Pseudomonas aeruginosa is a major cause of respiratory biofilm-related infections in patients with cystic fibrosis. We developed an in vitro pharmacodynamic model to study the activity of antipseudomonal antibiotics against PAO1 biofilms grown in artificial sputum medium with agar [ASM(+)] versus that against biofilms grown in Trypticase soy broth supplemented with glucose and NaCl (TGN). We measured bacterial counts, metabolic activity (fluorescein diacetate [FDA] hydrolysis), and biomass (crystal violet absorbance).
KEYWORDS: Pseudomonas aeruginosa, antibiotic, artificial sputum medium, biofilms, ceftazidime, ciprofloxacin, colistin, cystic fibrosis, meropenem, tobramycin
ABSTRACT
Pseudomonas aeruginosa is a major cause of respiratory biofilm-related infections in patients with cystic fibrosis. We developed an in vitro pharmacodynamic model to study the activity of antipseudomonal antibiotics against PAO1 biofilms grown in artificial sputum medium with agar [ASM(+)] versus that against biofilms grown in Trypticase soy broth supplemented with glucose and NaCl (TGN). We measured bacterial counts, metabolic activity (fluorescein diacetate [FDA] hydrolysis), and biomass (crystal violet absorbance). Biofilms grew slower in ASM(+) than in TGN but reached the same CFU counts and metabolic activity in both media and a slightly higher biomass after 48 h in ASM(+) than in TGN. The concentration-response curves of the antibiotics after 24 h of incubation with mature biofilms showed maximal effects ranging from a 3 (ciprofloxacin)- to a 1.5 (ceftazidime, meropenem)-log10-CFU decrease, with tobramycin and colistin showing intermediate values. These maximal reductions in the numbers of CFU were similar in both media for ciprofloxacin and β-lactams but lower in ASM(+) than in TGN for tobramycin and colistin; they were reached at concentrations lower than the human maximum concentration in plasma for ciprofloxacin and β-lactams only. The reductions in metabolic activity and in biomass were low in both media. Small-colony variants were selected by tobramycin in ASM(+) and by ciprofloxacin in both media. The model was then successfully applied to 4 isolates from patients with cystic fibrosis. These biofilms showed CFU counts similar to those of PAO1 biofilms in ASM(+) but a higher biomass than PAO1 biofilms in ASM(+) and moderate differences in their susceptibility to antibiotics from that of PAO1 biofilms grown in this medium. This model proved useful to establish the pharmacodynamic profile of drugs against P. aeruginosa biofilms in the context of cystic fibrosis.
TEXT
Pseudomonas aeruginosa is the most prevalent microorganism in the respiratory tract of adult patients suffering from cystic fibrosis (CF), being detected in 70% of adults aged 25 years or older (1). It is a major cause of mortality in this population thanks to its capacity to colonize the sputum, where it forms microcolonies embedded in an alginate matrix, strongly suggestive of biofilms (2). Biofilms are indeed defined as microbial communities living in a self-produced matrix essentially composed of polysaccharides, extracellular DNA, and proteins (3–5). These structures offer a protection against host defenses and antibiotics due to the barrier effect of the extracellular matrix as well as to metabolic changes in the bacterial populations, leading to dormant phenotypes less responsive to antibiotics (3, 6).
One of the determining factors in the capacity of P. aeruginosa to colonize the lungs and form biofilms therein is the accumulation of a thickened mucus as a consequence of the dysfunction of the cystic fibrosis transmembrane conductance regulator channel and the subsequent impaired mucociliary clearance of inhaled microbes (7, 8). In addition, P. aeruginosa may display a large variety of phenotypes, like mucoid or small-colony variants (SCVs). The latter can be selected by antibiotics, which inhibit their growth, adding another risk factor for both the persistence of the infection (9, 10) and biofilm formation, as SCVs overproduce matrix polysaccharides (5, 9, 10).
In spite of the clinical importance of these infections, only a few studies have examined in detail the activity of antibiotics in a medium that mimics the sputum of patients with CF (11–13). In the present study, we used a previously described medium (artificial sputum medium [ASM] with agar [ASM(+)]) that mimics much better the composition and rheological properties of the sputum of CF patients (14) than the so-called synthetic CF sputum medium (SCFM) (15). An improved version of SCFM containing major constituents of sputum, like DNA, N-acetyl-d-glucosamine, mucin, and dioleoylphosphatidylcholine, could also have been used (16), but its rheological properties have not been established so far. We study the activity of antibiotics in vitro against biofilms made by P. aeruginosa in this medium and on a pharmacodynamic basis, in order to establish their relative potency and maximal efficacy (17). We systematically compared ASM(+) with Trypticase soy broth (TSB) supplemented by glucose and NaCl (TGN), a medium optimized to favor the growth of biofilms of S. aureus and other bacterial species (17; unpublished data from our team). We selected 5 antibiotics frequently used in these patients, namely, the fluoroquinolone ciprofloxacin, the β-lactams ceftazidime and meropenem, the aminoglycoside tobramycin, and the polymyxin colistin (6).
RESULTS
Establishing experimental conditions for FDA assay with PAO1.
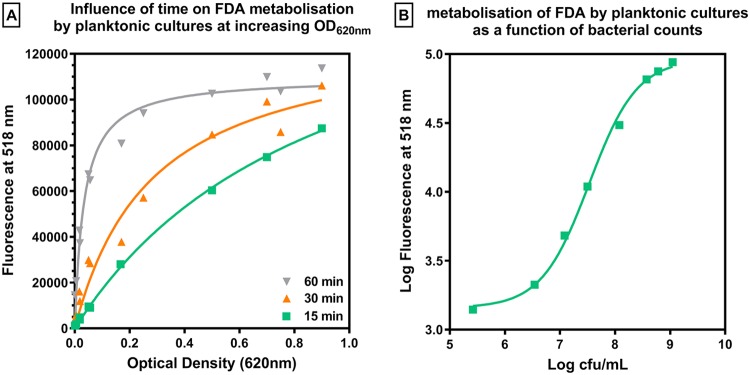
As a preliminary experiment, we determined the optimal conditions for assaying bacterial metabolic activity using the fluorescein diacetate (FDA) assay, in which FDA is cleaved into fluorescein by bacterial enzymes produced by metabolically active bacteria. To this end, we examined the influence of the time of incubation with FDA on fluorescein fluorescence for planktonic cultures of PAO1 at different inocula in cation-adjusted Mueller-Hinton broth (MHB-ca). The numbers of CFU were measured in parallel. Figure 1A shows the evolution of the fluorescence signal as a function of the optical density at 620 nm (OD620) at 3 different incubation times. After 15 min of incubation, the fluorescence signal increased almost linearly (R2 of the linear regression, 0.986) for OD620 values of ≤0.5 (corresponding to approximately 4 × 108 CFU/ml), after which a trend to a saturation of the signal was observed. When the time of incubation was prolonged to 30 or 60 min, the range over which the relationship between the OD620 and fluorescence was linear was restricted to OD620 values of <0.17 (corresponding to approximately 108 CFU/ml). Figure 1B shows the relationship between the fluorescence signal and bacterial counts after 15 min of incubation. This relationship was linear over counts ranging from 106.5 to 108.5 CFU/ml (R2 of the linear regression, 0.9985), covering the numbers recovered from biofilms (see below). This time of incubation was therefore adopted for all experiments.
FIG 1.
Establishing experimental conditions for fluorescein assay. (A) Fluorescein fluorescence signal measured for planktonic cultures of PAO1 at increasing OD620 values after 15, 30, and 60 min of incubation with 100-mg/liter fluorescein diacetate (FDA). (B) Correlation between the fluorescence signal measured after 15 min of incubation and CFU counts. Data are means ± SD from 3 independent determinations.
