Summary:
Pulmonary artery enlargement on chest CT imaging is independently associated with all-cause mortality in moderate-severe COPD, after adjustment for other known risk factors for COPD mortality and cardiovascular disease.
Keywords: COPD, mortality, CT, pulmonary artery, pulmonary hypertension, chest imaging
To the Editor:
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality worldwide (1). Pulmonary hypertension (PH) has been associated with reduced survival among individuals with COPD (2) and is an independent risk factor for mortality following acute exacerbations of COPD (AECOPD) (3). Measurement of the pulmonary artery to aorta (PA:A) ratio by computed tomography (CT) and assessment of PA enlargement (PA:A>1) outperforms echocardiography in identifying PH in severe COPD (4), and PA enlargement has been independently associated with risk for total and severe AECOPD in two large prospective COPD cohorts (5). Studies of population-based (6) and non-COPD (7) cohorts have indicated an association between PA enlargement on CT and mortality. However, these studies did not include adjustment for other factors associated with COPD severity, known risk factors for COPD mortality, and risk factors for atherosclerotic cardiovascular disease (ASCVD), which is a major cause of mortality in COPD.
We analysed CT imaging and clinical data from the COPDGene study and hypothesised that PA enlargement is associated with all-cause mortality, even after adjustment for other known predictors of mortality in COPD and risk factors for ASCVD. We analysed data from 3464 COPDGene participants with GOLD spirometry grades 2-4 COPD (5) who had CT imaging, and vital status assessment approximately six years later. This cohort underwent detailed baseline assessment (8), volumetric non-contrast CT scans of the chest with measurement of PA and aorta at the level of the PA bifurcation (5), and long-term longitudinal clinical follow-up (9) as previously described. All-cause mortality was determined via longitudinal follow-up, contact with next-of-kin, clinical records, death certificates, and information from the Social Security Death Index (10).
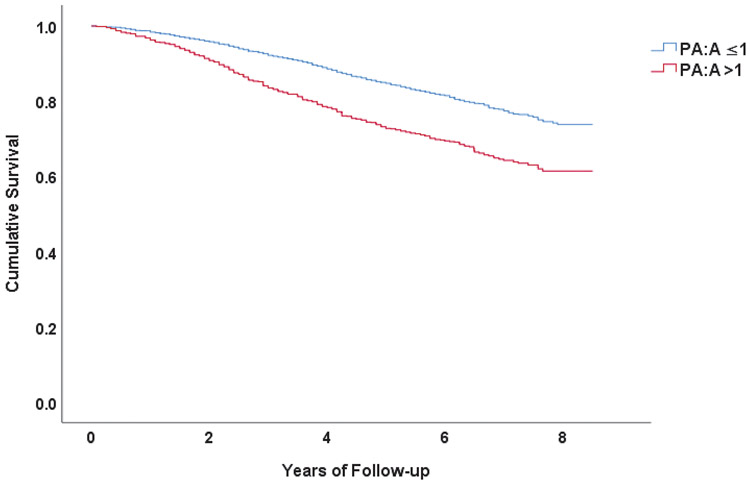
Participants had a median follow-up period of 6.3 years (IQR = 1.7 years, range = 8.4 years). Mean age was 63±9 years, 44% were female, and 78% were white. The mean FEV1 was 50±18% predicted, and 28% used supplemental oxygen. Mean PA:A was 0.90±0.14 and 810 (23.4%) participants had PA:A ratio >1. During the follow-up period, 755 (21.8%) participants died. In Kaplan-Meier analysis (SPSS), participants with PA:A >1 had shorter mean estimated survival (6.7 (95% CI [6.50, 6.89]) vs 7.4 (95% CI [7.35, 7.53]) years, P<0.001 (log-rank), Figure).
Figure: Association between PA enlargement and all-cause mortality in COPD.
Kaplan-Meier survival curves stratified by the presence or absence of PA enlargement (P<0.001, log-rank test).
Cox proportional hazards (CPH) models were used to investigate for potential association between PA enlargement (PA:A >1) and mortality. The multivariable CPH model included covariates that adjusted for other known factors associated with mortality and disease severity in COPD, as well as ASCVD. These included demographics (age, sex, race), body mass index (BMI), use of supplemental oxygen, six-minute walk distance (6MWD), Modified Medical Research Council (mMRC) dyspnoea score, FEV1 (% predicted), history of severe AECOPD within the preceding 12 months (self-reported using questions modified from standardised questionnaires), percent emphysema on CT (Slicer software), current smoking, and self-reported histories of coronary artery disease, diabetes mellitus, hypertension, and hyperlipidaemia. In the unadjusted CPH model, PA enlargement was associated with HR for mortality of 1.75 (95% CI [1.50, 2.04], P<0.001). In the multivariable CPH model, PA enlargement was associated with a 42% increase in mortality risk (HR 1.42; 95% CI [1.17, 1.72], P<0.001). Older age, male sex, lower BMI, lower FEV1 (% predicted), shorter 6MWD, supplemental oxygen use, higher mMRC dyspnoea scores, and current smoking were also associated with increased mortality risk in the adjusted CPH model. ASCVD-specific factors were not associated with risk for mortality in this cohort.
