Infections with enterohemorrhagic Escherichia coli (EHEC) cause disease ranging from mild diarrhea to hemolytic-uremic syndrome (HUS) and are the most common cause of renal failure in children in high-income countries. The severity of the disease derives from the release of Shiga toxins (Stx). The use of antibiotics to treat EHEC infections is generally avoided, as it can result in increased stx expression. Here, we systematically tested different classes of antibiotics and found that their influence on stx expression and release varies significantly.
KEYWORDS: EHEC, Citrobacter rodentium, Shiga toxin, antibiotics, mouse model
ABSTRACT
Infections with enterohemorrhagic Escherichia coli (EHEC) cause disease ranging from mild diarrhea to hemolytic-uremic syndrome (HUS) and are the most common cause of renal failure in children in high-income countries. The severity of the disease derives from the release of Shiga toxins (Stx). The use of antibiotics to treat EHEC infections is generally avoided, as it can result in increased stx expression. Here, we systematically tested different classes of antibiotics and found that their influence on stx expression and release varies significantly. We assessed a selection of these antibiotics in vivo using the Citrobacter rodentium ϕstx2dact mouse model and show that stx2d-inducing antibiotics resulted in weight loss and kidney damage despite clearance of the infection. However, several non-Stx-inducing antibiotics cleared bacterial infection without causing Stx-mediated pathology. Our results suggest that these antibiotics might be useful in the treatment of EHEC-infected human patients and decrease the risk of HUS development.
INTRODUCTION
Infection with enterohemorrhagic Escherichia coli (EHEC) can cause severe diarrhea and hemorrhagic colitis and mainly affects children and the elderly (1, 2). In approximately 10 to 15% of patients, the disease progresses to hemolytic-uremic syndrome (HUS) (2), which is characterized by the triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure (3). The reasons for HUS development are poorly understood, but it is dependent on the strain, its source, the host genotype, and the intestinal microbiota (4, 5). Moreover, it is evident that systemic manifestations are a result of the production and release of Shiga toxin (Stx) (6, 7).
Stx is considered the major virulence factor of EHEC (1, 2, 5–7). Two main types exist, Stx1 and Stx2, with several subtypes belonging to each group (8). Stx2 subtypes, especially Stx2a, are commonly associated with more severe human disease than Stx1 variants (9–11). The genes encoding either Stx are found on lambdoid prophages integrated into the bacterial chromosome (12, 13). The exact mechanism of Stx induction during infection is unclear, but it has been suggested to involve host factors, metabolites produced by the intestinal microbiota, and proinflammatory cytokines produced in response to the pathogen (5, 14, 15). Moreover, induction of the bacterial SOS response and hence the lytic phage cycle, e.g., by antibiotics, results in an increased production and release of Stx from the bacterial cells (13, 16, 17). Since EHEC has been reported to produce more Stx when treated with certain antibiotics, treatment of EHEC infections with antibiotics has been considered contraindicated due to concerns of increased risk of HUS development (5, 15, 18).
The effects observed for antibiotics from different classes on the induction of stx expression vary greatly and are dependent on the antibiotic mode of action, the Stx-producing E. coli (STEC) strain, and the Stx subtype (17, 19–26). While some antibiotics such as ansamycins, which inhibit bacterial transcription, and chloramphenicol, which blocks protein synthesis, yielded consistently favorable results in in vitro studies (1–5, 17, 20, 21), results for other antibiotics such as beta-lactams, which inhibit the synthesis of the peptidoglycan layer, are more conflicting (19, 20, 27–30). Several of the conducted in vitro studies have been confirmed in animal studies, but others reported contradictory results (5, 24, 30–33). Notably, the models used in these studies differed, as did the antibiotics and the timing and route of their application, making a comparison of the results between these studies difficult. Furthermore, the above models do not recapitulate the formation of attaching and effacing (A/E) lesions characterized by intimate attachment of the pathogen and actin rearrangement upon host cell binding, extensive mucosal colonization, and intestinal damage (34, 35). These models generally result in rapid clearance, as EHEC is unable to efficiently colonize mice and colonization is achieved only after infection with large doses of EHEC by use of immunodeficient or gnotobiotic mice, or after disruption of the microbiota by antibiotic pretreatment (34, 35), all of which reduces the amount of information that can be gained about the in vivo situation.
In contrast, murine infection with a Stx-producing strain of Citrobacter rodentium reflects the characteristic mode of intestinal colonization and Stx-mediated pathogenesis of the most prevalent EHEC strains (36). C. rodentium is a mouse pathogen that shares many of its virulence genes with EHEC, including the LEE pathogenicity island that confers the ability to form A/E lesions (37–39). Found on the surface of colonic epithelial cells, these lesions are indistinguishable from those that are observed in EHEC-infected humans (40, 41). A recent study introduced a Stx2dact-encoding prophage into the C. rodentium genome and showed that the resulting disease pathology observed in infected mice reflected EHEC infections in humans, including the formation of A/E lesions, production of inflammatory cytokines, and kidney injury (36).
Here, we assessed a panel of antibiotics from different classes for their abilities to induce stx2 expression and Stx2 release in vitro for both the EHEC O157:H7 strain EDL933 and C. rodentium ϕstx2dact. We identified several common antibiotics that showed no induction in either strain. Using the C. rodentium ϕstx2dact mouse model of infection, we found that Stx-inducing antibiotics such as enrofloxacin triggered the development of severe renal disease, whereas several non-Stx-inducing antibiotics led to colonization clearance and did not induce detrimental kidney damage. In summary, our data suggest that the C. rodentium ϕstx2dact model allows assessment of treatment options for EHEC infections and that several commonly used antibiotics may be safe to use in case of an outbreak.
RESULTS
Effects of subinhibitory antibiotic concentrations on stx expression.