Setting up the biofilm model with PAO1.
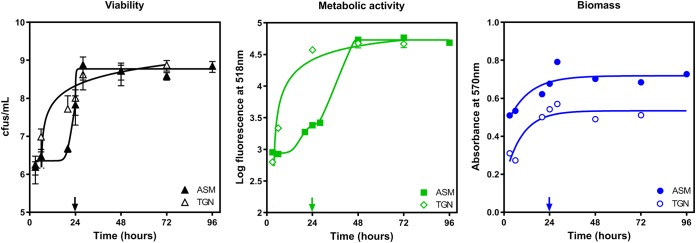
The kinetics of growth of PAO1 in a biofilm was followed over 72 h in TGN and ASM with agar [ASM(+)]. Preliminary experiments showed that PAO1 was unable to grow and form a biofilm if it was directly inoculated in ASM(+), which we attributed to the high viscosity of this medium. We therefore incubated the bacteria first in ASM without agar [ASM(−)] for 24 h to allow for adhesion, after which the medium was replaced by ASM(+). Figure 2 shows the evolution of the numbers of CFU (left); metabolic activity, as assessed by the FDA assay (middle); or biomass, as evaluated by crystal violet staining (right), over time. In TGN, the bacterial counts increased from 107 CFU/ml to 7 × 108 CFU/ml over the first 24 h, after which a plateau was reached. Fluorescein fluorescence and crystal violet absorbance increased in parallel. In ASM, bacterial growth was minimal during the first day of incubation in ASM(−), after which it rapidly recapitulated to reach a plateau at 7 × 108 CFU/ml in 8 h. The fluorescence signal followed a similar pattern, with, however, a slow increase in the fluorescence signal being seen in the first hours following the change of medium from ASM(−) to ASM(+). The biomass also increased over time at a rate similar to that observed in TGN but with slightly higher values all over the time of incubation. On this basis, an incubation time of 24 h in TGN and of 24 h in ASM(−) followed by 24 h in ASM(+) was considered to generate a stable biofilm (with respect to bacterial counts, metabolic activity, and biomass) that could be exposed to antibiotics.
FIG 2.
Kinetics of growth of biofilm in TGN and ASM. The initial inocula were 3 × 107 CFU/ml in TGN and 5.5 × 107 in ASM(−). In this case, attachment was allowed for 24 h in ASM(−), after which the medium was replaced by ASM(+) (highlighted by arrows on the x axes). The graphs show the evolution over time of the numbers of CFU (viability), the fluorescein fluorescence generated by the hydrolysis of fluorescein diacetate (FDA) by metabolically active bacteria (metabolic activity or vitality), or the crystal violet absorbance (biomass). Data are means ± SD from at least 3 independent experiments performed in 3 replicates. When not visible, the SD were smaller than the size of the symbols.
Antibiotic activity against planktonic cultures of PAO1.
Table 1 shows the MICs of the selected antibiotics against PAO1 in TGN and ASM(+) media compared to those in cation-adjusted Mueller-Hinton broth (MHB-ca). PAO1 was fully susceptible to all antibiotics tested. In TGN, MICs were similar (±1 doubling dilution) to those in MHB-ca for all antibiotics except tobramycin, the MIC of which was 8-fold (3 doubling dilutions) higher in TGN. The MICs were 2-fold (1 doubling dilution) lower in ASM(+) than in MHB-ca for ciprofloxacin and tobramycin and 4-fold (2 doubling dilutions) lower for β-lactams but 4-fold (2 doubling dilutions) higher for colistin.
TABLE 1.
Antibiotic susceptibility of PAO1 in different media
| Antibiotic | MIC (mg/liter) |
EUCAST resistance breakpoint (mg/liter)b | Human Cmax (mg/liter)c | ||
|---|---|---|---|---|---|
| MHB-ca | TGN | ASM(+)a | |||
| Ciprofloxacin | 0.125 | 0.125 | 0.06 | 0.5 | 3.7 |
| Ceftazidime | 1 | 0.5 | 0.25 | 8 | 87–170 |
| Meropenem | 0.5 | 0.5 | 0.125 | 8 | 50–115 |
| Tobramycin | 0.25 | 2 | 0.125 | 4 | 16–30 |
| Colistin | 0.5 | 0.5 | 2 | 2 | 2.7 |
MIC determined by counting of the number of CFU after plating due to the turbidity of the medium.
MIC value above which the strain is considered as resistant to the tested antibiotic.
Based on the Belgian summary of product characteristics (prescribing information) for each drug at the conventional and high (as recommended for P. aeruginosa infections) registered doses or based on reference 48 for colistin.
Antibiotic activity against biofilms of PAO1.
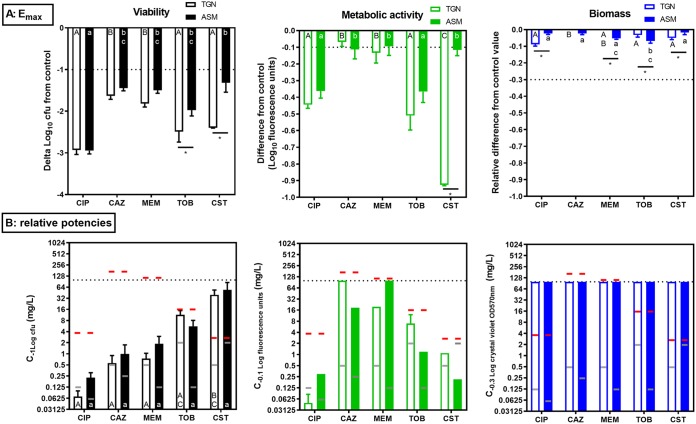
The activities of these five antibiotics against bacterial viability, vitality (metabolic activity), and biofilm biomass were then evaluated after 24 h of incubation of stable biofilms in TGN or in ASM(+), defined as biofilms precultivated for 24 h in TGN or for 24 h in ASM(−) followed by 24 h in ASM(+), respectively. The corresponding concentration-response curves are presented in Fig. 3, and the pertinent calculated pharmacodynamic parameters (the maximal effect [Emax] extrapolated for an infinitely large concentration or the concentration needed to achieve a reduction in the number of CFU of 1 log10 [viability] [C−1 log], which corresponds to the concentration needed for a 0.1-log10 reduction in fluorescein fluorescence [metabolic activity] [C−0.1] or a 0.3-log10 decrease in the crystal violet OD570 [biomass] [C−0.3]; see reference 14 for more explanations of these parameters) are presented in Fig. 4.
FIG 3.
