The purpose of this study was to evaluate the pharmacokinetics of ritonavir-boosted fosamprenavir during pregnancy and postpartum. Amprenavir (the active moiety of fosamprenavir) and ritonavir intensive pharmacokinetic evaluations were performed at steady state during the second and third trimesters of pregnancy and postpartum. Plasma concentrations of amprenavir and ritonavir were measured using high-performance liquid chromatography. The target amprenavir area under the concentration-versus-time curve (AUC) was higher than the 10th percentile (27.
KEYWORDS: AIDS, amprenavir, fosamprenavir, human immunodeficiency virus, pharmacokinetics, postpartum, pregnancy, ritonavir
ABSTRACT
The purpose of this study was to evaluate the pharmacokinetics of ritonavir-boosted fosamprenavir during pregnancy and postpartum. Amprenavir (the active moiety of fosamprenavir) and ritonavir intensive pharmacokinetic evaluations were performed at steady state during the second and third trimesters of pregnancy and postpartum. Plasma concentrations of amprenavir and ritonavir were measured using high-performance liquid chromatography. The target amprenavir area under the concentration-versus-time curve (AUC) was higher than the 10th percentile (27.7 μg · h/ml) of the median area under the curve for ritonavir-boosted fosamprenavir in adults receiving twice-daily fosamprenavir-ritonavir at 700 mg/100 mg. Twenty-nine women were included in the analysis. The amprenavir AUC from time zero to 12 h (AUC0–12) was lower (geometric mean ratio [GMR], 0.60 [confidence interval {CI}, 0.49 to 0.72] [P < 0.001]) while its apparent oral clearance was higher (GMR, 1.68 [CI, 1.38 to 2.03] [P < 0.001]) in the third trimester than postpartum. Similarly, the ritonavir AUC0–12 was lower in the second (GMR, 0.51 [CI, 0.28 to 0.91] [P = 0.09]) and third (GMR, 0.72 [CI, 0.55 to 0.95] [P = 0.005]) trimesters than postpartum, while its apparent oral clearance was higher in the second (GMR, 1.98 [CI, 1.10 to 3.56] [P = 0.06]) and third (GMR, 1.38 [CI, 1.05 to 1.82] [P = 0.009]) trimesters than postpartum. The amprenavir area under the curve exceeded the target for 6/8 (75%) women in the 2nd trimester, 18/28 (64%) in the 3rd trimester, and 19/22 (86.4%) postpartum, and the trough concentrations (Cmin) of amprenavir were 4- to 16-fold above the mean amprenavir-protein-adjusted 50% inhibitory concentration (IC50) of 0.146 μg/ml. Although amprenavir plasma concentrations in women receiving ritonavir-boosted fosamprenavir were lower during pregnancy than postpartum, the reduced amprenavir concentrations were still above the exposures needed for viral suppression.
INTRODUCTION
Fosamprenavir (FPV), a calcium phosphoester prodrug of amprenavir (APV), in combination with low-dose ritonavir (RTV), is a protease inhibitor (PI) that is not recommended for use in pregnant women living with HIV but may be an option in certain circumstances. FPV is available as 700-mg tablets and is currently dosed as FPV-RTV at 700 mg/100 mg twice daily (1). Although FPV-RTV is not routinely used in preventing perinatal transmission, it is still of benefit in people living with HIV in countries where novel PIs are currently unavailable or in FPV treatment-experienced adults living with HIV (2). APV has also been shown to be efficacious against breast cancer by inhibiting the activity of extracellular signal-regulated kinase 2 (ERK2), inhibiting tumor growth in human MCF-7 cancer cells, and inducing apoptosis both in vitro and in vivo, making APV a promising drug for future anticancer therapeutics (3).
Fosamprenavir, upon oral administration, is rapidly and extensively converted to the active drug APV in the intestinal mucosa (4–6). APV is subsequently metabolized in the liver by cytochrome P450 3A4 (CYP3A4), primarily by oxidation to two major metabolites, M2 and M3 (7). APV is an inhibitor of the HIV-1 protease enzyme: it binds to the HIV protease active site and blocks replication by inhibiting the cleavage of the HIV-1 55 Gag precursor protein into p17 and p24 core proteins, which are necessary for viral maturation (8). APV and its metabolites are excreted mainly in feces (75%) and urine (14%) (9). Due to physiological and immunological changes that occur during pregnancy (increased CYP3A activity [10], increased volume of distribution [V], and increased renal clearance), there is decreased exposure to many antiretrovirals (ARVs), particularly the PIs, during the second and third trimesters of pregnancy (11, 12).
The pharmacokinetics (PK) of FPV-RTV were studied previously in pregnant and postpartum women attending HIV pregnancy clinics in New York by Cespedes et al. (13). Amprenavir exposure decreased by 35% during the 2nd trimester of pregnancy and by 25% during the 3rd trimester with 700-mg/100-mg FPV-RTV twice-daily dosing compared to postpartum (13). Similarly, APV trough plasma concentrations (Cmin) decreased by 36% during the 2nd trimester and by 38% in the 3rd trimester of pregnancy with 700-mg/100-mg FPV-RTV twice-daily dosing compared to postpartum. However, the PK analysis by Cespedes et al. was limited to six patients in the second trimester and nine patients in the third trimester and postpartum (13). A larger sample size is critically important in PK studies, as it provides a better understanding of intra- and interindividual variability needed for robust PK predictions (14). Therefore, the goal of the present study was to evaluate the PK of FPV-RTV (700/100 mg twice daily) during pregnancy using a larger and diverse sample size of women living with HIV from multiple countries.
RESULTS
Demographic characteristics and clinical outcomes for the 29 participants are shown in Table 1. Of the 29 participants, 8 were sampled in the second trimester, 28 were sampled in the third trimester, and 22 were sampled postpartum. The median age at delivery of the mothers participating in this study was 31 years (interquartile range [IQR], 25.4, 34.1 years). Twelve (41%) women were black non-Hispanic, 15 (52%) were Hispanic, 1 participant (3%) was Asian, and 1 (3%) was white non-Hispanic. The median gestational ages at the time of sampling were 24.6 weeks (IQR, 21.2, 25.6 weeks) in the 2nd trimester and 32.7 weeks (IQR, 31.6, 35.0 weeks) in the 3rd trimester, and the median postpartum sampling time was 6.7 weeks after delivery (IQR, 6.0, 9.9 weeks) (Table 1).
