Dermatophytosis due to the Trichophyton mentagrophytes-Trichophyton interdigitale complex is being increasingly reported across India. Reports of therapeutic failure have surfaced recently, but there are no clinical break points (CBP) or epidemiological cutoffs (ECVs) available to guide the treatment of dermatophytosis. In this study, a total of 498 isolates of the T. mentagrophytes-interdigitale complex were collected from six medical centers over a period of five years (2014 to 2018).
KEYWORDS: MIC, UL-WT, antifungal resistance, dermatophytes, Trichophyton mentagrophyte
ABSTRACT
Dermatophytosis due to the Trichophyton mentagrophytes-Trichophyton interdigitale complex is being increasingly reported across India. Reports of therapeutic failure have surfaced recently, but there are no clinical break points (CBP) or epidemiological cutoffs (ECVs) available to guide the treatment of dermatophytosis. In this study, a total of 498 isolates of the T. mentagrophytes-interdigitale complex were collected from six medical centers over a period of five years (2014 to 2018). Antifungal susceptibility testing of the isolates was carried out for itraconazole, fluconazole, ketoconazole, voriconazole, luliconazole, sertaconazole, miconazole, clotrimazole, terbinafine, amorolfine, naftifine, ciclopirox olamine, and griseofulvin. The MICs (in mg/liter) comprising >95% of the modeled populations were as follows: 0.06 for miconazole, luliconazole, and amorolfine; 0.25 for voriconazole; 0.5 for itraconazole, ketoconazole, and ciclopirox olamine; 1 for clotrimazole and sertaconazole; 8 for terbinafine; 16 for naftifine; 32 for fluconazole; and 64 for griseofulvin. A high percentage of isolates above the upper limit of the wild-type MIC (UL-WT) were observed for miconazole (29%), luliconazole (13.9%), terbinafine (11.4%), naftifine (5.2%), and voriconazole (4.8%), while they were low for itraconazole (0.2%). Since the MICs of itraconazole were low against the T. mentagrophytes-interdigitale complex, this could be considered the choice of first-line treatment. The F397L mutation in the squalene epoxidase (SE) gene was observed in 77.1% of isolates with a terbinafine MIC of ≥1 mg/liter, but no mutation was detected in isolates with a terbinafine MIC of <1 mg/liter. In the absence of CBPs, evaluation of the UL-WT may be beneficial for managing dermatophytosis and monitoring the emergence of isolates with reduced susceptibility.
INTRODUCTION
An indisputable rise in the prevalence of dermatophytosis with an alarming rise in chronic, recalcitrant, relapse, and recurrent cases has been seen in India over the past few years (1–3). The main etiological agents implicated in these infections are members of three different genera, namely Trichophyton, Microsporum, and Epidermophyton, with various species within each genus (4). In contrast to the past, wherein T. rubrum was the most predominant among the Trichophyton species, a notable rise in the incidence of T. mentagrophytes-interdigitale complex-associated dermatophytosis has been observed in India in recent times (1, 5). Apart from the changing pathogen epidemiology, several atypical clinical presentations are being reported, perhaps due to the rampant use of corticosteroids and poor compliance to therapy, which results in significant disease-related morbidity in affected patients (4, 6).
In the current clinical scenario, experience-driven consensus guidelines for diagnosing and managing dermatophytosis are needed. Recently, the Expert Consensus on the management of Dermatophytosis (ECTODERM) was published in India to bridge the large void in research related to disease management (3). The current treatment recommendations include the use of terbinafine, itraconazole, sertaconazole, ciclopirox olamine, ketoconazole, naftifine, and newer drugs such as amorolfine and luliconazole, which have shown potent fungicidal activity against Trichophyton species (7). However, recent reports of resistance to terbinafine resulting in treatment failure in dermatophytosis caused by species belonging to the T. mentagrophytes-interdigitale complex is of great concern to the treating physicians (1, 2, 8). Apart from allylamine resistance, triazole resistance, especially to fluconazole, is high (35.4%) in the Indian isolates, which is also associated with treatment failure (1).
To guide the treatment choice of an appropriate antifungal agent, the Clinical and Laboratory Standards Institute (CLSI) has introduced guidelines for the antifungal susceptibility testing (AFST) for dermatophytes (9, 10). Clinical breakpoints (CBPs), which are based on information derived from clinical studies, help in predicting response to treatment. However, they depend on several factors such as drug MIC, pharmacokinetic (PK)/pharmacodynamic (PD) parameters, treatment outcome in clinical studies, and postmarketing susceptibility data. In view of the lack of CBPs, either the epidemiological cutoff values (ECVs or ECOFFs) or the upper limit of wild-type MIC (UL-WT) may be useful to differentiate between wild type (WT) and non-wild-type (NWT) isolates, as these depend only on the MIC (11). Unfortunately, to date there is no CBP, ECV, or UL-WT established for any of the Trichophyton species. Available guidelines for the determination of ECV focus on Candida, Cryptococcus, and Aspergillus species (12). In view of the veritable country-wide epidemic of dermatophytosis predominantly by a single species, the T. mentagrophytes-interdigitale complex, and the lack of CBPs, there is an urgent need to define either ECVs or the UL-WT to guide therapy.
In this study, a large collection of T. mentagrophytes-interdigitale complex isolates collected from six medical centers in India was tested against 13 antifungal drugs to elucidate the MIC distribution and to define the India-specific UL-WT for each antifungal agent.
RESULTS
The antifungal susceptibility profiles and UL-WT of 13 antifungal drugs against T. mentagrophytes-interdigitale complex are depicted in Table 1. The antifungal susceptibility testing (AFST) of all 498 isolates was performed against nine antifungals (fluconazole, itraconazole, voriconazole, clotrimazole, sertaconazole, terbinafine, griseofulvin, amorolfine, and ciclopirox olamine). For AFST to sertaconazole, luliconazole, ketoconazole, and naftifine, a total of 327, 415, 463, and 462 isolates were used, respectively. Among the azoles, itraconazole (geometric mean [GM] 0.08 mg/liter) and miconazole (GM 0.06 mg/liter) exhibited potent activity against the T. mentagrophytes-interdigitale complex, whereas among allylamines, terbinafine (GM 0.09 mg/liter) was more potent than naftifine (GM 0.133 mg/liter). Higher MIC90 values were observed for fluconazole (16 mg/liter), terbinafine (8 mg/liter), naftifine (8 mg/liter), and griseofulvin (32 mg/liter). The MIC90 of ketoconazole, clotrimazole, sertaconazole, and ciclopirox olamine were 0.5 mg/liter. Amorolfine, luliconazole, miconazole, itraconazole, and voriconazole had low MIC90 values of 0.06, 0.125, 0.25, 0.25, and 0.25 mg/liter, respectively.
TABLE 1.