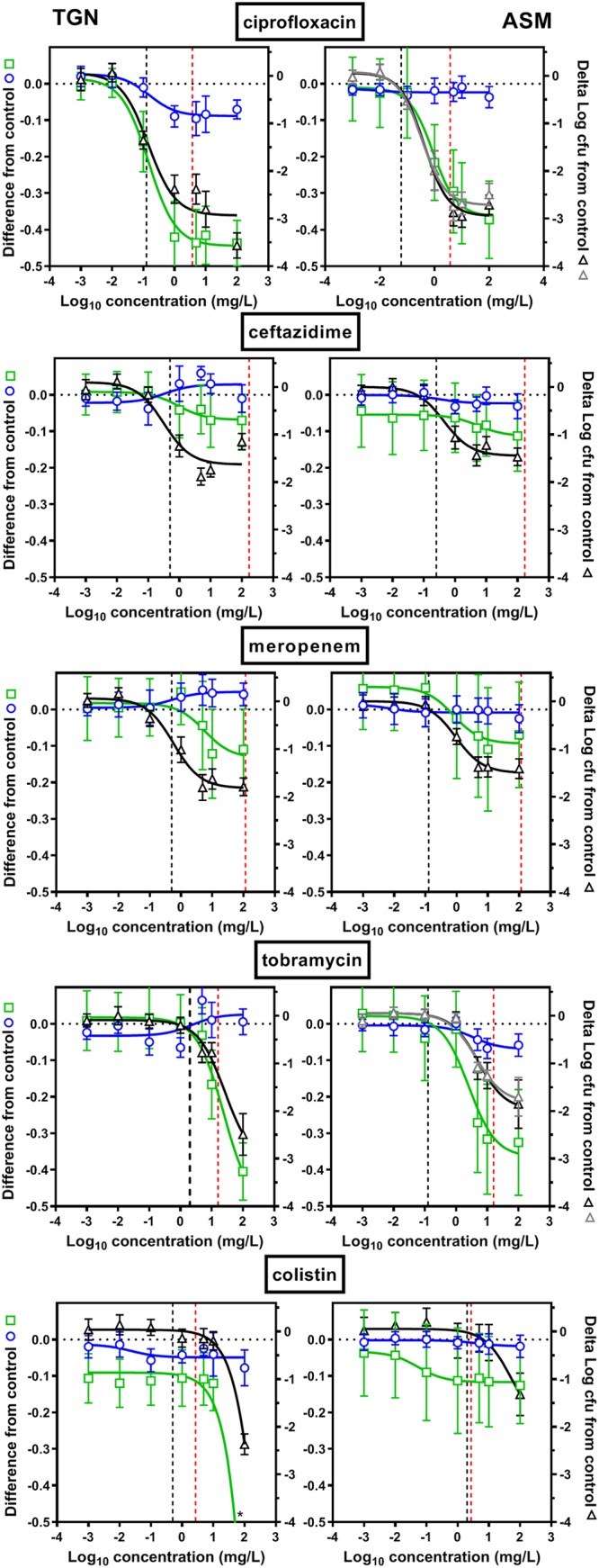
Activities of antibiotics against biofilms in TGN (left) or in ASM(+) (right). Concentration-responses curves of antibiotics against 24-h biofilms of PAO1. Biofilms were incubated with increasing concentration of antibiotics for 24 h (ciprofloxacin, ceftazidime, meropenem, tobramycin, and colistin). The left ordinate shows the decrease in metabolic activity (fluorescein assay, green open squares) or biofilm mass (crystal violet assay, blue open circles), expressed as the decrease from the control value (no antibiotic present; Δlog fluorescence units for the metabolic assay; Δlog OD570 for crystal violet staining). The right ordinate shows the change in viability (CFU counts; colonies were allowed to grow for 24 h [black open triangles] or 48 h [gray open triangles] on PIA plates), expressed as the reduction (on a log scale) from the control value (the value obtained with no antibiotic present). The vertical black dashed line is the MIC of the antibiotic in the corresponding medium, and the red dashed line is the human Cmax after the intravenous administration of a high dose (Table 1). All values are means ± SEM from 3 or 4 independent experiments performed in triplicate (when not visible, the error bars are smaller than the size of symbols). Note that the last data point is at a value lower than −0.5 for metabolic activity with colistin in TGN (−0.9, highlighted by an asterisk), but the scale was maintained to allow comparison with all the other graphs.
FIG 4.
Antibiotic maximal efficacies (Emax) (A) and antibiotic relative potencies (C−1 log, C−0.1, or C−0.3) (B) compared to the values obtained with no treatment for PAO1 in TGN (open bars) and in ASM(+) (closed bars). (A) The graphs show the maximal reduction in the numbers of CFU (left; viability), in log fluorescein fluorescence (middle; metabolic activity), or in log crystal violet OD570 (right; biofilm mass) compared to the values for the untreated controls and as extrapolated from the sigmoid regression of the concentration-response curve for an infinitely large concentration (Fig. 3). For tobramycin and colistin in TGN and for colistin in ASM(+), Emax could not be calculated based on the equation of the sigmoidal regression (the plateau was not reached); the graph therefore shows the effect observed at the highest concentration tested. In the other cases, the Emax value is very similar to the effect observed at a concentration of 100 mg/liter, as the plateau was already reached at this concentration. (B) The graphs show the antibiotic concentration (in milligrams per liter) causing a reduction in the number of CFU of 1 log10 (left; viability) (C−1 log), which corresponds to the concentration needed for a 0.1-log10 reduction in fluorescein fluorescence (middle; metabolic activity) (C−0.1) or a 0.3-log10 decrease in the crystal violet OD570 (right; biofilm mass) (C−0.3), compared to the values for the untreated controls and as intrapolated from the sigmoid regression of the concentration-response curve (Fig. 3). These effects are highlighted by the horizontal dotted line in the top row. Red horizontal lines correspond to the human Cmax after intravenous administration of a high dose; gray horizontal lines correspond to the MICs in the corresponding medium (Table 1). CIP, ciprofloxacin; CAZ, ceftazidime; MEM, meropenem; TOB, tobramycin; CST, colistin. Values are means ± SEM from 3 or 4 independent experiments performed in triplicate. Statistical analyses were performed by two-way analysis of variance with Tukey’s posttest for multiple comparisons; values with different letters are significantly different from each other (P < 0.05). Capital letters compare the results for the antibiotics in TGN; lowercase letters compare the results for the antibiotics in ASM(+) (statistics could not be performed on C−0.1 values [metabolic activity] because the confidence interval on the values was large due to the shape of the curves around this value). *, significant difference between media for a given antibiotic.
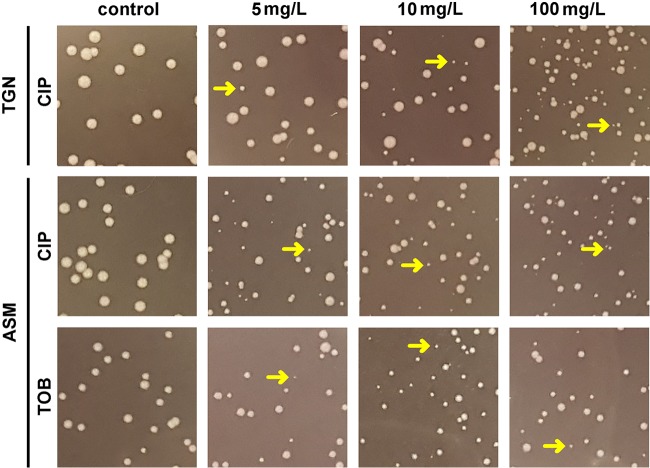
Considering antibiotic effects on CFU first, we observed a concentration-dependent reduction in all cases, which could be fitted to sigmoidal regressions. Minor differences between the two media were observed for all antibiotics except tobramycin and colistin, which were more active in TGN than in ASM(+) (Fig. 4A, left). The maximal effect ranged from a 3-log10-CFU decrease for ciprofloxacin, which was the most effective, to a 1.5- to 1.8-log10-CFU decrease for β-lactams, which were the least effective. For tobramycin and colistin, the maximal effect ranged from a 2- and 3-log10-CFU decrease (note that a plateau value was not reached at the highest concentration tested in these cases, so that we rather report the effect observed at the highest concentration tested [100 mg/liter]). This Emax was obtained at clinically achievable concentrations (lower than the maximum concentration in plasma [Cmax] in humans) for ciprofloxacin and β-lactams but not for tobramycin and colistin (see the dashed red lines in Fig. 3, which point to Cmax values). The concentrations needed to reduce the inoculum by 1 log10 (Fig. 4B, left) were close to the MIC in TGN for ciprofloxacin and β-lactams but higher than the MIC for tobramycin and colistin in TGN and for all drugs in ASM(+). They were lower than the human Cmax in both media for ciprofloxacin and β-lactams, close to this value for tobramycin, but higher for colistin. We noticed the presence of small colonies on plates from biofilms incubated with ciprofloxacin in both media or with tobramycin in ASM(+), the proportion of which increased with the antibiotic concentration (Fig. 5). We therefore compared the CFU counts obtained under these conditions after 24 h and 48 h of incubation of Pseudomonas isolation agar (PIA) plates (black and gray curves, respectively, on the corresponding graphs in Fig. 3) but did not find any difference in the global reduction in bacterial counts, suggesting the we did not underestimate the CFU counts after 24 h, despite the small size of some of them.