TABLE 1.
Demographic characteristics and outcomes (n = 29)
| Parameter | Value |
|---|---|
| Maternal characteristics | |
| Median age at delivery (yrs) (IQR) | 30.8 (25.4, 35.1) |
| Median wt at delivery (kg) (IQR) | 83.0 (77.7, 99.0) |
| No. (%) of subjects of race/ethnicity | |
| Black non-Hispanic | 12 (41) |
| Hispanic (regardless of race) | 15 (52) |
| White non-Hispanic | 1 (3) |
| Asian, Pacific Islander | 1 (3) |
| No. (%) of individuals from country | |
| Argentina | 2 (7) |
| Brazil | 6 (21) |
| USA | 21 (72) |
| Second-trimester PK evaluation | |
| Median gestational age (wks) (IQR) | 24.6 (21.2, 25.6) |
| Median duration of FPV before PK evaluations (wks) (IQR) | 7.2 (5.1, 63.1) |
| Median HIV-1 RNA load (copies/ml) (IQR) | 159.0 (44.0, 627.5) |
| No. (%) of mothers with viral load of <75 copies/ml | 3 (38) |
| Median CD4 count (cells/mm3) (IQR) | 484.5 (417.5, 571.0) |
| Third-trimester PK evaluation | |
| Median gestational age (wks) (IQR) | 32.7 (31.6, 35.0) |
| Median duration of FPV before PK evaluations (wks) (IQR) | 19.2 (10.7, 101.7) |
| Median HIV-1 RNA load (copies/ml) (IQR) | 50.0 (48.0, 120.0) |
| No. (%) of mothers with viral load of <75 copies/ml | 19 (70) |
| Median CD4 count (cells/mm3) (IQR) | 491.0 (356.0, 635.0) |
| PK evaluation at delivery | |
| Median HIV-1 RNA load (copies/ml) (IQR) | 50.0 (48.0, 77.5) |
| Median gestational age (wks) (IQR) | 38.7 (37.9, 39.4) |
| No. (%) of mothers with viral load of <75 copies/ml | 21 (75) |
| Median CD4 count (cells/mm3) (IQR) | 491.0 (358.0, 699.0) |
| Postpartum PK evaluation | |
| Median time postdelivery (wks) (IQR) | 6.7 (6.0, 9.9) |
| Median HIV-1 RNA load (copies/ml) (IQR) | 50.0 (48.0, 56.0) |
| No. (%) of mothers with viral load of <75 copies/ml | 13 (76) |
| Median CD4 count (cells/mm3) (IQR) | 590.0 (394.0, 794.0) |
| Pregnancy outcomes | |
| Median birth wt (g) (IQR) | 3,237.5 (2935.0, 3477.9) |
| No. (%) of infants with infection status | 26 (90) uninfected, 2 (7) indeterminate,a 1 (3) pending based on available data |
An infant was considered HIV infection indeterminate if nucleic acid tests were negative but were not sufficient to meet the definitively negative criterion (i.e., two negative nucleic acid tests, with one after 1 month and the other after 4 months of age), often because of withdrawal from the study before the age of 4 months.
The plasma HIV-1 RNA loads were ≤75 copies/ml in 38% (3/8) of participants in the second trimester, 70% (19/27) in the third trimester, and 76% (13/17) postpartum. The median CD4 counts (cells per milliliter) were 485 (IQR, 418, 571) in the second trimester, 491 (IQR, 356, 635) in the third trimester, and 590 (IQR, 394, 794) postpartum. The median gestational age at the time of delivery was 38.7 (IQR, 37.9, 39.4) weeks, and the median neonatal birth weight was 3,238 g (IQR, 2,935, 3,478 g) (Table 1).
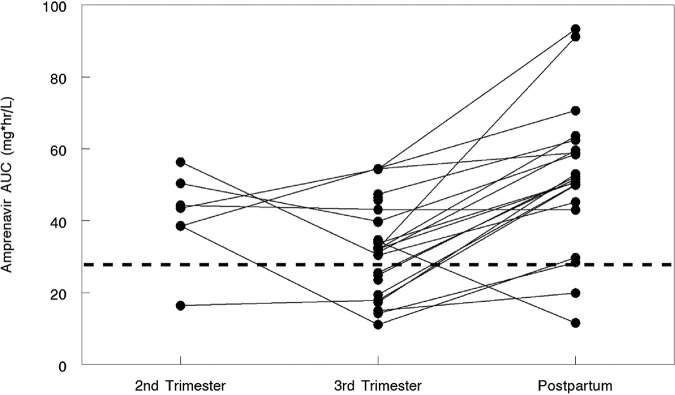
Amprenavir and ritonavir PK parameters with standard adult dosing (FPV at 700 mg and RTV at 100 mg twice daily) during the second trimester (n = 8) and third trimester (n = 28) and 2 weeks postpartum (n = 22) are presented in Tables 2 and 3. Since FPV is the prodrug for APV, APV exposure was measured. The APV area under the concentration-versus-time curve from time zero to 12 h postdose (AUC0–12) was lower in the 3rd trimester (geometric mean ratio, 0.60 [confidence interval {CI}, 0.49 to 0.72] [P < 0.001]) than postpartum (Fig. 1). The median APV AUC values (interquartile ranges) were 43.5 μg · h/ml (IQR, 38.5, 50.4 μg · h/ml) during the second trimester, 32.2 μg · h/liter (IQR, 21.5, 39.7 μg · h/ml) during the third trimester, and 51.6 μg · h/ml (IQR, 45.2, 59.6 μg · h/ml) postpartum (Table 2). The APV AUC exceeded the target for 6/8 (75%) participants in the 2nd trimester, 18/28 (64%) in the 3rd trimester, and 19/22 (86.4%) postpartum (Fig. 1).
TABLE 2.