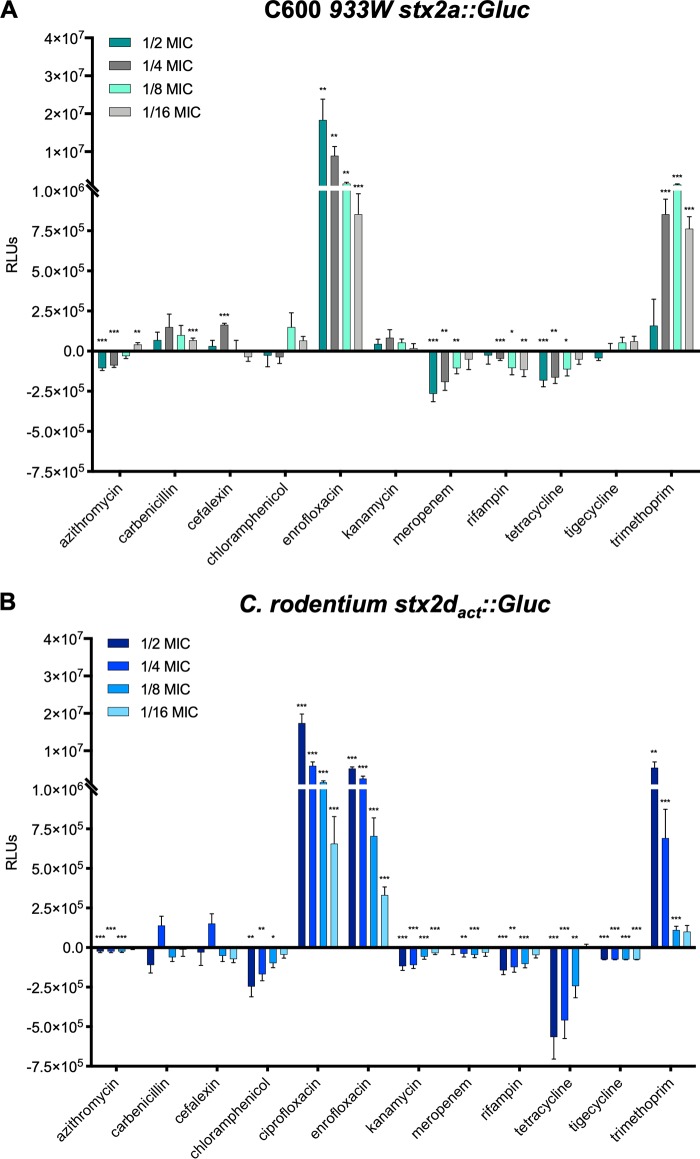
To compare how different antibiotics modulate the expression of stx genes in E. coli, we first used a stx-Gaussia luciferase (Gluc) reporter strain, C600 W34 ϕstx2a::Gluc, to determine stx2 promoter-mediated reporter gene induction at different subinhibitory concentrations (MICs, 1/2 to 1/16). Antibiotics of the quinolone family (enrofloxacin, ciprofloxacin), which interfere with DNA replication, and the antifolates (trimethoprim), which block folic acid synthesis, strongly induced reporter gene expression in a concentration-dependent manner (Fig. 1A). However, antibiotics belonging to other classes, interfering with cell wall, protein, and RNA synthesis (e.g., cephalexin, carbenicillin, chloramphenicol, kanamycin, tetracycline, rifampin), did not (Fig. 1A). Some antibiotics (e.g., tetracycline, chloramphenicol) even reduced the expression of the reporter. A similar expression pattern was observed in C. rodentium harboring the ϕstx2dact::Gluc reporter (Fig. 1B), demonstrating that the effects of subinhibitory antibiotic concentrations on stx2 expression in E. coli can be replicated in C. rodentium.
FIG 1.
Gaussia luciferase production is increased upon treatment with fluoroquinolone and antifolate antibiotics in both E. coli C600 ϕ933W and C. rodentium ϕstx2dact. C600 W34 ϕstx2a::Gluc and C. rodentium ϕstx2dact::Gluc strains were grown overnight at 37°C in LB broth supplemented with subinhibitory concentrations of the indicated antibiotics (1/2 to 1/16 MIC). Levels of Gaussia luciferase secreted from C600 W34 ϕstx2a::Gluc (A) and C. rodentium ϕstx2dact::Gluc (B) were measured by a Gaussia luciferase activity assay using equalized amounts of cleared bacterial supernatants. Shown are the means (± standard errors of the means [SEM]) of three independent experiments. Statistical analysis was performed by Student's t test, corrected using the Holm-Sidak method (*, P < 0.05; **, P < 0.01; ***, P < 0.001), with results compared to those of untreated bacteria. RLUs, relative light units.
Effects of subinhibitory and inhibitory concentrations of noninducing antibiotics on Stx secretion.
It is possible that the amount of Stx produced by bacteria in response to antibiotics differs from the amount of toxin released into the environment. To address this, we used inhibitory (1 MIC) and subinhibitory (1/2 MIC) concentrations of one antibiotic per family to treat C. rodentium ϕstx2dact and EHEC EDL933 cultures. Filter-sterilized supernatants were prepared and used for cytotoxicity assays. Vero cells are commonly used to evaluate Stx-mediated cytotoxicity, but these cells did not respond to the Stx2d subtype encoded by C. rodentium (see Fig. S1A in the supplemental material). Therefore, LLC-PK1 cells were used to study the cytotoxicity of C. rodentium ϕstx2dact supernatants, as these porcine renal cells responded to toxin treatment (Fig. S1B), while VeroB4 cells were used for EDL933 supernatants.
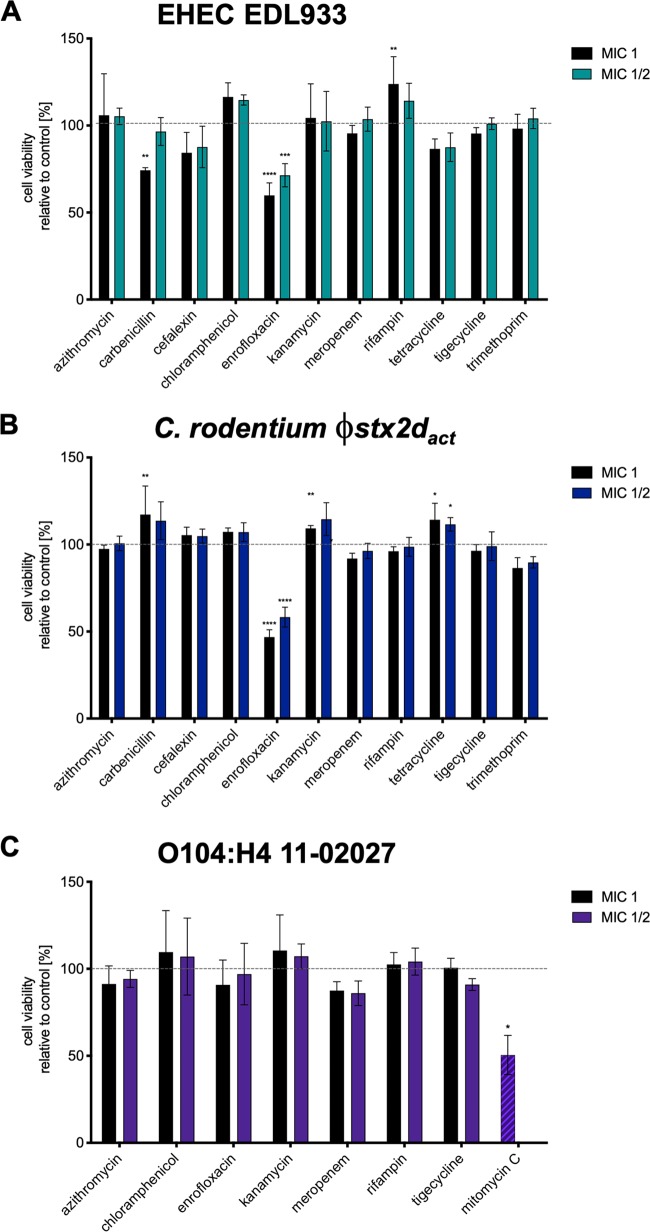
The cytotoxicity of supernatants from antibiotic-treated bacteria was compared to that of untreated controls, which was set as 100% cell viability. The results obtained for EDL933 (Fig. 2A) and C. rodentium ϕstx2dact (Fig. 2B) supported those of the reporter gene assays. Enrofloxacin-treated cell supernatants induced the release of Stx. Carbenicillin treatment at 1 MIC induced Stx release from EDL933 but not from C. rodentium ϕstx2dact, reducing Vero cell viability to ∼80% when treated with the respective supernatant. None of the other antibiotics increased toxin secretion by EDL933 or C. rodentium ϕstx2dact. Interestingly, while trimethoprim induced SOS response-dependent reporter gene expression in E. coli and C. rodentium, intoxication of cells with supernatants from EDL933 and C. rodentium ϕstx2dact treated with this antibiotic did not significantly alter cell viability (Fig. 2), suggesting that the concentration of this antibiotic might not be sufficient to induce bacterial cell lysis.
