of P. aeruginosa is limited to the pump substrates that target the ribosome and indirectly trigger operon mexXY expression through a sophisticated transcription attenuation mechanism involving an antirepressor protein named ArmZ (20, 21). Loss-of-function mutations in gene mexZ, which encodes the TetR-like local repressor of mexXY, are a common cause of decreased (2- to 16-fold) susceptibility to aminoglycosides in clinical strains of P. aeruginosa (19, 22). Besides mutations in mexZ (called agrZ in mutants) and various alterations in the ribosomal machinery that alleviate the transcription attenuation mechanism cited above (agrW1 mutants) (23, 24), a class of mutations in the TCS ParRS was discovered; the mutations activate expression of the operons mexXY and arn, with concomitant repression of the carbapenem-specific porin-encoding gene oprD (9). These findings highlighted a functional link between MexXY/OprM and the Ara4N-based modification of LPS, via the ParRS phosphorelay. Whether the mutationally activated response regulator ParR directly binds to the promoter region of operon mexXY could not be demonstrated by DNA footprinting experiments (K. Jeannot, unpublished data). In continuity with the previous results, here we show that the efflux system MexXY/OprM and the Arn modification pathway cooperate to provide P. aeruginosa with protection against colistin.
RESULTS AND DISCUSSION
Inoculum-dependent bactericidal activity of colistin.
Polymyxins exert rapid, concentration-dependent bactericidal activity on P. aeruginosa strains when used at concentrations near or above the MIC (25, 26). However, as previously demonstrated in pharmacodynamic studies, large bacterial inocula tend to attenuate this killing activity (25, 27). In order to analyze the impact of colistin under controlled experimental conditions, we carried out time-kill experiments with increasing inocula of the bioluminescent strain PAO1::lux. The bacterial suspensions were exposed to static concentrations of colistin from 0.25 to 16 μg ml−1 (0.5 to 32 times the MIC). As expected, the minimal concentrations needed to reduce the initial bacterial load by at least 2 log10 units after 2 h of contact varied with the cell density (16 μg ml−1 with 5 × 108 CFU ml−1, 4 μg ml−1 with 108 CFU ml−1, 2 μg ml−1 with 5 × 107 CFU ml−1, 1 μg ml−1 with 107 and 5 × 106 CFU ml−1, and 0.5 μg ml−1 with 106 CFU ml−1). The relationship between the inoculum size and the 2-h minimal bactericidal concentration is presented in Fig. 1. According to these data, a colistin concentration of 2 μg ml−1, which corresponds to the susceptibility breakpoints defined by both CLSI (28) and EUCAST (29), would remain bactericidal up to 5 × 107 bacteria but might fail to eradicate more dense populations, such as those found in the airways of some cystic fibrosis patients heavily colonized by P. aeruginosa.
FIG 1.
Relationship between the bacterial inoculum size and concentration of colistin required to reduce ≥2 log10 CFU ml−1 of strain PAO1::lux after 2 h of drug exposure. The exponential correlation between the two parameters is as follows: y = 0.001e1.3252x.
Role of ParRS in intrinsic resistance of P. aeruginosa to colistin-induced killing.
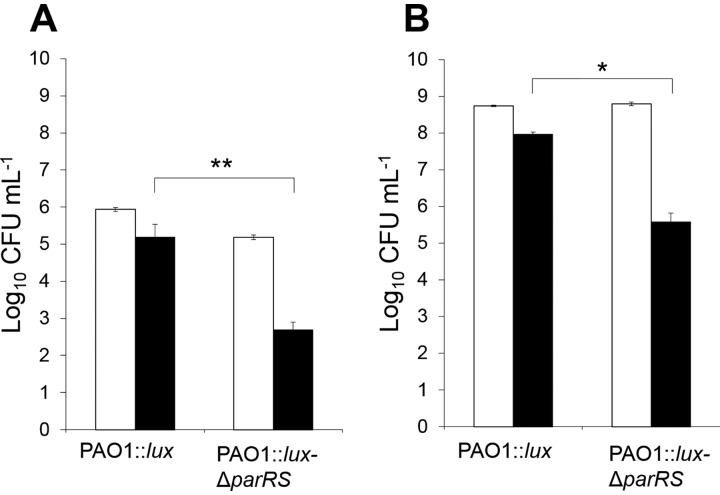
The TCSs PmrAB, CprRS, and ParRS are activated upon polymyxin exposure (5, 7, 9, 10). To better assess their respective roles in the protection of wild-type strains of P. aeruginosa against colistin, their encoding genes were deleted to construct single- and multiple-gene knockout mutants from strain PAO1::lux. Inocula of 5 × 108 CFU ml−1 were used to determine the extent of bacterial killing after 2 h of drug exposure at 8 μg ml−1 (Table 1). While suppression of PmrAB and CprRS had virtually no impact on the early bactericidal activity of colistin, compared with the parent PAO1::lux, ParRS inactivation resulted in ∼100-fold greater CFU decline (Table 1). Similar results were obtained at lower cell densities (106 CFU ml−1) with a drug concentration of 0.4 μg ml−1, consistent with the inoculum-dependent effects of polymyxins (Fig. 2). To evaluate the specificity of ParRS in response to colistin, we also measured the bactericidal effect of ciprofloxacin (2 μg ml−1) under similar conditions (5 × 108 CFU ml−1) (data not shown). After 2 h of drug exposure, the amounts of surviving bacteria were not different between the PAO1::lux strain and its mutant PAO1ΔparRS, indicating that the impact of ParRS on the bactericidal effect of colistin is specific to this molecule. Rather surprisingly, inactivation of the LPS modification operon arn sensitized PAO1::lux only 15-fold (Table 1). Moreover, impairment of this major mechanism of adaptation to AMPs in P. aeruginosa did not further increase the killing efficacy of the polymyxin in a parRS deletion mutant (compare PAO1::lux-ΔparRS with mutant PAO1::lux-Δ4TCS-Δarn, which lacks operons parRS, pmrAB, cprRS, PhoPQ, and arn, in Table 1). Finally, additional experiments showed that the MIC of colistin, which reflects the bacteriostatic potency of this antibiotic, was not affected by the deletion of any of the aforementioned loci except parRS, which was associated with a modest 2-fold decrease in the MIC, relative to PAO1::lux (0.25 versus 0.5 μg ml−1) (Table 1). Altogether, these data suggested a contribution of Arn-independent, ParRS-regulated mechanisms to the tolerance of P. aeruginosa to colistin.