Comparison of amprenavir pharmacokinetics in the 2nd trimester versus postpartum and in the 3rd trimester versus postpartuma
| PK parameter | Median value for group (IQR) |
2T/PP comparison |
3T/PP comparison |
||||
|---|---|---|---|---|---|---|---|
| 2T (n = 8) | 3T (n = 28) | PP (n = 22) | GMR (90% CI) | P value | GMR (90% CI) | P value | |
| APV AUC0–12 (μg · h/ml) | 43.50 (38.50, 50.40) | 32.15 (21.45, 39.70) | 51.60 (45.20, 59.60) | 0.68 (0.44, 1.04) | 0.22 | 0.60 (0.49, 0.72) | <0.001 |
| APV CL/F (liters/h) | 13.79 (11.91, 15.58) | 18.66 (15.11, 28.23) | 11.63 (10.07, 13.27) | 1.48 (0.96, 2.27) | 0.22 | 1.68 (1.38, 2.03) | <0.001 |
| APV T1/2 (h) | 8.67 (5.90, 13.57) | 12.98 (8.50, 31.62) | 14.26 (8.22, 28.25) | 0.41 (0.009, 17.97) | 1.00 | 1.02 (0.42, 2.49) | 0.637 |
| APV Cmin (μg/ml) | 1.91 (0.34, 2.39) | 1.48 (0.86, 1.80) | 2.42 (1.36, 3.08) | 0.37 (0.10, 1.40) | 0.16 | 0.97 (0.55, 1.71) | 0.01 |
| APV Clast (μg/ml) | 2.05 (1.56, 2.65) | 1.67 (1.13, 2.24) | 2.80 (1.93, 3.82) | 0.39 (0.10, 1.54) | 0.22 | 0.60 (0.45, 0.81) | 0.004 |
| APV Cmax (μg/ml) | 5.61 (4.47, 6.64) | 5.12 (3.60, 6.26) | 6.75 (4.31, 9.24) | 0.83 (0.56, 1.22) | 0.16 | 0.74 (0.58, 0.93) | 0.03 |
| APV C0 (μg/ml) | 2.19 (1.05, 3.13) | 1.70 (1.34, 2.28) | 3.14 (1.56, 4.94) | 0.71 (0.40, 1.27) | 0.47 | 0.91 (0.50, 1.65) | <0.001 |
| APV C12 (μg/ml) | 2.12 (1.39, 2.67) | 1.64 (1.16, 2.21) | 2.87 (2.34, 3.41) | 0.48 (0.14, 1.65) | 0.44 | 0.56 (0.43, 0.72) | <0.001 |
P values were determined by a Wilcoxon rank sum test. 2T, second trimester; 3T, third trimester; PP, postpartum; IQR, interquartile range; AUC0–12, area under the concentration-versus-time curve from time zero to 12 h postdose; CL/F, apparent oral clearance; Cmin, minimum concentration; Cmax, maximum concentration; T1/2, elimination half-life; Clast, last observed quantifiable concentration; C0, initial concentration at time zero; C12, concentration at 12 h postdose. Values in boldface type are significant P values (<0.1).
TABLE 3.
Comparison of ritonavir pharmacokinetics in the 2nd trimester versus postpartum and in the 3rd trimester versus postpartuma
| PK parameter | Median value for group (IQR) |
Comparison of 2T/PP |
Comparison of 3T/PP |
||||
|---|---|---|---|---|---|---|---|
| 2T (n = 8) | 3T (n = 28) | PP (n = 22) | GMR (90% CI) | P value | GMR (90% CI) | P value | |
| RTV AUC0–12 (μg · h/ml) | 2.52 (1.35, 4.10) | 3.68 (2.76, 5.64) | 4.86 (2.73, 6.60) | 0.51 (0.28, 0.91) | 0.09 | 0.72 (0.55, 0.95) | 0.005 |
| RTV CL/F (liters/h) | 47.16 (24.61, 74.15) | 27.20 (17.74, 36.22) | 20.63 (15.15, 36.72) | 1.98 (1.10, 3.56) | 0.06 | 1.38 (1.05, 1.82) | 0.009 |
| RTV T1/2 (h) | 3.71 (3.06, 10.92) | 4.08 (3.47, 6.65) | 4.92 (3.03, 6.64) | 1.05 (0.18, 6.14) | 1.00 | 1.45 (0.57, 3.69) | 0.520 |
| RTV Cmin (μg/ml) | 0.07 (0.05, 0.12) | 0.12 (0.05, 0.15) | 0.10 (0.05, 0.20) | 0.49 (0.24, 0.99) | 0.08 | 1.08 (0.87, 1.33) | 0.720 |
| RTV Clast (μg/ml) | 0.09 (0.06, 0.16) | 0.13 (0.06, 0.24) | 0.18 (0.08, 0.26) | 0.45 (0.20, 1.03) | 0.08 | 0.83 (0.63, 1.10) | 0.250 |
| RTV Cmax (μg/ml) | 0.41 (0.25, 0.73) | 0.64 (0.51, 1.07) | 0.77 (0.51, 1.08) | 0.65 (0.37, 1.12) | 0.30 | 0.83 (0.61, 1.11) | 0.475 |
| RTV C0 (μg/ml) | 0.13 (0.06, 0.24) | 0.16 (0.11, 0.31) | 0.19 (0.09, 0.41) | 0.59 (0.31, 1.10) | 0.30 | 0.87 (0.62, 1.22) | 0.134 |
| RTV C12 (μg/ml) | 0.12 (0.05, 0.29) | 0.16 (0.05, 0.20) | 0.19 (0.08, 0.26) | 0.71 (0.24, 2.14) | 0.69 | 0.70 (0.53, 0.91) | 0.029 |
P values were determined by a Wilcoxon rank sum test. 2T, second trimester; 3T, third trimester; PP, postpartum; IQR, interquartile range; AUC0–12, area under the concentration-versus-time curve from time zero to 12 h postdose; CL/F, apparent oral clearance; Cmin, minimum concentration; Cmax, maximum concentration; T1/2, elimination half-life; Clast, last observed quantifiable concentration; C0, initial concentration at time zero; C12, concentration at 12 h postdose. Values in boldface type are significant P values (<0.1).
FIG 1.
Amprenavir AUCs in women during the 2nd and 3rd trimesters and postpartum. The estimated 10th percentile for the AUC of amprenavir after FPV-RTV 700/100-mg twice-daily dosing is 27.7 μg · h/ml (represented by the dashed line). One, ten, and two women fell below the 10th-percentile line in the second- and third-trimester and postpartum states, respectively.