MIC and UL-WT distribution of T. mentagrophytes-interdigitale complex isolates against 13 antifungal drugs tested using the CLSI M38-A2 broth microdilution methoda
| Antifungal agent | Total no of isolates | No. of isolates with MIC (mg/liter) of: |
UL-WT 95% | UL-WT 97.5% | % NWT 95% | % NWT 97.5% | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0078 | 0.015 | 0.03 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ||||||
| Miconazole | 327 | 179 | 53 | 39 | 33 | 19 | 1 | 2 | 1 | 0.06 | 0.06 | 29 | 29 | |||||||
| Luliconazole | 415 | 227 | 130 | 34 | 13 | 10 | 1 | 0.06 | 0.125 | 13.9 | 5.7 | |||||||||
| Fluconazole | 498 | 1 | 2 | 26 | 8 | 24 | 17 | 119 | 139 | 92 | 37 | 32 | 1 | 32 | 64 | 0.2 | 0 | |||
| Itraconazole | 498 | 31 | 47 | 28 | 158 | 109 | 81 | 42 | 2 | 0.5 | 1 | 0.2 | 0 | |||||||
| Voriconazole | 498 | 61 | 17 | 83 | 185 | 85 | 43 | 18 | 4 | 1 | 1 | 0.25 | 0.5 | 4.8 | 1.2 | |||||
| Ketoconazole | 463 | 24 | 34 | 113 | 112 | 116 | 59 | 3 | 2 | 0.5 | 1 | 1 | 0.4 | |||||||
| Clotrimazole | 498 | 2 | 3 | 34 | 32 | 51 | 215 | 142 | 16 | 3 | 1 | 1 | 0.6 | 0.6 | ||||||
| Sertaconazole | 498 | 46 | 104 | 58 | 94 | 113 | 64 | 18 | 1 | 1 | 2 | 0.2 | 0 | |||||||
| Naftifine | 462 | 247 | 55 | 29 | 12 | 18 | 12 | 13 | 24 | 28 | 24 | 16* | 16* | 5.2 | 5.2 | |||||
| Terbinafine | 498 | 209 | 88 | 24 | 19 | 17 | 39 | 8 | 18 | 19 | 49 | 6 | 2 | 8* | 8* | 11.4 | 11.4 | |||
| Griseofulvin | 498 | 70 | 9 | 15 | 19 | 29 | 44 | 79 | 198 | 11 | 24 | 64* | 128* | ND | ND | |||||
| Amorolfine | 498 | 59 | 231 | 137 | 48 | 8 | 11 | 3 | 1 | 0.06 | 0.06 | 4.6 | 4.6 | |||||||
| Ciclopirox olamine | 498 | 19 | 2 | 5 | 381 | 41 | 20 | 30 | 0.5 | 0.5 | 10 | 10 | ||||||||
Most frequently obtained MIC or mode is indicated in boldface type. NWT, non-wild-type; ND, not determined; UL-WT, upper limit of wild-type MIC. The UL-WT for naftifine, terbinafine, and griseofulvin are indicated by * and were calculated based on MIC95 and MIC97 values.
The MIC range, MIC50, MIC90, and GM values for all 13 antifungal drugs against T. mentagrophytes-interdigitale complex are summarized in Table 2. Both the 95% and 97.5% MICs were calculated to determine the UL-WT. For the antifungals which showed multimodal, nonsymmetrical, or truncated MIC frequency distribution, both the MIC95 and MIC97 were documented as UL-WT. The highest 95% UL-WTs were observed for griseofulvin (64 mg/liter) and fluconazole (32 mg/liter), whereas the lowest value of 0.06 mg/liter was found for two azoles (miconazole and luliconazole) and morpholine (amorolfine). High percentages of isolates above the UL-WT were observed for miconazole (29%), luliconazole (13.9%), terbinafine (11.4%), naftifine (5.2%), and voriconazole (4.8%), but low percentages for itraconazole (0.2%), sertaconazole (0.2%), and fluconazole (0.2%).
TABLE 2.
Various studies representing the MIC (mg/liter) distribution of Trichophyton mentagrophytes-interdigitale complex isolates against antifungal drugs tested using the CLSI M38-A2 broth microdilution methoda
| Reference | Year | Total no. of drugs tested | No. of isolates | Drug | Range | MIC50 | MIC90 | GM |
|---|---|---|---|---|---|---|---|---|
| This study | 2020 | 13 | 498 (Tm/Ti complex) | Miconazoleb | 0.03–4 | 0.03 | 0.25 | 0.06 |
| Luliconazolec | 0.03–1 | 0.03 | 0.125 | 0.05 | ||||
| Fluconazole | 0.0625–64 | 4 | 16 | 3.28 | ||||
| Itraconazole | 0.0078–1 | 0.06 | 0.25 | 0.08 | ||||
| Voriconazole | 0.0078–4 | 0.06 | 0.25 | 0.05 | ||||
| Ketoconazoled | 0.0078–2 | 0.125 | 0.5 | 0.120 | ||||
| Clotrimazole | 0.0078–2 | 0.25 | 0.5 | 0.231 | ||||
| Sertaconazole | 0.0078–2 | 0.125 | 0.5 | 0.108 | ||||
| Naftifinee | 0.03–16 | 0.03 | 8 | 0.133 | ||||
| Terbinafine | 0.015–32 | 0.03 | 8 | 0.096 | ||||
| Griseofulvin | 0.25–128 | 16 | 32 | 9.27 | ||||
| Amorolfine | 0.0078–4 | 0.015 | 0.06 | 0.024 | ||||
| Ciclopirox olamine | 0.03–2 | 0.25 | 0.5 | 0.289 | ||||
| Rudramurthy et al. (1) | 2018 | 12 | 88 (Ti) | Fluconazole | 2–32 | 4 | 16 | 5.03 |
| Ketoconazole | 0.0625–2 | 0.125 | 0.5 | 0.17 | ||||
| Sertaconazole | 0.03–1 | 0.125 | 0.5 | 0.13 | ||||
| Clotrimazole | 0.125–2 | 0.25 | 0.5 | 0.36 | ||||
| Voriconazole | 0.0312–2 | 0.125 | 0.5 | 0.12 | ||||
| Itraconazole | 0.15–8 | 0.125 | 0.5 | 0.13 | ||||
| Terbinafine | 0.015–32 | 0.0312 | 4 | 0.06 | ||||
| Naftifine | 0.0312–16 | 0.0312 | 8 | 0.1 | ||||
| Amorolfine | 0.007–4 | 0.0156 | 0.0625 | 0.02 | ||||