FIG 2.
Cytotoxic activity of supernatants of STEC and stx2dact-expressing C. rodentium strain. Strains O157:H7 EDL933, C. rodentium ϕstx2dact, and O104:H4 11-02027 were grown at 37°C in the presence of the indicated antibiotics. Supernatants of the cultures were withdrawn and cleared from bacteria by centrifugation. Bacteria-free supernatant of the O157:H7 EDL933 (A) or the O104:H4 11-02027 (C) culture was added to VeroB4 cells, and that of the C. rodentium ϕstx2dact culture was added to LLC-PK1 cells (B). After incubation for 72 h, XTT solution was added to PBS-washed cells for 2 h. Cells treated with 0.01% Triton X-100 were used as the positive (dead) control, while untreated cells were used as the negative (live) control. After incubation, the absorbance of the samples was determined at 475 nm and the cell viability of untreated cells was set to 100%. Shown are the mean values (±SEM) of three independent experiments performed in quadruplicate in comparison to the untreated control. Statistical analysis was performed via unpaired Mann-Whitney test (*, P < 0.05; **, P < 0.01; ***, P < 0.001), with all treated groups compared to the untreated control.
Noninducing antibiotics identified using EDL933 and C. rodentium ϕstx2dact were also effective in the EHEC/EAEC O104:H4 strain 11-02027.
The severe STEC outbreak in 2011 in Germany was caused by an EHEC/enteroaggregative E. coli (EAEC) O104:H4 strain carrying a Stx2a-encoding phage. This strain has a genome related to enteroaggregative E. coli strains and the pAA plasmid responsible for the aggregative phenotype (42, 43). Due to the differences in their genetic backgrounds, it seemed possible that the response of this strain varies from those of EHEC O157:H7 strains. Here, we tested the O104:H4 strain 11-02027 that was isolated from a patient during the outbreak (44) for its response to antibiotics by using the Vero cytotoxicity assay. Since the outbreak strain possessed not only the Stx-encoding phage but also a resistance plasmid with similarity to pEC_Bactec (NCBI accession no. GU371927) harboring TEM-1 and CTX-M-15 β-lactamase genes (43, 45), it is resistant to a large number of antibiotics. These include tetracyclines, streptomycin, trimethoprim, sulfonamides, cephalosporins, and ampicillin (42). Therefore, we limited our studies to the investigation of the effects of the remaining antibiotics that had proven inhibitory in the initial study with EDL933 (Fig. 2A), which included antibiotics suggested as last-resort treatment options during the outbreak (23). We observed that none of the antibiotics increased Stx release from O104:H4 strain 11-02027 when used at the MIC or 1/2 MICs (Fig. 2C). Surprisingly, enrofloxacin had no effect on Stx release, while the common Stx inducer mitomycin C increased cytotoxicity (Fig. 2C).
Antibiotic treatment of mice infected with C. rodentium ϕstx2dact identified noninducing antibiotics that efficiently cleared the infection.
To evaluate whether the inhibitory effects observed in vitro resulted in clearance of infection and increased survival in vivo under more natural conditions, we tested six common, orally provided antibiotics in the C. rodentium ϕstx2dact mouse model. Chloramphenicol could not be used in the study, as C. rodentium ϕstx2dact DBS770 contains a resistance cassette for chloramphenicol integrated for selection (36). We orally infected mice with 5 × 108 CFU of C. rodentium ϕstx2dact. Mice were weighed daily (Fig. 3A; Fig. S2), and fecal colonization with C. rodentium ϕstx2dact was monitored every other day over the course of the experiment (Fig. 3B; Fig. S3). From day 4 postinfection, mice were provided with therapeutic concentrations of antibiotics in their drinking water. Mice treated with the quinolone antibiotic enrofloxacin rapidly cleared the bacterial infection (Fig. 3B). However, 40% of the infected, enrofloxacin-treated mice showed a marked weight loss of 20% of the initial body weight and required euthanization, similar to nontreated mice, with 50% of animals requiring euthanization over the course of the experiment (12 days) (Fig. 3C). Mice treated with ampicillin, kanamycin, or tetracycline all cleared infection within 2 days of the commencement of treatment. None of these mice lost a considerable amount of weight, resulting in a 100% survival rate for these groups (Fig. 3A to C; Fig. S2 and S3). Mice treated with trimethoprim showed a highly reduced ability to clear the infection. This resulted in an infection-induced weight loss from which the mice were able to recover after eradication of the bacteria. Rifampin, which targets bacterial transcription, caused only partial clearance of bacteria in the feces (Fig. 3B; Fig. S3). Interestingly, no weight loss was observed for this group, and all mice survived the infection (Fig. 3A and C; Fig. S2).
FIG 3.