TABLE 1.
Impact of the Ara4N synthesis pathway and genes belonging to the ParRS regulon on the bactericidal and bacteriostatic effects of colistin
| Strain | Killing of bacteria (log10 CFU ml−1)a | Colistin MIC (μg ml−1) |
|---|---|---|
| PAO1 | ND | 0.5 |
| PAO1::lux | −0.68 ± 0.07 | 0.5 |
| PAO1::lux-ΔpmrAB | −0.70 ± 0.19 | 0.5 |
| PAO1::lux-ΔcprRS | −0.78 ± 0.20 | 0.5 |
| PAO1::lux-ΔparRS | −3.06 ± 0.34 | 0.25 |
| PAO1::lux-Δarn | −1.85 ± 0.10 | 0.5 |
| PAO1::lux-Δ4TCS-Δarnb | −3.05 ± 0.28 | 0.25 |
| PAO1::lux-ΔPA4773 | −1.98 ± 0.02 | 0.5 |
| PAO1::lux-ΔPA4774 | −1.69 ± 0.46 | 0.5 |
| PAO1::lux-ΔPA4775 | −1.72 ± 0.53 | 0.5 |
| PAO1::lux-ΔmexXY | −1.77 ± 0.34 | 0.5 |
| PAO1::lux-ΔmexXY-Δarn | −1.66 ± 0.49 | 0.5 |
| PAO1::lux-ΔmexXY-ΔPA4773 | −1.99 ± 0.68 | 0.5 |
| PAO1::lux-ΔmexXY-ΔPA4773-Δarn | −1.76 ± 0.42 | 0.5 |
| PAO1::lux-ΔmexXY(pAK1900) | −1.19 ± 0.33 | 0.5 |
| PAO1::lux-ΔmexXY(pAGH97) | −0.77 ± 0.16 | 0.5 |
| PAO1::lux(pAK1900) | ND | 0.5 |
| PAO1::lux(pAKarn) | ND | 4 |
| PAO1::lux-ΔmexXY(pAKarn) | ND | 1 |
| PAO1::lux-Δarn(pAGH97) | ND | 0.5 |
| PAOW2 | ND | 2 |
| PAOW2Δarn | ND | 1 |
| PAOW2ΔmexXY | ND | 1 |
The indicated values correspond to the reduction of an initial inoculum of 5 × 108 CFU ml−1 after 2 h of colistin exposure (8 μg ml−1) and are the means of at least three independent experiments. Values in bold indicate bacterial killing at least 1.5 log10 units greater than that of PAO1::lux. Standard deviations are indicated. ND, not determined.
Mutant PAO1::lux-Δ4TCS-Δarn lacks the operons pmrAB, cprRS, parRS, and phoPQ, in addition to the operon arnBCADTEF-ugd.
FIG 2.
ParRS contribution to the protection of P. aeruginosa against colistin. Low (106 CFU ml−1) (A) and high (5 × 108 CFU ml−1) (B) inocula of strain PAO1::lux and mutant PAO1::lux-ΔparRS were exposed to 0.4 and 8 μg ml−1 of colistin, respectively. White and black bars represent the numbers of living cells at the beginning (time zero) and after 2 h of colistin treatment, respectively. Statistically significant differences between the strains were established with the paired Student's t test. *, P < 0.05; **, P < 0.005.
The PA4773-PA4774-PA4775 gene cluster mitigates the lethal effects of colistin.
By applying the same experimental conditions as described above (5 × 108 CFU ml−1 and 8 μg ml−1 colistin), we assessed the protective role of various loci belonging to the ParR regulon, through a similar gene deletion strategy (9). This approach showed that genes oprD, PA1797, PA2358, and PA2655 had no influence on the bactericidal and bacteriostatic activities of colistin (data not shown). In contrast, inactivation of PA4773, PA4774, or PA4775, which together form a cluster promoting the presence of norspermidine at the bacterial surface and subsequent aminoglycoside resistance in pmrB mutants (18, 30), reduced the number of surviving bacteria 10- to 20-fold after 2 h of drug treatment, although without significant changes in the colistin MIC (Table 1). To assess whether the lack of surface-localized norspermidine accounts for this sensitization effect, we exogenously added the polyamine, at a final concentration of 150 μM, to the growth medium of strain PAO1::lux. A similar experiment was carried out in parallel with spermidine. However, these polyamines failed to reduce colistin activity (data not shown), suggesting that other protective molecules are produced under the control of the three-gene locus. In the bacterium Thermus thermophilus, a triamine/agmatine aminopropyl transferase (TAAPT) is able to add an aminopropyl residue from decarboxylated S-adenosylmethionine to various precursors, thus giving rise to the synthesis of different polyamines (31). Interestingly, we discovered that gene PA4774 encodes a close homolog of TAAPT from T. thermophilus strain HB8 (60% amino acid sequence identity) (1UIR_A) (31). Thus, it is tempting to speculate that one or several polyamines in addition to norspermidine are produced upon colistin stress and, in contrast to norspermidine, contribute to the tolerance of P. aeruginosa to this AMP. However, further investigations are required to confirm this hypothesis.
