Five Candida krusei isolates (susceptible and resistant) recovered from the urine of a kidney transplant patient treated with voriconazole (VRC) 200 mg twice daily for 20 days were studied. Eight unrelated clinical isolates of C. krusei were exposed in vitro to VRC 0.001 μg/ml for 30 days. Development of VRC transient resistance occurred in vivo, and induction of permanent resistance occurred in vitro.
KEYWORDS: Candida krusei, underexposure to antifungal drugs, mechanisms of antifungal resistance, ABC1 ABC2 efflux pump genes, ERG11 gene mutations, candiduria, voriconazole, candidiasis
ABSTRACT
Five Candida krusei isolates (susceptible and resistant) recovered from the urine of a kidney transplant patient treated with voriconazole (VRC) 200 mg twice daily for 20 days were studied. Eight unrelated clinical isolates of C. krusei were exposed in vitro to VRC 0.001 μg/ml for 30 days. Development of VRC transient resistance occurred in vivo, and induction of permanent resistance occurred in vitro. Mostly, ABC1 and ERG11 genes were overexpressed, and a homozygous T418C mutation in the ERG11 gene was found.
INTRODUCTION
Candida species are unique in their ability to both colonize and cause invasive disease in the urinary tract (1). Candiduria is asymptomatic in up to 96% of patients and does not systematically require antifungal therapy (2). However, it can spark development of systemic candidiasis in critically ill patients. Candiduria may also be a sign of candidemia. Patients diagnosed with candiduria in the intensive care unit have higher mortality rates than similar patients without candiduria (3). Fluconazole (FLC) is the first choice for treatment because 60% to 80% is excreted unchanged in urine by the kidneys; also, it reaches high concentrations in urine, exceeding plasma levels by up to 20 times (4–7). Other azoles, such as voriconazole (VRC) and posaconazole (POS), achieve minimal urinary excretion, can concentrate well in kidney tissue, and may be effective in Candida renal parenchymal infections (7–9). Liposomal amphotericin B is not recommended for the treatment of lower urinary tract infections caused by Candida species because it is not found in significant concentrations in urine (10). However, amphotericin B bladder irrigation, although controversial, has recently been considered an alternative treatment in patients with cystitis and urinary fungus balls or those who cannot be treated with systemic antifungals (11–13). Two major mechanisms associated with azole resistance are described in C. krusei: the presence of Abc1 and Abc2 efflux pump proteins, encoded by the ABC1 and ABC2 genes (14), and reduced sensitivity of the Erg11 protein, encoded by the ERG11 gene, to azole antifungals (15).
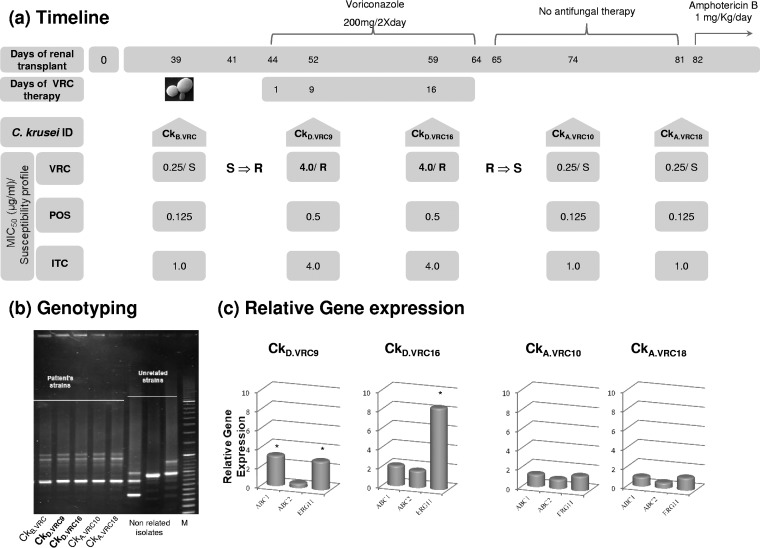
In this study, we describe a case of acquired resistance to VRC in a renal transplant patient with several complications, including candiduria due to C. krusei infection. A 20-year-old woman was submitted to renal transplantation and received immunosuppressive therapy. Twelve days after transplantation, bacteremia due to Escherichia coli developed; the patient was administered intravenous ceftriaxone and ciprofloxacin. On day 18 after transplantation, surgical procedures were performed to preserve the graft. At 25 days after transplantation, the first episode of candiduria occurred. On day 44 posttransplantation, oral VRC 200 mg twice daily was prescribed for 20 days despite its probable low urinary diffusion, associated with low expected efficacy against candiduria. However, the primary concerns were (i) to protect the parenchyma of the kidney transplant from Candida infection and (ii) to treat candiduria, because the patient was a transplant recipient with a high risk of rejecting the transplant. Fluconazole could not be administered due to C. krusei intrinsic resistance, and amphotericin B was not initially considered for treatment because of its nephrotoxicity. At 82 days after renal transplantation, the patient was readmitted to the emergency department with fever; a dissecting aneurysm of the renal artery was diagnosed by computed tomography scan. The kidney graft was resected, and the patient was started on antibiotics and intravenous amphotericin B 1 mg/kg daily