Tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF) are prodrugs of the HIV-1 nucleotide reverse transcriptase inhibitor tenofovir (TFV). In vivo, TAF achieves >4-fold-higher intracellular levels of TFV diphosphate (TFV-DP) compared to TDF. Since thymidine analog-associated mutations (TAMs) in HIV-1 confer reduced TFV susceptibility, patients with TAM-containing HIV-1 may benefit from higher TFV-DP levels delivered by TAF. Moreover, the presence of the M184V mutation increases TFV susceptibility during TDF- or TAF-based therapy.
KEYWORDS: HIV-1, M184V, TAF, antiretroviral resistance, tenofovir alafenamide, thymidine analog-associated mutations, TAMs
ABSTRACT
Tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF) are prodrugs of the HIV-1 nucleotide reverse transcriptase inhibitor tenofovir (TFV). In vivo, TAF achieves >4-fold-higher intracellular levels of TFV diphosphate (TFV-DP) compared to TDF. Since thymidine analog-associated mutations (TAMs) in HIV-1 confer reduced TFV susceptibility, patients with TAM-containing HIV-1 may benefit from higher TFV-DP levels delivered by TAF. Moreover, the presence of the M184V mutation increases TFV susceptibility during TDF- or TAF-based therapy. The susceptibilities to antiviral drugs of site-directed mutants (SDMs) and patient-derived mutants containing combinations of TAMs (M41L, D67N, K70R, L210W, T215Y, and K219Q) with or without the M184V mutation (TAMs±M184V) were evaluated using either 5-day multicycle (MC; n = 110) or 2-day single-cycle (SC; n = 96) HIV assays. The presence of M184V in TAM-containing HIV-1 SDMs (n = 48) significantly increased TAF sensitivity compared to SDMs without M184V (n = 48). The comparison of TAF and TDF resistance profiles was further assessed in viral breakthrough (VB) experiments mimicking clinically relevant drug concentrations. A total of 68 mutants were assayed at physiological concentration in VB experiments, with 15/68 mutants breaking through with TDF (TFV, the in vitro equivalent of TDF, was used in these experiments), and only 3 of 68 mutants breaking through under TAF treatment. Overall, in the VB assay mimicking the 4-fold-higher intracellular levels of TFV-DP observed clinically with TAF versus TDF, TAF inhibited viral breakthrough of most TAM-containing HIV-1, whereas TDF did not. These results indicate that TAF has a higher resistance threshold than TDF and suggest that higher resistance cutoffs should be applied for TAF compared to TDF in genotypic and phenotypic resistance algorithms.
INTRODUCTION
Combination antiretroviral therapies (ARTs) comprising three pharmaceutical agents have greatly improved the life of people living with HIV infection. Nucleoside reverse transcriptase inhibitors (NRTIs) have played a key role in ART throughout the HIV epidemics and constitute the backbone of most treatment regimens recommended guideline panels (1, 2). NRTIs, such as abacavir (ABC), emtricitabine (FTC), lamivudine (3TC), tenofovir (TFV), and zidovudine (ZDV/AZT), are analogues of the natural nucleoside bases (adenosine, cytidine, guanosine, and thymidine) that are missing the 3′ OH group. Upon phosphorylation to their triphosphate form (or equivalent), NRTIs act as chain terminators, thus preventing HIV-1 reverse transcriptase (RT) from carrying out HIV-1 DNA polymerization (reviewed in reference 3). The initial use of ZDV revolutionized HIV treatment, but ZDV monotherapy quickly led to the emergence of ZDV-resistant mutants characterized by the RT mutations D67N, K70R, T215Y/F, and K219Q (4), resulting in the loss of antiviral activity of ZDV. These four mutations, as well as two additional RT mutations identified subsequently (M41L and L210W), are collectively referred to as thymidine analog-associated mutations (TAMs). As a direct consequence of the emergence of these resistance mutations, transmitted ZDV-resistant viruses were detected in untreated individuals as early as 1993 (5), and these mutations have persisted to this day in treatment-naive individuals at a prevalence near 3% (6).
TAMs have been shown to confer various levels of resistance against ZDV, depending on the number and combination of TAMs present in the samples tested. In addition, the presence of TAMs in HIV has been associated with various levels of cross-resistance to most NRTIs, including 3TC, FTC, ABC, and TFV (7, 8). In the clinic, in addition to the effect of TAMs on ZDV described above, the presence of three or more TAMs inclusive of either M41L or L210W was associated with reduced response to tenofovir disoproxil fumarate (TDF, a prodrug of TFV) (9) and led to the establishment of reduced-susceptibility clinical resistance cutoffs of 1.4 to 4 (Monogram Biosciences PhenoSense assay). The impact of preexisting TAMs on response to treatment with tenofovir alafenamide (TAF, the new prodrug of TFV) has not been determined clinically, but since treatment with TAF leads to a >4-fold increase in the peripheral blood mononuclear cell (PBMC) intracellular concentration of tenofovir diphosphate (TFV-DP, the active moiety resulting from TDF and TAF dosing) in vivo compared to treatment with TDF (10, 11), one could anticipate an increased activity of TAF compared to TDF in patients with TAMs.
In this context, the M184V/I RT mutation is another key resistance mutation affecting NRTIs. M184V/I confers high-level resistance to FTC and 3TC in vitro (12, 13) and is the most common mutation that emerges in situations of virologic failure with ART that include FTC or 3TC (14, 15). M184V is also associated with reduced susceptibility to ABC (13) and hypersusceptibility to ZDV and TFV (16–18).
In order to further our understanding of the impact of TAMs and M184V/I on the resistance profile of TAF and other NRTIs, we generated a large set of site-directed mutant viruses representing most combinations of TAMs with or without M184V (n = 96) and collected patient-derived viruses with TAMs with or without M184V (TAMs±M184V) (n = 14). We determined the resistance profiles of these recombinant viruses in vitro in standard phenotypic assays, and we compared the activity of TAF and TDF in a subset of mutants (n = 68) in viral breakthrough assays reflective of in vivo TFV-DP intracellular concentrations obtained upon dosing with TAF or TDF.
(These findings were presented in part at the Conference on Retroviruses and Opportunistic Infections, 4 to 7 March 2018, in Boston, MA.)
RESULTS
Phenotypic characterization of recombinant viruses.