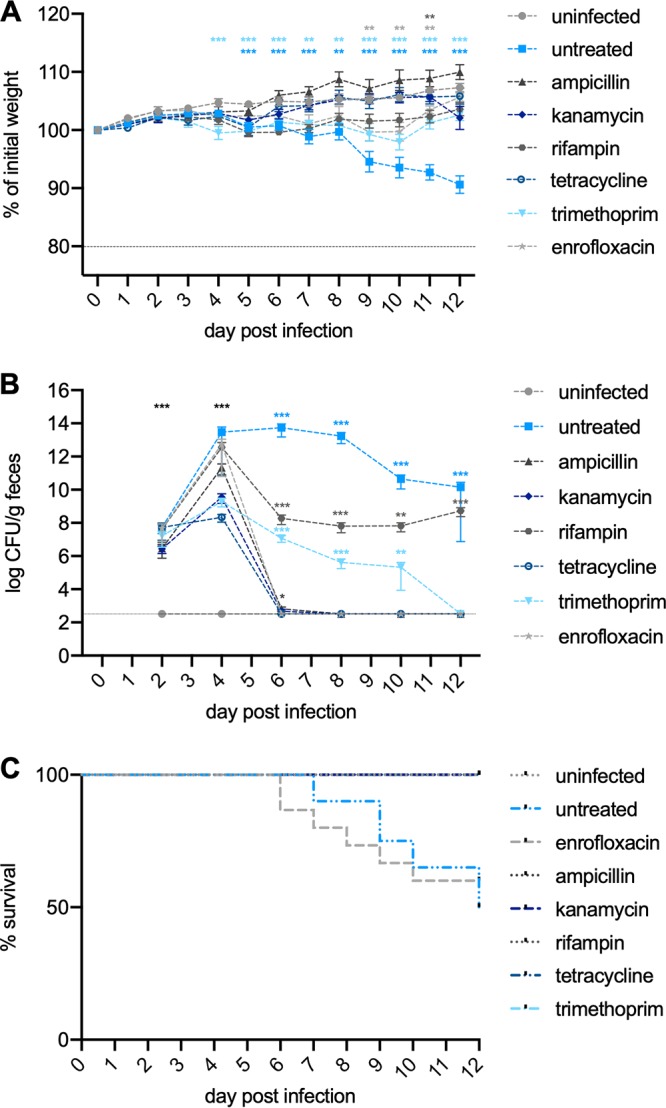
Influence of antibiotic treatment on weight loss, bacterial clearance, and survival of C. rodentium ϕstx2dact-infected mice. Six-week old female C57BL/6 mice were orally infected with 5 × 108 CFU of C. rodentium ϕstx2dact and supplied with the indicated antibiotics in the drinking water from day 4 postinfection. The infection was followed for 12 days. (A) Changes in body weight represent the mean (±SEM) for each group and are expressed as percent change from day 0. (B) Colonization was assessed by plating fecal samples for viable counts and are shown as mean CFU (±SEM) of 15 mice representing three separate experiments with 5 mice each. Statistical analysis was performed using the Mann-Whitney test, comparing weight change or colonization of uninfected mice to that of mice treated with antibiotics or untreated at each time point (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (C) The survival of the mice in each group was determined as a percentage of the whole (n = 15).
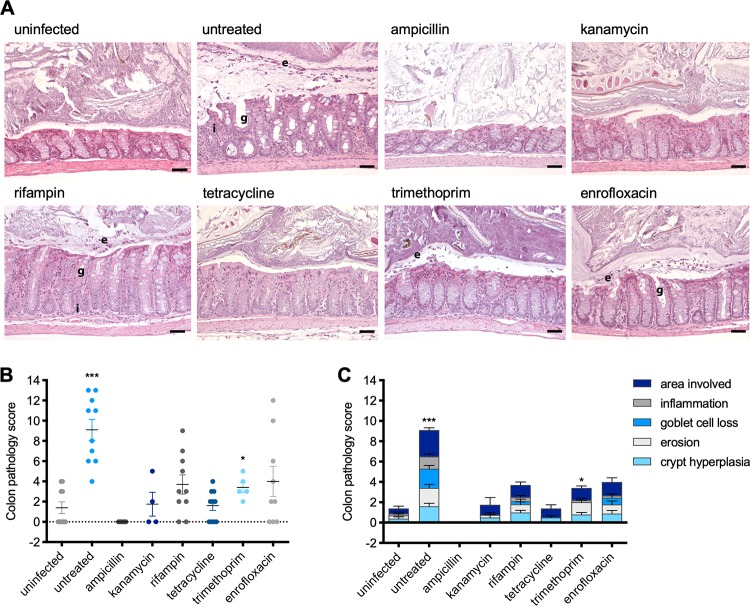
Various degrees of Stx-induced colon and kidney damage were observed in mice treated with antibiotics.
To analyze how antibiotic treatment influenced the colon and kidney pathology of C. rodentium ϕstx2dact, organs were removed from euthanized mice 12 days postinfection and colon and kidney pathology scores were determined on hematoxylin-and-eosin (H&E)-stained sections. As shown in Fig. 4, we found that the colons of infected untreated mice showed a high degree of inflammation, erosion, and crypt hyperplasia as well as goblet cell loss. The colon pathology scores of the enrofloxacin-, rifampin-, and trimethoprim-treated mice were increased but still significantly lower than the scores of the infected untreated mice (Fig. 4B and C). It is likely that not the toxin, but the C. rodentium itself, which causes inflammatory colitis in mice (37, 40, 46), is responsible for the pathological effect. In contrast, no inflammation or loss of goblet cells and only low levels of crypt hyperplasia were seen in the colons of infected kanamycin- and tetracycline-treated mice (Fig. 4A and B), which were immediately cleared.
FIG 4.
Influence of antibiotic treatment on intestinal damage during murine infection with C. rodentium ϕstx2dact. (A) Representative H&E-stained colon sections of uninfected mice or infected mice treated with the indicated antibiotic from day 4 after infection. Scale bars, 50 μm. Pathological changes are indicated as follows: e, epithelial erosion; g, goblet cell loss; i, inflammation. (B) The pathology of the colons of uninfected mice or infected mice with and without antibiotic treatment was scored, and the pathology scores of individual mice are shown as means (±SEM) for all assessed mice per group. Statistical analysis was performed by Student's t test, corrected using the Holm-Sidak method (*, P < 0.05; **, P < 0.01; ***, P < 0.001), with results for infected/treated mice compared to those for the untreated control group. (C) Summary of the pathological changes of each group.
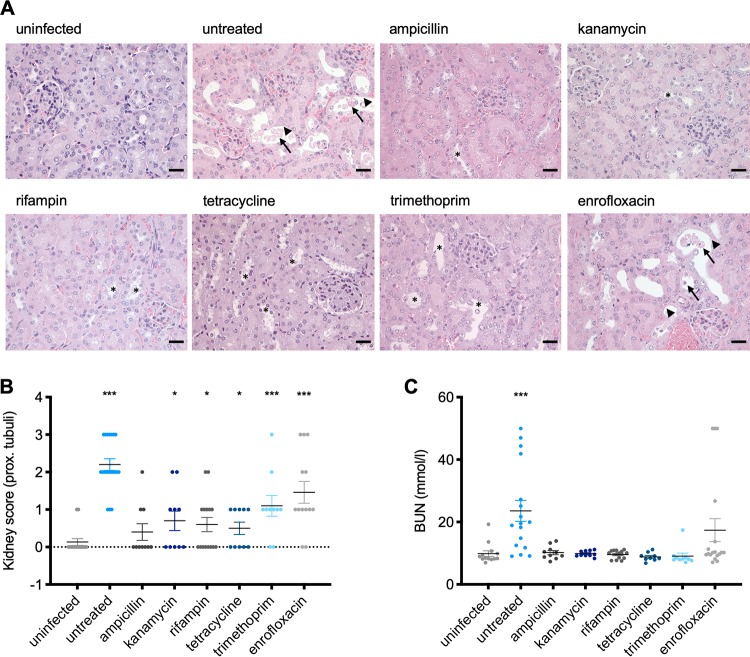
The kidneys of infected, untreated animals showed marked pathological changes of the proximal tubules (Fig. 5A and B). Pathological changes included moderate to severe tubular degeneration characterized by a loss of the lining epithelium and sloughing of necrotic cells in the lumina in 5 of 15 mice (Fig. 5A and B). In contrast, no pathological changes could be observed in mice treated with ampicillin, kanamycin, rifampin, and tetracycline (Fig. 5A and B). In contrast, some tubular degeneration with loss of the lining epithelium was also observed in the kidneys of trimethoprim-treated animals, which were only able to slowly clear the infection (Fig. 3B). Severe pathological changes, similar to those in infected untreated mice, were observed with enrofloxacin-treated mice (Fig. 5A and B). Tubular degeneration in these mice suggests that kidney damage was indeed a result of Stx release upon antibiotic treatment, because the bacteria themselves were eliminated by day 6 postinfection (Fig. 3B).