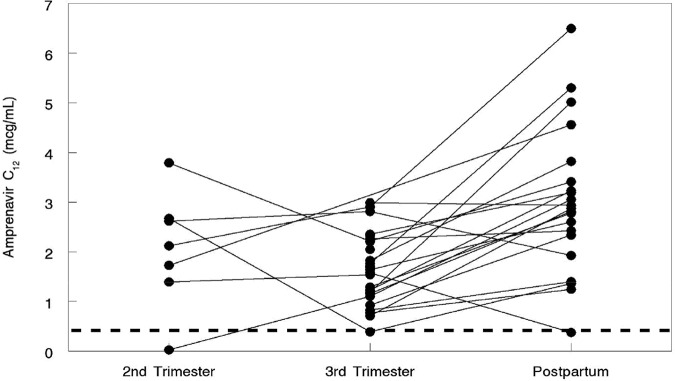
The amprenavir apparent oral clearance (CL/F) was higher in the 3rd trimester (GMR, 1.68 [CI, 1.38 to 2.03] [P < 0.001]) than postpartum (P < 0.001). The amprenavir minimum plasma concentration (Cmin) (0.97 [CI, 0.55 to 1.71] [P = 0.01]), the APV initial serum concentration (C0) (GMR, 0.91 [CI, 0.50 to 1.65]), and the APV last observable quantifiable plasma concentration (Clast) (GMR, 0.60 [CI, 0.45 to 0.81] [P = 0.004]) were lower in the 3rd trimester than postpartum. Similarly, the APV maximum plasma concentration (Cmax) (GMR, 0.74 [CI, 0.58 to 0.93] [P = 0.03]) and trough serum concentration at 12 h (C12) (GMR, 0.56 [CI, 0.43 to 0.72]) were lower in the 3rd trimester than postpartum (Fig. 2). The minimum APV target trough concentration for the wild-type virus of 0.4 μg/ml (15) was exceeded by 87.5% (7/8) of women in the 2nd trimester, 96.4% (27/28) in the 3rd trimester, and 95.5% (21/22) postpartum (Fig. 2).
FIG 2.
Amprenavir trough concentrations at 12 h in women during the 2nd and 3rd trimesters and postpartum. The dashed line represents 0.4 μg/ml, the minimum target trough concentration for wild-type virus. One woman had a trough concentration below this level at each evaluation period (2nd trimester, 3rd trimester, and postpartum).
Ritonavir PK data are shown in Table 3. The ritonavir AUC0–12 was lower in the 2nd (GMR, 0.51 [CI, 0.28 to 0.91] [P = 0.09]) and 3rd (GMR, 0.73 [CI, 0.55 to 0.95] [P = 0.005]) trimesters than postpartum. The ritonavir CL/F was higher in the 2nd (GMR, 1.98 [CI, 1.10 to 3.56] [P = 0.06]) and 3rd (GMR, 1.38 [CI, 1.05 to 1.82] [P = 0.005]) trimesters than postpartum. The ritonavir Clast (GMR, 0.45 [CI, 0.20 to 1.03] [P = 0.08]) and Cmin (GMR, 0.49 [CI, 0.24 to 0.99] [P = 0.08]) were lower in the 2nd trimester than postpartum. The ritonavir C12 (GMR, 0.70 [CI, 0.53 to 0.91] [P = 0.03]) was lower in the 3rd trimester than postpartum.
Third-trimester APV and RTV PK parameters by viral load (≤75 copies/ml versus >75 copies/ml) are shown in Table 4. No statistically significant associations between drug exposure and viral load suppression were detected (Table 4). Four women (13.8%) experienced adverse events that were possibly treatment related, including moderate to severe elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. All antiretrovirals received, and the number of mothers taking each one at the time of PK evaluations, are summarized in Table 5.
TABLE 4.
Third-trimester APV and RTV PK parameters by viral load categorya
| PK parameter | APV |
RTV |
||||||
|---|---|---|---|---|---|---|---|---|
| Value for group |
P value | Value for group |
P value | |||||
| Viral load of ≤75 copies/ml (n = 19) | Viral load of >75 copies/ml (n = 8) | Total (n = 27) | Viral load of ≤75 copies/ml (n = 19) | Viral load of >75 copies/ml (n = 8) | Total (n = 27) | |||
| Median AUC0–12 (μg · h/ml) (IQR) | 30.4 (17.3, 39.6) | 33.3 (31.4, 38.9) | 32.1 (19.4, 39.6) | 0.159 | 3.36 (1.85, 6.19) | 3.68 (3.37, 5.38) | 3.61 (2.76, 5.64) | 0.580 |
| >AUC0–12 median [no. (%) of participants] | 0.103 | 1.000 | ||||||
| Yes | 7 (54) | 6 (46) | 13 | 8 (67) | 4 (33) | 12 | ||
| No | 12 (86) | 2 (14) | 14 | 11 (73) | 4 (27) | 15 | ||
| Median C0 (μg/ml) (IQR) | 1.84 (1.03, 2.51) | 1.58 (1.41, 2.16) | 1.76 (1.26, 2.28) | 0.577 | 0.18 (0.10, 0.34) | 0.13 (0.12, 0.18) | 0.16 (0.11, 0.31) | 0.441 |
| >C0 median [no. (%) of participants] | 0.420 | 0.420 | ||||||
| Yes | 11 (79) | 3 (21) | 14 | 11 (79) | 3 (21) | 14 | ||
| No | 8 (62) | 5 (38) | 13 | 8 (62) | 5 (38) | 13 | ||
| Median Cmin (μg/ml) (IQR) | 1.29 (0.74, 2.20) | 1.48 (1.32, 1.72) | 1.42 (0.85, 1.83) | 0.958 | 0.13 (0.05, 0.16) | 0.12 (0.09, 0.15) | 0.12 (0.05, 0.16) | 0.872 |
| >Cmin median [no. (%) of participants] | 1.000 | 1.000 | ||||||
| Yes | 9 (69) | 4 (31) | 13 | 10 (71) | 4 (29) | 14 | ||
| No | 10 (71) | 4 (29) | 14 | 9 (69) | 4 (31) | 13 | ||
P values were determined by Fisher’s exact test for categorized PK parameters and a Wilcoxon rank sum test for continuous PK parameters. AUC0–12, area under the concentration-versus-time curve from time zero to 12 h postdose; Cmin, minimum (trough) drug concentration; C0, initial drug concentration at time zero.
TABLE 5.