for 3 weeks. Since the first episode of candiduria, several C. krusei isolates were recovered from the urine, and five were selected according to their VRC susceptibility profile. They were designated according to the day of isolation, as follows: CkB.VRC (isolated before VRC therapy), CkD.VRC9 and CkD.VRC16 (isolated during VRC therapy, on days 9 and 16), and CkA.VRC10 and CkA.VRC18 (isolated after VRC discontinuation, on days 10 and 18). VRC, POS, and itraconazole (ITC) susceptibility profiles were determined according to CLSI reference protocols M27-A3 and M27-S4, and the susceptibility profile was attributed according to the same protocols (16, 17). The timeline of isolation and susceptibility profiles of the C. krusei clinical isolates are detailed in Fig. 1a. All of the isolates exhibited the same CKRS-1 electrophoretic pattern (Fig. 1b), determined according to Carlotti et al. (18), and the same allelic CKTNR microsatellite profile, i.e., profile e-f/d, described by Shemer et al. (19). Previously, combination of these two typing methods showed a discriminatory power of 0.995 (F. Grenouillet, unpublished data). Therefore, with an error risk of 0.5% (20), we stated that the same C. krusei strain was colonizing the transplanted patient during this period despite exhibiting distinct antifungal susceptibility profiles. Three unrelated C. krusei strains were used as control, two clinical isolates recovered from other patients and C. krusei strain CBS 573 (18, 19).
FIG 1.
In vivo induction of resistance to voriconazole. (a) Timeline of the renal transplant procedure and antifungal therapy; identity of the C. krusei clinical isolates recovered from the kidney-transplant recipient and their respective susceptibility profiles. (b) Genotyping of C. krusei clinical isolates. M, molecular weight marker. (c) Relative gene expression profile of ABC1, ABC2, and ERG11 genes for the C. krusei clinical isolates. *, P ≤ 0.05.
The transient acquisition of resistance to VRC in vivo was intentionally replicated in vitro in the presence of subinhibitory concentrations of VRC: eight independent C. krusei clinical isolates susceptible to VRC obtained from distinct patients not previously submitted to VRC therapy (D0) were grown in brain heart infusion (BHI) containing VRC 0.001 μg/ml for 30 days (D30). The MICs of VRC, POS, and ITC were determined as described previously (16, 17) and are presented in Table 1. The MICs of VRC for the resistant strains were redetermined in accordance with CLSI M27-A3 and M27-S4 protocols in the absence and presence of FK506 (tacrolimus) 100 μg/ml, a blocker of ATP-dependent efflux pumps (21). The MIC values decreased by 2-fold up to 7-fold in all C. krusei strains assayed, and all reverted from resistant to susceptible phenotypes (Table 2). Analyses of resistance gene expression were performed according to Ricardo et al. (22), except for the SYBR green used in the quantitative real-time PCR mixture (SensiFAST SYBR No-Rox mix 1×, Bioline) and the primer annealing temperature of 60°C. Target genes exhibiting a ≥2-fold increase in the expression level and a P value of ≤0.05 were considered significantly overexpressed. The ERG11 gene (1,890 bp) was amplified by PCR and other sequencing reaction conditions and sequence analyses were according to Ricardo et al. (22). Resistant C. krusei clinical isolates CkD.VRC9 and CkD.VRC16 presented significant overexpression of ABC1 and ERG11 genes, which came to basal levels in the posttherapy susceptible isolates (Fig. 1c). No mutations in the ERG11 gene were found in these isolates, which is in accordance with the transient phenotype, where gene expression increases only in the presence of the antifungal. A transient resistant phenotype has already been described in Candida albicans due to the presence of instable aneuploidies, which often disappear when the strains grow in the absence of the drug together with loss of resistance (23, 24). A similar phenomenon may occur in the resistant C. krusei clinical isolates; albeit, the ineffective antifungal treatment due to the low concentration of VRC reaching the urinary tract was enough to induce a transient resistance in vivo. Besides, the acquisition of antifungal drug resistance due to short-term exposure or