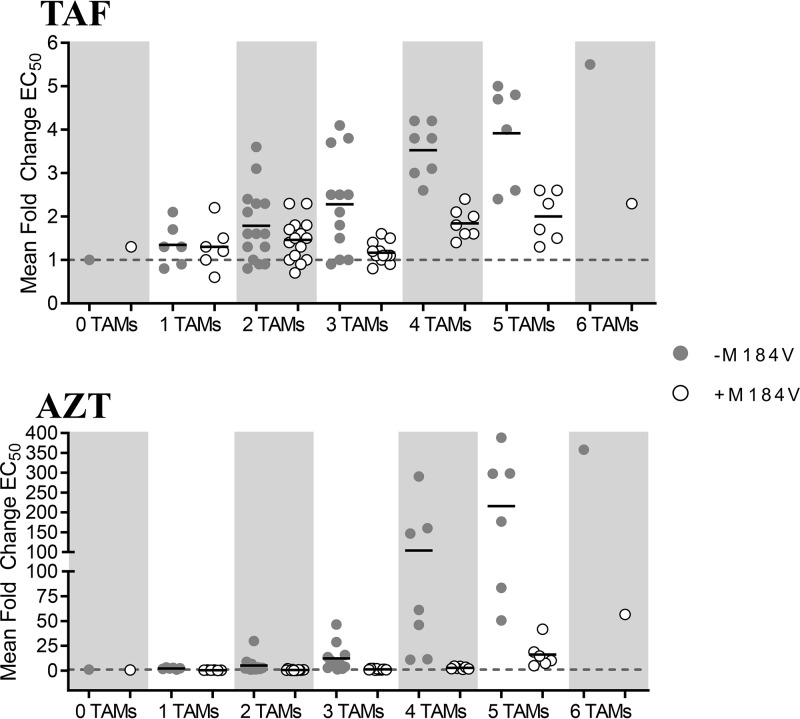
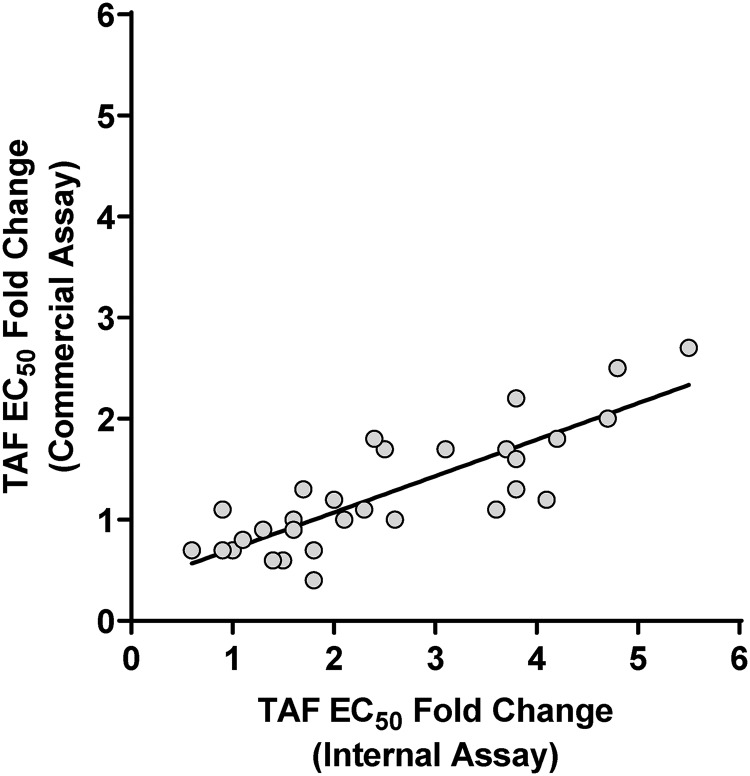
Single-cycle phenotypic 50% effective concentration (EC50) assays with TAF and AZT were performed with the SDMs (see the supplemental material). While a high level of reduced susceptibility to the control drug AZT was observed, ranging from 0.5- to >350-fold above the wild-type control as expected, reduced susceptibility to TAF was moderate and ranged from 1.3- to 5.5-fold above the wild-type control (Table 1 and Fig. 1), with most fold changes (FC) of ≥1.8 (27 of 42) showing a statistical difference from the wild type (P < 0.05, t test). The addition of M184V in viruses with one to two TAMs had only a marginal effect on susceptibility to TAF (1.2-fold increase); however, the presence of M184V in viruses with ≥3 TAMs increased virus susceptibility to TAF by an average of 2-fold (P < 0.01; paired t test comparing fold change in viruses with ≥3 TAMs with or without M184V). Though stronger in magnitude, a similar effect was noted for AZT in the presence of M184V, with an increase in susceptibility to AZT of 7.3-fold in mutants with one to three TAMs (P < 0.01; paired t test comparing fold change in viruses with or without M184V) and of 26-fold in mutants with four to six TAMs (P < 0.01). Comparison of the in-house single-cycle EC50 data to data from the commercial single-cycle EC50 assay from Monogram Biosciences (PhenoSense) for 30 viruses tested with TAF showed a good correlation between the two assays, with moderately higher fold changes obtained in the in-house assay (Fig. 2).
TABLE 1.
Single-cycle phenotypic sensitivities of site-directed TAM-containing mutants with or without M184V
| No. of TAMsa (n [per class]) | Mean phenotypic EC50 fold change (SD) compared to the wild typeb |
|||
|---|---|---|---|---|
| Without M184V |
With M184V |
|||
| TAF | AZT | TAF | AZT | |
| No TAMs (2) | 1.0 (0.7) | 1.0 (1.4) | 1.3 (0.4) | 0.6 (0.1) |
| 1 TAM (12) | 1.4 (0.5) | 2.2 (0.8) | 1.3 (0.5) | 0.5 (0.1) |
| 2 TAMs (30) | 1.8 (0.8) | 5.2 (7.2) | 1.5 (0.5) | 0.7 (0.4) |
| 3 TAMs (24) | 2.3 (1.1) | 12.3 (13.2) | 1.2 (0.2) | 1.2 (0.6) |
| 4 TAMs (14) | 3.5 (0.6) | 104 (102) | 1.8 (0.3) | 2.8 (1.3) |
| 5 TAMs (12) | 3.9 (1.1) | 216 (134) | 2.0 (0.6) | 16.3 (13.4) |
| 6 TAMs (2) | 5.5 (3.2) | 358 (176) | 2.3 (0.7) | 57 (22) |
| All mutants (96) | 2.4 (1.3) | 55 (103) | 1.5 (0.5) | 4.2 (10.3) |
The thymidine analog-associated mutations (TAMs) included M41L, D67N, K70R, L210W, T215Y/F, and K219E/N/Q/R in RT.
Mean values were calculated from ≥3 independent triplicate experiments for each mutant. The TAF wild-type EC50 was 5.1 nM in the single-cycle phenotypic assay. The AZT wild-type EC50 was 0.30 μM in the single-cycle phenotypic assay. The single-cycle phenotypic assay was performed using noninfectious virus over 2 days.
FIG 1.
Single-cycle phenotypic EC50 assays of HIV-1 site-directed mutants containing TAM±M184V against TAF and AZT. Viruses are grouped by number of TAMs with (open circle) or without (filled circle) M184V. Each circle represents a single virus. Mean EC50-fold changes compared to the wild type are shown for each TAM group (mean of ≥3 replicate experiments for each individual virus is indicated by a horizontal black line). The dashed line indicates a fold change of 1. Single-cycle antiviral assay in MT-2 cells with pKSHX backbone viruses, and 2- and 2.5-fold drug dilutions for TAF and AZT, respectively, were incubated for 2 days and read using a luciferase assay readout.
FIG 2.