Number of mothers taking each ARV at the time of PK evaluations
| Druga | No. of mothers taking ARVs at the 2nd-trimester PK visit | No. of mothers taking ARVs at the 3rd-trimester PK visit |
|---|---|---|
| 3TC | 4 | 15 |
| ABC | 0 | 2 |
| DDI | 0 | 1 |
| FPV | 8 | 28 |
| FTC | 4 | 12 |
| NVP | 0 | 1 |
| RTV | 8 | 27 |
| TDF | 4 | 15 |
| ZDV | 4 | 14 |
3TC, lamivudine; ABC, abacavir; DDI, didanosine; FPV, fosamprenavir; FTC, emtricitabine; NVP, nevirapine; RTV, ritonavir; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
DISCUSSION
Pregnancy is known to modify the actions of some drug-metabolizing enzymes, impacting drug exposure (16–18). Previous studies demonstrated decreases in exposure to PIs during pregnancy, including lopinavir, atazanavir, saquinavir, indinavir, darunavir, and nelfinavir (19–26). The largest decreases are notable in the third trimester, while second-trimester concentrations were generally decreased to a lesser extent (27, 28). However, boosting with RTV improves the PK and pharmacodynamic (PD) profiles of most PIs (29, 30). For example, when APV is used without RTV, trough concentration (Cmin) values (0.280 μg/ml) were found to be very close to the EC90 (concentration producing 90% of the maximal antiviral effect) value of 0.228 μg/ml (5). However, with RTV boosting, Cmin values were 8- to 9-fold higher (1.92 μg/ml) (31). These results have direct implications for perinatal transmission and HIV resistance.
Physiological changes during pregnancy can explain the decreased drug exposures to APV and RTV. FPV is rapidly and almost entirely hydrolyzed to APV and inorganic phosphate as it is absorbed from the gastrointestinal tract after oral administration (5, 6). APV is transported by P-glycoprotein (P-gp) and has a large apparent volume of distribution of over 430 liters (8). APV has an elimination half-life (T1/2) of 7.7 h when unboosted, but this increases to 15 to 23 h when boosted with RTV. APV is a substrate of cytochrome P450 (CYP3A) enzymes; an inhibitor of CYP3A4 (32), breast cancer resistant protein (BRCP) (32), P-gp (33), and organic anion transporters (OATS) (34); and almost exclusively metabolized by CYP3A isoforms (2, 8). Therefore, the large volume of distribution, increased clearance, and increases in CYP3A activity during pregnancy (35% to 38%) (35), especially during the third trimester, likely contribute to the lower drug exposures and enhanced clearance of APV from maternal plasma. APV is highly protein bound, with 90% of circulating plasma APV levels bound to plasma proteins (mainly alpha-1-acid glycoprotein) (36).
An understanding of known pharmacokinetic-pharmacodynamic (PKPD) relationships of APV (AUC, viral response, and protein-adjusted 50% inhibitory concentration [IC50]/IC90) in the context of lower exposures is needed to evaluate whether the decrease in APV exposure is clinically relevant during pregnancy. To effectively and consistently suppress HIV replication in people living with HIV, antiretroviral drugs must achieve certain concentrations and be maintained at concentrations that exceed the susceptibility of the virus to that medication (15). This requires that the minimum drug concentrations exceed the inhibitory concentrations for particular strains of HIV (wild type or resistant type) (37). Steady-state PKPD and efficacy relationships show that trough concentrations (Cmin) of APV are good predictors of a decrease in viral load (5). In this study, the minimum APV target trough concentration for wild-type virus of 0.4 μg/ml was exceeded by 87.5% (7/8) of participants in the 2nd trimester, 96.4% (27/28) in the 3rd trimester, and 95.5% (21/22) postpartum (Fig. 2). Also, the Cmin values of APV were 4- to 16-fold above the mean APV protein-adjusted IC50 of 0.146 μg/ml (5, 31) for wild-type HIV-1 (Table 2). The 10th-percentile median AUC for RTV-boosted FPV in adults on twice-daily FPV-RTV at 700/100 mg (27.7 μg · h/ml) was exceeded by 100% (8/8) of participants in the second trimester, 92.9% (26/28) in the third trimester, and 100% (22/22) postpartum (Table 2). In addition, using a cutoff value of ≤75 copies/ml versus >75 copies/ml for an undetectable viral load, we were not able to identify any statistically significant associations between drug exposure and viral load suppression (Table 4), although this was most likely due to the small sample size and lack of statistical power. Many women met the minimum trough concentrations during the second and third trimesters of pregnancy, as well as postpartum, suggesting that reductions in RTV-boosted APV exposures were not clinically significant.
While our current findings suggest that the use of FPV-RTV at 700 mg/100 mg twice daily in pregnant women does not provide exposure comparable to that in nonpregnant adults, a dose adjustment may not be necessary as the majority of women fell above the 10th-percentile AUC and had trough levels of >0.4 μg/ml. Those pregnant women whose APV troughs fell below this target may have an inadequate virological response, so close monitoring of the viral load in pregnant women receiving FPV is warranted. No participant in our study received an increased dose of FPV, so our data provide no information on the impact of dose adjustment on APV exposures during pregnancy. APV exposure may also be increased by increasing the RTV dose. Increased plasma RTV could provide higher exposure to the boosted PI, slowing down the metabolism of APV and increasing minimum trough concentration, half-life, and AUC values while minimizing adverse effects by concurrently decreasing the time to maximum plasma concentration (Tmax) and Cmax. However, RTV is often not well tolerated due to gastrointestinal side effects, possibly limiting enthusiasm for using an increased dose in pregnant women.
Our study has several strengths. First, pregnant patients in the FPV arm of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network 1026s study were monitored in a longitudinal pattern throughout pregnancy and postpartum, during which evaluation of clinical findings related to FPV exposure occurred at regular time intervals. Second, because this was a prospective cohort study, confounding, recall, and selection biases were minimized. Third, within-participant comparisons (second or third trimester versus postpartum) reduced concerns about heterogeneity during this PK study. Fourth, another strength of this study is the sample size: 29 participants. Fifth, complete PK data were available for 96.5% (28/29) of participants evaluated in the third trimester of pregnancy and for 76% (22/29) evaluated postpartum.