the presence of low doses of antifungals associated with therapeutic protocols has recently been described in Candida tropicalis and Candida auris isolates (25, 26). C. krusei strains incubated in vitro with very low concentrations of VRC revealed different gene expression profiles. All strains, except Ck24D30, Ck34D30, and Ck40D30, exhibited at least one of the resistance genes significantly overexpressed (>2-fold increase; P< 0.05) compared with the respective susceptible strain (D0). The most remarkable increase in both ABC efflux genes was registered in strain Ck21D30, which also presented the highest MIC value of VRC (Table 1). This fact can explain the highest reduction in the MIC value of VRC in the presence of FK506 (Table 2), and efflux was probably the main mechanism of resistance in this strain. Different mutations in the ERG11 gene sequence were found in these strains (Table 1). The mutations found in strains Ck21D30, Ck24D30, Ck32D30, and Ck42D30 were previously described in C. albicans (27); in particular, the T418C mutation found in strains Ck24D30 (homozygous) and Ck42D30 (heterozygous) was associated with VRC resistance in C. krusei (22) and C. albicans (28, 29). Strain Ck24D30, which had the lowest expression of resistance genes, was the only strain showing a homozygous T418C mutation translating into Tyr140→His. We can relate this mutation to its resistance phenotype, although other experiments, such as expression of the mutated allele in a susceptible yeast strain, could be performed to confirm this association. Concerning strain Ck42D30, because the evolution of resistance can occur in a stepwise manner (30), occurrence of the heterozygous T418C mutation may represent an intermediate step toward a more stable resistant phenotype. The other two heterozygous mutations in strains Ck21D30 and Ck32D30 (Table 1) translate the amino acid Ala122 to Ser or Val, respectively, located within 12 Å of the Erg11 protein binding site for VRC and FLC (31), thus interfering with azole affinity to the target protein. Strains Ck34D30 and Ck40D30 presented no mutation in the ERG11 gene or significant overexpression of efflux pumps encoding genes and ERG11, which may explain the resistance phenotype. Studies by Cuomo and colleagues (32), identified orthologues of genes in a C. krusei clinical isolate that were previously associated with drug resistance in C. albicans. Hence, these orthologue genes may be present in these strains conferring resistance and explaining the reversion of the phenotype in the presence of FK506. The presence of multiple mechanisms of resistance in C. albicans confers crossed resistance to azoles (33); therefore, the same may occur in our resistant C. krusei strains, explaining not only the resistance phenotype to VRC but also the elevated MIC values of POS and ITC.
TABLE 1.
Susceptibility profile; relative expression of ABC1, ABC2, and ERG11 genes; and ERG11 gene mutations of C. krusei strains incubated in vitro with VRC
| Strain ID | Biological sample | MIC50 (μg/ml)/susceptibility profilea for: |
Gene expression (fold increase [P value]) |
ERG11 gene sequence mutations |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VRC |
POS |
ITC |
ABC1 | ABC2 | ERG11 | D0 | D30 | |||||
| D0 | D30 | D0 | D30 | D0 | D30 | |||||||
| Ck1 | Sputum | 0.25/S | 2.0/R | 0.125 | 0.5 | 0.25 | 1.0 | 3.67 (0.0045) | b | b | ||
| Ck8 | Mouth | 0.25/S | 2.0/R | 0.125 | 1.0 | 0.5 | >16 | 5.55 (0.0132) | b | b | ||
| Ck21 | Digestive fistula | 0.50/S | 8.0/R | 0.25 | 1.0 | 0.5 | >16 | 8.89 (0.009) | 16.02 (0.0028) | b | Heterozygous nonsynonymous G364G/T (A122→S) | |
| Ck24 | Urine | 0.25/S | 4.0/R | 0.25 | 0.25 | 0.5 | >16 | b | b | b | Homozygous nonsynonymous T418C (Y140→H) | |
| Ck32 | Sputum | 0.125/S | 2.0/R | 0.125 | 1.0 | 0.25 | >16 | 2.15 (0.099) | b | 2.97 (0.0015) | Heterozygous nonsynonymous C365C/T (A122→V) | |
| Ck34 | Stools | 0.50/S | 4.0/R | 0.25 | 0.5 | 0.5 | >16 | b | b | b | ||
| Ck40 | Sputum | 0.25/S | 2.0/R | 0.25 | 0.5 | 0.5 | >16 | b | b | b | ||
| Ck42 | Ascites | 0.25/S | 4.0/R | 0.25 | 0.25 | 0.5 | >16 | 2.07 (0.0089) | b | 2.71 (0.0013) | Heterozygous nonsynonymous C1091T (A364→V) | Heterozygous nonsynonymous C1091T (A364→V) and T418C (Y140→H) |
S, susceptible; R, resistant.