Comparison of TAF EC50-fold change (FC) from wild-type between commercial single-cycle phenotypic assay (Monogram Biosciences; y axis) and internal single-cycle phenotypic assay (x axis). The commercial assay was performed for 30 site-directed mutants with one to six TAMs, with or without M184V, that showed a wide range of FCs in the internal assay. The linear regression parameters were as follows: R2 = 0.69 and y = 0.36x + 0.35.
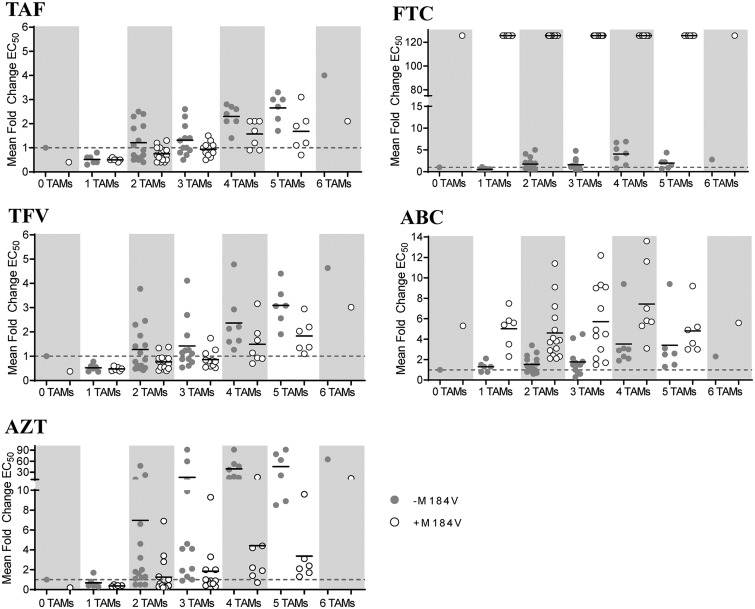
Multicycle phenotypic EC50 assays with NRTIs and control compounds were performed on SDMs and patient-derived clinical isolates. For SDMs, a gradual increase in resistance to TAF, TFV, and AZT was observed with increasing numbers of TAMs, with fold changes above wild-type control ranging from 0.3 to 4.0, 0.4 to 4.8, and 0.2 to > 91 for TAF, TFV, and AZT, respectively. Fold changes of ≤0.5 and ≥ 1.5 for TAF, ≤0.4 and ≥1.6 for TFV, and ≤0.3 and ≥1.7 for AZT were statistically different from wild-type (P < 0.05, t test), indicating the robustness of the assay to detect drug hypersusceptibility and low-level resistance. In the presence of M184V, lower levels of resistance were observed for all three drugs, with both TAF and TFV showing a statistically significant 1.5-fold increased drug susceptibility in viruses with one to six TAMs (P < 0.01, paired t test comparing fold changes in viruses with or without M184V), and AZT showing an average 7.8-fold increase in susceptibility in the presence of M184V in viruses with TAMs (P < 0.01, paired t test comparing fold changes in viruses with or without M184V) (Table 2 and Fig. 3). Resistance to ABC and FTC in the presence of TAMs increased overall gradually up to four TAMs, with fold changes from the wild type ranging from 0.3 to 9.4 for ABC and from 0.2 to 6.9 for FTC in the absence of M184V and from 1.5 to 13.6 for ABC and no antiviral activity noted for FTC in the presence of M184V (FC > 126). All ABC fold changes of ≥1.5 and FTC fold changes of ≥1.9 were significantly different from the wild type (P < 0.05, t test). The presence of M184V increased resistance to ABC by 2.6-fold overall across all SDMs (P < 0.01, paired t test comparing fold changes in viruses with or without M184V) (Table 2 and Fig. 3). Resistance levels were higher for patient-derived viruses compared to SDMs, likely as a result of the choice of samples used to generate the patient-derived isolates, most of which containing TAMs associated with higher levels of resistance to TFV (M41L, L210W, and T215Y). Overall, PD viruses followed a similar trend as the SDMs, with a gradual increase in FC with increasing number of TAMs (isolates with three to five TAMs were tested) for TAF, TFV, AZT, and ABC (Table 3). Fold changes compared to the wild type ranged from 0.9 to 12.6 for TAF (P < 0.01 for 13 of 14 isolates with FC ≥ 1.8), 1.0 to 14.2 for TFV (P < 0.05 for 12 of 14 isolates with FC ≥ 1.6), 5.1 to >91 for AZT (P < 0.01 for all 14 isolates), 1.4 to >126 for FTC (P < 0.01 for 12 of 14 isolates with FC ≥ 2.1), and 2.0 to 18.8 for ABC (P < 0.01 for all 14 isolates). Similar to SDMs, isolates with M184V showed an increase in susceptibility to TAF, TFV, and AZT and a decrease in susceptibility to ABC and no antiviral effect noted for FTC. Control compounds (DTG and DRV) showed near wild-type levels, reflective of the wild-type sequence in these RT mutants for integrase and protease, respectively.
TABLE 2.
Multicycle phenotypic sensitivities of site-directed mutants containing TAMs with or without M184V
| No. of TAMsa (n [per class]) | Mean phenotypic EC50 fold change (SD) compared to the wild typeb |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAF |
TDFc |
AZT |
ABC |
FTC |
DTG |
DRV |
||||||||
| – | + | – | + | – | + | – | + | – | + | – | + | – | + | |
| No TAMs (2) | 1.0 (0.4) | 0.4 (0.1) | 1.0 (0.5) | 0.4 (0.1) | 1.0 (0.5) | 0.2 (0.1) | 1.0 (0.2) | 5.3 (1.4) | 1.0 (0.5) | >126 (0) | 1.0 (0.6) | 0.6 (0.3) | 1.0 (0.4) | 0.6 (0.2) |
| 1 TAMs (12) | 0.5 (0.2) | 0.5 (0.1) | 0.5 (0.2) | 0.5 (0.1) | 0.7 (0.5) | 0.4 (0.1) | 1.3 (0.5) | 5.0 (1.8) | 0.6 (0.3) | >126 (0) | 0.6 (0.1) | 0.7 (0.1) | 0.7 (0.2) | 0.8 (0.2) |
| 2 TAMs (30) | 1.2 (0.8) | 0.8 (0.3) | 1.3 (1.0) | 0.8 (0.3) | 7.0 (12.5) | 1.2 (1.8) | 1.5 (0.8) | 4.6 (2.7) | 1.8 (1.4) | >126 (0) | 0.7 (0.1) | 0.7 (0.1) | 0.9 (0.4) | 0.9 (0.4) |
| 3 TAMs (24) | 1.3 (0.6) | 0.9 (0.3) | 1.4 (1.0) | 0.9 (0.4) | >16.0 (29) | 1.8 (2.5) | 1.8 (1.3) | 5.7 (3.5) | 1.6 (1.2) | >126 (0) | 0.7 (0.3) | 0.8 (0.2) | 1.0 (0.5) | 1.1 (0.3) |
| 4 TAMs (14) | 2.3 (0.5) | 1.6 (0.6) | 2.4 (1.2) | 1.5 (0.9) | >39 (28) | 4.4 (5.4) | 3.5 (2.6) | 7.4 (3.7) | 4.1 (2.4) | >126 (0) | 0.7 (0.2) | 0.7 (0.3) | 1.0 (0.2) | 1.0 (0.3) |
| 5 TAMs (12) | 2.7 (0.6) | 1.7 (0.9) | 3.1 (0.8) | 1.8 (0.7) | >45 (37) | 3.3 (3.1) | 3.4 (3.0) | 4.8 (2.3) | 2.0 (1.3) | >126 (0) | 0.5 (0.2) | 0.6 (0.2) | 0.7 (0.4) | 0.9 (0.4) |
| 6 TAMs (2) | 4.0 (1.6) | 2.1 (1.1) | 4.6 (2.0) | 3.0 (1.7) | >65 (23) | 13.2 (7.8) | 2.3 (0.4) | 5.6 (0.7) | 2.8 (1.4) | >126 (0) | 0.4 (0.1) | 0.7 (0.3) | 0.7 (0.2) | 1.5 (0.8) |
| All mutants (96) | 1.5 (1.0) | 1.0 (0.6) | 1.7 (1.2) | 1.0 (0.7) | 19 (28) | 2.2 (3.4) | 2.1 (1.8) | 5.4 (3.0) | 1.9 (1.7) | >126 (0) | 0.7 (0.2) | 0.7 (0.2) | 0.9 (0.4) | 0.9 (0.3) |
The TAMs included M41L, D67N, K70R, L210W, T215Y/F, and K219E/N/Q/R in RT.