Efflux system MexXY/OprM, a third player in bacterial tolerance to colistin.
Like the arn operon and the PA4773-PA4774-PA4775 gene cluster, the mexXY genes belong to the ParR regulon and are de facto overexpressed in polymyxin-exposed bacteria through activation of ParRS (9, 10). Unexpectedly, we found that the lack of operon mexXY sensitized strain PAO1::lux to the lethal effects of colistin 12-fold (Table 1); complementation of the mutant PAO1::lux-ΔmexXY with a plasmid-borne copy of the operon (pAK1900-derived construct pAGH97) suppressed this sensitization (Table 1). Pioneering works aiming to identify the substrates of MexXY/OprM were based on MIC determinations and thereby excluded polymyxins from the list of potentially transported molecules (32). Consistent with these findings, inactivation of that pump in PAO1::lux had no impact on the bacteriostatic activity of colistin (MIC of 0.5 μg ml−1) (Table 1). Reinforcing the notion that this molecule is not a good substrate for MexXY/OprM, no increase in the drug MIC was noted when the mexXY genes were overexpressed from plasmid pAGH97 in PAO1::lux-ΔmexXY (Table 1). In comparison, introduction of vector pAK1900 carrying the entire arn operon (construct pAKArn) into PAO1::lux caused a 8-fold increase in colistin resistance (4 μg ml−1 versus 0.5 μg ml−1). Clearly indicating that, while MexXY/OprM is not thought to export colistin significantly, it cooperates with the Arn LPS modification pathway to protect P. aeruginosa against this antibiotic, transformation of PAO1::lux-ΔmexXY with plasmid pAKArn rendered this mutant only 2-fold more resistant to the antibiotic (1 μg ml−1 versus 0.5 μg ml−1) (Table 1). A possible explanation for these results might be that colistin is actually a substrate for MexXY/OprM, with its active export becoming evident in terms of resistance only when its permeation across the outer membrane is strongly decreased by Ara4N-based modification of LPS, which would increase the efflux rate/influx rate ratio. Other examples of RND pumps involved in AMP resistance have been reported for Klebsiella pneumoniae (AcrAB-TolC and H239_3064) and Neisseria gonorrhoeae (MrtCDE) (33–35). Alternatively, MexXY/OprM might export some constituents of LPS, as previously envisaged (36), or might actively transfer specific molecules from the periplasm to the bacterial surface to counteract the electrostatic binding of colistin. To rule out the possibility that the lack of a MexXY efflux pump is counterbalanced by the overproduction of another one, we measured, by real-time quantitative PCR (RT-qPCR), the expression levels of genes mexA and mexC in strain PAO1::lux and its mexXY deletion mutant (PAO1ΔmexXY::lux). Neither mexB (1.9 ± 0.31-fold) nor mexC (1.6 ± 0.36-fold) was upregulated in the absence of MexXY, indicating that MexAB-OprM and MexCD-OprJ do not relay MexXY/OprM under these conditions. Finally, the impact of the triple deletion ΔmexXY-ΔPA4773-Δarn was also tested, yielding findings similar to those for single mutants regarding both the killing (12-fold CFU reduction, compared with PAO1::lux) and bacteriostatic (MIC of 0.5 μg ml−1) aspects of colistin action (Table 1). The absence of additive effects among these gene deletions supports the idea that the physiological functions determined by the three loci are intimately overlapped in protecting wild-type strains of P. aeruginosa against colistin. As mentioned previously, additional protective mechanisms regulated by ParRS still remain to be identified.
Interplay between MexXY/OprM and PmrAB.
As shown previously, exposure of P. aeruginosa to ribosome-targeting antibiotics (e.g., chloramphenicol and spectinomycin) induces operon mexXY expression while downregulating that of the arn locus and genes PA4773 and PA4774 (35). As a result, these inhibitors increase (2- to 4-fold) the susceptibility of P. aeruginosa to colistin (36). In line with these observations, we carried out RT-qPCR experiments to assess the transcript levels of the genes arnA (as a representative gene of the arn operon), mexY (for the mexXY operon), and PA4773 (for the PA4773-PA4774-PA4775 cluster) in strain PAO1::lux and mutants PAO1::lux-Δarn, PAO1::lux-ΔmexXY, and PAO1::lux-ΔPA4773 after they had been subjected to 8 μg ml−1 colistin for 2 h. Suppression of operon arn and gene PA4773 did not modify mexY expression in colistin-treated or untreated PAO1::lux cells (Fig. 3A). In contrast, inactivation of mexXY caused significant upregulation of arnA (9.5-fold more than in PAO1::lux) and PA4773 (5.3-fold more) upon colistin exposure exclusively (Fig. 3B and C), suggesting that the Ara4N modification pathway and polyamine biosynthetic pathway PA4773-PA4774-PA4775 somehow compensate for the lack of MexXY/OprM in colistin-treated bacteria. Because genes PA4773 and arnA belong to the regulon of the response regulator PmrA (12), we quantified pmrA gene transcripts in PAO1::lux-ΔmexXY and PAO1::lux cells exposed to colistin (Fig. 3D). Levels of these transcripts appeared to be 4.8-fold higher in the deletion mutant, which confirms that the absence of MexXY/OprM generates overactivation of the TCS PmrAB upon colistin treatment, which in turn enhances the expression of Arn and the polyamine pathway. While the role of MexXY/OprM in the rapid adaptation of P. aeruginosa to colistin stress remains unclear, it could be assumed that the efflux system clears the periplasm of molecules or ions that are sensed by the sensor PmrB, an hypothesis that is fully consistent with the observation by Poole and coworkers that the upregulation of MexXY upon aminoglycoside exposure (i.e., independently of the ParRS signaling pathway) is associated with lower arn expression levels and diminished polymyxin resistance (36).