This study had its limitations. First, this is an observational PK/safety study of a heterogeneous group of pregnant women receiving FPV-RTV for clinical care. There was variation in their background characteristics, and pregnant women who began FPV-RTV but did not tolerate it or demonstrated inadequate initial efficacy would have been taken off the drug and would not have been eligible for the study. Second, we did not assess the pharmacogenomic relationship between FPV-RTV dosing and genetic resistance to HIV in pregnancy.
In conclusion, our findings confirm that RTV-boosted FPV exposure is decreased during pregnancy. Although exposure was lower during pregnancy, few women were found to have a trough level below the recommended trough level of 0.4 μg/ml, and the majority of women met the 10th-percentile AUC of 27.7 μg · h/liter during the second and third trimesters of pregnancy and postpartum. Most participants achieved a viral load of <75 copies/ml, further suggesting adequate viral suppression despite decreased ARV exposure. However, our sample size was small, and further investigation of methods to achieve APV exposure during pregnancy equivalent to that in nonpregnant adults, such as increasing the ritonavir dose, is warranted.
MATERIALS AND METHODS
The study protocol, the informed-consent documents, and all subsequent modifications were reviewed and approved by the local institutional review board (IRB)/ethics committee responsible for oversight of the study. The study followed all relevant human subject research guidelines. All participants provided signed informed consent before participation, and the study was registered in ClinicalTrials.gov (identifier NCT00042289). This study was done as part of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network P1026s, Pharmacokinetic Properties of Antiretroviral and Related Drugs during Pregnancy and Postpartum (ClinicalTrials.gov identifier NCT00042289), an ongoing, multicenter, nonblind, prospective phase IV study of the PK and safety of selected ARVs in women living with HIV. The study included an arm for pregnant women receiving FPV at 700 mg with ritonavir at 100 mg twice daily.
Pregnant women living with HIV were eligible for enrollment if they were receiving 700 mg/100 mg FPV-RTV as part of clinical care for at least 2 weeks and planned to continue the regimen. Exclusion criteria were the concurrent use of medications known to interfere with the absorption, metabolism, or clearance of FPV or RTV, including multiple gestation and clinical or laboratory toxicity that, in the opinion of the site investigator, would likely require a change in the medication regimen during the study. Medications were prescribed by the participant’s health care provider, who remained responsible for her clinical management throughout the study. Participants continued on study until the completion of postpartum PK sampling. For women enrolling during the second trimester of pregnancy, APV PK were determined in real time between weeks 20 and 26 of gestation and repeated between weeks 30 and 36 of gestation. Women enrolling in the third trimester had PK sampling performed between weeks 30 and 36 of gestation. PK sampling was repeated between weeks 6 and 12 postpartum. Infants were enrolled at the same time as their mothers, with maternal consent. An infant was considered HIV negative if at least two nucleic acid tests were negative, with one after 1 month and the other after 4 months of age. An infant was considered HIV infection indeterminate if nucleic acid tests were negative but were not sufficient to meet the definitively negative criterion (i.e., two negative nucleic acid tests, with one after 1 month and the other after 4 months of age), often because of withdrawal from the study before the age of 4 months.
Clinical and laboratory monitoring.
Maternal data obtained for this analysis were maternal age, ethnicity, weight, concomitant medications, CD4 counts, and plasma viral load assay results. Plasma viral load assays were done locally and had lower limits of detection of as high as 75 copies/ml, so all viral load measurements of 75 copies/ml or lower were set to 75 copies/ml for data analyses. Maternal clinical and laboratory toxicities were assessed through clinical evaluations (history and physical examination) and laboratory assays (alanine aminotransferase, aspartate aminotransferase, creatinine, blood urea nitrogen [BUN], albumin, bilirubin, and hemoglobin) on each PK sampling day and at delivery. Infant data included birth weight, gestational age at birth, and HIV status, if available. The study team reviewed toxicity reports on monthly conference calls, although the participant’s provider was responsible for clinical management. The Division of AIDS (DAIDS)/National Institute of Allergy and Infectious Diseases toxicity table for grading the severity of adult adverse experiences was used to report adverse events for study participants (38). All toxicities were monitored through resolution.
Sample collection and drug assays.
Participants were stable on their ARV regimen for at least 2 weeks before PK sampling. Seven plasma samples were drawn at the second-trimester, third-trimester, and postpartum PK evaluation visits, starting immediately before an oral FPV-RTV dose and at 1, 2, 4, 6, 8, and 12 h postdose. Fosamprenavir-ritonavir was given as an observed dose. Other information collected included the time of the two prior doses, the two most recent meals, and maternal height and weight. A single maternal plasma sample and an umbilical cord sample after the cord was clamped were collected at delivery. The University of California, San Diego (UCSD), Pediatric Clinical Pharmacology Laboratory, using a validated, reversed-phase, multiplex, high-performance liquid chromatography method, measured APV and RTV. The lower limits of quantitation were 0.047 μg/ml for APV and 0.094 μg/ml for RTV. The University of California, San Diego, laboratory has been enrolled in the AIDS Clinical Trials Group Quality Assurance/Quality Control Proficiency Testing Program since 2001, which performs standardized interlaboratory testing twice a year.
Pharmacokinetic and statistical analyses.
The maximum plasma concentration (Cmax), minimum plasma concentration (Cmin), and 12-h-postdose concentration (C12) were determined by direct inspection. For concentrations below the assay limit of detection, a value of one-half of the detection limit (0.024 μg/ml for amprenavir and 0.047 μg/ml for ritonavir) was used in summary calculations. The AUC0–12 values during the dosing interval (from time zero to 12 h postdose) for APV and RTV were estimated using the trapezoidal rule. The apparent oral clearance (CL/F) from plasma was calculated as dose divided by AUC0–12. The terminal slope of the curve (λz) was estimated from the last two measurable and declining concentrations between 6 and 12 h postdose. The half-life was calculated as dose divided by λz, and the apparent volume of distribution (V/F) was determined as CL/F divided by λz. The amprenavir AUC0–12 was calculated for each woman and compared with the APV AUC0–12 in nonpregnant adults. Each participant’s provider was notified of the participant’s plasma concentrations and AUC0–12 within 2 weeks. If the APV AUC0–12 was below the target of 27.7 μg · h/ml (the 10th percentile in nonpregnant adult populations), the provider was offered the option of discussing the results and possible dose modifications with a study team pharmacologist.