Mean values were calculated from ≥3 independent triplicate experiments for each mutant. TAMs with (+) or without (–) M184V were evaluated. The wild-type EC50 values were as follows: TAF, 0.015 μM; TFV, 3.0 μM; AZT, 0.16 μM; ABC, 0.77 μM; FTC, 0.42 μM; DTG, 0.001 μM; and DRV, 0.01 μM. A “>” symbol indicates that at least one EC50 value was greater than the highest drug concentration. The multicycle phenotypic assay was performed using infectious virus over 5 days.
TFV, the in vitro equivalent of TDF, was used in these experiments.
FIG 3.
Multicycle phenotypic EC50 assays of HIV-1 site-directed mutants containing TAM±M184V against TAF, TFV, AZT, FTC, and ABC. Multicycle antiviral assay in MT-2 cells with pXXLAI backbone viruses and 5-fold drug dilutions were incubated for 5 days, and then the cell viability was measured using a luciferase-based readout (CellTiterGlo). Viruses are grouped by number of TAMs with (open circles) or without (filled circles) M184V. Each circle represents a single virus. Mean EC50-fold changes compared to the wild type are shown for each TAM group (mean of ≥3 replicate experiments for each individual virus is indicated by a horizontal black line). The dashed line indicates a fold change of 1.
TABLE 3.
Multicycle phenotypic sensitivities of patient-derived TAM-containing mutants with or without M184V
| No. of TAMsa | n | Mean EC50 fold change (SD) compared to the wild typeb |
||||||
|---|---|---|---|---|---|---|---|---|
| TAF | TDFc | AZT | ABC | FTC | DTG | DRV | ||
| 3 TAMs | 3 | 2.9 (1.3) | 3.0 (1.4) | >72 (27) | 2.4 (0.4) | 2.0 (0.5) | 0.6 (0.3) | 2.2 (2.7) |
| 3 TAMs + M184V | 2 | 2.1 (0.4) | 2.1 (0.9) | 14.3 (13.0) | 5.1 (0.1) | >126 (0) | 0.8 (0.2) | 0.8 (0.0) |
| 4 TAMs | 2 | 5.4 (3.8) | 6.4 (4.4) | >91 (0) | 4.3 (3.2) | 13.9 (17) | 0.6 (0.1) | 0.9 (0.3) |
| 4 TAMs + M184V | 2 | 1.6 (1.0) | 1.8 (1.1) | >36 (41) | 11.7 (10.2) | >126 (0) | 0.6 (0.1) | 1.0 (0.5) |
| 5 TAMs | 3 | 10.0 (3.1) | 9.3 (4.6) | >91 (0) | 10.0 (3.9) | 7.1 (4.5) | 1.1 (0.5) | 0.7 (0.2) |
| 5 TAMs + M184V | 2 | 3.8 (0.6) | 4.3 (0.8) | >82 (12.6) | 8.3 (1.5) | >126 (0) | 0.9 (0.4) | 1.0 (0.2) |
The TAMs included M41L, D67N, K70R, L210W, T215Y/F, and K219E/N/Q/R in RT.
Wild-type EC50 values were as follows: TAF, 0.015 μM; TFV, 3.0 μM; AZT, 0.16 μM; ABC, 0.77 μM; FTC, 0.42 μM; DTG, 0.001 μM; and DRV, 0.01 μM. A “>” symbol indicates that at least one EC50 value was greater than the highest drug concentration. Mean values were calculated from ≥3 independent triplicate experiments for each mutant. The multicycle phenotypic assay was performed using infectious virus over 5 days.
TFV, the in vitro equivalent of TDF, was used in these experiments.
Similar trends were observed between single and multicycle phenotypic EC50 assays for TAF and AZT (Fig. 1 and 3). An increase in the number of TAMs led to decreased susceptibility to the drugs, and the addition of M184V increased the virus susceptibility to drug in both single-cycle and multicycle phenotypic EC50 assays.
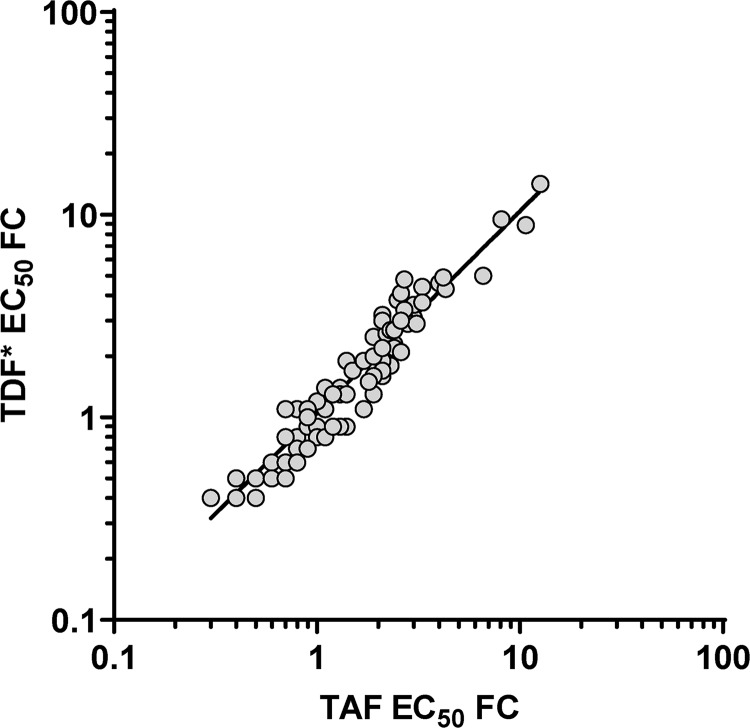
TAF and TFV fold changes in the multicycle EC50 assay were strongly correlated (R2 = 0.93, Fig. 4). These results were expected since TAF and TFV both lead to the same active moiety (TFV-DP) that is responsible for the antiviral effect with both drugs. However, these in vitro phenotypic EC50 assays measure drug inhibition in a nonphysiological model system that does not reproduce the complex pharmacological events (drug loading, metabolism, elimination, etc.) associated with the >4-fold increase in intracellular TFV-DP loading achieved physiologically with TAF compared to TFV/TDF. We evaluated the effect of the higher TFV-DP concentration achieved physiologically with TAF in comparison to TFV/TDF by assessing the growth of TAM-containing mutants at physiological concentrations of TAF and TFV in the viral breakthrough assay described below.