FIG 3.
Influence of pump MexXY on the expression of the genes arnA, PA4773, and pmrA. Transcript levels of the genes mexY (A), arnA (B), PA4773 (C), and pmrA (D) were assessed by RT-qPCR in PAO1::lux and various deletion mutants. Log-phase bacteria were grown in the absence (white bars) or presence (black bars) of 8 μg ml−1 colistin for 2 h. Error bars indicate standard deviations of three biological replicates. Statistically significant differences between the strains were established with the paired Student's t test. *, P < 0.05; **, P < 0.005; NS, not significant.
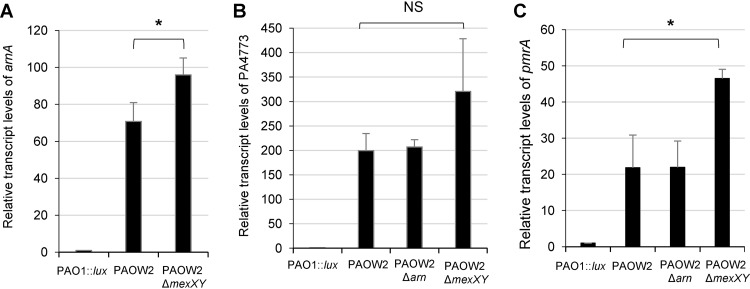
In a previous work, we showed that P. aeruginosa cells exposed to sub-MICs of colistin upregulate operon mexXY as a result of the activation of TCS ParRS (9). Thus, it was potentially interesting in this work to determine whether the pmrA gene is upregulated in a mutant exhibiting stable activation of ParRS in the absence of colistin exposure. We used the PAO1-derived mutant PAOW2, which constitutively overexpresses the arn and mexXY operons together with gene cluster PA4773-PA4774-PA4775, subsequent to a mutation (M59I) in the regulator ParR (9). Compared to PAO1::lux, PAOW2 exhibited much higher transcript levels of genes arnA (70-fold), PA4773 (200-fold), pmrA (21-fold), and mexY (21-fold) (Fig. 4). While the loss of operon arn had no effects on PA4773 and pmrA transcriptional activities, suppression of operon mexXY was associated with significant increases in arnA and pmrA mRNA levels in the activated ParRS background of PAOW2. The absence of colistin in the growth medium of these various mutants explicitly highlights ParRS-mediated interactions between MexXY/OprM and the Arn LPS modification pathway. It remains to be clarified, however, whether ParR directly (i.e., through direct binding to promoter sequences) or indirectly activates PmrAB in PAOW2 and in the colistin-treated wild-type strain PAO1. Of note, the colistin MIC for mutant PAOW2 (2 μg ml−1) was reduced 2-fold upon operon mexXY deletion (1 μg ml−1), a result consistent with MexXY/OprM contributing to both the tolerance of P. aeruginosa to early bactericidal effects of colistin and the acquired resistance to this compound (Table 1).
FIG 4.
Impact of mutationally activated ParR on the expression of the genes arnA (A), PA4773 (B), and pmrA (C). RT-qPCR experiments were performed with strain PAO1::lux, PAOW2 (which carries the M59I substitution in regulator ParR), and various deletion mutants grown in the presence of 8 μg ml−1 colistin for 2 h. Error bars indicate standard deviations of three biological replicates. Statistically significant differences between the strains were established with the paired Student's t test. *, P < 0.05; NS, not significant.
MexXY/OprM and the Arn LPS modification pathway are both involved in the high colistin resistance of pmrB mutants.