FIG 5.
Morphology of colonies of bacteria recovered from biofilms of PAO1 cultivated in TGN medium or ASM(+) and exposed for 24 h to ciprofloxacin (CIP) or tobramycin (TOB) at 5, 10, or 100 mg/liter or under control conditions and plated on PIA. The arrows point to typical colonies of small-colony variants.
Considering, then, the effects of antibiotics on metabolic activity, we observed globally low reductions in the fluorescence signal (compared to those observed in planktonic cultures for equivalent reductions in CFU counts [a 0.1-log10 difference versus a 0.8-log10 difference in the fluorescence signal over a 1-log10 change in the numbers of CFU in biofilms and planktonic cultures, respectively]; see Fig. S1 in the supplemental material), but the range of concentrations over which a reduction in the fluorescence signal was observed matched that over which the antibiotics reduced the CFU counts.
Regarding biomass, all antibiotics failed to cause a significant decrease in crystal violet absorbance in both media.
Adaptability of the model to CF clinical isolates.
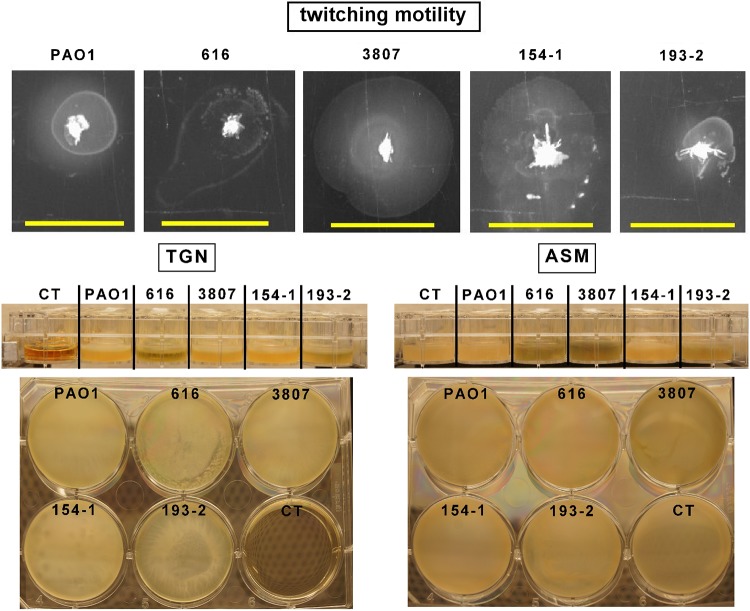
Clinical isolates collected from patients with CF markedly differ from PAO1 and are usually considered higher biofilm producers, partially due to their greater twitching motility (18). We therefore examined whether the model that we set up with PAO1 could also be used for CF clinical isolates. To this effect, we selected 4 clinical isolates fully susceptible to the antibiotics that we were testing here (Table 2) and compared them to PAO1 for their twitching motility, their capacity to form biofilms, and their susceptibility to antibiotics in planktonic cultures and biofilms grown in ASM(+). As illustrated in Fig. 6, these isolates showed higher twitching motility than PAO1 (except for isolate 193-2) and formed a well-visible biofilm below the surface of the culture medium. We then quantified the CFU and biomass for biofilms grown in ASM(+). We did not study the metabolization of fluorescein diacetate for these isolates, because we showed that it was poorly sensitive to the detection of changes in viability after antibiotic exposure for PAO1. Figure 7 shows that, while the CFU counts were similar in clinical isolates and PAO1, biofilm biomass was significantly higher for these isolates than for the reference strain.
TABLE 2.
Antibiotic susceptibility of clinical isolates in MHB-ca and ASM(+)
| Antibiotic | MIC (mg/liter) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 616 (PA921)a |
3807 (PA970)a |
154-1 (PA1024)b |
193-2 (PA1060)b |
|||||
| MHB-ca | ASM(+)c | MHB-ca | ASM(+) | MHB-ca | ASM(+) | MHB-ca | ASM(+) | |
| Ciprofloxacin | 0.5 | 0.5 | 0.032 | 0.064 | 0.5 | 0.5 | 2 | 2 |
| Ceftazidime | 2 | 0.5 | 1 | 1 | 2 | 0.5 | 2 | 1 |
| Meropenem | 0.125 | 0.064 | 0.125 | 0.064 | 0.5 | 1 | 2 | 4 |
| Tobramycin | 4 | 4 | 2 | 1 | 4 | 8 | 1 | 2 |
| Colistin | 2 | 2 | 2 | 4 | 2 | 4 | 1 | 8 |
Laboratoire de Bactériologie, Hôpital Jean Minjoz, Besançon, France.
University Hospital Münster, Münster, Germany.
MICs were determined by counting of the number of CFU after plating due to the turbidity of the medium.
FIG 6.
(Top) Twitching motility of PAO1 and of the 4 CF isolates under study. Bars, 1 cm. (Middle and bottom) Lateral view (middle) and bottom view (obtained using a mirror; bottom) of biofilms made by PAO1 and the 4 CF isolates under study in TGN or ASM(+). The biofilms were cultured in 24-well (lateral view) or 6-well (bottom view) plates for 24 h (TGN) or 24 h in ASM(−) followed by 24 h in ASM(+) (ASM), and pictures were taken under control conditions. The control (CT) is a well containing the culture medium only.
FIG 7.
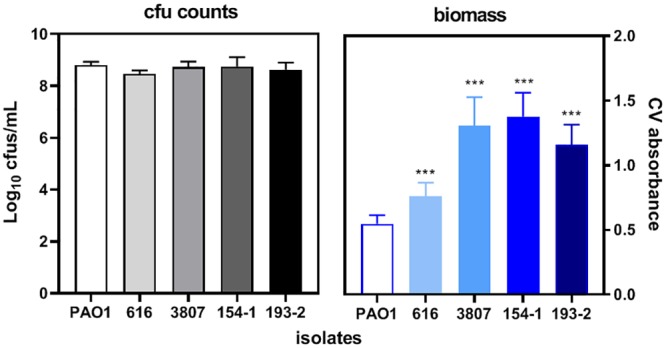
Quantification of CFU (left) and biomass (right; evaluated by crystal violet absorbance) in biofilms made by 4 CF clinical isolates in comparison with those made by PAO1 after 24 h of growth in ASM(−) followed by 24 h of incubation in ASM(+). Data are means ± SEM from 2 independent experiments in 3 replicates for CFU and from 2 experiments in 2 replicates for biomass. Statistical analyses were performed by 1-way analysis of variance with Dunnett’s post hoc test comparing the results for each isolate with those for PAO1. ***, P < 0.001.
In a next step, we examined the activity of the five antibiotics tested previously against these 4 isolates. The MICs in ASM(+) were similar (in most of the cases, ±1 or 2 doubling dilutions, as for PAO1) to those in MHB-ca (Table 2). We then studied the antibiotic activity against biofilms grown in ASM(+), focusing on concentrations corresponding to their respective human Cmax and on the maximal concentration tested against PAO1 (100 mg/liter). Considering the effects of the antibiotics on viability within the biofilms, ciprofloxacin was the most active and colistin was the least active against all strains, as already reported for PAO1 (Fig. 8, left). Some variations in the reduction in the number of CFU were noticed among isolates for each individual antibiotic. They were the most important for ciprofloxacin at 100 mg/liter, which was significantly more active against 2 out of the 4 isolates than against PAO1 (see Fig. S2 for a picture of the corresponding biofilms). As observed for PAO1, drug effects on biomass were minimal (Fig. 8, right). SCVs were observed under the same conditions observed for PAO1 (not shown).