FIG 4.
Comparison of TAF and TDF EC50-fold change (FC) from the wild type in the multicycle phenotypic assay. All 110 pXXLAI backbone viruses containing TAMs±M184V (96 site-directed mutants + 14 patient-derived isolates) are plotted. The linear regression parameters are as follows: R2 = 0.93 and y = 1.04x – 0.006. *, TFV, the in vitro equivalent of TDF, was used in these experiments.
Viral breakthrough assays.
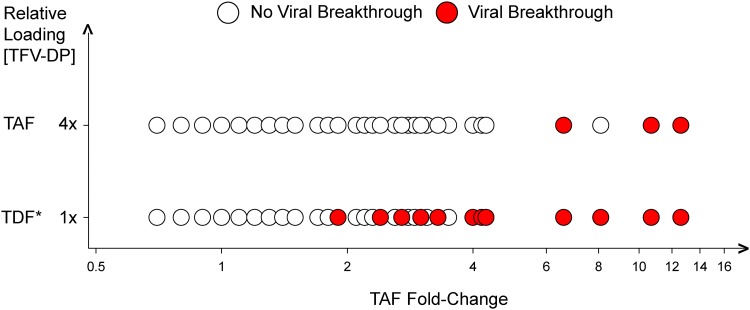
MT-2 cells were incubated with concentrations of TAF (0.8 μM) or TFV (50 μM) that mimicked physiological concentrations in vivo and were infected with various SDMs or patient isolates (8). A total of 68 viruses with various levels of TAF/TDF resistance and various numbers of TAMs were used in the viral breakthrough experiments (54 SDMs and 14 patient-derived isolates). Of the 68 viruses tested, 15 viruses broke through with TFV/TDF, with only 3/15 also breaking through with TAF (Fig. 5). The 15 viruses that broke through with TDF contained three or more TAMs (with or without M184V), with TAF fold changes ranging from 1.9 to 12.6 (Table 4). In contrast, the only three viruses that broke through with TAF contained five TAMs, and their TAF fold changes ranged from 6.6 to 12.6. These data indicate that TAF has a higher resistance threshold compared to TDF at concentrations mimicking physiological concentrations.
FIG 5.
Viral breakthrough of selected viruses (n = 68, see the supplemental material) in the presence of physiological concentrations of TAF and TDF. MT-2 cells were incubated overnight with either TDF or TAF at concentrations reflective of in vivo loading achieved with the two prodrugs of TFV (TDF, 50 μM [1×EC95 corresponding to an IQ95 = 1]; TAF, 0.8 μM [4×EC95 corresponding to an IQ95 = 4]) and infected with HIV-1 mutants. Mutant viruses tested (each represented by a circle) are plotted against the TAF EC50-fold changes measured for the mutants. *, TFV, the in vitro equivalent of TDF, was used in these experiments.
TABLE 4.
Time to viral breakthrough at physiological concentrations of TAF or TFV
| Isolate ID | TAF FCa | Mutant RT sequence | No. of TAMsb | Mutant typec | Time to viral breakthrough (days)d |
|
|---|---|---|---|---|---|---|
| TFV | TAF | |||||
| WT | 1.0 | No mutations | WT | WT | >28 | >28 |
| 101 | 1.9 | M41L, L210W, T215Y | 3 TAMs | PD | 13 | >28 |
| 110 | 2.4 | D67N, T69N, K70R, T215V, K219Q, M184V | 3 TAMs + M184V | PD | 22 | >28 |
| 114 | 2.7 | D67N, T69TA, K70R, T215F, K219Q | 4 TAMs | PD | 4 | >28 |
| 67 | 2.7 | M41L, D67N, L210W, T215Y | 4 TAMs | SDM | 14 | >28 |
| 85 | 3.0 | M41L, D67N, K70R, L210W, T215Y | 5 TAMs | SDM | 19 | >28 |
| 87 | 3.0 | M41L, D67N, K70R, T215Y, K219Q | 5 TAMs | SDM | 13 | >28 |
| 108 | 3.3 | M41L, D67N, K70R, T215F, K219Q, M184V | 5 TAMs + M184V | PD | 20 | >28 |
| 81 | 3.3 | M41L, D67N, L210W, T215Y, K219Q | 5 TAMs | SDM | 18 | >28 |
| 95 | 4.0 | M41L, D67N, K70R, L210W, T215Y, K219Q | 6 TAMs | SDM | 19 | >28 |
| 112 | 4.2 | M41L, D67N, T69N, K70R, T215F, K219E, M184V | 5 TAMs + M184V | PD | 10 | >28 |
| 111 | 4.3 | M41L, L210W, T215Y | 3 TAMs | PD | 4 | >28 |
| 120 | 6.6 | M41L, D67N, L210W, T215Y, K219R | 5 TAMs | PD | 10 | 20 |
| 113 | 8.1 | M41L, D67N, L210W, T215Y | 4 TAMs | PD | 10 | >28 |
| 121 | 10.7 | M41L, D67N, T69D, L74I, L210W, T215Y, K219R | 5 TAMs | PD | 4 | 13 |
| 117 | 12.6 | M41L, D67N, T69D, L210W, T215Y, K219R | 5 TAMs | PD | 5 | 10 |
FC, fold change. The EC50 for TAF was 0.015 μM.
WT, wild-type virus. The TAMs included M41L, D67N, K70R, L210W, T215Y/F, and K219E/N/Q/R.
Patient-derived (PD) mutants were cloned from clinical plasma samples. SDM, site-directed mutants.
TFV was used at a concentration of 50 μM; TAF was used at a concentration of 0.8 μM.
DISCUSSION
We conducted experiments with the aim of gaining a better understanding of the resistance profile of TAF (the second-generation prodrug of TFV) in comparison with TDF (first-generation prodrug of TFV). Since treatment with TAF leads to a >4-fold increase in the PBMC intracellular concentration of the anti-HIV active moiety TFV-DP compared to treatment with TDF (10, 11), we hypothesized that the resistance threshold for TAF should be higher than for TDF.