| Ciclopirox olamine | 0.25–0.5 | 0.25 | 0.25 | 0.25 | ||||
| Griseofulvin | 2–128 | 32 | 64 | 26.31 | ||||
| Luliconazole | 0.0312–0.25 | 0.0312 | 0.125 | 0.05139 | ||||
| Pathania et al. (15) | 2018 | 4 | 36 (Tm) | Griseofulvin | 0.5–128 | 16 | 128 | |
| Fluconazole | 0.12–32 | 4 | 16 | |||||
| Terbinafine | 0.015–8 | 0.125 | 8 | |||||
| Itraconazole | 0.015–1 | 0.063 | 0.5 | |||||
| Khurana et al. (8) | 2018 | 11 | 64 (Ti) | Terbinafine | 0.25–≥32 | 1 | 32 | 2.813 |
| Itraconazole | 0.06–≥16 | 0.25 | 1 | 0.287 | ||||
| Fluconazole | 0.5–≥64 | 16 | 32 | 13.205 | ||||
| Voriconazole | 0.06–2 | 0.25 | 0.5 | 0.202 | ||||
| Ketoconazole | 0.25–≥32 | 0.5 | 0.5 | 0.666 | ||||
| Amphotericin B | 0.25–1 | 0.5 | 0.5 | 0.38 | ||||
| Griseofulvin | 0.5–≥8 | 2 | 4 | 2.874 | ||||
| Miconazole | 0.25–≥16 | 2 | 4 | 1.978 | ||||
| Econazole | 0.5–8 | 2 | 4 | 1.836 | ||||
| Luliconazole | 0.0035–0.125 | 0.007 | 0.007 | 0.005 | ||||
| Sertaconazole | 0.25–≥16 | 1 | 2 | 0.842 | ||||
| Rezaei-Matehkolaei et al. (34) | 2018 | 6 | 66 (Ti) | Efinaconazole | 0.002–0.006 | 0.008 | 0.016 | 0.008 |
| Lanoconazole | 0.001–0.008 | 0.002 | 0.004 | 0.002 | ||||
| Luliconazole | 0.0002–0.004 | 0.0005 | 0.001 | 0.0006 | ||||
| Fluconazole | 4–64 | 8 | 16 | 11.07 | ||||
| Itraconazole | 0.03–0.5 | 0.12 | 0.25 | 0.111 | ||||
| Terbinafine | 0.004–0.12 | 0.015 | 0.03 | 0.013 | ||||
| Salehi et al. (19) | 2018 | 8 | 27 (Ti) | Terbinafine | 0.003–0.125 | 0.01 | 0.06 | 0.01 |
| Griseofulvin | 0.03–64 | 0.12 | 35.2 | 0.41 | ||||
| Itraconazole | 0.01–4 | 0.06 | 1.3 | 0.07 | ||||
| Voriconazole | 0.01–16 | 0.37 | 8.8 | 0.41 | ||||
| Ketoconazole | 0.03–4 | 0.25 | 2.2 | 0.32 | ||||
| Econazole | 0.03–0.5 | 0.06 | 0.5 | 0.08 | ||||
| Lanoconazole | 0.03–0.5 | 0.06 | 0.5 | 0.09 | ||||
| Butenafine | 0.03–0.5 | 0.06 | 0.5 | 0.09 | ||||
| Singh et al. (2) | 2018 | 11 | 63 (Ti) | Terbinafine | 0.06–32 | 1 | 32 | 2.7 |
| Itraconazole | 0.06–16 | 0.5 | 2 | 0.51 | ||||
| Voriconazole | 0.06–16 | 0.25 | 2 | 0.32 | ||||
| Fluconazole | 0.5–64 | 32 | 64 | 16.7 | ||||
| Luliconazole | 0.003–0.5 | 0.015 | 0.06 | 0.014 | ||||
| Sertaconazole | 0.125–16 | 2 | 16 | 2.3 | ||||
| Miconazole | 0.5–16 | 2 | 8 | 2.44 | ||||
| Ketoconazole | 0.125–64 | 1 | 32 | 1.37 | ||||
| Clotrimazole | 0.008–32 | 4 | 4 | 2.83 | ||||
| Amphotericin B | 0.25–8 | 0.5 | 1 | 0.56 | ||||
| Griseofulvin | 1–32 | 4 | 8 | 4.09 | ||||
| Sadeghi-Nejad et al. (20) | 2017 | 2 | 3 (Tm) | Ketoconazole | 0.78—6.25 | 1.56 | 6.25 | 3.52 |
| Griseofulvin | 12.5—100 | 25 | 100 | 56.25 | ||||
| Baghi et al. (22) | 2016 | 12 | 52 (Ti) | Luliconazole | 0.016–0.032 | 0.016 | 0.032 | 0.02 |
| Itraconazole | 0.031–0.5 | 0.25 | 0.5 | 0.18 | ||||
| Micafungin | 0.5–8 | 4 | 8 | 3.31 | ||||
| Fluconazole | 2–64 | 32 | 64 | 20.8 | ||||
| Griseofulvin | 0.5–4 | 1 | 2 | 0.98 | ||||
| Terbinafine | 0.008–0.125 | 0.063 | 0.25 | 0.071 | ||||
| Lanoconazole | 0.063–0.5 | 0.25 | 0.25 | 0.17 | ||||
| Econazole | 0.031–0.5 | 0.5 | 0.5 | 0.3 | ||||
| butenafine | 0.031–1 | 0.25 | 1 | 0.26 | ||||
| Caspofungin | 0.008–0.032 | 0.016 | 0.032 | 0.016 | ||||
| Anidulafungin | 0.008–0.016 | 0.008 | 0.016 | 0.01 | ||||
| Tolnaftate | 0.008–0.125 | 0.063 | 0.25 | 0.076 | ||||
| Ansari et al. (35) | 2015 | 4 | 156 (Ti) | Fluconazole | 4–128 | 32 | 64 | 27.47 |
| Itraconazole | 0.008–0.25 | 0.063 | 0.125 | 0.062 | ||||
| Terbinafine | 0.004–0.25 | 0.016 | 0.125 | 0.017 | ||||
| Griseofulvin | 0.25–4 | 1 | 4 | 0.93 | ||||
| Badali et al. (36) | 2015 | 13 (Tm) | Amphotericin B | 0.125–1 | 1 | 2 | 0.68 | |
| Fluconazole | 16–64 | 32 | 64 | 41.77 | ||||
| Itraconazole | 0.5–16 | 2 | 16 | 2.11 | ||||
| Voriconazole | 0.25–2 | 1 | 2 | 1.41 | ||||
| Posaconazole | 0.5–1 | 1 | 1 | 0.85 | ||||
| Isavuconazole | 0.25–2 | 1 | 2 | 0.94 | ||||
| Caspofungin | 0.5–2 | 1 | 2 | 1.05 | ||||
| Anidulafungin | 0.008–0.063 | 0.031 | 0.063 | 0.03 | ||||
| Terbinafine | 0.016–0.31 | 0.031 | 0.031 | 0.03 | ||||
| Adimi et al. (30) | 2013 | 10 | 136 (Tm) | Terbinafine | 0.0156–16 | 0.0312 | 16 | 0.093 |
| Griseofulvin | 0.0312–56 | 2 | 256 | 2.31 | ||||
| Itraconazole | 0.0009–4 | 0.0625 | 0.5 | 0.035 | ||||
| Ketoconazole | 0.0312–32 | 2 | 8 | 0.43 | ||||
| Fluconazole | 0.0625–256 | 64 | 256 | 10.44 | ||||
| Voriconazole | 0.0156–8 | 0.5 | 4 | 0.174 | ||||
| Clotrimazole | 0.0312–32 | 0.125 | 2 | 0.33 | ||||
| Ciclopirox olamine | 0.0312–32 | 2 | 16 | 1.81 | ||||