Gene expression fold increase <2; P ≥ 0.05.
TABLE 2.
MIC and susceptibility profile to VRC alone and with FK506
| Resistance induction | Strain | MIC50 (μg/ml)/susceptibility profile for: |
|
|---|---|---|---|
| VRC | VRC + FK506 100 μg/ml | ||
| In vivo | CkD.VRC9 | 4/R | 0.125/S |
| CkD.VRC16 | 4/R | 0.125/S | |
| In vitro | Ck1D30 | 2/R | 0.25/S |
| Ck8D30 | 2/R | 0.06/S | |
| Ck21D30 | 8/R | 0.06/S | |
| Ck24D30 | 4/R | 1.0/I | |
| Ck32D30 | 2/R | 0.125/S | |
| Ck34D30 | 4/R | 0.125/S | |
| Ck40D30 | 2/R | 0.06/S | |
| Ck42D30 | 4/R | 0.25/S | |
In summary, the development of resistance in C. krusei strains from different backgrounds was studied, even in the presence of very low doses of VRC, both in vivo and in vitro. This shows that candiduria should not be considered an innocent situation, and physicians should pay particular attention to such cases. Different mechanisms of resistance were found, i.e., increased efflux pump expression and the presence of mutations in the ERG11 gene conferring increased resistance to VRC and promoting the high MIC values of POS and ITC.
ACKNOWLEDGMENTS
We thank Isabel Santos for the excellent lab technical support.
I.M.M. was supported by FCT (Fundação para a Ciência e a Tecnologia) Ciência 2008 and cofinanced by the European Social Fund. R.M.S. was partially funded by FEDER (Programa Operacional Factores de Competitividade-COMPETE) and by National Funds through FCT (projects UID/BIM/04501/2013 and POCI-01-0145-FEDER-007628 and grant SFRH/BPD/111148/2015). The remaining authors have no conflicts of interest to report.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Fisher JF. 2011. Candida urinary tract infections—epidemiology, pathogenesis, diagnosis, and treatment: executive summary. Clin Infect Dis 52:S429–432. doi: 10.1093/cid/cir108. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman CA. 2014. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am 28:61–74. doi: 10.1016/j.idc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Bougnoux M-E, Kac G, Aegerter P, d'Enfert C, Fagon J-Y, CandiRea Study Group . 2008. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med 34:292–299. doi: 10.1007/s00134-007-0865-y. [DOI] [PubMed] [Google Scholar]
- 4.Brammer KW, Coakley AJ, Jezequel SG, Tarbit MH. 1991. The disposition and metabolism of [14C]fluconazole in humans. Drug Metab Dispos 19:764–767. [PubMed] [Google Scholar]
- 5.Thomas L, Tracy CR. 2015. Treatment of fungal urinary tract infection. Urol Clin North Am 42:473–483. doi: 10.1016/j.ucl.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Sobel JD, Kauffman CA, McKinsey D, Zervos M, Vazquez JA, Karchmer AW, Lee J, Thomas C, Panzer H, Dismukes WE, The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group . 2000. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. Clin Infect Dis 30:19–24. doi: 10.1086/313580. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JF, Sobel JD, Kauffman CA, Newman CA. 2011. Candida urinary tract infections—treatment. Clin Infect Dis 52:S457–S466. doi: 10.1093/cid/cir112. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LB, Kauffman CA. 2003. Voriconazole: a new triazole antifungal agent. Clin Infect Dis 36:630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 9.Raad II, Graybill JR, Bustamante AB, Cornely OA, Gaona-Flores V, Afif C, Graham DR, Greenberg RN, Hadley S, Langston A, Negroni R, Perfect JR, Pitisuttithum P, Restrepo A, Schiller G, Pedicone L, Ullmann AJ. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis 42:1726–1734. doi: 10.1086/504328. [DOI] [PubMed] [Google Scholar]
- 10.Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP. 2007. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother 59:941–951. doi: 10.1093/jac/dkm077. [DOI] [PubMed] [Google Scholar]
- 11.Malani AN, Kauffman CA. 2007. Candida urinary tract infections: treatment options. Expert Rev Anti Infect Ther 5:277–284. doi: 10.1586/14787210.5.2.277. [DOI] [PubMed] [Google Scholar]
- 12.Tuon FF, Amato VS, Penteado Filho SR. 2009. Bladder irrigation with amphotericin B and fungal urinary tract infection–systematic review with meta-analysis. Int J Infect Dis 13:701–706. doi: 10.1016/j.ijid.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KA, Caylor MM, Lin FC, Campbell-Bright S. 2017. Comparison of amphotericin B bladder irrigations versus fluconazole for the treatment of Candiduria in intensive care unit patients. J Pharm Pract 30:347–352. doi: 10.1177/0897190016645032. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar SK, Edlind TD. 2001. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med Mycol 39:109–116. doi: 10.1080/mmy.39.1.109.116. [DOI] [PubMed] [Google Scholar]