FIG 8.
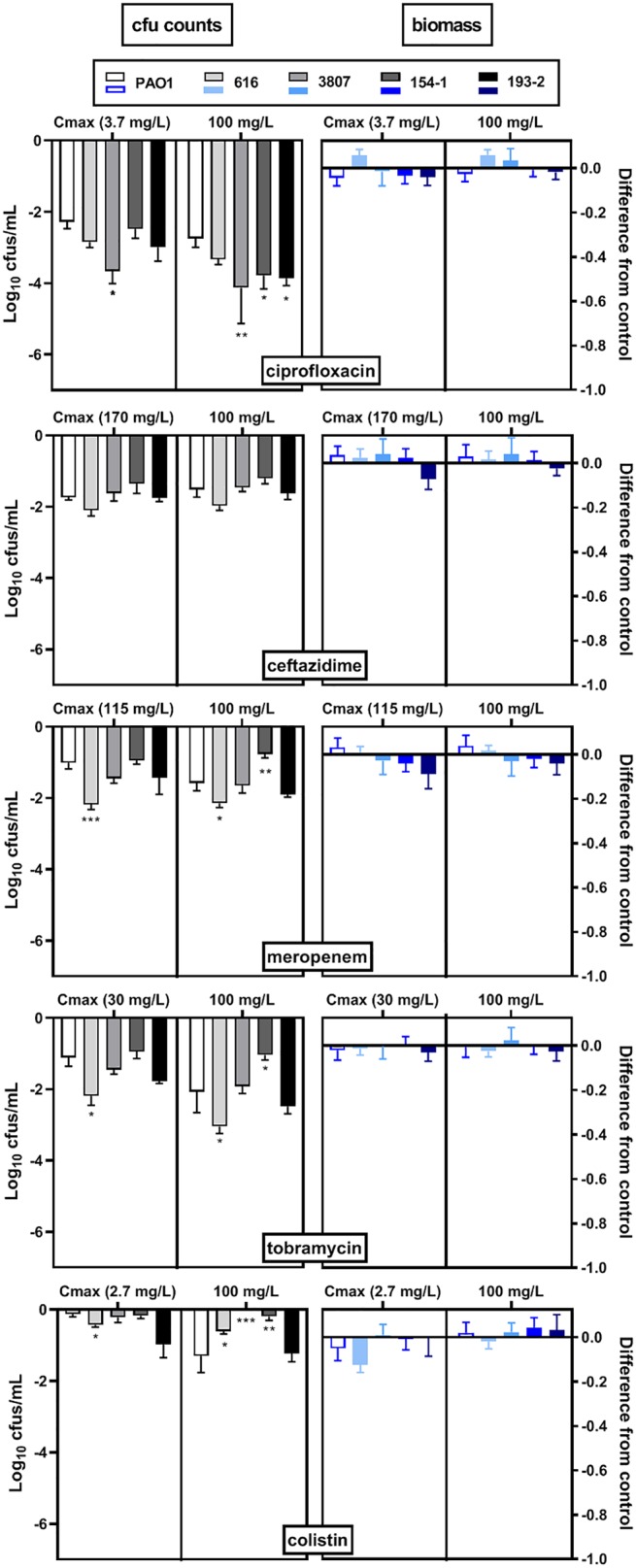
Activity of antibiotics against biofilms grown in ASM(+) for 4 CF clinical isolates in comparison with that for PAO1 and thereafter incubated with antibiotics (ciprofloxacin, ceftazidime, meropenem, tobramycin, and colistin) at their human Cmax (Table 1) or at 100 mg/liter (the maximal concentration tested in the concentration-response curves for PAO1 in Fig. 3) for 24 h. The graphs show the reduction in the log10 number of CFU (left) or in the log10 OD570 of crystal violet compared to the values for the untreated control. Data are means ± SEM from 2 independent experiments in 3 replicates for CFU and from 2 experiments in 2 replicates for biomass. Statistical analyses were performed by 1-way analysis of variance with Dunnett’s post hoc test comparing each isolate with PAO1. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
DISCUSSION
This study is, to the best of our knowledge, the first one to present a comparative view of the pharmacodynamics of antibiotics against P. aeruginosa biofilms in the context of cystic fibrosis. Of interest also, we used here in vitro models comparable to those previously published for Staphylococcus aureus (14), allowing for drawing a point-by-point parallel between these two pathogens that play an important role in lung infections in patients with cystic fibrosis.
A first point of interest is thus to compare the kinetics of biofilm development for both bacterial species. While a plateau value (corresponding to a stable biofilm) was obtained after 24 h of incubation for S. aureus or for P. aeruginosa in TGN, we noticed a delay in CFU growth as well as in increase in metabolic activity for P. aeruginosa biofilms cultivated in ASM(+), since the plateau was reached after 48 h only. We attribute this delay to the need for a 24-h adhesion phase in medium without agar to ensure further P. aeruginosa growth. In addition, although the number of CFU was similar for S. aureus and P. aeruginosa in both media for mature biofilms, the biomass, evaluated by crystal violet staining, was lower for P. aeruginosa than for S. aureus, as well as in TGN versus ASM(+). As previously suggested (19), this could possibly be explained by an inadequate fixation of crystal violet to the biofilms, especially in TGN, because of the larger amounts of water present in the rather slimy matrix built up by P. aeruginosa.
Another salient observation concerns the major difference in the rate of metabolization of FDA by P. aeruginosa in biofilms versus planktonic cultures. Like Peeters et al. (19), we found a linear relationship between the fluorescence signal and the CFU counts over a range of approximately 105 to 108 CFU for both planktonic and biofilm-embedded bacteria, but with much higher signals being seen in biofilms at low bacterial counts, suggesting higher metabolic activity under these conditions than in planktonic cultures. Although this observation is counterintuitive if taking into account the sessile character of bacteria in biofilms, it has already been reported and interpreted as denoting higher levels of hydrolytic enzymes in biofilms (20). Corroborating this hypothesis, secreted esterases are known to be overexpressed during biofilm formation by P. aeruginosa (21), yet this high rate of conversion of FDA in biofilms considerably reduces the sensitivity and suitability of the method to evaluate antibiotic activity in biofilms, pleading for the systematic use of CFU counting.
Considering these antibiotic effects on CFU counts, we observed maximal efficacies ranging from a 1.5- to a 3-log10 decrease in the numbers of CFU, which is similar to what we previously described for S. aureus in TGN (14). Very few in vitro studies have looked into the details of the pharmacodynamics of antibiotic activity against P. aeruginosa in the context of cystic fibrosis, generally concentrating on the determination of minimal biofilm eradication concentration (MBEC) values against clinical isolates. These MBEC have been determined in TSB supplemented with 1% glucose and found to be 300- to 600-fold higher than the MICs for ciprofloxacin, tobramycin, and colistin and more than 2,500-fold higher than the MICs for β-lactams (22), which is coherent with our finding that the maximal efficacy of β-lactams is lower than that of the other drugs.