| Amorolfine | 0.0078–32 | 0.25 | 8 | 0.245 | ||||
| Naftifine | 0.0625–6 | 0.25 | 12 | 0.326 | ||||
| Yenişehirli et al. (37) | 2013 | 6 | 49 (Tm) | Terbinafine | 0.015–0.125 | 0.06 | 0.125 | 0.06 |
| Amphotericin B | 0.03–2 | 0.125 | 0.5 | 0.14 | ||||
| Miconazole | 0.03–2 | 0.125 | 2 | 0.16 | ||||
| Itraconazole | 0.03–0.5 | 0.25 | 0.5 | 0.13 | ||||
| Ketoconazole | 0.03–4 | 0.25 | 1 | 0.2 | ||||
| Griseofulvin | 0.03–16 | 0.5 | 2 | 0.49 | ||||
| Carrillo-Muñoz et al. (21) | 2013 | 10 | 19 (Ti) | Amorolfine | 0.015 | 0.062 | ||
| Bifonazole | 0.015 | 16 | ||||||
| Clotrimazole | 0.015 | 1 | ||||||
| Econazole | 0.015 | 1 | ||||||
| Ketoconazole | 0.015 | 4 | ||||||
| Miconazole | 0.015 | 4 | ||||||
| Oxiconazole | 0.015 | 4 | ||||||
| Sertaconazole | 0.015 | 1 | ||||||
| Ticonazole | 0.015 | 8 | ||||||
| Terbinafine | 0.015 | 0.5 | ||||||
| 26 (Tm) | Amorolfine | 0.015 | 0.031 | |||||
| Bifonazole | 0.061 | 16 | ||||||
| Clotrimazole | 0.015 | 1 | ||||||
| Econazole | 0.015 | 2 | ||||||
| Ketoconazole | 0.015 | 0.5 | ||||||
| Miconazole | 0.015 | 8 | ||||||
| Oxiconazole | 0.031 | 2 | ||||||
| Sertaconazole | 0.031 | 16 | ||||||
| Ticonazole | 0.015 | 0.5 | ||||||
| Terbinafine | 0.015 | 0.015 | ||||||
| Silva et al. (38) | 2014 | 6 | 24 (Ti) | Ketoconazole | 0.031–16 | 0.25 | 2 | 0.46 |
| Fluconazole | 2–64 | 8 | 64 | 5.9 | ||||
| Griseofulvin | 0.125–64 | 2 | 32 | 1.6 | ||||
| Itraconazole | 0.0312–2 | 0.125 | 0.5 | 0.19 | ||||
| Terbinafine | 0.031–2 | 0.0312 | 0.0312 | 0.03 | ||||
| Voriconazole | 0.0312–1 | 0.062 | 0.5 | 0.24 | ||||
| Ataides et al. (39) | 2012 | 4 | 7 (Tm) | Itraconazole | 0.125–16 | 0.25 | 16 | 0.41 |
| Ketoconazole | 0.5–4 | 0.5 | 4 | 1.48 | ||||
| Griseofulvin | 1–8 | 1 | 8 | 0.61 | ||||
| Terbinafine | 0.015–0.062 | 0.015 | 0.062 | 0.01 | ||||
| Zalacain et al. (40) | 2011 | 5 | 29 (Tm) | Ciclopirox olamine | 0.032–0.500 | 0.25 | 0.5 | 0.18 |
| Fluconazole | 32 | - | - | ND | ||||
| Itraconazole | 0.032–1.000 | 0.5 | 1 | 0.35 | ||||
| Terbinafine | 0.016–0.500 | 0.125 | 0.5 | 0.082 | ||||
| Eberconazole | 0.016–1.000 | 0.25 | 1 | 0.18 | ||||
| Bueno et al. (41) | 2010 | 4 | 18 (Tm) | Itraconazole | 0.03–0.5 | 0.25 | 0.5 | 0.2 |
| Fluconazole | 16–64 | 64 | 64 | 50.37 | ||||
| Terbinafine | 0.007–0.06 | 0.015 | 0.03 | 0.014 | ||||
| Voriconazole | 0.03–0.5 | 0.125 | 0.5 | 0.19 | ||||
| Mota et al. (42) | 2009 | 5 | 14 (Tm) | Fluconazole | 4–16 | 16 | 16 | |
| Itraconazole | 0.03–0.25 | 0.125 | 0.25 | |||||
| Ketoconazole | 0.03–1 | 0.125 | 0.25 | |||||
| Terbinafine | 0.03–0.5 | 0.06 | 0.25 | |||||
| Griseofulvin | 0.25–1 | 0.5 | 0.5 | |||||
| Valverde et al. (43) | 2008 | 3 | 30 (Tm) | Itraconazole | 0.25 | 0.5 | ||
| Fluconazole | 16 | 64 | ||||||
| Voriconazole | 0.12 | 0.25 | ||||||
GM, geometric mean; Ti, Trichophyton interdigitale; Tm, Trichophyton mentagrophytes.
327 isolates screened.
415 isolates screened.
463 isolates screened.
462 isolates screened.
The phylogenetic analysis by internal transcribed spacer (ITS) dendrogram revealed that all our isolates belong to T. mentagrophytes type VIII. Therefore, genotype-based differences in MIC distribution could not be analyzed. Molecular screening for the mechanism of allylamine resistance was performed by sequencing the squalene epoxidase (SE) gene of all isolates with terbinafine at a MIC of ≥1 mg/liter and representative isolates with <1 mg/liter. Of 133 isolates sequenced, the F397L mutation was seen in 74 isolates (55.6%). The MIC range of the isolates with this mutation were between 1 and 32 mg/liter for terbinafine and 0.06 to 16 mg/liter for naftifine. Distribution of the F397L mutation in relation to the terbinafine MICs is provided in Fig. 1. Among 57 isolates with a high terbinafine MIC (≥8 mg/liter), the mutation was observed in 43 isolates (75.4%). Of the 39 isolates with terbinafine MICs between 1 and 4 mg/liter, 31 isolates (79.5%) had the F397L mutation. No mutation was observed in the 37 isolates with MIC values of <1 mg/liter (Fig. 1). While calculating the UL-WT based on the mutation analysis, a lower UL-WT was observed for terbinafine (1 mg/liter versus 8 mg/liter) and naftifine (0.06 mg/liter versus 16 mg/liter) compared to broth microbroth dilution-based results. Thus, the percentage of isolates above the UL-WT was higher both for naftifine (46.5% versus 5.2%) and terbinafine (20.5% versus 11.4%) on mutation analysis versus microbroth dilution method.