Mutational activation of TCS PmrAB is a major cause of resistance to polymyxins in clinical strains of P. aeruginosa. To assess whether MexXY/OprM contributes to the phenotype conferred by mutations activating sensor PmrB (V28G, ΔL172, Q105P, and G188D in the following strains, respectively), we compared two PAO1-derived pmrB spontaneous mutants named AB8.2 (colistin MIC of 128 μg ml−1) and AB16.2 (MIC of 128 μg ml−1), as well as two resistant pmrB clinical isolates named 2243 and 3795 (MICs of 128 μg ml−1), with their respective mexXY knockout counterparts (18). As indicated in Table 2, suppression of this efflux system led to strong reductions (16-fold to >128-fold) in colistin MICs in all of these bacteria but left residual resistance (MICs of 4 or 8 μg ml−1), relative to the wild-type strain PAO1 (0.5 μg ml−1). A more pronounced effect was obtained by deleting the whole arn operon in mutants AB8.2 and AB16.2 (Table 2). Indeed, impairment of Ara4N-based LPS modification almost completely restored the parental susceptibility in the two strains (MICs of 1 μg ml−1). Confirming that MexXY/OprM somehow reinforces this outer membrane impermeability mechanism, ectopic expression of mexXY genes from plasmid pAGH97 fully complemented the four ΔmexXY mutants (Table 2). Because mexXY expression is repressed in P. aeruginosa (37), rapid activation of the operon may occur upon colistin stress, to allow the synthesis of protective amounts of the MexXY/OprM pump. Consistent with that, the expression of mexY was not upregulated in the pmrB mutants (AB8.2 and AB16.2) in the absence of colistin. However, it remained strongly inducible (8-fold) when the bacteria were exposed to colistin, similarly to strain PAO1 (see Fig. S1 in the supplemental material). As demonstrated previously, the low basal level of mexXY transcription is increased when wild-type P. aeruginosa cells are subjected to sub-MIC concentrations of colistin, as a result of ParRS activation (9). In support of the notion that similar upregulation occurs in pmrB mutants through the activation of ParRS, suppression of these genes in mutant AB16.2 partially reversed the resistance to this antibiotic. Compared with AB16.2, mutant AB16.2ΔparRS exhibited 32-fold greater susceptibility to colistin (MICs of 4 μg ml−1 versus 128 μg ml−1) (Table 2). Conversely, a slight but reproducible 2-fold increase in the drug MIC was noticed in AB8.2 and AB16.2 upon deletion of the repressor MexZ-encoding gene and subsequent overexpression of the mexXY operon. All of these data agree with MexXY/OprM reinforcing the outer membrane permeability barrier of pmrB mutants toward colistin, through active efflux of still undetermined substrates. To rule out the possibility that this pump might export Ara4N to the cell surface or promote its addition to LPS molecules via the Arn pathway, lipid A fractions from AB16.2 and AB16.2ΔmexXY were extracted and compared by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (38). The MALDI-TOF MS mass spectra showed similar patterns of molecular species substituted with 4AraN, thereby indicating that the LPS modification process is independent of the activity of MexXY/OprM (data not shown). Finally, neither the deletion of gene armZ, which encodes the antirepressor protein of MexZ, named ArmZ, nor that of genes encoding the TCSs PhoPQ and CprRS had an impact on the resistance phenotypes of mutants AB8.2 and AB16.2 (data not shown). Therefore, these results demonstrate that the high level of resistance of pmrB mutants to colistin relies on concomitant overexpression of the arn and mexXY operons, induced by dual activation of the TCSs PmrAB and ParRS, respectively.
TABLE 2.
Contribution of MexXY/OprM to the acquired resistance of pmrB mutants to colistin
| Strain | MIC (μg ml−1) |
|||
|---|---|---|---|---|
| Colistin | Amikacin | Ciprofloxacin | Cefepime | |
| PAO1 | 0.5 | 2 | 0.06 | 1 |
| AB8.2 | 128 | 4 | 0.06 | 1 |
| AB8.2ΔmexXY | 4 | 0.25 | 0.03 | 0.5 |
| AB8.2ΔmexXY(pAK1900) | 4 | 0.25 | 0.03 | 0.5 |
| AB8.2ΔmexXY(pAGH97) | 128 | 16 | 0.25 | 4 |
| AB8.2Δarn | 1 | 4 | 0.12 | 1 |
| AB8.2ΔmexZ | 256 | 16 | 0.25 | 4 |
| AB16.2 | 128 | 8 | 0.12 | 2 |
| AB16.2ΔmexXY | 8 | 0.25 | 0.06 | 1 |
| AB16.2 ΔmexXY(pAK1900) | 8 | 0.25 | 0.06 | 1 |
| AB16.2ΔmexXY(pAGH97) | 256 | 32 | 0.25 | 4 |
| AB16.2Δarn | 1 | 8 | 0.12 | 2 |
| AB16.2ΔparRS | 4 | 8 | 0.06 | 2 |
| AB16.2ΔmexZ | 256 | 32 | 0.25 | 4 |
| 2243 | 256 | 8 | 16 | 2 |
| 2243ΔmexXY | 8 | 0.25 | 8 | 0.5 |
| 2243ΔmexXY(pAK1900) | 8 | 0.25 | 8 | 0.5 |
| 2243ΔmexXY(pAGH97) | 512 | 16 | >16 | 4 |
| 3795 | >512 | 32 | 4 | 4 |
| 3795ΔmexXY | 4 | 0.5 | 1 | 2 |
| 3795ΔmexXY(pAK1900) | 4 | 0.5 | 1 | 2 |
| 3795ΔmexXY(pAGH97) | >512 | 64 | 8 | 16 |
Conclusion.
Polymyxins exert straightforward and potent antibacterial activity against susceptible Gram-negative species. Recent metabolomic and transcriptomic studies have demonstrated that, in addition to their well-known membrane-disrupting effects, these agents induce important perturbations in multiple cellular functions, including lipid metabolism, LPS production, and peptidoglycan synthesis (39).