FIG 5.
Influence of antibiotic treatment on Stx-mediated renal damage during murine infection with C. rodentium ϕstx2dact. (A) Kidney sections of uninfected mice or infected mice treated with the indicated antibiotic were stained with H&E and scored. Representative kidney sections for each group are shown, with scale bars indicating 25 μm. Pathological changes are indicated as follows: arrow, sloughing of necrotic cells in lumen; arrowhead, loss of lining epithelium; *, tubular dilation. (B) Pathology scores of proximal tubules of the individual animals in each group. (C) Serum BUN (blood urea nitrogen) levels were determined as a marker for kidney damage. Shown are the means (±SEM) for all assessed mice per group. Statistical analysis was performed by Student's t test, corrected using the Holm-Sidak method (*, P < 0.05; **, P < 0.01; ***, P < 0.001), with results for infected/treated mice compared to those for the untreated control group.
Serological analysis of available samples from mice treated with enrofloxacin showed a strong increase in levels of blood urea nitrogen (BUN), a marker of kidney damage, from 9.9 ± 3.3 mmol/liter in uninfected untreated mice to 35.3 ± 11.6 mmol/liter in infected untreated mice, with three samples reaching the detection limit (49.98 mmol/liter) (Fig. 5B). Strikingly, BUN levels were not altered in animals from the ampicillin-, kanamycin-, rifampin-, and trimethoprim-treated groups compared to the untreated group (Fig. 5C). Notably, while rifampin-treated mice were still colonized (Fig. 3B), the bacteria seem unable to induce Stx release, as the serum levels of BUN were similar to those of untreated uninfected mice (9.6 ± 1.3 mmol/liter compared to 9.9 ± 3.3 mmol/liter), and the histological analysis of the kidneys showed only very mild tubular dilation (Fig. 5A to C).
DISCUSSION
The complications associated with EHEC infections represent a serious global public health problem, and their prevention is the prime goal in the treatment of patients. Unfortunately, treatment is currently limited to supportive therapy, as the use of antibiotics is thought to enhance Stx production, increasing the risk of HUS development. However, the necessity for antibiotic treatment might arise for a subset of patients with life-threatening manifestations, as the outbreak in Germany in 2011 has shown. Therefore, more detailed investigations into possible antibiotic options are important. While this has been attempted with subsets of antibiotics in vitro before, the confirmation of the noninducing effect of promising antibiotics in adequate animal models has not been fully explored.
In this study, we systematically investigated the effect of antibiotics from a number of different classes on reporter gene expression from the Stx2d-encoding phage ϕ1720a-02 introduced into the chromosome of C. rodentium (36). We showed that, similar to a stx2a gene fusion in the 933W phage in E. coli, the reporter gene induction occurred when bacteria were treated with antibiotics belonging to the fluoroquinolones (enrofloxacin) or antifolates (trimethoprim), confirming the results of earlier studies (17, 19–21, 23, 47). Reporter gene induction by quinolones correlated with the release of biologically active toxins produced and released by the pathogens (Fig. 1 and 2). Using C. rodentium ϕstx2dact for infection of mice, we further observed that enrofloxacin-treated mice rapidly cleared the infection but displayed severe Stx-mediated pathology (Fig. 3, 4, and 5). This indicates that phage induction by quinolones is particularly hazardous. This is supported by studies in which an increase in fecal Stx and death in 70 to 80% of infected mice treated with ciprofloxacin or levofloxacin were observed despite a decrease in fecal STEC (30, 32, 48). Other studies have reported a significantly lower occurrence of death of STEC-infected mice after intraperitoneal or oral administration of norfloxacin, but in these studies, the antibiotics were administered 2 to 3 h postinfection (29, 49). It has been discussed that rapid synthesis of Stx may facilitate the entry of the toxin into the bloodstream and the subsequent effects on kidneys and other organs (17). This strongly suggests that administration of fluoroquinolones such as ciprofloxacin or enrofloxacin as treatment against diarrheal infections should be used only in cases where infection with Stx-producing E. coli can be ruled out, especially since ciprofloxacin is commonly prescribed for patients with severe diarrhea and enteric infections of unknown etiology (17). Similar to what has been observed in mice, it is possible that early treatment with quinolones might produce different results, as has been described by Geerdes-Fenge et al. (50) for patients treated with ciprofloxacin in the early days of infection. The onset of bloody diarrhea in patients usually occurs between 3 and 8 days after infection (2). In this study, we commenced antibiotic treatment on day 4 postinfection, as published previously (32). However, as mice do not develop diarrhea, the different stages of infection in mice are harder to distinguish and disease progression may be more rapid.
Also, the use of trimethoprim was found to increase stx2 induction in E. coli and C. rodentium. This antibiotic did not clear the infection rapidly and did not reduce or reduced only slightly Stx-mediated kidney damage in mice. This confirmed a previous study with trimethoprim-treated STEC-infected mice (51) and supports observations from clinical studies indicating that the use of trimethoprim increases the risk of HUS development in humans (52), although others report no higher risk (5).
In contrast, antibiotics from other classes had no effect on or reduced reporter gene expression. These results are also in agreement with those of Bielaszewska et al. (23), who showed that Stx synthesis of EHEC strains increased in the presence of ciprofloxacin while chloramphenicol inhibited Stx production, as did azithromycin, rifaximin, meropenem, and tigecycline. Here, we showed that three of the antibiotics used in our animal study (ampicillin, kanamycin, and tetracycline) cleared bacterial infection and rifampin reduced the bacterial load without any effects on colon or kidney health. It is unclear whether the concentration of rifampin was too low to clear the infection, but the induced blockage of transcription appeared sufficient to inhibit the development of Stx-mediated disease. No induction of stx expression by rifampin, kanamycin, and tetracycline confirms previous reports with different STEC strains (17, 20, 21, 23, 26, 29, 31, 54), suggesting that these antibiotics may provide promising treatment options. In fact, the few patients who developed HUS and were treated with rifaximin, a rifampin derivative, had fewer seizures and excreted E. coli for a shorter time period than those not treated (53). However, ampicillin has yielded opposing results in in vitro studies (19, 20), and frequent resistance of EHEC to beta-lactams suggests that other classes of antibiotics may be a better treatment option. In particular, care has to be taken, as the timing of antibiotic administration, e.g., whether shortly after infection (0 to 2 days postinfection) or after the appearance of first symptoms such as weight loss (7 to 8 days postinfection), appears to play an important role on the development of HUS (5, 17). Nonetheless, interstrain differences in the susceptibility to phage activation and possible other induction mechanisms cannot be ruled out and require rapid identification of the response of STEC outbreak strains to antibiotics. A more promising treatment strategy may be a combination therapy, in which two antibiotics which had no effect on stx induction are given either simultaneously or with a slight delay. Moreover, a combination of an inhibitory antibiotic such as rifampin, tetracycline, or chloramphenicol with an antibiotic that quickly eradicates the infection may be advantageous, as might be a combination of an antibiotic and neutralizing antibodies. We conclude that some of the non-Stx-inducing antibiotics seem to be able to avert disease progression in Stx-expressing C. rodentium-infected mice and are promising candidates to minimize HUS development in humans.