FIG 1.
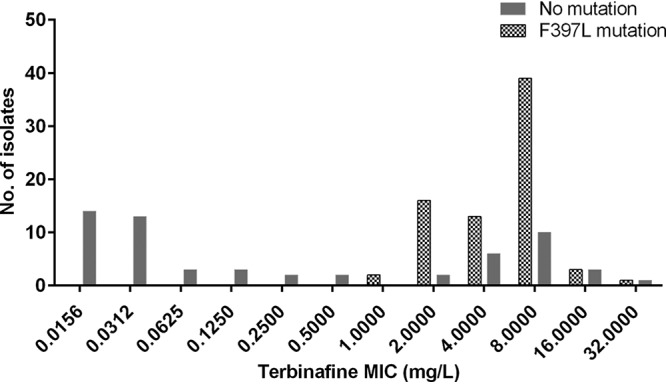
Relationship between mutation in the squalene epoxidase gene and the MIC for terbinafine in 133 T. mentagrophytes-interdigitale complex isolates.
DISCUSSION
The testing for susceptibility to any drug is extremely valuable for defining the likely response of infection by a particular organism. CBPs enable us to predict therapeutic outcomes and are based on clinical trial data, global surveys of susceptibility, and PK/PD parameters (13). Either the ECV or the UL-WT offers an alternative for guiding the choice of optimum treatment by virtue of the MIC data. Since neither the CBPs nor the ECVs or UL-WT for T. mentagrophytes-interdigitale complex have been defined, we conducted this study to determine the UL-WT to antifungal agents commonly used in the management of dermatophytosis. To the best of our knowledge, this is the first multicenter study to propose the UL-WT for the T. mentagrophytes-interdigitale complex isolates of Indian origin.
An alarming rise in prevalence and incidence of dermatophytosis has been observed across India (14). Recent reports indicate an epidemic-like situation, with the chief etiological agent being T. mentagrophytes followed by T. rubrum (1, 2, 15). T. mentagrophytes is a complex group containing different species. The taxonomy of this species is still not well delineated. Based on the ITS sequences, two major studies from India report that the T. mentagrophytes complex causing the epidemic in India belongs to T. interdigitale (1, 2). Later, on the basis of multigene sequences like ITS, beta tubulin, translation elongation factor, and calmodulin gene, all the T. mentragrophytes complex strains of India were found to be distinct from other clades and were described as T. mentragrophytes type VIII. The whole-genome sequence of Indian isolates shares similarity with the recent common ancester, T. mentagrophytes-interdigitale complex, and these could not be conclusively differentiated as two species (16). Thus, we characterized all of our isolates as T. mentagrophytes-interdigitale complex to preclude any misidentification (1, 16–18). Experts recommend that accurate identification of the implicated fungal pathogen and its AFST may be necessary for the management of the recurrent, relapse, or chronic cases (3). Six medical centers from both North India and South India participated in the present study and a large number of T. mentagrophytes-interdigitale complex isolates were evaluated against 13 antifungal agents. The MICs obtained in this study compared with the results of previous studies are provided in Table 2. Previously reported MIC50 ranges for T. mentagrophytes-interdigitale of various antifungal drugs were similar to those observed in the present study (8, 19–22). The MIC distributions of itraconazole, miconazole, luliconazole, voriconazole, ketoconazole, sertaconazole, naftifine, ciclopirox olamine, and clotrimazole show good susceptibility of T. mentagrophytes-interdigitale complex compared to terbinafine, fluconazole, and griseofulvin. Recent reports in Indian literature have revealed the high MIC of terbinafine for Trichophyton spp. (1, 2). Clinical evidence of relapse and incomplete mycological cure after standard (250 mg, twice daily for 2 weeks) oral terbinafine therapy have also been reported (23). Since the pharmacodynamic properties of terbinafine remain incompletely described, a cumulative percentage (%T>MIC) of 100% was used as a conservative approach to describe its dosing interval in an animal study (24). According to that study, the use of 250 mg of terbinafine twice daily was appropriate for treatment of dermatophytic infections caused by T. mentagrophytes with a MIC of 0.01 mg/liter (24). However, since the tissues infected by dermatophytes are avascular components of the skin and adenexa, the time to attain therapeutic concentrations in them may differ greatly from plasma (24). In the present study, we observed that >40% of the T. mentagrophytes-interdigitale complex isolates had a terbinafine MIC of >0.01 mg/liter. A higher dose with a multidosing strategy may be required to treat infections by T. mentagrophytes-interdigitale complex isolates with higher MICs. Unfortunately, this may not be clinically practical due to the possibility of drug-related side effects. These findings emphasize that the use of terbinafine as the first line drug against dermatophytic infections is largely unsubstantiated. In view of the contemporary disease epidemiology, it may have lost clinical efficacy against Indian T. mentagrophytes-interdigitale complex and requires further evaluation. However, although serum levels of terbinafine follow a predictive PK/PD, these levels may not parallel the site-specific drug levels. In fact, some studies show that the skin levels of terbinafine exceed trough plasma levels by nearly 10 to 40 times the MIC, indicating little rationale for higher doses (25).
In clinical practice, CBPs are needed to optimize appropriate antifungal treatment. However, CBPs are not defined for any antifungal agent against dermatophytes. In such a scenario, ECVs may help in identifying isolates with antifungal resistance, as has been seen for other molds such as Aspergillus spp., Mucorales, and Sporothrix spp. (13, 26–28). According to CLSI guidelines, the evaluation of ECVs should follow certain criteria, i.e., (i) the MIC should be determined as per standard guidelines, either by following the protocol of CLSI or the European Committee on Antimicrobial Susceptibility Testing (EUCAST); (ii) large numbers of isolates (n ≥100) should be included; (iii) the MIC of isolates from different laboratories (≥3) should be used; and (iv) the AFST should be performed by many investigators (≥3) (13). Our study fulfils the above criteria (except for partial fulfillment of the last criteria), and thus we propose the UL-WT instead of an ECV for the T. mentagrophytes-interdigitale complex of Indian origin against the antifungal agents in Table 1. For UL-WT determination, isolate identification to the species level is important according to CLSI guidelines (12). In our study, three antifungal agents (terbinafine, naftifine, and griseofulvin) had nonsymmetric, multimodal, or truncated MIC distribution due to which the ECOFF could not be determined using the software. Thus, the UL-WT (in mg/liter) calculated based on the MIC95 (27) for terbinafine, naftifine, and griseofulvin was 8, 16, and 64, respectively. Interestingly, the mutation analysis of the SE gene revealed that even isolates with MICs at less than 1 mg/liter for terbinafine and 0.06 mg/liter for naftifine (both allylamines) exhibited the F397L mutation that was absent in all isolates with MICs of <1 mg/liter for terbinafine and 0.03 mg/liter for naftifine. This finding suggests that the UL-WT of these drugs is probably lower than that estimated based on MIC95 and MIC97. These findings substantiate our interpretation that the use of terbinafine for first-line management of T. mentagrophytes-interdigitale complex infections needs further evaluation. Overall, we noted low MIC and UL-WT values for miconazole, luliconazole, itraconazole, voriconazole, and ketoconazole. Itraconazole has also been found to have a lower MIC than terbinafine against a majority of T. mentagrophytes complex isolates and the use of itraconazole as a first line drug in the management of dermatophytosis may be beneficial (29).