Within-participant comparisons (second or third trimester versus postpartum) were performed for continuous outcome measures using the Wilcoxon signed-rank test and for dichotomous outcome measures using McNemar’s test, with a P value of <0.1 considered statistically significant. The 90% confidence limits for the geometric mean ratios of the PK exposure parameters were calculated to describe the range of values that were consistent with the observed data to assess whether there was a clinically significant difference in exposure. Data analysis was done using SAS (version 9.4; SAS Institute, Cary, NC).
ACKNOWLEDGMENTS
The participation sites of the P1026s Protocol Team include the following: 2802, New Jersey Medical School CRS (Linda Bettica, Charmane Calilap-Bernardo, and Arlene Bardeguez); 3801, Texas Children’s Hospital CRS (Shelley Buschur, Chivon Jackson, and Mary Paul); 4101, Columbia CRS (Philip La Russa); 4201, University of Miami Pediatric Perinatal HIV/AIDS CRS (Claudia Florez, Patricia Bryan, and Monica Stone); 5003, Metropolitan Hospital, New York CRS; 5011, Boston Medical Center CRS (Debra McLaud, Christina Yarrington, and Diana Clarke); 5012, New York University, New York NICHD CRS (Nagamah Deygoo and William Borkowsky); 5048, University of Southern California School of Medicine-Los Angeles County NICHD CRS (Françoise Kamer, LaShonda Spencer, and James Homans); 5072, Hospital dos Servidores Rio de Janeiro NICHD CRS (Esau C. Joao and Camille Medeiros Braga); 5082, Hospital General de Agudos Buenos Aires NICHD CRS (Marcelo H. Losso, Silvina A. Ivalo, and Alejandro Hakim); 5083, Rush U. Cook County Hospital Chicago CRS (Julie Schmidt, Maureen McNichols, and Mariam Aziz); 5112, David Geffen School of Medicine at UCLA CRS (Jaime G. Deville, Karin Nielsen, and Bonnie Ank); 5113, The Children’s Hospital of Philadelphia, Philadelphia CRS; and 6501, St. Jude CRS (Katherine M. Knapp, Nina Sublette, and Edwin Thorpe, Jr.).
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Collaborators: Linda Bettica, Charmane Calilap-Bernardo, Arlene Bardeguez, Shelley Buschur, Chivon Jackson, Mary Paul, Philip La Russa, Claudia Florez, Patricia Bryan, Monica Stone, Debra McLaud, Christina Yarrington, Diana Clarke, Nagamah Deygoo, William Borkowsky, Françoise Kamer, LaShonda Spencer, James Homans, Esau C. Joao, Camille Medeiros Braga, Marcelo H. Losso, Silvina A. Ivalo, Alejandro Hakim, Julie Schmidt, Maureen McNichols, Mariam Aziz, Jaime G. Deville, Karin Nielsen, Bonnie Ank, Katherine M. Knapp, Nina Sublette, and Edwin Thorpe, Jr.
REFERENCES
- 1.Office of AIDS Research, NIH. 2018. Fosamprenavir (Lexiva, FPV). Office of AIDS Research, NIH, Bethesda, MD: https://aidsinfo.nih.gov/guidelines/html/3/perinatal/210/fosamprenavir-lexiva-fpv. Accessed 1 November 2019. [Google Scholar]
- 2.Arvieux C, Tribut O. 2005. Amprenavir or fosamprenavir plus ritonavir in HIV infection: pharmacology, efficacy and tolerability profile. Drugs 65:633–659. doi: 10.2165/00003495-200565050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Li X, Li T, Wang H, Shi W, Qi P, Li C, Chen J, Bao J, Huang G, Wang Y. 2017. Repositioning of amprenavir as a novel extracellular signal-regulated kinase-2 inhibitor and apoptosis inducer in MCF-7 human breast cancer. Int J Oncol 50:823–834. doi: 10.3892/ijo.2017.3860. [DOI] [PubMed] [Google Scholar]
- 4.Furfine ES, Baker CT, Hale MR, Reynolds DJ, Salisbury JA, Searle AD, Studenberg SD, Todd D, Tung RD, Spaltenstein A. 2004. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother 48:791–798. doi: 10.1128/aac.48.3.791-798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler BM, Gillotin C, Lou Y, Stein DS. 2001. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob Agents Chemother 45:30–37. doi: 10.1128/AAC.45.1.30-37.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadler BM, Stein DS. 2002. Clinical pharmacology and pharmacokinetics of amprenavir. Ann Pharmacother 36:102–118. doi: 10.1345/aph.10423. [DOI] [PubMed] [Google Scholar]
- 7.Borrajo A, Ranazzi A, Pollicita M, Bruno R, Modesti A, Alteri C, Perno CF, Svicher V, Aquaro S. 2017. Effects of amprenavir on HIV-1 maturation, production and infectivity following drug withdrawal in chronically-infected monocytes/macrophages. Viruses 9:277. doi: 10.3390/v9100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wire MB, Shelton MJ, Studenberg S. 2006. Fosamprenavir: clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet 45:137–168. doi: 10.2165/00003088-200645020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chapman TM, Plosker GL, Perry CM. 2004. Fosamprenavir: a review of its use in the management of antiretroviral therapy-naive patients with HIV infection. Drugs 64:2101–2124. doi: 10.2165/00003495-200464180-00014. [DOI] [PubMed] [Google Scholar]
- 10.Aweeka FT, Hu C, Huang L, Best BM, Stek A, Lizak P, Burchett SK, Read JS, Watts H, Mirochnick M, Capparelli EV, International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1026s Protocol Team . 2015. Alteration in cytochrome P450 3A4 activity as measured by a urine cortisol assay in HIV-1-infected pregnant women and relationship to antiretroviral pharmacokinetics. HIV Med 16:176–183. doi: 10.1111/hiv.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feghali M, Venkataramanan R, Caritis S. 2015. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 39:512–519. doi: 10.1053/j.semperi.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eke AC, Dooley KE, Sheffield J. 2019. Pharmacologic research in pregnant women—time to get it right. N Engl J Med 380:1293–1295. doi: 10.1056/NEJMp1815325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cespedes MS, Castor D, Ford SL, Lee D, Lou Y, Pakes GE, Aberg JA. 2013. Steady-state pharmacokinetics, cord blood concentrations, and safety of ritonavir-boosted fosamprenavir in pregnancy. J Acquir Immune Defic Syndr 62:550–554. doi: 10.1097/QAI.0b013e318285d918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam VH, Kabbara S, Yeh RF, Leary RH. 2006. Impact of sample size on the performance of multiple-model pharmacokinetic simulations. Antimicrob Agents Chemother 50:3950–3952. doi: 10.1128/AAC.00337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta EP, Limoli KL, Trinh L, Parkin NT, King JR, Weidler JM, Ofotokun I, Petropoulos CJ. 2012. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother 56:5938–5945. doi: 10.1128/AAC.00691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong H. 2010. Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol 6:689–699. doi: 10.1517/17425251003677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isoherranen N, Thummel KE. 2013. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos 41:256–262. doi: 10.1124/dmd.112.050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke AB, Greupink R, Abduljalil K. 2018. Drug dosing in pregnant women: challenges and opportunities in using physiologically based pharmacokinetic modeling and simulations. CPT Pharmacometrics Syst Pharmacol 7:103–110. doi: 10.1002/psp4.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, Rossi SS, Smith E, Read JS, Capparelli EV, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 1026s Study Team . 2010. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr 54:381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, Elgie C, Holland DT, Smith E, Tuomala R, Cotter A, Read JS. 2006. Reduced lopinavir exposure during pregnancy. AIDS 20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 21.Kreitchmann R, Best BM, Wang J, Stek A, Caparelli E, Watts DH, Smith E, Shapiro DE, Rossi S, Burchett SK, Hawkins E, Byroads M, Cressey TR, Mirochnick M. 2013. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr 63:59–66. doi: 10.1097/QAI.0b013e318289b4d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirochnick M, Best BM, Stek AM, Capparelli EV, Hu C, Burchett SK, Rossi SS, Hawkins E, Basar M, Smith E, Read JS, IMPAACT 1026s Study Team . 2011. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr 56:412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittweger M, Arasteh K. 2007. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet 46:739–756. doi: 10.2165/00003088-200746090-00002. [DOI] [PubMed] [Google Scholar]
- 24.Colbers A, Hawkins D, Hidalgo-Tenorio C, van der Ende M, Gingelmaier A, Weizsacker K, Kabeya K, Taylor G, Rockstroh J, Lambert J, Molto J, Wyen C, Sadiq ST, Ivanovic J, Giaquinto C, Burger D, PANNA Network . 2015. Atazanavir exposure is effective during pregnancy regardless of tenofovir use. Antivir Ther 20:57–64. doi: 10.3851/IMP2820. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Rebollar M, Lonca M, Perez I, Soy D, Brunet M, Martin R, Coll O, Hernandez S, Laguno M, Milinkovic A, Larrousse M, Calvo M, Blanco JL, Martinez E, Gatell JM, Mallolas J. 2011. Pharmacokinetic study of saquinavir 500 mg plus ritonavir (1000/100 mg twice a day) in HIV-positive pregnant women. Ther Drug Monit 33:772–777. doi: 10.1097/FTD.0b013e318236376d. [DOI] [PubMed] [Google Scholar]
- 26.von Hentig N, Nisius G, Lennemann T, Khaykin P, Stephan C, Babacan E, Staszewski S, Kurowski M, Harder S, Haberl A. 2008. Pharmacokinetics, safety and efficacy of saquinavir/ritonavir 1,000/100 mg twice daily as HIV type-1 therapy and transmission prophylaxis in pregnancy. Antivir Ther 13:1039–1046. [PubMed] [Google Scholar]
- 27.Eke AC, Chakhtoura N, Kashuba A, Best BM, Sykes C, Wang J, Stek AM, Smith E, Calabrese S, Capparelli EV, Mirochnick M, IMPAACT P1026s Protocol Team . 2018. Rilpivirine plasma and cervicovaginal concentrations in women during pregnancy and postpartum. J Acquir Immune Defic Syndr 78:308–313. doi: 10.1097/QAI.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eke AC, McCormack SA, Best BM, Stek AM, Wang J, Kreitchmann R, Shapiro D, Smith E, Mofenson LM, Capparelli EV, Mirochnick M, IMPAACT P1026s Protocol Team . 2019. Pharmacokinetics of increased nelfinavir plasma concentrations in women during pregnancy and postpartum. J Clin Pharmacol 59:386–393. doi: 10.1002/jcph.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eke AC, Mirochnick M. 2019. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expert Opin Drug Metab Toxicol 15:523–525. doi: 10.1080/17425255.2019.1628947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eke AC, Mirochnick MH. 2019. Cobicistat as a pharmacoenhancer in pregnancy and postpartum: progress to date and next steps. J Clin Pharmacol 59:779–783. doi: 10.1002/jcph.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goujard C, Vincent I, Meynard JL, Choudet N, Bollens D, Rousseau C, Demarles D, Gillotin C, Bidault R, Taburet AM. 2003. Steady-state pharmacokinetics of amprenavir coadministered with ritonavir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother 47:118–123. doi: 10.1128/aac.47.1.118-123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. 2007. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother 59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 33.Janneh O, Jones E, Chandler B, Owen A, Khoo SH. 2007. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother 60:987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 34.Ye ZW, Augustijns P, Annaert P. 2008. Cellular accumulation of cholyl-glycylamido-fluorescein in sandwich-cultured rat hepatocytes: kinetic characterization, transport mechanisms, and effect of human immunodeficiency virus protease inhibitors. Drug Metab Dispos 36:1315–1321. doi: 10.1124/dmd.107.019398. [DOI] [PubMed] [Google Scholar]
- 35.Tracy TS, Venkataramanan R, Glover DD, Caritis SN, National Institute for Child Health and Human Development Network of Maternal-Fetal-Medicine Units . 2005. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Noble S, Goa KL. 2000. Amprenavir: a review of its clinical potential in patients with HIV infection. Drugs 60:1383–1410. doi: 10.2165/00003495-200060060-00012. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz A, Price RW, Gisslen M. 2012. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother 67:299–311. doi: 10.1093/jac/dkr492. [DOI] [PubMed] [Google Scholar]
- 38.Division of AIDS. 2014. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Division of AIDS, NIAID, NIH, Bethesda, MD: https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf. Accessed 31 January 2019. [Google Scholar]