In the present work, we show that the wild-type susceptibility, as well as mutation-driven resistance, of P. aeruginosa to colistin depends mainly on synergistic interactions between the outer membrane impermeability mechanism based on Ara4N-based LPS decoration and the active efflux system MexXY/OprM. TCS ParRS definitely appears as a key element in the adaptive (i.e., drug-induced) response of the pathogen to colistin, as ParRS inactivation strongly affects (i) bacterial survival in the presence of lethal drug concentrations and (ii) drug MICs in mutants constitutively overproducing the Arn LPS modification pathway (e.g., pmrB mutants). In apparent contradiction to this pivotal role, mutations activating ParRS poorly affect the resistance of P. aeruginosa to colistin, with a modal 2-fold increase in the MIC (9). Likely accounting for these results, we noticed that mutational activation of ParRS is associated with relatively modest overexpression of the arn locus (i.e., ∼50-fold greater than the wild-type PAO1 level), compared with similar mutations in other TCSs such as PmrAB (e.g., 400- to 600-fold greater) (9, 18). MS experiments (MALDI-TOF MS) would surely be useful to study the correlation between the degree of substitution of LPS molecules with Ara4N and colistin MICs. More puzzling was our observation that, while deletion of parRS genes causes a 2-fold reduction in the colistin MIC (this study and reference 9), it otherwise quite strongly sensitizes P. aeruginosa to early drug killing (Table 1). In addition, neither the suppression of the mexXY operon nor that of PA4773, PA4774, and PA4775 loci further sensitizes the PAO1::lux-ΔparRS mutant to colistin. While our experiments finally demonstrate a functional interdependence of the Arn LPS modification process, the efflux pump MexXY/OprM, and the polyamine biosynthetic pathway determined by PA4773, PA4774, and PA4775, the poor impact of the inactivation of ParRS on colistin MICs may appear somewhat surprising. Actually, as shown in Fig. S2 in the supplemental material, exposure of the mutant PAO1::lux-ΔparRS to colistin triggers a rapid but transient decline in viable cell counts, a phase that is followed by regrowth after 4 h of contact with the drug. Such growth rebound likely involves complex adaptive mechanisms developed by surviving bacteria to counteract the pleiotropic effects of colistin. This delayed adaptation process tends to mitigate the early killing effects of colistin and would explain the relatively low impact of ParRS inactivation on drug MICs (i.e., because the MICs are determined after 18 h of drug contact). Finally, a scenario emerges in which, upon colistin exposure, wild-type cells of P. aeruginosa rapidly decorate their LPS with Ara4N, increase the amounts of polyamines at their surface, and activate the MexXY/OprM-dependent efflux of still unknown molecules in a coordinated way involving TCS ParRS. In addition, the present study highlights the important contribution of the MexXY/OprM pump to the increased colistin resistance of pmrB mutants, a finding that opens new therapeutic perspectives for efflux inhibitors. Such inhibitors would potentially reverse the resistance of pmrB mutants (and possibly pmrA, PhoPQ, and cprRS mutants) to colistin and prevent their emergence under therapy.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. All bacterial cultures were performed at 35 ± 1°C in Mueller-Hinton broth (MHB) (Bio-Rad), on Mueller-Hinton agar (MHA) (Becton, Dickinson) with adjusted concentrations of Ca2+ (from 20 to 25 mg liter−1) and Mg2+ (from 10 to 12.5 mg liter−1), or in M9 minimal medium (42 mM Na2HPO4, 22 mM KH2PO4, 19 mM NH4Cl, 8.5 mM NaCl) supplemented with 5% sucrose as the sole carbon source. The plasmid-containing Escherichia coli strains were selected on MHA supplemented with kanamycin (50 μg ml−1), gentamicin (5 μg ml−1), ampicillin (100 μg ml−1), or streptomycin (50 μg ml−1). Single- and multiple-knockout mutants lacking parRS, pmrAB, phoPQ, cprRS, arnBCADTEF-ugD (arn), PA4773, PA4774, PA4775, PA1797, PA2359, PA2655, mexXY, and/or mexZ genes were constructed by using overlapping PCR and recombination events, as reported previously (9, 40), and the primers listed in Table S2.
Construction of bioluminescent strain PAO1::luxCDABE.
The wild-type reference strain P. aeruginosa PAO1 was rendered strongly bioluminescent by insertion of the luxCDABE operon into the chromosomal site attTn7, using recombinant plasmid pUC18-mini-Tn7T-Gmr-lux modified as follows. The lac promoter region of plasmid pCR2.1-TOPO (Invitrogen) was PCR amplified with primers PlacA1 and PlacA2 (Table S2). The resulting amplicon was digested with SpeI and BamHI endonucleases and then was cloned upstream of the luxCDABE coding sequence in pUC18-mini-Tn7T-Gmr-lux. The new construct, pUC18-mini-Tn7T-Gmr-Placlux, was transferred into strain PAO1 and various derivative mutants by electroporation, allowing the integration of mini-Tn7 into the bacterial chromosome, as described previously (41). To determine the correlation between viable cell counts (CFU) and emitted bioluminescence (relative light units [RLU]), strain PAO1::lux was grown in MHB up to an A600 of 1 (∼109 CFU ml−1). Then, the culture was serially diluted 1:10 (from 108 to 10 CFU ml−1) in MHB, and aliquots of 0.2 ml were removed from each dilution. In parallel, 100 μl was spread onto MHA plates. Colonies of survivors were counted after overnight incubation at 35 ± 2°C. The data presented in the text are means of three independent experiments. The correlation between the number of living bacteria (log10 CFU per milliliter) and bioluminescence (log10 RLU) was linear for inocula greater than 106 CFU ml−1 and is defined by the equation y = 0.8993x − 1.966, with R2 = 0.992.
Strain complementation with plasmid pAKArn.
Genomic DNA of strain PAO1 was extracted by using the Wizard genomic DNA purification kit (Promega Corp., Charbonnières-les-Bains, France). A 8,953-bp fragment containing the whole operon arnBCADTEF-ugd was then amplified by PCR with specific primers PA3552Fw and PA3559Rv (Table S2). This amplicon was first cloned into vector pCR-Blunt II-TOPO. Next, a 9,059-bp fragment was subcloned into the HindIII-XbaI plasmid pAK1900 (to yield pAKArn) and transferred by electroporation into strain PAO1::lux and its deletion mutant PAO1::lux-ΔmexXY. The transformants were selected on MHA supplemented with 150 μg ml−1 ticarcillin.