The major limitation of this study was that the majority of the isolates were from a single center, the Postgraduate Institute of Medical Education and Research (PGIMER). Also, AFST was performed at a single institute (PGIMER) independently by laboratory persons belonging to all 6 centers. Another concern is that being a tertiary care center, most patients presenting to us might have prior exposure to antifungals, antibiotics, and/or steroids. This could be responsible for the high MIC values to some antifungals (terbinafine, griseofulvin, and fluconazole) and the UL-WT may artificially encompass resistant (non-wild-type) isolates. Additionally, using the Indian T. mentagrophytes-interdigitale complex to determine the UL-WT may have limited global applicability, as some antifungals have been shown to have high UL-WT values compared to global data. Thus, future multicentric studies involving isolates with more geographical diversity must be designed to further define the ECVs or UL-WT. The inclusion of isolates belonging to different genotypes from diverse geographic locations will enable the analysis of genotype/clade-related differences in MIC distribution, which could not be performed in the present study. The lack of facilities for AFST of dermatophytes at most Indian institutes limited the testing of isolates at more than one center in the present study. Regular training programs and capacity building at multiple centers could enable the determination of ECVs and CBPs in future studies.
In conclusion, UL-WT values are an important tool for distinguishing WT from NWT isolates and provide a preliminary idea to the treating physician in optimizing antifungal therapy. However, the UL-WT values are based solely on the in vitro data of isolates collected from various centers and are not an interpretative breakpoint. Nonetheless, since breakpoints are not available for dermatophyte infections, these values can assist the clinician to predict whether an isolate is likely to respond to a specific antifungal. Future studies assessing the clinical response to treatment and monitoring the PK and PD of antifungal therapy could be beneficial in establishing CBPs.
MATERIALS AND METHODS
Isolates.
A total of 498 clinical isolates of T. mentagrophytes-interdigitale complex were collected over a period of 5 years (2014 to 2018) from six tertiary care hospitals in India. All clinical isolates were recovered from skin (n = 481), hair (n = 13), and nail (n = 4) samples at the following medical centers: Postgraduate Institute of Medical Education and Research, Chandigarh (n = 354), Government Medical College Haldwani, Nainital (n = 44), Jawaharlal Nehru Medical College, Belagavi, Karnataka (n = 41), Father Muller Medical College, Mangalore (n = 32), Bhasker Medical College & General Hospital, Telangana (n = 15), and St. John’s Medical College & Hospital, Bangalore (n = 12). In cases of multiple isolates from the same patient, only unique clinical isolates were used. The identification of all strains as T. mentagrophytes-interdigitale complex was based on culture morphology and microscopic characteristics and confirmed by sequencing of the internal transcribed spacer (ITS) region and 28S region of ribosomal DNA, and beta tubulin (1, 16, 17).
Antifungal susceptibility testing.
The AFST of all the isolates was performed at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, and the tests were performed independently by laboratory personnel of respective institutes contributing the isolates. The strains were subcultured on potato dextrose agar (PDA) medium prior to AFST. The broth microdilution technique was performed according to Clinical and Laboratory Standards Institute (CLSI) M38 A2 protocol with minor modifications, as previously described (1, 10, 30). Inoculum suspension with approximately 106 CFU/ml conidia was collected from 7 to 14 days culture grown on PDA and quantified microscopically by hemocytometer. The suspension was then diluted to 1:100 according to primary concentration. Double the final concentration (103 CFU/ml) of the conidia was adjusted before adding to the drug plates. An initial inoculum corresponding to 65 to 70% transmittance at 530 nm in a spectrophotometer was used. Inoculated plates were incubated at 28°C for 4 days prior to interpretation. In all the isolates, the MICs of azoles, griseofulvin, and amorolfine were documented as the concentration showing prominent inhibition of growth (approximately 80%) compared to that in growth-control wells. For terbinafine, naftifine, luliconazole, ciclopirox olamine, and miconazole, a 100% growth inhibition was documented, as described by Rudramurthy et al. (1). To check the purity of the inoculated plates, 10 μl of the growth from the growth-control well was inoculated onto Sabouraud dextrose agar (10). Panels of 13 topical or systemically applied antifungals commonly used for the treatment of dermatophytosis were tested. The panel included azoles (fluconazole, voriconazole, itraconazole, ketoconazole, sertaconazole, luliconazole, clotrimazole, and miconazole), allylamines (terbinafine and naftifine), a morpholine (amorolfine), an oxaborole/hydroxamic acid (ciclopirox olamine), and an antifungal antibiotic (griseofulvin), all purchased from Sigma-Aldrich, Bengaluru, India. The drugs were dissolved in dimethyl sulfoxide and the final concentrations (in mg/liter) ranged from 0.0625 to 32 for fluconazole; 0.0078 to 4 for voriconazole, itraconazole, and amorolfine; 0.0312 to 16 for ketoconazole, luliconazole, sertaconazole, clotrimazole, ciclopirox olamine, and naftifine; 0.0156 to 64 for terbinafine; 0.0156 to 8 for sertaconazole; and 0.25 to 128 for griseofulvin. T. mentagrophytes-interdigitale complex (NCCPF 800035), Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), and Aspergillus flavus (ATCC 204304) strains were used as quality control measures. The AFST was performed in triplicate for each isolate to ensure quality and reproducibility.
Distribution of MIC and UL-WT determination.
The data analysis was performed using an Excel 2018 spreadsheet. The MIC50 and MIC90 were obtained by a descriptive statistics analysis. In statistical analysis, the modeled population was established based on fitting a normal distribution at the lower end of the MIC and the mean and standard deviation of the normal distribution were calculated to determine the MIC capturing at least 95%, 97.5%, and 99% of the modeled WT population. The Microsoft Excel spreadsheet calculator (ECOFFinder program version XL2000+, http://www.eucast.org/mic_distributions_and_ecoffs/) was used for statistical determination of the UL-WT for the 13 antifungal drugs (26, 31, 32).
The isolate with no mechanisms of acquired resistance or mutational resistance for the antifungal agent tested is defined as the WT. The NWT isolates are those with presumed or known mechanisms of resistance for the antifungal agent being tested. The isolates were classified as WT or NWT depending on whether the MIC was ≤UL-WT or >UL-WT for the above-mentioned antifungals (26, 31, 32).
Phylogenetic analysis.
