Highlights
-
•
COVID-19 is spreading globally.
-
•
Evaluate the clinical characteristics and outcomes of pregnant women confirmed with COVID-19 in Wuhan, China.
-
•
Pulmonary CT screening on admission may be necessary to reduce the transmission of COVID-19
-
•
COVID-19 is not an the indication of cesarean section.
Keywords: COVID-19, pregnant women, pulmonary CT screening, Infection, Public health
Abstract
Background
COVID-19 is spreading globally. This study aims to evaluate the clinical characteristics and outcomes of pregnant women confirmed with COVID-19 to provide reference for clinical work.
Methods
The clinical features and outcomes of 10 pregnant women confirmed with COVID-19 at Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, a tertiary- care teaching hospital in Hubei province, Wuhan, China from January 23 to February 23, 2020 were retrospectively analyzed.
Results
All the 10 observed pregnant women including 9 singletons and 1 twin were native people in Wuhan. All of them were diagnosed mild COVID-19, and none of the patients developed severe COVID-19 or died. Among the 10 patients, two patients underwent vaginal delivery, two patients underwent intrapartum cesarean section, and the remaining six patients underwent elective cesarean section. All of 10 patients showed lung abnormalities by pulmonary CT images after delivery. Their eleven newborns were recorded and no neonatal asphyxia was observed.
Conclusions
Pulmonary CT screening on admission may be necessary to reduce the risk of nosocomial transmission of COVID-19 during the outbreak period. And COVID-19 is not an indication of cesarean section.
1. Introduction
Since December 2019, the outbreak of the 2019 novel coronavirus disease (COVID-19) infection has progressed to a pandemic in China, especially in Wuhan (Zhu et al., 2020a). At the beginning, a series of unknown viral pneumonia cases were found in Wuhan, the capital city of Hubei province, and spread rapidly throughout China and other countries, including Thailand, Republic of Korea, Japan, United States, Philippines, Viet Nam (Wu et al., 2020). Further investigation revealed a novel coronavirus, termed 2019-nCoV at first and subsequently SARS-CoV-2 (Cordes and Heim, 2020), and COVID-19 was the term agreed internationally for the name of the acute respiratory disease syndrome caused by the pathogen ultimately. As of April 5, 2020, according to data released by the National Health Commission of China, the cumulative number of confirmed cases in mainland China has reached 81708, including 77078 cured cases, 3331 death cases, and 88 suspected cases (National Health Commission of the People's Republic of China, 2020). A recent study by Huang and colleagues (Huang et al., 2020) focused on the epidemiological, clinical characteristics, treatment and clinical outcomes of non-pregnant patients with laboratory-confirmed COVID-19. However, there are only some reports on pregnant women with COVID-19 infection at present (Chen et al., 2020, Zhu et al., 2020b, Karimi-Zarchi et al., 2020, Liu et al., 2020). In order to address the clinical features of pregnant women with confirmed COVID-19, we retrospectively reviewed clinical records, laboratory findings and chest CT scan of 10 pregnant women with laboratory-confirmed COVID-19 (tested positive on maternal throat swab), who were admitted to Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, from January 23th to February23th, 2020.
2. Methods
2.1. Ethical approval
The study was reviewed and approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology (Record number 2020001). Written informed consent was obtained from enrolled patients.
2.2. Study design and patients
We retrospectively reviewed the clinical features and outcomes of 10 pregnant women with laboratory-confirmed COVID-19 at Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China from January 23 to February 23, 2020. Diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program (4th edition) published by the National Health Commission of China (NHC, in press). By collecting throat swab samples of all ten pregnant women, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were tested positive by use of quantitative RT-PCR (qRT-PCR) (WHO, 2020).