In contrast to our previous observations with S. aureus (14), the only drugs for which we observed a reduction in maximal efficacy in ASM(+) versus TGN were tobramycin and colistin, i.e., the two drugs which are hydrophilic polycations. This result is coherent with the results of previous studies. First, tobramycin and, to a lesser extent, colistin were found to be more active against biofilms grown in Luria-Bertani broth than against biofilms grown in ASM(−), but at concentrations higher than those tested here (23). These authors attributed this difference to an enrichment of the biofilm matrix in polysaccharides in ASM(−). This explanation may, however, not apply to our study because TGN is supplemented by glucose, which upregulates the expression of the extracellular polysaccharide-related gene pslA, required for biofilm formation (24). Second, the addition of frozen mucus from patients with CF to preformed biofilms was shown to decrease the activity of tobramycin, which was related to a reduced transport of this polycationic drug in the biofilm (25) due to its strong ionic interactions with components of the biofilm matrix and/or mucus (26). Likewise, colistin strongly binds to mucin in vitro, which considerably decreases its activity (27).
In our previous work with S. aureus, we evidenced SCVs specifically in biofilms cultivated in ASM(+) and exposed to tobramycin (14). The same observation was made here, but it was extended to ciprofloxacin in both culture media. While the selection of SCVs by aminoglycosides was described a long time ago in different species, including S. aureus (28), P. aeruginosa (29), or Enterobacteriaceae (30), less is known about those selected by fluoroquinolones. They were described in Salmonella enterica as a transient response to stress (31), but also in P. aeruginosa biofilms grown in TSB (32) or in ASM(−) (33). No specific auxotrophism could be determined for these SCVs (33). P. aeruginosa SCVs are generally considered high biofilm producers (34), less motile (33) and hyperpiliated (35), to be adapted to oxidative stress (35), and producers of smaller amounts of siderophores (36). They are frequently isolated from the sputum of patients with CF but revert quickly to a normal growing phenotype (9, 37). Similar to the SCVs selected in vitro, those isolated from patients do not show any auxotrophism (37). Thus, these SCVs are considered as participating to the physiopathology of biofilm-related infections in the lungs of patients with CF (38).
In contrast to many studies which use extremely high antibiotic concentrations in order to measure MBEC, our concentration-response curves cover the range of concentrations that can be achieved in vivo after intravenous administration. This allows us to conclude that, in both media, a 1-log10 decrease in CFU counts could be achieved at concentrations below the human Cmax for all drugs except colistin. For the β-lactams and ciprofloxacin, decreases of approximately 1.5 log10 and 3 log10, respectively, were obtained at this Cmax, which would argue in favor of the use of these drugs. Yet tobramycin and colistin are also administered by inhalation, generating higher local concentrations, but it should be mentioned that SCVs were more frequently recovered in the sputum from patients receiving aerosolized antibiotics than in patients receiving intravenous antibiotics (37).
Importantly, the biofilm model that we set up here is applicable to isolates from patients suffering from CF. Of interest, these biofilms all form below the surface of the medium, as previously described, and show the presence of bacterial aggregates similar to those found in the sputum of patients with CF (13, 39). We confirm that these isolates globally show a higher twitching motility, recognized as being necessary for exploration and attachment to surfaces and well as for the formation and development of the biofilm architecture (18). They also produce more biofilm biomass than the reference strain. In spite of this larger matrix amount, however, they do not show a lower susceptibility to antibiotics than PAO1, and they even sometimes show a higher susceptibility. Further studies are thus needed to establish the respective role of ASM(+) components and matrix constituents in limiting antibiotic access to bacteria embedded in biofilms.
Our study suffers from some limitations. We included only a few clinical isolates in our work, which may not represent the diversity encountered in clinical isolates, especially because we selected on purpose only fully susceptible ones. We did not modulate the pH or the oxygenation of the medium, which may differ from the physiological conditions in the CF lungs, possibly affecting bacterial growth and antibiotic activity (40, 41). We exposed our biofilms to fixed antibiotic concentrations and for a specific incubation time, which do not mimic the pharmacokinetic variations observed in patients but keep the advantage of facilitating a direct comparison of pharmacodynamic profiles among drugs.
Taking these drawbacks into account, our data nevertheless contribute to rationalize therapeutic failures in the treatment of pulmonary infections by P. aeruginosa in cystic fibrosis, while our model paves the way to further studies aiming at examining in more detail clinical isolates, including those harboring multiresistance; other drugs or drug combinations; as well as innovative therapeutic options.
MATERIALS AND METHODS
Bacterial strain and antibiotics.
P. aeruginosa PAO1 was used as a reference strain. Four isolates from patients with cystic fibrosis previously characterized to be fully susceptible to the antibiotics used in this study were included in specific experiments (42). These strains were routinely grown on Trypticase soy agar (TSA; VWR, Radnor, PA) for overnight culture and on Pseudomonas isolation agar (PIA; Sigma-Aldrich, St. Louis, MO) for determination of the numbers of CFU (colonies were easier to count [round shape] and contaminations could be excluded on this medium).
Antibiotics were obtained as microbiological standards as follows: ciprofloxacin HCl (potency, 93.9%) was from Bayer (Leverkusen, Germany), tobramycin (potency, 100%) was from Galephar (Marche-en-Famenne, Belgium), ceftazidime pentahydrated (potency, 72.5%) was from Panpharma (Luitré-Dompierre, France), and colistin sulfate salt (potency, 79.6%) was from Sigma-Aldrich. Meropenem (potency, 74%) was procured as a generic drug branded product distributed for human parenteral use in Belgium by Sandoz (Holzkirchen, Germany).
Culture media for biofilm cultures.
Three media were used in parallel, namely, Trypticase soy broth (VWR) supplemented with 1% glucose and 2% NaCl (TGN) (17, 43) and two types of artificial sputum media (adapted from references 11, 44, and 45), differing by the fact one is supplemented in agar [ASM(+)] and the other one is not [ASM(−)]. ASM(−) contains, per liter, 10 g mucin (Sigma-Aldrich), 4 g DNA (Sigma-Aldrich), 5.9 mg diethylenetriaminepentaacetic acid (DTPA; Sigma-Aldrich), 5 g NaCl (VWR), 2.2 g KCl (Sigma-Aldrich), 5 g amino acids (Becton, Dickinson, Franklin Lakes, NJ), 1.81 g Tris (Calbiochem, San Diego, CA), and 5 ml egg yolk emulsion (Sigma-Aldrich). ASM(+) contains, in addition, 3 g/liter agar (Becton, Dickinson). All constituents except egg yolk emulsion were autoclaved (egg yolk emulsion was added aseptically to the autoclaved medium), after which the pH was adjusted to 7 with NaOH. We previously showed that ASM(+) better mimics the composition and viscoelastic properties of the mucus found in the respiratory tract of patients with cystic fibrosis (14).
Macroscopic twitching assay.
Twitching motility was determined as previously described (46). Briefly, bacteria were plated onto 1.5% lysogeny broth (LB) agar plates (prepared using lysogeny broth [Alfa Aesar, Ward Hill, MA] and 1.5% agar) and incubated overnight at 37°C, after which a small portion of the bacterial streak was collected with a toothpick and stabbed in the center of a 1% LB agar plate, which was then incubated at 37°C for 24 h. To better visualize the twitching motility, the plates were flooded with a small volume of cold twitching motility (TM) developer solution (400 ml deionized water, 100 ml glacial acetic acid [Merck-Millipore, Burlington, MA], 500 ml methanol [Merck-Millipore], stored at 4°C) for 30 min, after which the top colonies were gently scraped with a plastic disposable loop. The TM developer solution and scraped colonies were carefully decanted into a beaker, and images of the plates were taken using a Bio-Rad Molecular imager (Gel Doc XR+ system; Bio-Rad, Hercules, CA).
Development of the biofilm model.