MATERIALS AND METHODS
Ethics disclosure.
C57BL/6Rj mice (Janvier) were housed under pathogen-free conditions in accordance with FELASA recommendations in biosafety level 3 animal facilities of the Helmholtz Centre for Infection Research, Braunschweig, Germany The protocol was approved by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (permission no. 33.19-42502-04-16/2124). Animals were treated with appropriate care, and all efforts were made to minimize suffering.
Bacterial strains and cell lines.
Bacterial strains and cell lines are given in Table S1 in the supplemental material. Bacteria were grown in Luria-Bertani broth (LB; Carl Roth, Germany) or Mueller-Hinton broth (MH; Sigma-Aldrich/Merck, Germany) broth as required. Cell lines were cultured at 37°C with 5% CO2 in a HeraCell 150 cell culture incubator (Thermo Fisher Scientific, Germany).
MIC determination.
The antibiotics used are given in Table S3. The MICs of the antibiotics were determined according to the Clinical and Laboratory Standards Institute guidelines for all strains used in this study (https://clsi.org). The MICs for all tested strains are provided in Table 1.
TABLE 1.
MICs of antibiotics determined for bacterial strains used in this studya
| Antibiotic | MIC (μg/ml) |
|||
|---|---|---|---|---|
| C. rodentium DBS100 | E. coli EDL933 | E. coli 11-02027 |
E. coli C600 |
|
| Ampicillin | 32 | R | 5 | |
| Azithromycin | 5 | (4.0) | 10 | 5 |
| Carbenicillin | 24 | 3 | R | 2.4 |
| Cephalexin | 0.15 | 0.15 | R | 6 |
| Chloramphenicol | 1.5 | 1.5 (8.0) | 3 | 3 |
| Ciprofloxacin | 0.004 | 0.004 (0.06) | 0.004 | 0.08 |
| Enrofloxacin | 0.04 | 0.04 | 0.04 | 0.04 |
| Kanamycin | 0.6 | 0.3 (0.25) | 2.5 | 2.5 |
| Meropenem | 0.03 | (0.016) | 0.1 | 0.03 |
| Rifampin | 1 | 2 | 2 | 8 |
| Tetracycline | 1.5 | 1.5 | R | 0.4 |
| Tigecycline | 0.16 | (0.125) | 0.15 | 0.08 |
| Trimethoprim | 0.06 | 0.06 | R | 0.25 |
| Vancomycin | 40 | 80 | 78 | 80 |
R, resistance (reference). Published concentrations for EHEC EDL933 are given in parentheses.
Reporter gene assays.
For reporter gene assays, one colony of the respective bacterial strain was inoculated into 5 ml of LB (Carl Roth) and supplemented with the appropriate amounts of each antibiotic to yield 1/2 to 1/16 MIC. Cultures were then grown at 37°C, 200 rpm, for 15 h. The optical density (OD) of each culture was determined, and a volume corresponding to an OD at 600 nm (OD600) of 1 (109 cells) was pelleted at 13,900 × g (Eppendorf Minispin Plus) for 1 min. Twenty microliters of supernatant was mixed with 50 μl BioLux Gaussia luciferase assay (New England Biolabs) substrate, luciferase activity was determined in a VarioSkan Flash plate reader (Thermo Fisher Scientific) according to the manufacturer’s recommendations, and values were compared to those of untreated culture supernatant. LB medium (Carl Roth) was used as a negative control.
Antibiotic induction of Stx production.
For cytotoxicity assays, bacterial strains were grown to an OD600 of 0.5 in 100 ml LB. Each culture was divided into 5-ml cultures, which were supplemented with the appropriate antibiotics of 1/2 to 1/16 MIC or left untreated. After incubation at 37°C for 15 h, the cultures were pelleted at 3,000 × g for 5 min, and culture supernatants were subsequently sterilized using filters with a 0.22-μm pore size and used for cytotoxicity assays.
XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt] cytotoxicity assays.
A total of 4 × 104 cells (VeroB4 or LLC-PK1) per ml were seeded into 96-well plates 24 h prior to treatment with supernatants. On the day of treatment, dilution series of the supernatants were prepared in the respective cell culture medium and 100 μl of the suspensions was added to each well and incubated under cell culture conditions (37°C, 5% CO2) for 72 h. Afterwards, the medium was removed and cells were washed twice in Dulbecco’s phosphate-buffered saline (Sigma-Aldrich/Merck). The medium was mixed with reagents of the cell proliferation kit II (Sigma-Aldrich/Merck) and added to the cells for 2 h. Cell viability was determined in a VarioSkan Flash plate reader (Thermo Fisher Scientific) by measuring the absorbance at 475 nm. Untreated cells were used as a positive (live) control, and cells treated with 0.1% Triton X-100 (Carl Roth) were used as a negative (dead) control.
Animal infections.
Six-week-old C57BL/6Rj mice purchased from Janvier Labs (Le Genest-Saint-Isle, France) were infected with 5 × 108 CFU of C. rodentium DBS770 in accordance with the feeding protocol described previously (36). From day 4 postinfection, drinking water was supplemented with 2% glucose and any one of the following antibiotics (for more information, see Table S3): ampicillin (10 mg/ml), enrofloxacin (0.25 mg/ml), kanamycin (2.6 mg/ml), tetracycline (1 mg/ml), rifampin (1 mg/ml), or trimethoprim (Trimetotat oral suspension, 48% [Livisto]). Supplemented water was exchanged daily to ensure continuously high levels of the antibiotics. Mice were weighed daily, and fecal colonization was determined every other day.
Histology and pathological evaluation.