2.3. Introduction of our birth center during the COVID-19 out-breaking period
During the out-breaking of COVID-19, the hospitals were divided into designated hospitals for COVID-19 and other non-designated hospitals (Health Commission of Hubei Province, 2020). Our birth center is a big center handling about 30,000 deliveries per year in the last three years. It belongs to a non-designated hospital, so non-COVID-19 pregnant women can delivery in our center. If the confirmed cases and suspected cases were in labor, they were allowed to deliver their babies in an isolation suite. After delivery, the confirmed cases were transferred to designated hospitals for further treatment and the suspected cases continued to be observed in our isolation suites. Additionally, if the cases were not timely diagnosed for COVID-19 on admission, they could be transferred to an isolation suite anytime for observation based on symptoms such as fever and/or cough, chest CT scan, and laboratory findings. The delivered babies were transferred to an isolation suite of NICU.
2.4. Collection of clinical data
We reviewed demographic data, time of onset of disease, clinical symptoms of fever, cough, fatigue and diarrhea, delivery methods, laboratory findings, and chest CT scans. The neonates’ information including birth weight, clinical symptoms, Apgar score, and outcomes were collected. Maternal and neonatal throat swab samples were collected and sent to the local Chinese Center for Disease Control and Prevention (CDC) for detection of COVID-19 by qRT-PCR. Throat swab samples of all pregnant women were collected for nucleic acid detection of COVID-19. Of all 11 babies, five babies underwent a throat swab sample test for COVID-19 by qRT-PCR within 24 hours.
2.5. Statistical analysis
Statistical analysis was carried out using SPSS, version 19.0. Continuous variables were directly expressed as a range. Categorical variables were expressed as number (n).
3. Results
All the 10 pregnant women were native people in Wuhan, who had a history of epidemiological exposure to COVID-19. The age of the pregnant women ranged from 29 to 35 years old. The gestational weeks of those patients ranged from 33 + 6 to 40 + 5 weeks on admission, and 4 babies were from 3 pregnant women (1 twin) were premature births (33 + 6, 34 + 6, 34 + 6 weeks). Of all cases, there were 4 cases of premature rupture of membranes(n = 4), 1 case of gestational diabetes(n = 1), 3 cases of preeclampsia(n = 3), 1 case of placental abruption(n = 1), 2 cases of fetal distress(n = 2), 1case of hypothyroidism(n = 1), and 1 case of anemia(n = 1). Patient 3 and 10 underwent vaginal delivery, patient 5 and 6 underwent intrapartum cesarean section for fetal distress, and the remaining six patients underwent elective cesarean section. (Table 1 ).
Table 1.
The complication and delivery method of the ten patients with COVID-19
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| complication | |||||||||||
| premature | Yes | Yes | Yes | 3 | |||||||
| PROM | Yes | - | Yes | - | Yes | Yes | - | - | - | 4 | |
| Placental abruption | Yes | - | - | - | - | - | - | - | - | - | 1 |
| Preeclampsia | Yes | - | - | - | - | Yes | - | - | Yes | - | 3 |
| Fetal distress | - | Yes | - | - | Yes | - | - | - | - | - | 2 |
| Twins | - | - | - | - | - | - | Yes | - | - | - | 1 |
| Hypothyroidism | - | - | - | - | - | - | - | Yes | - | - | 1 |
| Anemia | - | - | - | - | - | - | - | Yes | - | - | 1 |
| GDM | - | - | - | - | - | - | - | - | - | Yes | 1 |
| Delivery method | |||||||||||
| Vaginal birth | - | - | Yes | - | - | - | - | - | - | Yes | 2 |
| Elective CD | Yes | Yes | - | Yes | - | - | Yes | Yes | Yes | - | 8 |
| Intrapartum CD | Yes | Yes | |||||||||
| Indications for surgery | Placental abruption | Fetal distress | - | Previous CD | Fetal distress | Fetal distress | Twins | Previous CD | Preeclampsia | - |
Among those cases, two patients presented fever which lasted from prenatal to postpartum, five patients presented with postpartum fever, only one patient had a high fever (body temperature > 39.1 °C), and three confirmed COVID-19 patients had a normal body temperature during hospitalization. Other symptoms such as an upper respiratory tract infection were not clinically significant too, only one patient had an occasional cough and at the same time felt slight chest tightness. All of them were diagnosed mild COVID-19, and none of them developed severe COVID-19 with severe respiratory distress, or required mechanical ventilation, or died during the treatment period in designated hospitals or home quarantine period. All cases were followed up by telephone until reported fully recovered. During the observation period, no patients had myalgia, chill, sore throat, diarrhea or chest pain. (Table 2 )
Table 2.