Antibiotic susceptibility testing.
The MIC of colistin was determined by the standard serial 2-fold microdilution method in MHB, following the CLSI recommendations (28).
Drug bactericidal activities.
Overnight cultures of strain PAO1 and derived mutants were diluted into fresh prewarmed MHB to yield an A600 of 0.5 ± 0.05. The bacteria (5 × 108 CFU ml−1) were incubated for 30 min at 35 ± 2°C, with constant shaking (250 rpm). Further dilutions were performed to obtain appropriate inocula, from 5 × 108 to 106 CFU ml−1, prior to addition of colistin at final concentrations ranging from 0.25 to 16 μg ml−1; 200-μl volumes of culture were removed and transferred into the wells of a microtiter plate at selected time points. Bioluminescence (RLU) emitted by living cells was measured on a multilabel plate luminometer (Victor 2; PerkinElmer). The results were expressed as means of at least three independent experiments, each including two measures at each time point. Because of insufficient sensitivity of bioluminescence for inocula of ≤106 CFU ml−1, colony counting was performed on MHA plates for the highest dilution sample. Briefly, at selected time points after colistin addition, 100 μl of culture was spread onto a MHA plate (dilution of 10−1), and 100 μl was added to 900 μl of MHB in a sterile microtube, to achieve serial 1:10 dilutions from 10−2 to 10−6 in MHB. One hundred microliters of each suspension was spread on MHA plates (n = 3), and the survivors were counted after overnight incubation at 35°C.
RT-qPCR experiments.
Total RNA was extracted from bacteria cultivated in drug-free MHB or in MHB supplemented with 8 μg ml−1 colistin, up to an A600 of 0.8 ± 0.05. mRNA of target genes was reverse transcribed into cDNA and quantified on a Rotor-Gene RG-6000 RT-PCR instrument (Qiagen) by using the primers listed in Table S2 and a Fast Sybr green kit (Qiagen). Gene expression was normalized to that of untreated strain PAO1 (set at 1) after internal normalization with gene uvrD, taken as a reference, as described previously (9). The data presented are means of at least three independent experiments.
Lipid A isolation and analysis by MALDI-TOF MS.
Lipid A was directly extracted from bacterial cells by hydrolysis, as described previously (38, 42). Briefly, lyophilized bacteria were suspended in a mixture of 200 μl of isobutyric acid and 1 M ammonium hydroxide and maintained for 1.5 h at 100°C. The suspension was cooled and centrifuged at 2,000 × g for 10 min. The supernatant was diluted in water and dried. The sample was solubilized in 200 μl of methanol and centrifuged at 2,000 × g for 10 min. The lipid A was extracted from the pellet with a mixture of chloroform, methanol, and water (3:1.5:0.25 [vol/vol/vol]). The lipid A was then analyzed by MALDI-TOF MS (Shimadzu AXIMA Performance), in linear mode, by the LPS-BioSciences partner (Evry, France). Negative-ion and positive-ion mass spectra were recorded. Aliquots of 1 μl of the solution were deposited on the target and covered with the matrix dihydroxybenzoic acid (DHB). The results were compared with data from previous publications (43).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Loïs Andrey for his technical assistance.
This work was funded by the French Ministry of Health through the Santé Publique France agency.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Shlaes DM, Sahm D, Opiela C, Spellberg B. 2013. The FDA reboot of antibiotic development. Antimicrob Agents Chemother 57:4605–4607. doi: 10.1128/AAC.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos A, Falagas ME. 2008. Colistin and polymyxin B in critical care. Crit Care Clin 24:377–391. doi: 10.1016/j.ccc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 6.Yan A, Guan Z, Raetz CR. 2007. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J Biol Chem 282:36077–36089. doi: 10.1074/jbc.M706172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPhee JB, Lewenza S, Hancock RE. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol 50:205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock RE. 2010. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother 54:3372–3382. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller C, Plésiat P, Jeannot K. 2011. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and β-lactams in Pseudomonas aeruginosa. Antimicrob Agents Chemother 55:1211–1221. doi: 10.1128/AAC.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez L, Jenssen H, Bains M, Wiegand I, Gooderham WJ, Hancock RE. 2012. The two-component system CprRS senses cationic peptides and triggers adaptive resistance in Pseudomonas aeruginosa independently of ParRS. Antimicrob Agents Chemother 56:6212–6222. doi: 10.1128/AAC.01530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macfarlane EL, Kwasnicka A, Ochs MM, Hancock RE. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol Microbiol 34:305–316. doi: 10.1046/j.1365-2958.1999.01600.x. [DOI] [PubMed] [Google Scholar]