Colon and kidneys were removed and fixed in Roti-Histofix (Carl Roth) for 24 h and stored in 70% ethanol until further use. Samples were embedded in paraffin, and 3-μm-thick sections were stained with hematoxylin-eosin according to standard laboratory procedures. Sections were analyzed in a randomized fashion and deidentified with respect to the experimental groups. Tubular necrosis was graded as follows: 1, sporadic tubular necrosis or dilation; 2, 30 to 50% of the tubuli show necrosis; 3, more than 50% of the tubuli show necrosis. The colon was scored by five markers, namely inflammation, epithelial erosion, goblet cell loss, epithelial hyperplasia, and area involved. Of these markers, inflammation was scored as follows: 1, few inflammatory cells in lamina propria; 2, clearly visible inflammatory cells reaching submucosa; 3, transmural invasion of inflammatory cells. The marker epithelial erosion was scored as follows: 1, sporadic erosion of epithelial cells; 2, clearly visible erosion of epithelial cells, with moderate amounts of sloughed cells in lumen; 3, thinning of crypt walls and large amounts of sloughed cells in lumen. The marker goblet cell loss was scored as follows: 1, slightly reduced number of goblet cells; 2, moderate loss of goblet cells with sporadic increase in size; 3, severe loss of goblet cells with marked increase in size of goblet cells. The marker epithelial hyperplasia was scored as follows: 1, up to 100% increase in thickness; 2, more than 100% increase in thickness; 3, more than 100% increase in thickness and altered morphology. The marker area involved was scored as follows: 1, up to 30%; 2, 40 to 70%; 3, more than 70%.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Wolf, Amrei Rolof, Tanja Krause, and Karin Paduch for technical assistance. We are grateful to Roman Gerlach (Robert-Koch-Institut, Wernigerode, Germany) for supplying pWRG701.
Petra Dersch, Sabrina Mühlen, Martin Koeppel, and Bärbel Stecher are supported by the Deutsche Zentrum für Infektionsforschung (DZIF). Petra Dersch and Sabrina Mühlen were supported by the Niedersachsen Vorab−Coalition.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Karch H, Tarr PI, Bielaszewska M. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol 295:405–418. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 3.Andreoli SP, Trachtman H, Acheson DWK, Siegler RL, Obrig TG. 2002. Hemolytic uremic syndrome: epidemiology, pathophysiology, and therapy. Pediatr Nephrol 17:293–298. doi: 10.1007/s00467-001-0783-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Laing C, Zhang Z, Hallewell J, You C, Ziebell K, Johnson RP, Kropinski AM, Thomas JE, Karmali M, Gannon V. 2010. Lineage and host source are both correlated with levels of Shiga toxin 2 production by Escherichia coli O157:H7 strains. Appl Environ Microbiol 76:474–482. doi: 10.1128/AEM.01288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakoullis L, Papachristodoulou E, Chra P, Panos G. 2019. Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: a global overview. J Infect 79:75–94. doi: 10.1016/j.jinf.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Karmali MA, Steele BT, Petric M, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien AD, Lively TA, Chang TW, Gorbach SL. 1983. Purification of Shigella dysenteriae 1 (Shiga)-like toxin from Escherichia coli O157:H7 strain associated with haemorrhagic colitis. Lancet 2:573. doi: 10.1016/S0140-6736(83)90601-3. [DOI] [PubMed] [Google Scholar]
- 8.Melton-Celsa AR. 2014. Shiga toxin (Stx) classification, structure, and function. Microbiol Spectr 2:EHEC-0024-2013. doi: 10.1128/microbiolspec.EHEC-0024-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrich AW, Bielaszewska M, Zhang W-L, Pulz M, Kuczius T, Ammon A, Karch H. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich AW, Borell J, Bielaszewska M, Fruth A, Tschäpe H, Karch H. 2003. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J Clin Microbiol 41:2448–2453. doi: 10.1128/jcm.41.6.2448-2453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson S, Olsen KEP, Ethelberg S, Scheutz F. 2007. Subtyping method for Escherichia coli Shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J Clin Microbiol 45:2020–2024. doi: 10.1128/JCM.02591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newland JW, Strockbine NA, Miller SF, O'Brien AD, Holmes RK. 1985. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science 230:179–181. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 14.de Sablet T, Chassard C, Bernalier-Donadille A, Vareille M, Gobert AP, Martin C. 2009. Human microbiota-secreted factors inhibit Shiga toxin synthesis by enterohemorrhagic Escherichia coli O157:H7. Infect Immun 77:783–790. doi: 10.1128/IAI.01048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panos GZ, Betsi GI, Falagas ME. 2006. Systematic review: are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection?. Aliment Pharmacol Ther 24:731–742. doi: 10.1111/j.1365-2036.2006.03036.x. [DOI] [PubMed] [Google Scholar]
- 16.Mühldorfer I, Hacker J, Keusch GT, Acheson DW, Tschäpe H, Kane AV, Ritter A, Olschläger T, Donohue-Rolfe A. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect Immun 64:495–502. doi: 10.1128/IAI.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis 6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerhackl LB. 2000. E. coli, antibiotics, and the hemolytic-uremic syndrome. N Engl J Med 342:1990–1991. doi: 10.1056/NEJM200006293422611. [DOI] [PubMed] [Google Scholar]
- 19.Grif K, Dierich MP, Karch H, Allerberger F. 1998. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis 17:761–766. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]
- 20.McGannon CM, Fuller CA, Weiss AA. 2010. Different classes of antibiotics differentially influence Shiga toxin production. Antimicrob Agents Chemother 54:3790–3798. doi: 10.1128/AAC.01783-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochoa TJ, Chen J, Walker CM, Gonzales E, Cleary TG. 2007. Rifaximin does not induce toxin production or phage-mediated lysis of Shiga toxin-producing Escherichia coli. Antimicrob Agents Chemother 51:2837–2841. doi: 10.1128/AAC.01397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen MG, Hansen C, Riise E, Persson S, Olsen K. 2008. Subtype-specific suppression of Shiga toxin 2 released from Escherichia coli upon exposure to protein synthesis inhibitors. J Clin Microbiol 46:2987–2991. doi: 10.1128/JCM.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. 2012. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother 56:3277–3282. doi: 10.1128/AAC.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Donohue-Rolfe A, Krautz-Peterson G, Sevo M, Parry N, Abeijon C, Tzipori S. 2009. Gnotobiotic piglet infection model for evaluating the safe use of antibiotics against Escherichia coli O157:H7 infection. J Infect Dis 199:486–493. doi: 10.1086/596509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassar FJ, Rahal EA, Sabra A, Matar GM. 2013. Effects of subinhibitory concentrations of antimicrobial agents on Escherichia coli O157:H7 Shiga toxin release and role of the SOS response. Foodborne Pathog Dis 10:805–812. doi: 10.1089/fpd.2013.1510. [DOI] [PubMed] [Google Scholar]
- 26.Walterspiel JN, Ashkenazi S, Morrow AL, Cleary TG. 1992. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin I. Infection 20:25–29. doi: 10.1007/bf01704889. [DOI] [PubMed] [Google Scholar]
