Acinetobacter baumannii infections are difficult to treat and have limited treatment options. Carbapenems, including meropenem, are currently considered the first-line agents for the treatment of infections caused by Acinetobacter spp. The percentage of a 24-hour period that the concentration of free drug in plasma is above the MIC (%24-h fT>MIC) to achieve stasis, 1 log CFU, or 2 log CFU of bacterial killing against A. baumannii has not been studied previously for meropenem.
KEYWORDS: Acinetobacter baumannii, meropenem, thigh model
ABSTRACT
Acinetobacter baumannii infections are difficult to treat and have limited treatment options. Carbapenems, including meropenem, are currently considered the first-line agents for the treatment of infections caused by Acinetobacter spp. The percentage of a 24-hour period that the concentration of free drug in plasma is above the MIC (%24-h fT>MIC) to achieve stasis, 1 log CFU, or 2 log CFU of bacterial killing against A. baumannii has not been studied previously for meropenem. The objective of this study was to determine these parameters for meropenem against A. baumannii in a neutropenic mouse thigh infection model. Six A. baumannii clinical isolates with MICs ranging from 0.25 to 16 mg/liter were tested. Meropenem produced a bacteriostatic effect with a %24-h fT>MIC of 7 to 24% and produced 1 log CFU of bacterial killing with a %24-h fT>MIC of 15 to 37%.
TEXT
Acinetobacter baumannii colonizes and infects critically ill patients in intensive care units and long-term care units, especially those who are immunocompromised or have chronic and/or underlying disease. A. baumannii has as an uncanny ability to survive on environmental surfaces within the hospital setting and can be transmitted via the hands of health care providers and via contaminated tools and equipment (1–4). Currently, there are limited treatment options for A. baumannii infection, which has prompted the U.S. Centers for Disease Control (CDC) to consider A. baumannii a “serious” antimicrobial resistance threat (5).
Despite the challenges this pathogen poses to the community at large, there are limited scientific data, either in vivo or in vitro, describing the pharmacodynamics of the limited number of agents that are used to treat A. baumannii infections. This absence of data increases the difficulty clinicians face in selecting the optimal dosage regimen for treatment and the prevention of emerging resistance. One such agent, meropenem, is considered one of the first-line agents of choice for the treatment of infections caused by Acinetobacter spp.
Despite meropenem being a first-line agent for the treatment of A. baumannii infections and in clinical use for over a decade, the exposures required for bacterial killing have only been described in detail for Enterobacteriaceae and Pseudomonas aeruginosa (6–9). The purpose of these studies was to determine for meropenem the percentage of a 24-hour period that the concentration of free drug in plasma is above the MIC (%24-h fT>MIC) required for stasis, 1 log CFU, or 2 log CFU of bacterial killing against A. baumannii in a neutropenic mouse thigh infection model.
RESULTS
Susceptibility.
The MICs of the A. baumannii strains used in these studies are shown in Table 1. Three of the strains were susceptible to meropenem using current breakpoints and dosage regimens, and three were resistant to meropenem.
TABLE 1.
Comparative MICs of A. baumannii isolates used in these studies
| Strain | MIC (mg/liter) ofa: |
|||
|---|---|---|---|---|
| Meropenem | Minocycline | Tigecycline | Levofloxacin | |
| AB1148 | 0.25 | 0.03 | 0.25 | 0.13 |
| AB1036 | 0.5 | 0.06 | 0.25 | 4 |
| AB1022 | 2 | 1 | 2 | ND |
| AB1157 | 4 | 2 | 8 | 16 |
| AB1137 | 8 | 8 | ND | ND |
| AB1129 | 16 | 4 | 16 | >64 |
ND, not determined.
Pharmacokinetics.
The plasma pharmacokinetic parameters for meropenem are shown in Table 2. The total clearances were 1.88, 1.74, and 1.96 liters/h/kg for doses of 30, 100, and 300 mg/kg, respectively. Area under the concentration-time curve (AUC) values increased proportionally with increasing dose and ranged from 15.97 to 153.03 mg · h/liter for 30- and 300-mg/kg single doses, respectively.