Except when stated otherwise, biofilms were grown in 96-well plates (European catalog number 655 180; VWR) as previously described (14) when using TGN but with some modifications in ASM(+). In brief, a bacterial suspension was prepared in cation-adjusted Mueller-Hinton broth (MHB-ca; Sigma-Aldrich) starting from overnight cultures on TSA. When using TGN for biofilm growth, the 96-well plates were inoculated (200 μl/well) at approximately 3 × 107 CFU/ml (the OD620 was adjusted to 0.05) and then incubated at 37°C for 24 h (unless stated otherwise) to obtain a mature biofilm. When ASM(+) was used, 200 μl of a suspension at 5.5 × 107 CFU/ml in ASM(−) was inoculated in the 96-well plates and the plates were incubated for 24 h at 37°C to allow for bacterial attachment, after which the medium was removed and replaced by 200 μl of ASM(+) and the plates were reincubated for 24 h at 37°C (unless stated otherwise). In all cases, the plates were covered by Breathe-Easy sealing membranes, which are permeable to gas but which limit evaporation (Sigma-Aldrich). All changes of media or washing steps were carefully performed by slow pipetting from the bottom and close to the edge of the well to avoid removing biofilms growing below the surface of the medium.
Susceptibility testing.
MICs were determined by microdilution following the guidelines of the Clinical and Laboratory Standards Institute using cation-adjusted Mueller-Hinton broth (MHB-ca) (47) and compared to those measured in TGN and ASM(+) following the same protocol. As previously described (14), direct visual reading of the MICs was not possible in ASM(+) due to the intrinsic turbidity of this medium. Aliquots were therefore spread on PIA and incubated overnight, with MICs being defined as the lowest concentration for which there was no change in the number of CFU compared to the initial values. As bacteria tended to form aggregates in ASM(+), the content of each well was carefully homogenized by back-and-forth pipetting before taking aliquots, diluting them, and spreading them on agar plates.
Activity of antibiotics against biofilms.
After reaching maturity {24 h for TGN, 48 h for ASM [24 h in ASM(−), followed by 24 h in ASM(+)]}, the culture medium of the biofilms was removed and replaced with fresh medium (control) or medium supplemented with antibiotics at concentrations ranging from 10−3 to 102 mg/liter in order to obtain full concentration-response curves. The biofilms were reincubated for 24 h at 37°C. At the end of the incubation period, the medium was removed and the biofilm was washed once with 200 μl of 3-morpholinopropane-1-sulfonic acid (MOPS) buffer (20.9 g/liter of MOPS [Sigma-Aldrich], 5.6 g/liter NaCl; the pH was adjusted to 7 with NaOH) (19). Biofilm biomass was quantified using crystal violet, a cationic dye that nonspecifically stains negatively charged constituents in biofilms. The washed biofilms were fixed by heat at 60°C for about 24 h and incubated for 10 min at room temperature with 200 μl of crystal violet (final concentration, 0.2 g/liter; VWR). After removing the excess crystal violet, the plates were washed under running water and dried. The dye fixed to the biofilm was resolubilized in 200 μl of 66% acetic acid and incubated for 1 h at room temperature. The absorbance at 570 nm was measured using a SPECTRAmax Gemini XS microplate spectrophotometer (Molecular Devices LLC, Sunnyvale, CA) (14). The metabolic activity (vitality) in the biofilms was quantified using the fluorescein diacetate (FDA) assay. It is based on the hydrolysis by living bacteria of the nonfluorescent white dye fluorescein diacetate in the yellow highly fluorescent fluorescein (19). This dye is preferred to resazurin (which we used in S. aureus biofilms) to evaluate the metabolic activity of P. aeruginosa because it generates much higher fluorescence signals (19). The washed biofilms were incubated with 100 mg/liter fluorescein diacetate (Sigma-Aldrich) for 15 min (unless stated otherwise) at 37°C in the dark. Fluorescein fluorescence was measured at a wavelength of 518 nm with an excitation wavelength of 494 nm using a SPECTRAmax Gemini XS microplate spectrofluorometer. Bacterial counts were determined in washed biofilms resuspended in 1 ml of sterile phosphate-buffered saline in microcentrifugation tubes. The tubes were vortexed, placed for 5 min in a sonication bath (Bransonic Ultrasonic cleaner 3510E-MT; frequency, 40 kHz; Danbury, CT) to disrupt the biofilm, and vortexed again, after which aliquots were taken and diluted before spreading on PIA plates. Unless stated otherwise, the colonies were counted after 24 h of incubation at 37°C.
Curve-fitting and statistical analyses.
Curve-fitting analyses were made using GraphPad Prism (version 8.02) software (GraphPad Software, San Diego, CA). Data were used to fit a sigmoid function, which allowed us to calculate the maximal effect (Emax; the maximal reduction in viability, vitality, or biomass for an infinitely large concentration of antibiotic) and relative potencies (the antibiotic concentration needed for a reduction in the number of CFU in the biofilms of 1 log10 [C−1 log], which corresponds to the concentration needed for a 0.1-log10 reduction in fluorescein fluorescence [vitality] [C−0.1] or a 0.3-log10 decrease in the crystal violet OD570 [biomass] [C−0.3]). Statistical analyses were performed with GraphPad Instat (version 3.06) software (GraphPad Software) or GraphPad Prism (version 8.02) software.
Supplementary Material
ACKNOWLEDGMENTS
Y.D.I. received a Ph.D. fellowship from the Belgian Fonds pour la Recherche dans l’Industrie et l’Agriculture (FRIA), followed by a bourse du patrimoine of the Université catholique de Louvain. This work was supported by the FNRS (grants T.0189.16, J.0018.17, and J.0162.19) and the Fonds Spéciaux de Recherche from the UCLouvain.
F.V.B. is research director from the Fonds National belge de la Recherche scientifique (FRS-FNRS).
We are grateful to A. Mangin, A. Chaniotacos, and V. Yfantis for helpful technical assistance.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Cystic Fibrosis Foundation. 2018. 2017 patient registry annual data report. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf. Last updated 1 August 2018 Accessed 16 November 2018.