- 12.McPhee JB, Bains M, Winsor G, Lewenza S, Kwasnicka A, Brazas MD, Brinkman FS, Hancock RE. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J Bacteriol 188:3995–4006. doi: 10.1128/JB.00053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrow K, Kwon DH. 2009. Alterations in two-component regulatory systems of phoPQ and pmrAB are associated with polymyxin B resistance in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:5150–5154. doi: 10.1128/AAC.00893-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham N, Kwon DH. 2009. A single amino acid substitution in PmrB is associated with polymyxin B resistance in clinical isolate of Pseudomonas aeruginosa. FEMS Microbiol Lett 298:249–254. doi: 10.1111/j.1574-6968.2009.01720.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, Miller SI, Hoiby N, Moskowitz SM. 2011. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz SM, Brannon MK, Dasgupta N, Pier M, Sgambati N, Miller AK, Selgrade SE, Miller SI, Denton M, Conway SP, Johansen HK, Hoiby N. 2012. PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 56:1019–1030. doi: 10.1128/AAC.05829-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutu AD, Sgambati N, Strasbourger P, Brannon MK, Jacobs MA, Haugen E, Kaul RK, Johansen HK, Hoiby N, Moskowitz SM. 2013. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob Agents Chemother 57:2204–2215. doi: 10.1128/AAC.02353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolard A, Schniederjans M, Haussler S, Triponney P, Valot B, Plésiat P, Jeannot K. 2019. Production of norspermidine contributes to aminoglycoside resistance in pmrAB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother 63:e01044-19. doi: 10.1128/AAC.01044-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeannot K, Sobel ML, El Garch F, Poole K, Plésiat P. 2005. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J Bacteriol 187:5341–5346. doi: 10.1128/JB.187.15.5341-5346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita Y, Sobel ML, Poole K. 2006. Antibiotic inducibility of the MexXY multidrug efflux system of Pseudomonas aeruginosa: involvement of the antibiotic-inducible PA5471 gene product. J Bacteriol 188:1847–1855. doi: 10.1128/JB.188.5.1847-1855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guénard S, Muller C, Monlezun L, Benas P, Broutin I, Jeannot K, Plésiat P. 2014. Multiple mutations lead to MexXY-OprM-dependent aminoglycoside resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:221–228. doi: 10.1128/AAC.01252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westbrock-Wadman S, Sherman DR, Hickey MJ, Coulter SN, Zhu YQ, Warrener P, Nguyen LY, Shawar RM, Folger KR, Stover CK. 1999. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother 43:2975–2983. doi: 10.1128/AAC.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caughlan RE, Sriram S, Daigle DM, Woods AL, Buco J, Peterson RL, Dzink-Fox J, Walker S, Dean CR. 2009. Fmt bypass in Pseudomonas aeruginosa causes induction of MexXY efflux pump expression. Antimicrob Agents Chemother 53:5015–5021. doi: 10.1128/AAC.00253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. 2010. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother 54:3783–3789. doi: 10.1128/AAC.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3624–3630. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, D'Hondt RE, Jusko WJ, Forrest A, Tsuji BT. 2010. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob Agents Chemother 54:2051–2062. doi: 10.1128/AAC.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-S27 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 30.Johnson L, Mulcahy H, Kanevets U, Shi Y, Lewenza S. 2012. Surface-localized spermidine protects the Pseudomonas aeruginosa outer membrane from antibiotic treatment and oxidative stress. J Bacteriol 194:813–826. doi: 10.1128/JB.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohnuma M, Ganbe T, Terui Y, Niitsu M, Sato T, Tanaka N, Tamakoshi M, Samejima K, Kumasaka T, Oshima T. 2011. Crystal structures and enzymatic properties of a triamine/agmatine aminopropyltransferase from Thermus thermophilus. J Mol Biol 408:971–986. doi: 10.1016/j.jmb.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother 44:3322–3327. doi: 10.1128/aac.44.12.3322-3327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla E, Llobet E, Doménech-Sánchez A, Martínez-Martínez L, Bengoechea JA, Albertí S. 2010. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother 54:177–183. doi: 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng YH, Lin TL, Lin YT, Wang JT. 2018. A putative RND-type efflux pump, H239_3064, contributes to colistin resistance through CrrB in Klebsiella pneumoniae. J Antimicrob Chemother 73:1509–1516. doi: 10.1093/jac/dky054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole K, Lau CH, Gilmour C, Hao Y, Lam JS. 2015. Polymyxin susceptibility in Pseudomonas aeruginosa linked to the MexXY-OprM multidrug efflux system. Antimicrob Agents Chemother 59:7276–7289. doi: 10.1128/AAC.01785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo Y, Eda S, Gotoh N, Yoshihara E, Nakae T. 2004. MexZ-mediated regulation of mexXY multidrug efflux pump expression in Pseudomonas aeruginosa by binding on the mexZ-mexX intergenic DNA. FEMS Microbiol Lett 238:23–28. [DOI] [PubMed] [Google Scholar]
- 38.El Hamidi A, Tirsoaga A, Novikov A, Hussein A, Caroff M. 2005. Microextraction of bacterial lipid A: easy and rapid method for mass spectrometric characterization. J Lipid Res 46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Han ML, Zhu Y, Creek DJ, Lin YW, Gutu AD, Hertzog P, Purcell T, Shen HH, Moskowitz SM, Velkov T, Li J. 2019. Comparative metabolomics and transcriptomics reveal multiple pathways associated with polymyxin killing in Pseudomonas aeruginosa. mSystems 4:e00149-18. doi: 10.1128/mSystems.00149-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 41.Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 42.Ciornei CD, Novikov A, Beloin C, Fitting C, Caroff M, Ghigo JM, Cavaillon JM, Adib-Conquy M. 2010. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun 16:288–301. doi: 10.1177/1753425909341807. [DOI] [PubMed] [Google Scholar]
- 43.Hoiby N, Krogh Johansen H, Moser C, Song Z, Ciofu O, Kharazmi A. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 3:23–35. doi: 10.1016/s1286-4579(00)01349-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.