TABLE 2.
Meropenem pharmacokinetic parameters in Swiss-Webster mice
| Dose (mg/kg) | Pharmacokinetic parametera |
|||
|---|---|---|---|---|
| Total CL (liters/h/kg) | Total AUC (mg · h/liter) | Cmax (mg/liter) | t1/2 (h) | |
| 30 | 1.88 | 15.97 | 52.06 | 0.09 |
| 100 | 1.74 | 57.57 | 198.10 | 0.15 |
| 300 | 1.96 | 153.03 | 244.51 | 0.32 |
CL, clearance; AUC, area under the concentration-time curve; Cmax, maximum concentration in plasma; t1/2, half-life.
Thigh infection model.
The bacterial load in the thighs of untreated control mice at the start of treatment for A. baumannii strains AB1148, AB1036, AB1022, AB1157, AB1137, and AB1129 was 7.29 ± 0.13, 7.00 ± 0.26, 7.32 ± 0.27, 7.56 ± 0.12, 6.64 ± 0.11, and 7.04 ± 0.12 log CFU/thigh, respectively. All untreated control groups were sacrificed at 12 h postinfection due to physical distress, and bacterial counts were determined. By 12 h, all untreated strains grew by between 1.16 to 2.08 log CFU/thigh.
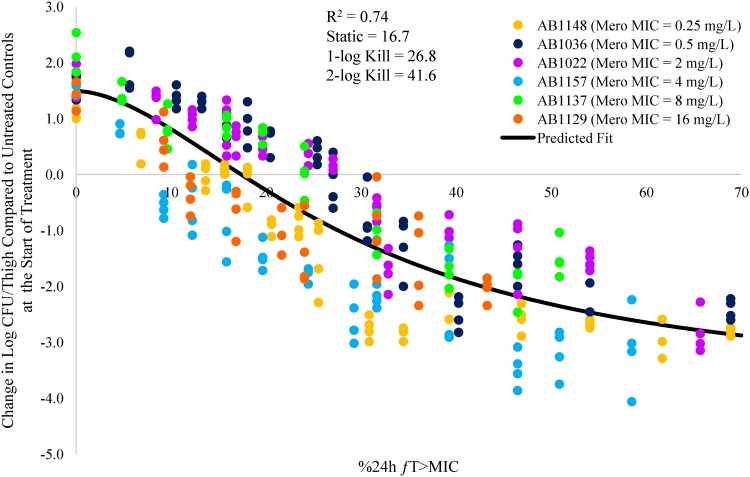
The individual meropenem %24-h fT>MIC versus change in log CFU/thigh for the six A. baumannii strains AB1148, AB1036, AB1022, AB1157, AB1137, and AB1129 are shown in Fig. 1 to 6. Figure 7 shows the pooled data from all six strains.
FIG 1.
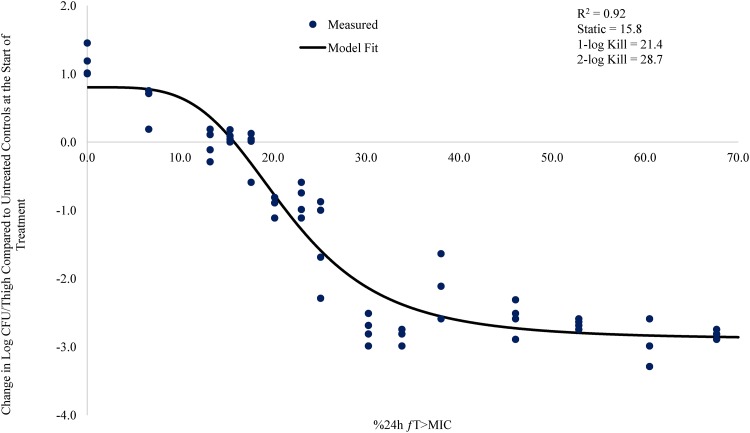
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1148 (meropenem MIC = 0.25 mg/liter) in a neutropenic mouse thigh infection model.