- 2.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 3.Ciofu O, Tolker-Nielsen T. 2019. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol 10:913. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/cmr.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann EE, Wozniak DJ. 2012. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev 36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciofu O, Rojo-Molinero E, Macià MD, Oliver A. 2017. Antibiotic treatment of biofilm infections. APMIS 125:304–319. doi: 10.1111/apm.12673. [DOI] [PubMed] [Google Scholar]
- 7.Ciofu O, Tolker-Nielsen T, Jensen PØ, Wang H, Høiby N. 2015. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev 85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Cohen TS, Prince A. 2012. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med 18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans TJ. 2015. Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis. Future Microbiol 10:231–239. doi: 10.2217/fmb.14.107. [DOI] [PubMed] [Google Scholar]
- 10.Malone JG. 2015. Role of small colony variants in persistence of Pseudomonas aeruginosa infections in cystic fibrosis lungs. Infect Drug Resist 8:237–247. doi: 10.2147/IDR.S68214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sriramulu DD, Lunsdorf H, Lam JS, Romling U. 2005. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol 54:667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 12.Hoiby N, Bjarnsholt T, Moser C, Jensen PO, Kolpen M, Qvist T, Aanaes K, Pressler T, Skov M, Ciofu O. 2017. Diagnosis of biofilm infections in cystic fibrosis patients. APMIS 125:339–343. doi: 10.1111/apm.12689. [DOI] [PubMed] [Google Scholar]
- 13.Kirchner S, Fothergill JL, Wright EA, James CE, Mowat E, Winstanley C. 2012. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J Vis Exp 2012:e3857. doi: 10.3791/3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz Iglesias Y, Wilms T, Vanbever R, Van Bambeke F. 2019. Activity of antibiotics against Staphylococcus aureus in an in vitro model of biofilms in the context of cystic fibrosis: influence of the culture medium. Antimicrob Agents Chemother 63:e00602-19. doi: 10.1128/AAC.00602-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. 2015. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci U S A 112:4110–4115. doi: 10.1073/pnas.1419677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer J, Siala W, Tulkens PM, Van Bambeke F. 2013. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother 57:2726–2737. doi: 10.1128/AAC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 19.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Tribedi P, Gupta AD, Sil AK. 2015. Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresour Bioprocess 2:14. doi: 10.1186/s40643-015-0044-x. [DOI] [Google Scholar]
- 21.Tielen P, Rosenau F, Wilhelm S, Jaeger KE, Flemming HC, Wingender J. 2010. Extracellular enzymes affect biofilm formation of mucoid Pseudomonas aeruginosa. Microbiology 156:2239–2252. doi: 10.1099/mic.0.037036-0. [DOI] [PubMed] [Google Scholar]
- 22.Dosler S, Karaaslan E. 2014. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 62:32–37. doi: 10.1016/j.peptides.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Rozenbaum RT, van der Mei HC, Woudstra W, de Jong ED, Busscher HJ, Sharma PK. 2019. Role of viscoelasticity in bacterial killing by antimicrobials in differently grown Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 63:e01972-18. doi: 10.1128/AAC.01972-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She P, Wang Y, Liu Y, Tan F, Chen L, Luo Z, Wu Y. 2019. Effects of exogenous glucose on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. Microbiologyopen 8:e933. doi: 10.1002/mbo3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller L, Murgia X, Siebenburger L, Borger C, Schwarzkopf K, Sewald K, Haussler S, Braun A, Lehr CM, Hittinger M, Wronski S. 2018. Human airway mucus alters susceptibility of Pseudomonas aeruginosa biofilms to tobramycin, but not colistin. J Antimicrob Chemother 73:2762–2769. doi: 10.1093/jac/dky241. [DOI] [PubMed] [Google Scholar]
- 26.Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL, Penterman J, Singh PK, Chopp DL, Packman AI, Parsek MR. 2013. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol 15:2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang JX, Blaskovich MAT, Pelingon R, Ramu S, Kavanagh A, Elliott AG, Butler MS, Montgomery AB, Cooper MA. 2015. Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother 59:5925–5931. doi: 10.1128/AAC.00808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaaff F, Bierbaum G, Baumert N, Bartmann P, Sahl HG. 2003. Mutations are involved in emergence of aminoglycoside-induced small colony variants of Staphylococcus aureus. Int J Med Microbiol 293:427–435. doi: 10.1078/1438-4221-00282. [DOI] [PubMed] [Google Scholar]
- 29.Gerber AU, Craig WA. 1982. Aminoglycoside-selected subpopulations of Pseudomonas aeruginosa: characterization and virulence in normal and leukopenic mice. J Lab Clin Med 100:671–681. [PubMed] [Google Scholar]
- 30.Musher DM, Baughn RE, Merrell GL. 1979. Selection of small-colony variants of Enterobacteriaceae by in vitro exposure to aminoglycosides: pathogenicity for experimental animals. J Infect Dis 140:209–214. doi: 10.1093/infdis/140.2.209. [DOI] [PubMed] [Google Scholar]
- 31.Drescher SPM, Gallo SW, Ferreira PMA, Ferreira CAS, de Oliveira SD. 2019. Salmonella enterica persister cells form unstable small colony variants after in vitro exposure to ciprofloxacin. Sci Rep 9:7232. doi: 10.1038/s41598-019-43631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belfield K, Bayston R, Hajduk N, Levell G, Birchall JP, Daniel M. 2017. Evaluation of combinations of putative anti-biofilm agents and antibiotics to eradicate biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother 72:2531–2538. doi: 10.1093/jac/dkx192. [DOI] [PubMed] [Google Scholar]
- 33.Sousa AM, Monteiro R, Pereira MO. 2018. Unveiling the early events of Pseudomonas aeruginosa adaptation in cystic fibrosis airway environment using a long-term in vitro maintenance. Int J Med Microbiol 308:1053–1064. doi: 10.1016/j.ijmm.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Kirisits MJ, Prost L, Starkey M, Parsek MR. 2005. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 71:4809–4821. doi: 10.1128/AEM.71.8.4809-4821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Gotz F, Haussler S, Jordan D, Saravanamuthu SS, Wehmhoner D, Strussmann A, Lauber J, Attree I, Buer J, Tummler B, Steinmetz I. 2004. Expression analysis of a highly adherent and cytotoxic small colony variant of Pseudomonas aeruginosa isolated from a lung of a patient with cystic fibrosis. J Bacteriol 186:3837–3847. doi: 10.1128/JB.186.12.3837-3847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabra W, Haddad AM, Zeng AP. 2014. Comparative physiological study of the wild type and the small colony variant of Pseudomonas aeruginosa 20265 under controlled growth conditions. World J Microbiol Biotechnol 30:1027–1036. doi: 10.1007/s11274-013-1521-z. [DOI] [PubMed] [Google Scholar]
- 37.Haussler S, Tummler B, Weissbrodt H, Rohde M, Steinmetz I. 1999. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin Infect Dis 29:621–625. doi: 10.1086/598644. [DOI] [PubMed] [Google Scholar]
- 38.Drenkard E, Ausubel FM. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 39.Haley CL, Colmer-Hamood JA, Hamood AN. 2012. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol 12:181. doi: 10.1186/1471-2180-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pompilio A, Crocetta V, Pomponio S, Fiscarelli E, Bonaventura GD. 2015. In vitro activity of colistin against biofilm by Pseudomonas aeruginosa is significantly improved under “cystic fibrosis-like” physicochemical conditions. Diagn Microbiol Infect Dis 82:318–325. doi: 10.1016/j.diagmicrobio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Quinn RA, Comstock W, Zhang T, Morton JT, da Silva R, Tran A, Aksenov A, Nothias LF, Wangpraseurt D, Melnik AV, Ackermann G, Conrad D, Klapper I, Knight R, Dorrestein PC. 2018. Niche partitioning of a pathogenic microbiome driven by chemical gradients. Sci Adv 4:eaau1908. doi: 10.1126/sciadv.aau1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mustafa MH, Chalhoub H, Denis O, Deplano A, Vergison A, Rodriguez-Villalobos H, Tunney MM, Elborn JS, Kahl BC, Traore H, Vanderbist F, Tulkens PM, Van Bambeke F. 2016. Antimicrobial susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients in northern Europe. Antimicrob Agents Chemother 60:6735–6741. doi: 10.1128/AAC.01046-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siala W, Mingeot-Leclercq MP, Tulkens PM, Hallin M, Denis O, Van Bambeke F. 2014. Comparison of the antibiotic activities of daptomycin, vancomycin, and the investigational fluoroquinolone delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother 58:6385–6397. doi: 10.1128/AAC.03482-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghani M, Soothill JS. 1997. Ceftazidime, gentamicin, and rifampicin, in combination, kill biofilms of mucoid Pseudomonas aeruginosa. Can J Microbiol 43:999–1004. doi: 10.1139/m97-144. [DOI] [PubMed] [Google Scholar]
- 45.Tavernier S, Coenye T. 2015. Quantification of Pseudomonas aeruginosa in multispecies biofilms using PMA-qPCR. PeerJ 3:e787. doi: 10.7717/peerj.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnbull L, Whitchurch CB. 2014. Motility assay: twitching motility. Methods Mol Biol 1149:73–86. doi: 10.1007/978-1-4939-0473-0_9. [DOI] [PubMed] [Google Scholar]
- 47.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 28th informational supplement. CLSI document M100-S28 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 48.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.