Our results are the first to demonstrate an association between PA enlargement on CT and mortality in moderate to severe COPD, even when adjusted for factors including AECOPD, components of the BODE index, and ASCVD. Several studies have previously reported associations between CT-based PA:A ratio and mortality. Using coronary CT angiography from patients with suspected coronary artery disease, Nakanishi et al. demonstrated an association between PA enlargement (defined as PA:A >0.9 in that study) and mortality after adjustment for cardiovascular risk factors and left ventricular function. However, this study neither reported the prevalence of, nor performed adjustment for factors associated with chronic lung disease (7). ASCVD is a major cause of mortality in COPD, and several factors such as age, sex, and smoking are related to both ASCVD risk and COPD severity/mortality. However, the results of our adjusted model suggest that the increased risk is unlikely attributable to ASCVD.
Shin et al. found an association between PA:A ≥1 and mortality over a shorter follow-up interval, in a smaller cohort of COPD patients referred for lung transplant/lung volume reduction evaluation, after adjustment for age, BMI, and FEV1 (% predicted) (11). Terzikhan et al. also reported an independent association between PA:A ratio and mortality among participants with COPD in a large, predominantly white population in the Netherlands. However, only 5% of the study population had moderate-severe COPD, and 17 of 2197 total participants had PA:A ≥1 (6). In contrast to prior studies, COPDGene is a multicentre, disease-specific study that included larger numbers of African-American participants (approximately 1/3 of total enrolment) (8) as well large numbers of subjects with moderate to severe disease. Our results also indicate a high prevalence of PA enlargement in the study cohort, with PA enlargement in nearly 1/4 of participants. Our findings add to the mounting body of evidence to indicate an association between PA:A ratio and adverse outcomes in COPD including severe acute exacerbations (5), reduced exercise tolerance (12), risk for development of resting hypoxaemia (13), as well as cardiac injury and more complicated hospital course during severe acute exacerbations (14).
Our results demonstrate increased mortality risk after adjustment for supplemental oxygen use, FEV1, and percent emphysema. This suggests that COPD severity cannot completely account for the observed associations, and supports the importance of mechanisms other than chronic hypoxia and capillary dropout due to emphysema in the pathophysiology of pulmonary vascular disease in COPD. Direct pulmonary vascular injury as a result of cigarette smoke exposure could also contribute to the development of PH in early or mild disease, in the absence of emphysema or hypoxaemia (15). Genetic variants in IREB2 and GALC that were identified through a genome-wide association study of COPDGene and ECLIPSE participants with PA:A>1 (16) might also account for earlier onset of PH in certain individuals.
The strengths of our study include use of a well-defined cohort, length of follow-up, and adjustment for other known predictors of mortality. PA:A measurement represents a reproducible, practical method for evaluation of patients with COPD for pulmonary vascular disease. Our study has several limitations. First, our results should not be used to imply a direct causal relationship between PA enlargement and mortality. Since we evaluated all-cause mortality, there is insufficient evidence to reach conclusions regarding specific causes of death among individuals with concurrent COPD and PA enlargement. Measurement of disease-specific outcomes and study of PA:A ratio in common comorbid conditions such as obesity, obstructive sleep apnoea, and congestive heart failure would be valuable in elucidating the nature of the associations observed in this study. Our conclusions are also based on a single cohort with moderate to severe disease, using noncontrast, non-gated CT imaging, though others have reached similar conclusions using different study populations and CT imaging protocols (6, 7, 17).
These findings extend the known utility of PA enlargement on CT scan as an imaging-based biomarker for morbidity and mortality in COPD. The increased use of CT imaging for clinical indications (i.e. lung cancer screening) coupled with the relative ease in ascertaining PA enlargement make this a practical metric for aiding in COPD prognosis.
Acknowledgments:
The authors acknowledge the COPDGene Core Teams and COPDGene Investigators. A complete list of these personnel is available at COPDGene.org.