FIG 2.
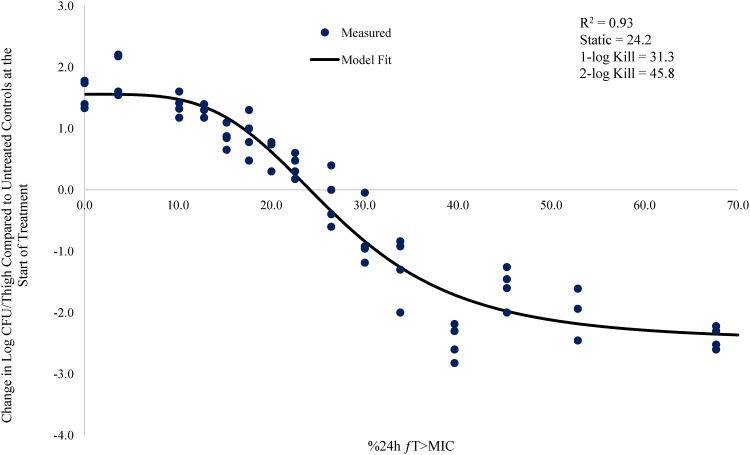
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1036 (meropenem MIC = 0.5 mg/liter) in a neutropenic mouse thigh infection model.
FIG 3.
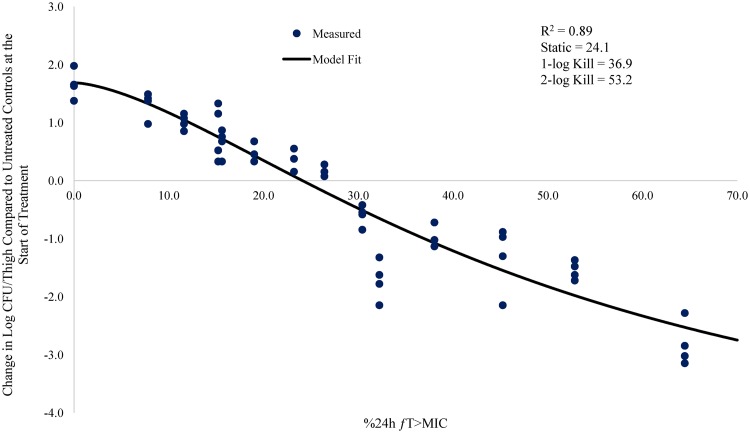
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1022 (meropenem MIC = 2.0 mg/liter) in a neutropenic mouse thigh infection model.
FIG 4.
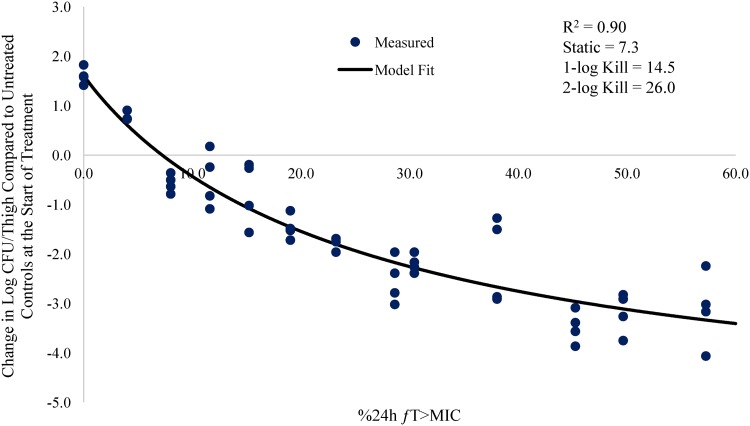
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1157 (meropenem MIC = 4.0 mg/liter) in a neutropenic mouse thigh infection model.
FIG 5.