Here, we established the in vitro resistance profile of TAF and TDF in phenotypic assays using 110 viruses containing TAMs in the presence or absence of M184V. We showed that in vitro both TAF and TDF have virtually the same resistance profile (1-to-1 correlation, Fig. 4), which was expected since both prodrugs lead to the same active moiety (TFV-DP). However, standard in vitro phenotypic assays do not capture the >4-fold increase in intracellular TFV-DP concentration obtained with TAF dosing compared to TDF in vivo. This limitation was overcome by performing viral breakthrough assays at physiologically relevant concentrations of TAF and TDF that mimicked the >4-fold increase of TFV-DP that TAF provides compared to TDF in vivo. In these assays, the 15 viruses that broke through with TFV/TDF contained three or more TAMs with phenotypic resistance of ≥1.9-fold resistance, while the 3 viruses that broke through with TAF contained five TAMs with phenotypic resistance of ≥6.6-fold resistance, similar to previously published findings (8). This suggests that the resistance threshold for TAF may be 3.5-fold (ratio of 6.6 to 1.9) higher than for TDF and that TAF can inhibit resistant viruses that carry up to four TAMs in the absence of M184V. The pharmacologic enhancement of TAF compared to TDF that leads to higher in vivo intracellular concentration of TFV-DP is a clear differentiation factor between the two prodrugs of TFV, and these findings should be factored into the genotypic and phenotypic algorithms used to estimate the level of drug resistance, which we have shown to be different between TAF and TDF. This pharmacological effect is analogous to the increased atazanavir (ATV) drug levels obtained in patients upon pharmacological boosting with ritonavir (RTV) (19), which led to the establishment of different resistance cutoffs for ATV compared to RTV-boosted ATV (2.2 and 5.2, respectively, in the Monogram Biosciences assay). Furthermore, we did not observe viral breakthrough of SDM viruses with up to six TAMs in the presence of M184V, suggesting that the known hypersusceptibility to TAF and TFV associated with the decreased RT processivity and viral fitness in the presence of M184V (20) also contributes to the higher resistance threshold for TAF and should be taken into account when predicting treatment response to TAF clinically.
Clinical validation of the higher resistance threshold for TAF compared to TDF has not been established, since doing so would knowingly put participants at risk of virologic failure and would be inconsistent with the current standard of care. However, evidence suggesting that TAF may be active against NRTI-resistant HIV in the clinic have been obtained in two clinical studies investigating virologically suppressed patients with NRTI resistance (including TAMs). One ongoing study (GS-US-292-1824) has enrolled treatment-experienced (TE) patients with historical evidence of the presence of M184V/I with or without one to two TAMs who switched to the single tablet regimen composed of elvitegravir/cobicistat/emtricitabine/TAF (E/C/F/TAF). Participants in that study experienced 100% efficacy (pure virologic failure algorithm) in maintaining viral suppression up to week 24 (latest time point available) (21). The other study (GS-US-292-0119) enrolled suppressed heavily TE patients with known HIV resistance mutations, including a number of patients with three TAMs (23 of 135). In that study, patients switched from a complex regimen containing multiple pills to a simplified regimen of DRV plus E/C/F/TAF or stayed on their baseline regimen. Switching to DRV + E/C/F/TAF was found to be superiorly efficacious through week 48 (94% success at maintaining viral suppression below detectable level) compared to staying on baseline regimen (22–24). Taken together, the high efficacy data observed in these studies suggests that TAF is likely playing an active role in suppressing the virus in these treatment-experienced patients with HIV harboring TAMs and other mutations, including M184V.
In conclusion, the data presented here have shown that the >4-fold increase in intracellular TFV-DP concentration upon dosing with TAF compared to TDF is associated with an increase in the resistance threshold for TAF compared to TDF. The results suggest that TAF can inhibit viruses with up to four TAMs (compared to only up to two TAMs with TDF), which was further increased to six TAMs in the presence of the relatively common M184V mutation. The resistance threshold for TAF was estimated to be 3.5-fold higher than that for TDF. Similar analyses with a large number of K65R-containing viruses are ongoing and will complement this study (C. Callebaut et al., unpublished data). Altogether, these data suggest that algorithmic prediction of clinical activity of TAF should not follow the same rules as TDF and should consider the pharmacological enhancement of intracellular TFV-DP concentration achieved with TAF dosing compared to TDF dosing.
MATERIALS AND METHODS
Reagents.
TAF, TFV, ABC, FTC, dolutegravir (DTG), and darunavir (DRV) were synthesized at Gilead Sciences (Foster City, CA). TFV was used instead of TDF in our in vitro experiments due to the limitations of the lack of stability of TDF in culture media. ZDV was purchased from Sigma-Aldrich (St. Louis. MO). HEK293T cells (293T cells) used for virus production were purchased from American Type Culture Collection (ATCC; Manassas, VA). MT-2 cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD).
The viral plasmid pXXLAI used to generate the infectious viruses for the multicycle assay was a gift from John Mellors. The plasmid was derived from the infectious clone pLAI3.2 (GenBank accession number K03455), which was modified to contain an XmaI and an XbaI restriction site within the HIV RT portion of the pol gene to facilitate cloning, as previously published (25). The viral plasmid pKSHX was used to generate the single-cycle luciferase reporter viruses for the single-cycle assay. pKSHX was generated from an NL4.3 vector (GenBank accession number AF324493) by knocking out vpr and env expression and introducing a luciferase firefly gene in place of nef, as previously published under the name pKS13 (26). This single-cycle virus was pseudotyped with VSV-G (27).
GlutaMAX RPMI 1640 culture medium, Gibco DMEM high glucose, penicillin (10,000 U/ml), streptomycin (10,000 μg/ml), and 100× HEPES were purchased from Thermo Fisher Scientific (Waltham, MA). Fetal bovine serum (FBS) was purchased from HyClone Laboratories (Logan, UT). Cell culture media were supplemented with 10% heat-inactivated FBS, 5 ml of penicillin (10,000 U/ml) and Streptomycin (10,000 μg/ml) and 5 ml of 100× HEPES per 500 ml of medium.
Virus cloning and production.
Recombinant mutant HIV-1 viruses were created either by site directed mutagenesis (SDM) of wild-type HIV-1 DNA, or by direct PCR of ART-experienced patient isolates (8). DNA fragments containing most combinations of the most frequent TAMs (M41L, D67N, K70R, L210W, T215Y, and K219Q in RT) with or without the M184V RT mutation were generated by SDM and cloned into both pKSHX and pXXLAI plasmids (Genewiz, South Plainfield, NJ) (see the supplemental material). Plasmid DNA was transfected into 293T cells using the transfection reagent TransIT-LT1 (Mirus Bio Corporation, Madison, WI). For pXXLAI plasmid transfection, 7 μg of the pXXLAI vector was transfected in 2 million 293T cells seeded in T-25 cell culture flasks in a 6-ml volume. Virus was harvested at 24 and at 48 h posttransfection and then combined and used for infectivity assays. For pKSHX plasmid transfection, 35 μg of the pKSHX vector and 8 μg of the envelope vector pCMV-VSVG were transfected in 12 million 293T cells seeded in T-225 cell culture flasks in a 46-ml volume. Culture medium was replaced with fresh media after 5 to 6 h. Virus was harvested at 24 h posttransfection.