Phylogenetic analysis was performed using the ITS sequences of representative isolates of the present study and sequences of all genotypes described previously (sequences retrieved from NCBI database) (33). T. quinckeanum was used as the outgroup due to its high divergence. Sequences were aligned using the multiple sequence alignment mode in ClustalX2 software. The aligned sequences were exported to Molecular Evolutionary Genetics Analysis software version 7 (MEGA 7) and a neighbor joining tree was constructed with 1,000 bootstrapping replicates by using Kimura 2 parameter model.
Mutation analysis for terbinafine.
A total of 133 isolates were screened for mutation in the squalene epoxidase gene. The complete gene was sequenced by using the primer pairs SE1aF-5′-CAGAGATAATGCAGCCATCG-3′ and SE1aR-5′-CCGGATTGATGTTCCTAGGT-3′; SE2aF-5′-CCACCAGCGGCGAATATAGA-3′ and SE2aR-5′-AGTCCAGTGCCAGACTGATG-3′; and SE3aF-5′-AGTCTGGCACTGGACTCCAA-3′ and SE3aR-5′-ATGATGCAGCGACGGTGACA-3′ (Sigma) as described earlier (1). Bionumerics software (Applied Maths, Ghent, Belgium) was used for consensus and concatenation of the sequences. The sequences and amino acid sequences depicted by using the ExPASy online tool (https://web.expasy.org/translate/) were aligned.
Data availability.
Sequences of the representative isolates have been deposited in GenBank under accession numbers MH517546 to MH517560, MN822738 to MN822771, MN824042 to MN824085, MN830960 to MN831002, MN830945 to MN830959, and MN831003 to MN831103 for the ITS region of ribosomal DNA; MK967531 to MK967551 for the 28S region of ribosomal DNA; MK982906 to MK982926 for BT; and MN836335 to MN836372 for SE.
ACKNOWLEDGMENTS
This work was supported by the Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) and the Indian Council of Medical Research (ICMR).
REFERENCES
- 1.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, Narang T, Chakrabarti A. 2018. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother 62:e02522-17. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, Meis JF, Chowdhary A. 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan M, Inamadar A, Mittal A, Miskeen AK, Srinivas CR, Sardana K, Godse K, Patel K, Rengasamy M, Rudramurthy S, Dogra S. 2018. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol 18:6–11. doi: 10.1186/s12895-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma S, Madhu R. 2017. The great Indian epidemic of superficial dermatophytosis: an appraisal. Indian J Dermatol 62:227–236. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler U-C, Wittig F, Krüger C, Nenoff K, Wiegand C, Saraswat A, Madhu R, Panda S, Das A, Kura M, Jain A, Koch D, Gräser Y, Uhrlaß S. 2019. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes-a molecular study. Mycoses 62:336–356. doi: 10.1111/myc.12878. [DOI] [PubMed] [Google Scholar]
- 6.Verma S. 2017. Steroid modified tinea. BMJ 356:j973. doi: 10.1136/bmj.j973. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo AK, Mahajan R. 2016. Management of tinea corporis, tinea cruris, and tinea pedis: a comprehensive review. Indian Dermatol Online J 7:77–86. doi: 10.4103/2229-5178.178099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, Gautam RK, Sharma PK, Jain D. 2018. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother 62:e01038-18. doi: 10.1128/AAC.01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2010. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A2, 2nd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Espinel-Ingroff A, Turnidge J. 2016. The role of epidemiological cutoff values (ECVs/ECOFFs) in antifungal susceptibility testing and interpretation for uncommon yeasts and moulds. Rev Iberoam Micol 33:63–75. doi: 10.1016/j.riam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2016. Epidemiological cutoff values for antifungal susceptibility testing. CLSI supplement M59. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Lockhart SR, Ghannoum MA, Alexander BD. 2017. Establishment and use of epidemiological cutoff values for molds and yeasts by use of the Clinical and Laboratory Standards Institute (CLSI) M57 standard. J Clin Microbiol 55:1262–1268. doi: 10.1128/JCM.02416-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dogra S, Uprety S. 2016. The menace of chronic and recurrent dermatophytosis in India: is the problem deeper than we perceive? Indian Dermatol Online J 7:73–76. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. 2018. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol 84:678–684. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Masih A, Monroy-Nieto J, Kumar Singh P, Bowers J, Travis J, Khurana A, Engelthaler DM, Meis JF, Chowdhary A. 2019. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol 3:103266. doi: 10.1016/j.fgb.2019.103266. [DOI] [PubMed] [Google Scholar]
- 17.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y. 2017. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhary A, Singh A, Singh PK, Khurana A, Meis JF. 2019. Perspectives on misidentification of Trichophyton interdigitale/Trichophyton mentagrophytes using internal transcribed spacer region sequencing: urgent need to update the sequence database. Mycoses 62:11–15. doi: 10.1111/myc.12865. [DOI] [PubMed] [Google Scholar]
- 19.Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. 2018. Antifungal drug susceptibility profile of clinically important dermatophytes and determination of point mutations in terbinafine-resistant isolates. Eur J Clin Microbiol Infect Dis 37:1841–1846. doi: 10.1007/s10096-018-3317-4. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi-Nejad B, Rezaei-Matehkolaei A, Yusef Naanaie S. 2017. Isolation and antifungal activity evaluation of Satureja khuzestanica jamzad extract against some clinically important dermatophytes. J Mycol Med 27:554–560. doi: 10.1016/j.mycmed.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Carrillo-Muñoz AJ, Tur-Tur C, Cárdenes D, Rojas F, Giusiano G. 2012. Sertaconazole antifungal profile determined by a microdilution method versus nine topical substances against dermatophyte fungi. Chemotherapy 58:399–404. doi: 10.1159/000345704. [DOI] [PubMed] [Google Scholar]
- 22.Baghi N, Shokohi T, Badali H, Makimura K, Rezaei-Matehkolaei A, Abdollahi M, Didehdar M, Haghani I, Abastabar M. 2016. In vitro activity of new azoles luliconazole and lanoconazole compared with ten other antifungal drugs against clinical dermatophyte isolates. Med Mycol 54:757–763. doi: 10.1093/mmy/myw016. [DOI] [PubMed] [Google Scholar]