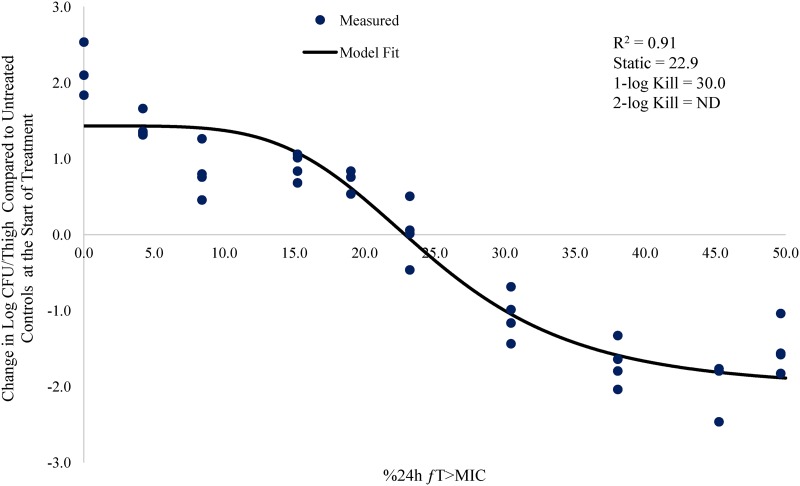
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1137 (meropenem MIC = 8.0 mg/liter) in a neutropenic mouse thigh infection model.
FIG 6.
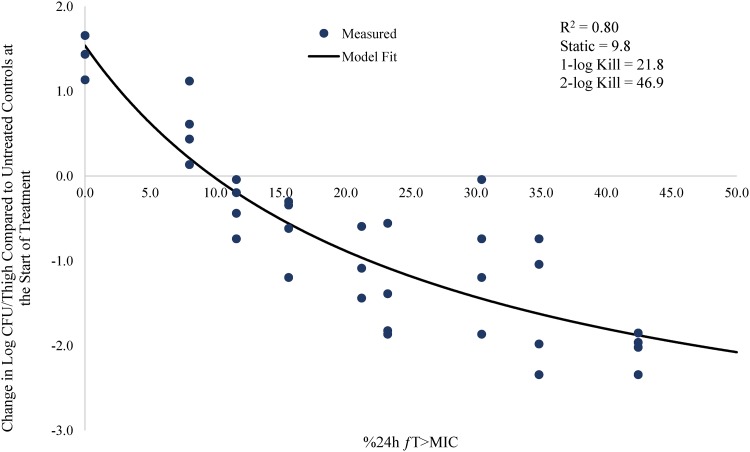
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for A. baumannii AB1129 (meropenem MIC = 16 mg/liter) in a neutropenic mouse thigh infection model.
FIG 7.
Relationship between meropenem %24-h fT>MIC and change in log CFU/thigh for all six A. baumannii strains.
The % 24-h free meropenem time above MIC for stasis, 1 log CFU, and 2 log CFU of bacterial killing for each strain are shown in Table 3. The meropenem %24-h fT>MIC required to achieve a bacteriostatic effect ranged from 7.3 to 24.2. The meropenem %24-h fT>MIC required to achieve 1 log CFU of bacterial killing ranged from 14.5 to 36.9. The meropenem %24-h fT>MIC to achieve 2 log CFU of bacterial killing ranged from 26.0 to 53.2. The mean meropenem %24-h fT>MIC required to achieve stasis, 1 log CFU, and 2 log CFU of bacterial killing was 17.3, 26.0, and 41.7, respectively.
TABLE 3.
Meropenem pharmacodynamic parameters against A. baumannii strainsa
| Strain | MIC (mg/liter) | R2 | %24-h fT>MIC to achieve: |
||
|---|---|---|---|---|---|
| Stasis | 1 log CFU killing | 2 log CFU killing | |||
| AB1148 | 0.25 | 0.92 | 15.8 | 21.4 | 28.7 |
| AB1036 | 0.5 | 0.93 | 24.2 | 31.3 | 45.8 |
| AB1022 | 2 | 0.89 | 24.1 | 36.9 | 53.2 |
| AB1157 | 4 | 0.90 | 7.3 | 14.5 | 26.0 |
| AB1137 | 8 | 0.93 | 22.9 | 30.1 | ND |
| AB1129 | 16 | 0.80 | 9.8 | 21.8 | 46.6 |
| Avg | NA | 0.90 | 17.3 | 26.0 | 41.7 |
NA, not applicable; ND, not determined.