- 27.Yoh M, Frimpong EK, Honda T. 1997. Effect of antimicrobial agents, especially fosfomycin, on the production and release of Vero toxin by enterohaemorrhagic Escherichia coli O157:H7. FEMS Immunol Med Microbiol 19:57–64. doi: 10.1111/j.1574-695X.1997.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoh M, Frimpong EK, Voravuthikunchai SP, Honda T. 1999. Effect of subinhibitory concentrations of antimicrobial agents (quinolones and macrolide) on the production of verotoxin by enterohemorrhagic Escherichia coli O157:H7. Can J Microbiol 45:732–739. doi: 10.1139/w99-069. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura K, Fujii J, Taniguchi H, Yoshida S. 1999. Chemotherapy for enterohemorrhagic Escherichia coli O157:H infection in a mouse model. FEMS Immunol Med Microbiol 26:101–108. doi: 10.1111/j.1574-695X.1999.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 30.Amran MY, Fujii J, Suzuki SO, Kolling GL, Villanueva S, Kainuma M, Kobayashi H, Kameyama H, Yoshida S. 2013. Investigation of encephalopathy caused by Shiga toxin 2c-producing Escherichia coli infection in mice. PLoS One 8:e58959. doi: 10.1371/journal.pone.0058959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahal EA, Kazzi N, Kanbar A, Abdelnoor AM, Matar GM. 2011. Role of rifampicin in limiting Escherichia coli O157:H7 Shiga-like toxin expression and enhancement of survival of infected BALB/c mice. Int J Antimicrob Agents 37:135–139. doi: 10.1016/j.ijantimicag.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis 181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 33.Zangari T, Melton-Celsa AR, Panda A, Smith MA, Tatarov I, De Tolla L, O'Brien AD. 2014. Enhanced virulence of the Escherichia coli O157:H7 spinach-associated outbreak strain in two animal models is associated with higher levels of Stx2 production after induction with ciprofloxacin. Infect Immun 82:4968–4977. doi: 10.1128/IAI.02361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohawk KL, O'Brien AD. 2011. Mouse models of Escherichia coli O157:H7 infection and Shiga toxin injection. J Biomed Biotechnol 2011:258185. doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie JM. 2014. Animal models of enterohemorrhagic Escherichia coli infection. Microbiol Spectr 2:EHEC-0022-2013. doi: 10.1128/microbiolspec.EHEC-0022-2013. [DOI] [PubMed] [Google Scholar]
- 36.Mallick EM, McBee ME, Vanguri VK, Melton-Celsa AR, Schlieper K, Karalius BJ, O'Brien AD, Butterton JR, Leong JM, Schauer DB. 2012. A novel murine infection model for Shiga toxin-producing Escherichia coli. J Clin Invest 122:4012–4024. doi: 10.1172/JCI62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. 2005. Citrobacter rodentium of mice and man. Cell Microbiol 7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 38.Borenshtein D, McBee ME, Schauer DB. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol 24:32–37. doi: 10.1097/MOG.0b013e3282f2b0fb. [DOI] [PubMed] [Google Scholar]
- 39.Crepin VF, Collins JW, Habibzay M, Frankel G. 2016. Citrobacter rodentium mouse model of bacterial infection. Nat Protoc 11:1851–1876. doi: 10.1038/nprot.2016.100. [DOI] [PubMed] [Google Scholar]
- 40.Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, Frankel G. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 41.Silberger DJ, Zindl CL, Weaver CT. 2017. Citrobacter rodentium: a model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol 10:1108–1117. doi: 10.1038/mi.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis 11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 43.Mellmann A, Harmsen D, Cummings C, Zentz E, Leopold S, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, McLaughlin S, Henkhaus J, Leopold B, Bielaszewska M, Prager R, Brzoska P, Moore R, Guenther S, Rothberg JM, Karch H. 2011. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 6:e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aurass P, Prager R, Flieger A. 2011. EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol 13:3139–3148. doi: 10.1111/j.1462-2920.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- 45.Smet A, Nieuwerburgh F, Van Vandekerckhove TT, Martel A, Deforce D, Butaye P, Haesebrouck F. 2010. Complete nucleotide sequence of CTX-M-15-plasmids from clinical Escherichia coli isolates: insertional events of transposons and insertion sequences. PLoS One 5:e11202. doi: 10.1371/journal.pone.0011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schauer DB, Falkow S. 1993. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun 61:2486–2492. doi: 10.1128/IAI.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JH, Stein BD. 2009. Antimicrobials effective for inhibition of enterohaemorrhagic Escherichia coli strains O26, O111, and O157 and their effects on Shiga toxin releases. J Microbiol Biotechnol 19:1238–1243. [PubMed] [Google Scholar]
- 48.Hiramatsu K, Murakami J, Kishi K, Hirata N, Yamasaki T, Kadota J, Shibata T, Nasu M. 2003. Treatment with rokitamycin suppresses the lethality in a murine model of Escherichia coli O157:H7 infection. Int J Antimicrob Agents 21:471–477. doi: 10.1016/S0924-8579(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 49.Sawamura S, Tanaka K, Koga Y. 1999. Therapeutic effects of antibiotics against enterohemorrhagic Escherichia coli (EHEC) O157:H7 (O157) infection: in vivo analysis using germfree mice. Kansenshogaku Zasshi 73:1054–1063. (In Japanese.) doi: 10.11150/kansenshogakuzasshi1970.73.1054. [DOI] [PubMed] [Google Scholar]
- 50.Geerdes-Fenge HF, Löbermann M, Nürnberg M, Fritzsche C, Koball S, Henschel J, Höhn R, Schober HC, Mitzner S, Podbielski A, Reisinger EC. 2013. Ciprofloxacin reduces the risk of hemolytic uremic syndrome in patients with Escherichia coli O104:H4-associated diarrhea. Infection 41:669–673. doi: 10.1007/s15010-012-0387-6. [DOI] [PubMed] [Google Scholar]
- 51.Kurioka T, Yunou Y, Harada H, Kita E. 1999. Efficacy of antibiotic therapy for infection with Shiga-like toxin-producing Escherichia coli O157:H7 in mice with protein-calorie malnutrition. Eur J Clin Microbiol Infect Dis 18:561–571. doi: 10.1007/s100960050348. [DOI] [PubMed] [Google Scholar]
- 52.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, Bremer JP, Brunkhorst R, Busch V, Dengler R, Deuschl G, Fellermann K, Fickenscher H, Gerigk C, Goettsche A, Greeve J, Hafer C, Hagenmüller F, Haller H, Herget-Rosenthal S, Hertenstein B, Hofmann C, Lang M, Kielstein JT, Klostermeier UC, Knobloch J, Kuehbacher M, Kunzendorf U, Lehnert H, Manns MP, Menne TF, Meyer TN, Michael C, Münte T, Neumann-Grutzeck C, Nuernberger J, Pavenstaedt H, Ramazan L, Renders L, Repenthin J, Ries W, Rohr A, Rump LC, Samuelsson O, Sayk F, Schmidt BMW, Schnatter S, Schöcklmann H, Schreiber S, von Seydewitz CU, Steinhoff J, Stracke S, Suerbaum S, van de Loo A, Vischedyk M, Weissenborn K, Wellhöner P, Wiesner M, Zeissig S, Büning J, Schiffer M, Kuehbacher T, EHEC-HUS Consortium . 2012. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 345:e4565. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fadlallah SM, Rahal EA, Sabra A, Kissoyan KA, Matar GM. 2015. Effect of rifampicin and gentamicin on Shiga toxin 2 expression level and the SOS response in Escherichia coli O104:H4. Foodborne Pathog Dis 12:47–55. doi: 10.1089/fpd.2014.1824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.