Multicycle phenotypic assay.
The phenotype of each virus was determined in a 5-day multicycle antiviral assay in MT-2 cells using a luciferase-based viability readout (CellTiterGlo; Promega, Madison, WI), as previously described (28). Briefly, MT-2 cells (2.4 million) were incubated with virus for 2 to 3 h at 37°C in 1.5-ml screw-cap tubes with gentle rocking. The amount of virus used was normalized to yield a signal-to-noise (S/N) ratio in the range of 4 to 7, which was equivalent to a multiplicity of infection of 0.005 based on the provided titer for the commercially available wild-type isolate HIV-1IIIB. The S/N ratio was calculated from the 300 nM DTG control (200-fold EC50; maximum cell survival) and the no-drug control (minimum cell survival). Black Corning assay plates (96 well; Fisher Scientific, Hampton, NH) were prepared with 5-fold dilutions of the drugs of interest in triplicate. Drug concentrations tested were as follows: TAF, 1 μM to 0.16 nM; TFV (in vitro equivalent of TDF), 300 μM to 48 nM; AZT, 15 μM to 2.4 nM; ABC, 20 μM to 6.4 nM; FTC, 50 μM to 16 nM; DTG, 188 to 0.06 nM; and DRV, 630 to 0.2 nM. After a 3-h incubation, infected MT-2 cells were diluted 1:14 to a concentration of 0.17 million cells/ml with tissue culture medium. Then, 50 μl of cell suspension was transferred to all wells in the assay plates to a final concentration of 8,500 cells/well. After 5 days of incubation (37°C, 5% CO2, 95% humidity), cultures were resuspended, 100 μl of CellTiterGlo reagent was added to each well, and the luminescence was measured using an Envision plate reader (Perkin-Elmer, Shelton, CT). The percent inhibition in the drug-containing wells in comparison to the fully protected DTG control were plotted using Excel (Microsoft, Redmond, WA) and XL Fit (IDBS, Alameda, CA), and the associated effective concentration to inhibit 50% of viral replication (EC50) was calculated. The statistical significance (P < 0.05) of the fold changes for the mutants compared to the wild-type control was calculated using Excel (two-tailed Student t test).
Single-cycle phenotypic assay.
The phenotype of each isolate was determined in a single-cycle 2-day antiviral assay in MT-2 cells using a luciferase-assay readout (Bright-Glo; Promega). MT-2 cells were plated in triplicate, along with pKSHX virus and diluted drug. The pKSHX vector contains a reporter luciferase gene (in place of nef), which was used to measure the quantity of virus produced. The optimal volume of pKSHX virus to use was determined in a virus titration assay with the goal to yield a readout between 10 and 40 million relative light units (RLU). The S/N ratio was calculated from the virus control in the absence of inhibitor (maximum luminescence signal) and the no-virus well control (minimum luminescence signal). White Corning assay plates (96 well; Fisher Scientific) were prepared with 50-μl dilutions of the virus and the drugs of interest in triplicate. TAF concentrations ranged from 7 μM to 0.013 nM with 2-fold dilutions. AZT concentrations ranged from 7.5 mM to 0.21 nM with 2.5-fold dilutions. MT-2 cells were diluted in culture medium, and 20,000 cells/well were added to the assay plates in a volume of 100 μl.
After 2 days of incubation (37°C, 5% CO2, 95% humidity), the cells were resuspended, 75 μl of Bright-Glo reagent was added to each well, and the luminescence was measured using an Envision plate reader (Perkin-Elmer). Viral infection luminescence signals in the drug-containing wells were plotted using Prism 7 (GraphPad Software, Inc., La Jolla, CA), and the EC50 was calculated. The statistical significance (P < 0.05) of the fold changes for the mutants compared to the wild-type control was calculated using Excel (two-tailed Student t test).
Thirty viruses containing one to six TAMs with or without M184V covering a wide FC range were selected and tested with Monogram Biosciences’ (South San Francisco, CA) phenotypic EC50 assay (PhenoSense) in order to compare the data obtained from both single-cycle assays.
Drug loading in viral breakthrough assay.
The viral breakthrough experiments were conducted as previously published (8, 29). MT-2 cells at a concentration of 0.4 million cells/ml were incubated (37°C, 5% CO2, 95% humidity) in the presence of TAF or TFV for 24 h at concentrations corresponding to TAF and TDF clinical exposure expressed as multiples of the EC95 for each drug (with EC95 estimated at 10×EC50). Cells were loaded with three TFV concentrations corresponding to inhibitory quotient (IQ) equivalents: 25 μM (0.5×EC95 corresponding to an IQ95 = 0.5), 50 μM (1×EC95 corresponding to an IQ95 = 1), and 100 μM (2×EC95 corresponding to an IQ95 = 2). For TAF, the concentrations used were adjusted to reflect the 4-fold increase in the intracellular concentration of TFV-DP observed in vivo upon dosing with TAF compared to dosing with TDF (10, 11). As a result, the three TAF concentrations used to load the cells were as follows: 0.4 μM (2×EC95 corresponding to an IQ95 = 2), 0.8 μM (4×EC95 corresponding to an IQ95 = 4), and 1.6 μM (8×EC95 corresponding to an IQ95 = 8). Cells loaded with either no drug or 300 nM dolutegravir (∼200×EC50) plus 100 nM efavirenz (∼100×EC50) were set up as controls.
Viral breakthrough assay.
As previously described (8, 29), tubes containing virus with 0.4 million MT-2 cells from each drug loading condition were incubated with gentle rocking at 37°C for 3 h. The amount of virus used was determined from the phenotypic EC50 assays to give an S/N value between 4 and 7. Infected cells were then plated at 10,000 cells per well in 96-well plates containing a final volume of 200 μl of cell culture medium with the same drug concentrations that were used at the loading step. Infections were scored after 4 or 5 days of incubation (37°C, 5% CO2, 95% humidity). Cultures were inspected under the microscope for signs of virus-induced cytopathic effect associated with viral breakthrough. New passages were carried out by transferring 50 μl of cells to new plates containing 150 μl of fresh medium with the appropriate drug concentrations. This process was repeated every 4 to 5 days up to 4 weeks.