DISCUSSION
The discovery and development of new treatment options for Gram-negative pathogens has become increasingly difficult and is hampered with a high development cost. Given the limited treatment options available today and the dearth of new treatment options in development, optimizing the dosage regimens of currently available antibacterial agents to maximize the exposure-response relationship is essential.
Recent efforts have been directed toward using pharmacokinetic and pharmacodynamic (PK/PD) parameters to optimize dosage regimens to maximize activity against specific pathogens (10). Meropenem PK/PD have been studied extensively in vitro, in animals, and in humans for susceptible and resistant strains of Enterobacteriaceae and Pseudomonas aeruginosa (6–10). As previously noted by MacVane et al. (11), the PK/PD profile of carbapenems against A. baumannii strains is not well defined. In an effort to help define the pharmacodynamics of carbapenems against A. baumannii, MacVane et al. simulated 500 mg of doripenem every 8 h by 1- or 4-h infusion, a mouse standardized regimen of imipenem 55 mg/kg every 8 h, and 1 g meropenem every 8 h by 1-h infusion in a neutropenic mouse thigh infection model against 14 A. baumannii isolates. They found that the %24-h fT>MIC needed to achieve a static effect and 1-log and 2-log CFU reductions was 23.67, 32.82, and 47.53%, respectively.
While those studies combined the data for three different carbapenems, the current study focused solely on meropenem. In the current study, the meropenem %24-h fT>MIC values needed to achieve a static effect and 1-log and 2-log CFU reductions were 17.3, 26.0, and 41.7, respectively. Overall, the exposure targets and the range of MICs tested are very similar across the two studies. The agreement between the two studies suggests that a PK/PD target for 1 log CFU of bacterial killing can be achieved with free meropenem concentrations above the MIC for 30% of the dosing interval, which is in contrast with the generally accepted PK/PD target for meropenem against other Gram-negative organisms of 40% time above MIC (10). Based on this target, a 0.5-g dose of meropenem administered by a standard 0.5-h infusion every 8 h will achieve 30% time above MIC for MICs of up to 4 mg/liter (12) which is 2-fold higher than the current breakpoint (CLSI and EUCAST) for meropenem and A. baumannii of ≤2 mg/liter (13, 14). When the dose is increased to 2 g every 8 h by 3-h infusion, free meropenem concentrations were found to be above 16 mg/liter for 30% or more of the dosing interval (12, 15). These data suggest that an optimized meropenem dosage regimen of 2 g every 8 h by 3-h infusion could be effective against A. baumannii isolates with MICs of up to 16 mg/liter and that the PK/PD target for A. baumannii is lower than that required for other Gram-negative bacteria.
MATERIALS AND METHODS
Animals.
All studies using animals were performed under protocols approved by an institutional animal care and use committee (IACUC). Female Swiss Webster mice (5 to 6 weeks of age) were obtained from Envigo (Placentia, CA). Animals were acclimated to laboratory conditions for at least 24 h prior to the initiation of any study.
Antimicrobial agents.
Meropenem (Sandoz) was reconstituted in 0.9% saline as described in the prescribing information, then further diluted in 0.9% saline to achieve target concentrations.
Strains and susceptibility testing.
Six A. baumannii clinical isolates were used in these studies. MICs were determined using a broth microdilution assay according to CLSI reference methods (16). Antibiotics were prepared at a concentration equivalent to 2-fold the highest desired final concentration in culture medium and were then diluted directly into 96-well microtiter plates. The microtiter plates were incubated for 17 to 18 h at 37°C and were read by using a microtiter plate reader (Molecular Devices, Sunnyvale, CA) at 600 nm (optical density [OD] value of less than 0.065 = no growth), as well as by visual observation using a reading mirror. The MIC was defined as the lowest concentration of antibiotic at which the visible growth of the organism was completely inhibited.