Sources of funding support: This work was supported by grants from the National Institutes of Health (NIH): 5T32HL105346-9 (D.C.L.), K08HL123940 (J.M.W.), The Genetic Epidemiology of COPD Program is supported by National Institutes of Health Grants U01 HL089897 and U01 HL089856.
Footnotes
Publisher's Disclaimer: This is an author-submitted, peer-reviewed version of a manuscript that has been accepted for publication in the European Respiratory Journal, prior to copy-editing, formatting and typesetting. This version of the manuscript may not be duplicated or reproduced without prior permission from the copyright owner, the European Respiratory Society. The publisher is not responsible or liable for any errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final, copy-edited, published article, which is the version of record, is available without a subscription 18 months after the date of issue publication.
References
- 1.Burney PG, Patel J, Newson R, Minelli C, Naghavi M. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748–55. [DOI] [PubMed] [Google Scholar]
- 4.Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145(4):824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E, Beaty TH, Curran-Everett D, Curtis JL, Hokanson JE, Lynch DA, Make BJ, Crapo JD, Silverman EK, Bowler RP, Dransfield MT, COPDGene Investigators, ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzikhan N, Bos D, Lahousse L, Wolff L, Verhamme KMC, Leening MJG, Felix JF, Gall H, Ghofrani HA, Franco OH, Ikram MA, Stricker BH, van der Lugt A, Brusselle G. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J. 2017;49(6). [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi R, Rana JS, Shalev A, Gransar H, Hayes SW, Labounty TM, Dey D, Miranda-Peats R, Thomson LE, Friedman JD, Abidov A, Min JK, Berman DS. Mortality risk as a function of the ratio of pulmonary trunk to ascending aorta diameter in patients with suspected coronary artery disease. Am J Cardiol. 2013;111(9):1259–63. [DOI] [PubMed] [Google Scholar]
- 8.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, Crapo JD, Zeldin RK, Make BJ, Regan EA, For The COPDGene Investigators. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. Copd. 2012;9(5):466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criner RN, Labaki WW, Regan EA, Bon JM, Soler X, Bhatt SP, Murray S, Hokanson JE, Silverman EK, Crapo JD, Curtis JL, Martinez FJ, Make BJ, Han MK, Martinez CH, COPDGene Investigators. Mortality and exacerbations by Global Initiative for Chronic Obstructive Lung Disease groups ABCD: 2011 versus 2017 in the COPDGene cohort. Chronic Obstr Pulm Dis. 2019;6(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin S, King CS, Brown AW, Albano MC, Atkins M, Sheridan MJ, Ahmad S, Newton KM, Weir N, Shlobin OA, Nathan SD. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med. 2014;108(11):1626–32. [DOI] [PubMed] [Google Scholar]
- 12.Wells JM, Iyer AS, Rahaghi FN, Bhatt SP, Gupta H, Denney TS, Lloyd SG, Dell'Italia LJ, Nath H, Estepar RS, Washko GR, Dransfield MT. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circulation Cardiovascular imaging. 2015;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JM, Estepar RS, McDonald MN, Bhatt SP, Diaz AA, Bailey WC, Jacobson FL, Dransfield MT, Washko GR, Make BJ, Casaburi R, van Beek EJ, Hoffman EA, Sciurba FC, Crapo JD, Silverman EK, Hersh CP, COPDGene Investigators. Clinical, physiologic, and radiographic factors contributing to development of hypoxemia in moderate to severe COPD: a cohort study. BMC Pulm Med. 2016;16(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JM, Morrison JB, Bhatt SP, Nath H, Dransfield MT. Pulmonary Artery Enlargement Is Associated With Cardiac Injury During Severe Exacerbations of COPD. Chest. 2016;149(5):1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voelkel NF, Gomez-Arroyo J, Mizuno S. COPD/emphysema: The vascular story. Pulm Circ. 2011;1(3):320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Cho MH, Hersh CP, McDonald ML, Wells JM, Dransfield MT, Bowler RP, Lynch DA, Lomas DA, Crapo JD, Silverman EK, COPDGene Investigators, ECLIPSE Investigators. IREB2 and GALC are associated with pulmonary artery enlargement in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2015;52(3):365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de-Torres JP, Ezponda A, Alcaide AB, Campo A, Berto J, Gonzalez J, Zulueta JJ, Casanova C, Rodriguez-Delgado LE, Celli BR, Bastarrika G. Pulmonary arterial enlargement predicts long-term survival in COPD patients. PLoS One. 2018;13(4):e0195640. [DOI] [PMC free article] [PubMed] [Google Scholar]