- 23.Majid I, Sheikh G, Kanth F, Hakak R. 2016. Relapse after oral terbinafine therapy in dermatophytosis: a clinical and mycological study. Indian J Dermatol 61:529–533. doi: 10.4103/0019-5154.190120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai MR, May ER, Imerman PM, Felz C, Day TA, Carlson SA, Noxon JO. 2011. Terbinafine pharmacokinetics after single dose oral administration in the dog. Vet Dermatol 22:528–534. doi: 10.1111/j.1365-3164.2011.00985.x. [DOI] [PubMed] [Google Scholar]
- 25.Faergemann J, Zehender H, Jones T, Maibach I. 1991. Terbinafine levels in serum, stratum corneum, dermis-epidermis (without stratum corneum), hair, sebum and eccrine sweat. Acta Derm Venereol 71:322–326. [PubMed] [Google Scholar]
- 26.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, Govender NP, Guarro J, Johnson EM, Kidd SE, Pereira SA, Rodrigues AM, Rozental S, Szeszs MW, Ballesté Alaniz R, Bonifaz A, Bonfietti LX, Borba-Santos LP, Capilla J, Colombo AL, Dolande M, Isla MG, Melhem MSC, Mesa-Arango AC, Oliveira MME, Panizo MM, Pires de Camargo Z, Zancope-Oliveira RM, Meis JF, Turnidge J. 2017. Multicenter, international Study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother 61:1–8. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ, Clinical and laboratory standards institute antifungal testing subcommittee . 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol 47:3142–3146. doi: 10.1128/JCM.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tortorano AM, Ghannoum M, Chowdhary A, Chakrabarti A, Lass-Flörl C, Guarro J, Gonzalez GM, Kidd S, Turnidge J, Dannaoui E, Espinel-Ingroff A, Meis JF, Pelaez T, Fothergill A, Dufresne P, Cordoba S. 2015. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob Agents Chemother 59:1745–1750. doi: 10.1128/AAC.04435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia V, Sharma P. 2015. Determination of minimum inhibitory concentrations of itraconazole, terbinafine and ketoconazole against dermatophyte species by broth microdilution method. Indian J Med Microbiol 33:533–537. doi: 10.4103/0255-0857.167341. [DOI] [PubMed] [Google Scholar]
- 30.Parvaneh A, Hashemi Seyed J, Mahmood M, Hossein M, Mohammad RS, Masood E, Ali R-M. 2013. In-vitro activity of 10 antifungal agents against 320 dermatophyte strains using micro dilution method in Tehran. Iran J Pharm Res 12:537–545. [PMC free article] [PubMed] [Google Scholar]
- 31.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 32.Lopez B, Siqueira de Oliveira R, Pinhata JMW, Chimara E, Pacheco Ascencio E, Puyén Guerra ZM, Wainmayer I, Simboli N, Del Granado M, Palomino JC, Ritacco V, Martin A. 2019. Bedaquiline and linezolid MIC distributions and epidemiological cut-off values for Mycobacterium tuberculosis in the latin american region. J Antimicrob Chemother 74:373–379. doi: 10.1093/jac/dky414. [DOI] [PubMed] [Google Scholar]
- 33.Nenoff P, Verma SB, Uhrlaß S, Burmester A, Gräser Y. 2019. A clarion call for preventing taxonomical errors of dermatophytes using the example of the novel Trichophyton mentagrophytes genotype VIII uniformly isolated in the Indian epidemic of superficial dermatophytosis. Mycoses 62:6–10. doi: 10.1111/myc.12848. [DOI] [PubMed] [Google Scholar]
- 34.Rezaei-Matehkolaei A, Khodavaisy S, Alshahni MM, Tamura T, Satoh K, Abastabar M, Shokoohi GR, Ahmadi B, Kord M, Taghipour S, Makimura K, Badali H. 2018. In vitro antifungal activity of novel triazole efinaconazole and five comparators against dermatophyte isolates. Antimicrob Agents Chemother 62:e02423-17. doi: 10.1128/AAC.02423-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansari S, Hedayati MT, Zomorodian K, Pakshir K, Badali H, Rafiei A, Ravandeh M, Seyedmousavi S. 2016. Molecular characterization and in vitro antifungal susceptibility of 316 clinical isolates of dermatophytes in Iran. Mycopathologia 181:89–95. doi: 10.1007/s11046-015-9941-y. [DOI] [PubMed] [Google Scholar]
- 36.Badali H, Mohammadi R, Mashedi O, de Hoog GS, Meis JF. 2015. In vitro susceptibility patterns of clinically important Trichophyton and Epidermophyton species against nine antifungal drugs. Mycoses 58:303–307. doi: 10.1111/myc.12315. [DOI] [PubMed] [Google Scholar]
- 37.Yenişehirli G, Tunçoğlu E, Yenişehirli A, Bulut Y. 2013. In vitro activities of antifungal drugs against dermatophytes isolated in Tokat, Turkey. Int J Dermatol 52:1557–1560. doi: 10.1111/ijd.12100. [DOI] [PubMed] [Google Scholar]
- 38.Silva LB, De Oliveira DB, Da Silva BV, De Souza RA, Da Silva PR, Ferreira-Paim K, Andrade-Silva LE, Silva-Vergara ML, Andrade AA. 2014. Identification and antifungal susceptibility of fungi isolated from dermatomycoses. J Eur Acad Dermatol Venereol 28:633–640. doi: 10.1111/jdv.12151. [DOI] [PubMed] [Google Scholar]
- 39.Ataides FS, Chaul MH, El Essal FE, Costa CR, Souza LKH, Fernandes OFL, Silva M. 2012. Antifungal susceptibility patterns of yeasts and filamentous fungi isolated from nail infection. J Eur Acad Dermatology Venereol 26:1479–1485. doi: 10.1111/j.1468-3083.2011.04315.x. [DOI] [PubMed] [Google Scholar]
- 40.Zalacain A, Obrador C, Martinez JP, Viñas M, Vinuesa T. 2011. Characterization of the antimicrobial susceptibility of fungi responsible for onychomycosis in Spain. Med Mycol 49:495–499. doi: 10.3109/13693786.2010.541949. [DOI] [PubMed] [Google Scholar]
- 41.Bueno JG, Martinez C, Zapata B, Sanclemente G, Gallego M, Mesa AC. 2010. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis. Clin Exp Dermatol 35:658–663. doi: 10.1111/j.1365-2230.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 42.Mota CRA, Miranda KC, Lemos J. d A, Costa CR, Hasimoto e Souza LK, Passos XS, Meneses e Silva H, Silva M. d R R. 2009. Comparison of in vitro activity of five antifungal agents against dermatophytes, using the agar dilution and broth microdilution methods. Rev Soc Bras Med Trop 42:250–254. doi: 10.1590/s0037-86822009000300003. [DOI] [PubMed] [Google Scholar]
- 43.Valverde A, Martín-Mazuelos E, Serrano MC, Almeida C, Castro C, Pemán J. 2008. Comparison of the Sensititre YeastOne colorimetric antifungal panel with the modified Clinical and Laboratory Standards Institute broth microdilution (M38-A) method for antifungal susceptibility testing of dermatophytes. Chemotherapy 54:427–430. doi: 10.1159/000158661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences of the representative isolates have been deposited in GenBank under accession numbers MH517546 to MH517560, MN822738 to MN822771, MN824042 to MN824085, MN830960 to MN831002, MN830945 to MN830959, and MN831003 to MN831103 for the ITS region of ribosomal DNA; MK967531 to MK967551 for the 28S region of ribosomal DNA; MK982906 to MK982926 for BT; and MN836335 to MN836372 for SE.