Pharmacokinetics.
The pharmacokinetics of meropenem were determined in uninfected neutropenic mice at doses of 30, 100, and 300 mg/kg. Neutropenia in mice was established by the administration of 150 mg/kg cyclophosphamide (Baxter, Deerfield, IL) by the intraperitoneal route 4 days and 1 day prior to the start of the study. At the designated time points, mice were humanely euthanized, and their blood was collected by cardiac puncture and transferred to EDTA-containing tubes. Blood samples were centrifuged within 5 min of collection at 12,000 × g for 5 min to obtain plasma. An equal volume of 1 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (pH 7) was added to plasma samples in order to stabilize meropenem, and samples were stored at −80°C until analyzed.
Bioanalytical assay.
Meropenem standard curves were prepared in plasma at concentrations of 0.04 to 50.0 μg/ml. Aliquots (25 μl) of sample were placed in 1.5-ml microcentrifuge tubes containing 200 μl of 4.0 μg/ml doripenem (internal standard) in 10%:45%:45% water-methanol-acetonitrile (vol/vol/vol). The samples were mixed using a vortex mixer, then centrifuged for 10 min at 15,000 × g using a tabletop centrifuge. The supernatant (∼150 μl) was removed and added to 400 μl of water in a 96-well plate. The samples were mixed again using a vortex mixer. An aliquot (20 μl) of each sample was injected onto a high-performance liquid chromatography mass spectrometer (HPLC-MS) for quantification. The lower limit of quantitation was 0.04 μg/ml. Plasma concentrations were fitted using a one-compartment, first-order model (Phoenix WinNonlin 8.0; Certara USA, Inc., Princeton, NJ).
Bacterial preparation for thigh model study.
Strains were grown in Mueller-Hinton broth (MHB) at 37°C under constant aeration overnight (∼20 h). The overnight culture was subcultured into fresh MHB and allowed to regrow for 3 h at 37°C under constant aeration to reach an absorbance of 0.30 to 0.35 at 600 nm (∼3 × 108 CFU/ml). The bacterial suspensions were then diluted in fresh MHB to yield ∼107 CFU/ml.
Neutropenic thigh infection model.
Mice were rendered neutropenic by the administration of 150 mg/kg cyclophosphamide (Sandoz) by the intraperitoneal route 4 days and 1 day prior to the start of the study. Mice were infected by intramuscular injection of 0.1 ml of inoculum (107 CFU/ml) into both thigh muscles while under isoflurane anesthesia (5% isoflurane in oxygen running at 4 liters/min).
Treatment regimens.
Starting 2 h postinfection, meropenem was administered every 2, 4, or 6 h over 24 h by the intraperitoneal route. Meropenem was administered at total daily doses ranging from 3.75 to 600 mg/kg (n = 4 thighs/dose). Meropenem protein binding of 10% was used to calculate free drug (17).
CFU determination.
Untreated control animals were euthanized at the start of treatment to establish the baseline bacterial burden. Two hours after the last treatment, treated groups and additional untreated control animals were euthanized via CO2 asphyxiation. Thighs were aseptically harvested and placed in sterile saline. The tissues were then homogenized using a tissue homogenizer (Pro Scientific, Oxford, CT), serially diluted 1:10 in sterile saline, and plated on Mueller-Hinton agar to determine bacterial counts.
Pharmacodynamic modeling.
The relationship between the %24-h fT>MIC and the change in log CFU compared to the start of treatment was fitted using the following inhibitory effect (Emax) model (Phoenix WinNonlin v 6.3; Certara, Mountain View, CA):
where E0 is the effect when X is equal to 0 (i.e., for the untreated control animals), Imax is the maximum reduction in the log number of CFU/lung, X is the %24-h fT>MIC, IC50 is the %24-h fT>MIC (X) corresponding to 50% of the maximum bacterial reduction, and γ is the steepness of the curve.