Supplementary Material
ACKNOWLEDGMENT
All authors are employees and stockholders of Gilead Sciences, Inc., which funded the study.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.The Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. 2018. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. DHHS/National Institutes of Health, Bethesda, MD: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.European AIDS Clinical Society. 2018. Guidelines, version 9.1 (English). European AIDS Clinical Society, Brussels, Belgium. [Google Scholar]
- 3.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. 2009. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol 385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larder BA, Kemp SD. 1989. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science 246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 5.Erice A, Mayers DL, Strike DG, Sannerud KJ, McCutchan FE, Henry K, Balfour HH Jr.. 1993. Brief report: primary infection with zidovudine-resistant human immunodeficiency virus type 1. N Engl J Med 328:1163–1165. doi: 10.1056/NEJM199304223281605. [DOI] [PubMed] [Google Scholar]
- 6.Margot NA, Wong P, Kulkarni R, White K, Porter D, Abram ME, Callebaut C, Miller MD. 2017. Commonly transmitted HIV-1 drug resistance mutations in reverse-transcriptase and protease in antiretroviral treatment-naive patients and response to regimens containing tenofovir disoproxil fumarate or tenofovir alafenamide. J Infect Dis 215:920–927. doi: 10.1093/infdis/jix015. [DOI] [PubMed] [Google Scholar]
- 7.Johnston E, Dupnik KM, Gonzales MJ, Winters MA, Rhee SY, Imamichi T, Shafer RW. 2005. Panel of prototypical infectious molecular HIV-1 clones containing multiple nucleoside reverse transcriptase inhibitor resistance mutations. AIDS 19:731–733. doi: 10.1097/01.aids.0000166098.54564.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margot NA, Liu Y, Miller MD, Callebaut C. 2016. High resistance barrier to tenofovir alafenamide is driven by higher loading of tenofovir diphosphate into target cells compared to tenofovir disoproxil fumarate. Antiviral Res 132:50–58. doi: 10.1016/j.antiviral.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Miller MD, Margot N, Lu B, Zhong L, Chen S-S, Cheng A, Wulfsohn M. 2004. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis 189:837–846. doi: 10.1086/381784. [DOI] [PubMed] [Google Scholar]
- 10.Sax PE, Zolopa A, Brar I, Elion R, Ortiz R, Post F, Wang H, Callebaut C, Martin H, Fordyce MW, McCallister S. 2014. Tenofovir alafenamide versus tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 67:52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 11.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, Pozniak A, Thompson M, Podzamczer D, Molina JM, Oka S, Koenig E, Trottier B, Andrade-Villanueva J, Crofoot G, Custodio JM, Plummer A, Zhong L, Cao H, Martin H, Callebaut C, Cheng AK, Fordyce MW, McCallister S, Team G-U-S. 2015. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 12.Tisdale M, Kemp SD, Parry NR, Larder BA. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A 90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margot NA, Waters JM, Miller MD. 2006. In vitro human immunodeficiency virus type 1 resistance selections with combinations of tenofovir and emtricitabine or abacavir and lamivudine. Antimicrob Agents Chemother 50:4087–4095. doi: 10.1128/AAC.00816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MD, Haddad M, Su C, Gibbs C, McColl DJ, Guyer B. 2012. Trends in HIV-1 reverse transcriptase resistance-associated mutations and antiretroviral prescription data from 2003-2010. Antivir Ther 17:993–999. doi: 10.3851/IMP2266. [DOI] [PubMed] [Google Scholar]
- 15.Margot N, Cox S, Das M, McCallister S, Miller MD, Callebaut C. 2018. Rare emergence of drug resistance in HIV-1 treatment-naive patients receiving elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide for 144 weeks. J Clin Virol 103:37–42. doi: 10.1016/j.jcv.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Miller MD, Anton KE, Mulato AS, Lamy PD, Cherrington JM. 1999. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J Infect Dis 179:92–100. doi: 10.1086/314560. [DOI] [PubMed] [Google Scholar]
- 17.Wolf K, Walter H, Beerenwinkel N, Keulen W, Kaiser R, Hoffmann D, Lengauer T, Selbig J, Vandamme A-M, Korn K, Schmidt B. 2003. Tenofovir resistance and resensitization. Antimicrob Agents Chemother 47:3478–3484. doi: 10.1128/aac.47.11.3478-3484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diallo K, Gotte M, Wainberg MA. 2003. Molecular impact of the M184V mutation in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 47:3377–3383. doi: 10.1128/aac.47.11.3377-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertz RJ, Persson A, Chung E, Zhu L, Zhang J, McGrath D, Grasela D. 2013. Pharmacokinetics and pharmacodynamics of atazanavir-containing antiretroviral regimens, with or without ritonavir, in patients who are HIV-positive and treatment-naive. Pharmacotherapy 33:284–294. doi: 10.1002/phar.1205. [DOI] [PubMed] [Google Scholar]
- 20.Turner D, Brenner B, Wainberg MA. 2004. Relationships among various nucleoside resistance-conferring mutations in the reverse transcriptase of HIV-1. J Antimicrob Chemother 53:53–57. doi: 10.1093/jac/dkh009. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Valero I, Llibre JM, Lazzarin A, di Perri G, Pulido F, Molina JM, Esser S, McNicholl IR, Lorgeoux RP, Margot N, Shao Y, Piontkowsky D, Das M, Haubrich R. 2018. A phase 3b open-label pilot study to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) single-tablet regimen in virologically suppressed HIV-1 infected adults harboring the NRTI resistance mutation M184V and/or M1841 (GS-US-292–1824): week 24 results, presentation TUAB0104. International AIDS Conference, Amsterdam, the Netherlands. [Google Scholar]
- 22.Huhn GD, Tebas P, Gallant J, Wilkin T, Cheng A, Yan M, Zhong L, Callebaut C, Custodio JM, Fordyce MW, Das M, McCallister S. 2017. A randomized, open-label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment-experienced HIV-1-infected adults. J Acquir Immune Defic Syndr 74:193–200. doi: 10.1097/QAI.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huhn G, Tebas P, Gallant J, Wilkin T, Cheng A, Yan M, Callebaut C, Fordyce M, Das M, McCallister S. 2015. Strategic simplification: the efficacy and safety of switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) plus darunavir (DRV) in treatment-experienced HIV-1-infected adults, NCT01968551, oral presentation 726. IDWeek, San Diego, CA. [Google Scholar]
- 24.Callebaut C. 2016. Resistance profile analysis of treatment-experienced HIV-1-infected patients switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) plus darunavir (DRV), presentation. HIV Glasgow 2016, Glasgow, United Kingdom. [Google Scholar]
- 25.Shi C, Mellors JW. 1997. A recombinant retroviral system for rapid in vivo analysis of human immunodeficiency virus type 1 susceptibility to reverse transcriptase inhibitors. Antimicrob Agents Chemother 41:2781–2785. doi: 10.1128/AAC.41.12.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balakrishnan M, Yant SR, Tsai L, O’Sullivan C, Bam RA, Tsai A, Niedziela-Majka A, Stray KM, Sakowicz R, Cihlar T. 2013. Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells. PLoS One 8:e74163. doi: 10.1371/journal.pone.0074163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee JK, Friedmann T, Burns JC. 1994. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 43(Pt A):99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- 28.Margot NA, Hluhanich RM, Jones GS, Andreatta KN, Tsiang M, McColl DJ, White KL, Miller MD. 2012. In vitro resistance selections using elvitegravir, raltegravir, and two metabolites of elvitegravir M1 and M4. Antiviral Res 93:288–296. doi: 10.1016/j.antiviral.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Cox S, Margot N, Miller M, Callebaut C. 2018. Short communication: antiviral activity of tenofovir alafenamide against HIV-1 subtypes and emergence of K65R. AIDS Res Hum Retroviruses 34:456–458. doi: 10.1089/AID.2017.0248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.