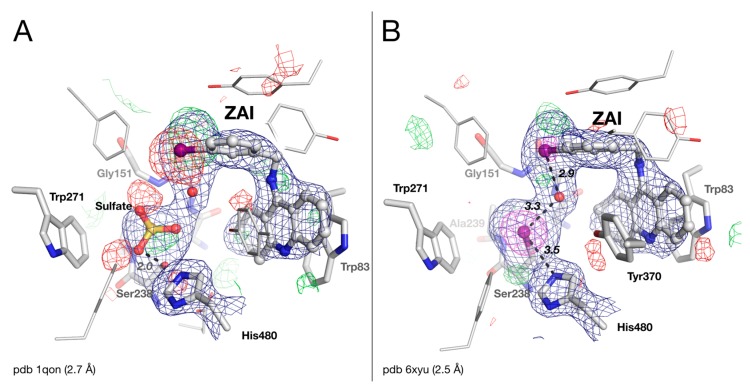
Figure 4.
Active-site gorge of the original (A) and updated (B) structures of DmAChE in complex with ZAI (iodobenzyltacrine). Residues of the catalytic triad (His480/Ser238), of the oxyanion hole (Gly150/Gly151/Ala239), and key residues of the acyl-binding pocket (Trp271) and choline-binding pocket (Trp83), are represented as sticks, with carbons in white, nitrogens in blue, and oxygens in red. The bound sulfate, iodine and ZAI are represented as ball-and-stick models. H-bonds are shown as black dashes, with distances in Å. The meshes represent the 2|Fo| – |Fc| map (1 σ blue / 5 σ magenta) and the |Fo| − |Fc| difference map (3 σ green /−3 σ red).