ACKNOWLEDGMENTS
This work, including the efforts of Mojgan Sabet, Ziad Tarazi, and David C. Griffith, was funded in part by the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA), under contract HHSO100201600026C.
We have no conflicts to declare.
REFERENCES
- 1.Munoz-Price LS, Arheart K, Nordmann P, Boulanger AE, Cleary T, Alvarez R, Pizano L, Namias N, Kett DH, Poirel L. 2013. Eighteen years of experience with Acinetobacter baumannii in a tertiary care hospital. Crit Care Med 41:2733–2742. doi: 10.1097/CCM.0b013e318298a541. [DOI] [PubMed] [Google Scholar]
- 2.Masse J, Elkalioubie A, Blazejewski C, Ledoux G, Wallet F, Poissy J, Preau S, Nseir S. 2017. Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: a prospective observational study. Eur J Clin Microbiol Infect Dis 36:797–805. doi: 10.1007/s10096-016-2863-x. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Namias N, Cleary T, Fajardo-Aquino Y, Depascale D, Arheart KL, Rivera JI, Doi Y. 2013. Acinetobacter baumannii: association between environmental contamination of patient rooms and occupant status. Infect Control Hosp Epidemiol 34:517–520. doi: 10.1086/670209. [DOI] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J, Timsit JF. 2019. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis 32:69–76. doi: 10.1097/QCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2019. Antibiotic resistance threats in the United States, 2019. CDC, Atlanta, GA. [Google Scholar]
- 6.Bowker KE, Holt HA, Lewis RJ, Reeves DS, MacGowan AP. 1998. Comparative pharmacodynamics of meropenem using an in-vitro model to simulate once, twice and three times daily dosing in humans. J Antimicrob Chemother 42:461–467. doi: 10.1093/jac/42.4.461. [DOI] [PubMed] [Google Scholar]
- 7.Bulik CC, Christensen H, Li P, Sutherland CA, Nicolau DP, Kuti JL. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 54:804–810. doi: 10.1128/AAC.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker R, Andes D, Conklin J, Ebert S, Craig WA. 1994. Pharmacodynamic activities of meropenem in an animal infection model, abstr A91 Abstr 34th Interscience Conference on Antimicrobial Agents and Chemotherapy, Orlando, FL. [Google Scholar]
- 9.Louie A, Liu W, Fikes S, Brown D, Drusano GL. 2013. Impact of meropenem in combination with tobramycin in a murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 57:2788–2792. doi: 10.1128/AAC.02624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drusano GL. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis 36:S42–S50. doi: 10.1086/344653. [DOI] [PubMed] [Google Scholar]
- 11.Macvane SH, Crandon JL, Nicolau DP. 2014. Characterizing in vivo pharmacodynamics of carbapenems against Acinetobacter baumannii in a murine thigh infection model to support breakpoint determinations. Antimicrob Agents Chemother 58:599–601. doi: 10.1128/AAC.02029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP. 2003. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 23:988–991. doi: 10.1592/phco.23.8.988.32878. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing: twenty-fifth informational supplement. CLSI document M100-S25 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretation of MICs and zone diameters, version 5.0. http://www.eucast.org/clinical_breakpoints.
- 15.Rubino CM, Bhavnani SM, Loutit JS, Morgan EE, White D, Dudley MN, Griffith DC. 2018. Phase 1 study of the safety, tolerability, and pharmacokinetics of vaborbactam and meropenem alone and in combination following single and multiple doses in healthy adult subjects. Antimicrob Agents Chemother 62:e02228-17. doi: 10.1128/AAC.02228-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 10th ed CLSI document M7-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Mattie H, Zhang LC, van Strijen E, Sekh BR, Douwes-Idema AE. 1997. Pharmacokinetic and pharmacodynamic models of the antistaphylococcal effects of meropenem and cloxacillin in vitro and in experimental infection. Antimicrob Agents Chemother 41:2083–2088. doi: 10.1128/AAC.41.10.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]