- 15.Orozco AS, Higginbotham LM, Hitchcock CA, Parkinson T, Falconer D, Ibrahim AS, Ghannoum MA, Filler SG. 1998. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother 42:2645–2649. doi: 10.1128/AAC.42.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standard Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. Document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Clinical and Laboratory Standard Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. Approved standard M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Carlotti A, Chaib F, Couble A, Bourgeois N, Blanchard V, Villard J. 1997. Rapid identification and fingerprinting of Candida krusei by PCR-based amplification of the species-specific repetitive polymorphic sequence CKRS-1. J Clin Microbiol 35:1337–1343. doi: 10.1128/JCM.35.6.1337-1343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shemer R, Weissman Z, Hashman N, Kornitzer D. 2001. A highly polymorphic degenerate microsatellite for molecular strain typing of Candida krusei. Microbiology 147:2021–2028. doi: 10.1099/00221287-147-8-2021. [DOI] [PubMed] [Google Scholar]
- 20.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol 26:2465–2466. doi: 10.1128/JCM.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao US, Scarborough GA. 1994. Direct demonstration of high affinity interactions of immunosuppressant drugs with the drug binding site of the human P-glycoprotein. Mol Pharmacol 45:773–776. [PubMed] [Google Scholar]
- 22.Ricardo E, Miranda IM, Faria-Ramos I, Silva RM, Rodrigues AG, Pina-Vaz C. 2014. In vivo and in vitro acquisition of resistance to voriconazole by Candida krusei. Antimicrob Agents Chemother 58:4604–4611. doi: 10.1128/AAC.02603-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selmecki AM, Forche A, Berman J. 2006. Aneuploidy and isochromosome formation in drug resistant Candida albicans. Science 313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coste A, Selmecki A, Forche A, Diogo D, Bougnoux M-E, d'Enfert C, Berman J, Sanglard D. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell 6:1889–1904. doi: 10.1128/EC.00151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 6:ofz262. doi: 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan Z, Ahmad S, Mokaddas E, Meis J, Joseph L, Abdullah A, Vayalil S. 2018. Development of echinocandin resistance in Candida tropicalis following short-term exposure to caspofungin for empiric therapy. Antimicrob Agents Chemother 62:e01926-17. doi: 10.1128/AAC.01926-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morio F, Loge C, Besse B, Hennequin C, Le Pape P. 2010. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn Microbiol Infect Dis 66:373–384. doi: 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Sanglard D, Ischer F, Koymans L, Bille J. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother 42:241–253. doi: 10.1128/AAC.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chau AS, Mendrick CA, Sabatelli FJ, Loebenberg D, McNicholas PM. 2004. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob Agents Chemother 48:2124–2131. doi: 10.1128/AAC.48.6.2124-2131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasse C, Dunkel N, Schäfer T, Schneider S, Dierolf F, Ohlsen K, Morschhäuser J. 2012. The stepwise acquisition of fluconazole resistance mutations causes a gradual loss of fitness in Candida albicans. Mol Microbiol 86:539–556. doi: 10.1111/j.1365-2958.2012.08210.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukuoka T, Johnston DA, Winslow CA, de Groot MJ, Burt C, Hitchcock CA, Filler SG. 2003. Genetic basis for differential activities of fluconazole and voriconazole against Candida krusei. Antimicrob Agents Chemother 47:1213–1219. doi: 10.1128/aac.47.4.1213-1219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuomo CA, Shea T, Yang B, Rao R, Forche A. 2017. Whole genome sequence of the heterozygous clinical isolate Candida krusei 81-B-5. G3 (Bethesda) 7:2883–2889. doi: 10.1534/g3.117.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford CB, Funt JM, Abbey D, Issi L, Guiducci C, Martinez DA, Delorey T, Li BY, White TC, Cuomo C, Rao RP, Berman J, Thompson DA, Regev A. 2015. The evolution of drug resistance in clinical isolates of Candida albicans. Elife 4:e00662. doi: 10.7554/eLife.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]