Clinical features of mothers with COVID-19 infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of admission | 11-Feb | 10-Feb | 8-Feb | 1-Feb | 26-Jan | 25-Jan | 5-Feb | 28-Jan | 26-Jan | 23-Jan | |
| Age (years) | 31 | 29 | 30 | 35 | 29 | 31 | 30 | 29 | 30 | 29 | |
| Gestational age on admission(w) | 34 + 6 | 40 + 3 | 38 + 0 | 33 + 6 | 39 + 6 | 38 + 2 | 34 + 6 | 39 + 1 | 39 + 1 | 40 + 5 | |
| Epidemiological history | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Date of delivery | 11-Feb | 10-Feb | 9-Feb | 1-Feb | 27-Jan | 26-Jan | 6-Feb | 29-Jan | 26-Jan | 24-Jan | |
| Signs and symptoms | |||||||||||
| Fever | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 7 |
| Prenatal fever (on admission) | Yes | No | No | Yes | No | No | No | No | No | No | 2 |
| Post-partum fever | No | No | No | No | Yes | Yes | No | Yes | Yes | Yes | 5 |
| Maximum body temperature | |||||||||||
| Low grade fever (37.3 -38.0 °C) | Yes | No | No | Yes | No | No | No | No | Yes | No | 3 |
| Moderate grade fever (38.1 -39.0 °C) | No | No | No | No | Yes | Yes | No | No | No | Yes | 3 |
| High grade fever (>39.1 °C) | No | No | No | No | No | No | No | Yes | No | No | 1 |
| Fever duration | 3 h | - | - | 12 h | 14d | 6 d | - | 7 d | 2 d | 6 d | |
| Cough | No | No | No | No | No | No | No | No | No | Yes | 1 |
| Chest tightness | No | No | No | No | No | No | No | No | No | Yes | 1 |
| Fatigue | No | No | No | No | No | No | No | No | No | Yes | 1 |
| Laboratory characteristics | |||||||||||
| Lymphocyte count (× 109) (admission) | 2.33 | 1.82 | 1.33 | 1.12 | 1.15 | 2.19 | 1.35 | 1.27 | 1.46 | 0.97 | 1 |
| Lymphopenia (<1.0 × 109) (after delivery) | 1.76 | 0.78 | 0.65 | 1.12 | 0.63 | 2.36 | 1.89 | 0.54 | 0.68 | 0.98 | 6 |
| White blood cell count (× 109) | 9.7 | 9.75 | 10.47 | 5.9 | 11.51 | 8.65 | 9.35 | 9.23 | 6.62 | 8.12 | |
| CRP (mg/L) | 3.72 | 8.37 | 5.61 | 1.96 | 20.48 | 0.93 | 3.12 | 74.6 | 19.56 | 7.5 | |
| ALT (U/L) | 14.9 | 10.4 | 8.7 | 9.1 | 5.3 | 3.9 | 9.2 | 6.9 | 17.7 | 9.1 | |
| AST (U/L) | 22.5 | 14.7 | 16.5 | 23.7 | 12.6 | 10.4 | 21 | 12.6 | 28.2 | 16.9 | |
| Lactate dehydrogenase (U/L) | 239.3 | 192.0 | 170.3 | 195.0 | 169.8 | 153.0 | 242.0 | 195.5 | 196.3 | 150.9 | |
| D-dimer (ug/L) | 4.77 | 2.16 | 2.00 | 1.05 | 1.31 | 0.93 | 6.48 | 1.94 | 1.31 | 0.99 | |
| Mixed infection with other pathogens | Mycoplasma | Mycoplasma1 | Mycoplasma | - | - | - | Mycoplasma | Mycoplasma | - | Mycoplasma | 6 |
Data from laboratory tests showed that only one of ten patients had lymphopenia (<1.0 × 109/L) on admission. After delivery, six patients exhibited lymphopenia and six patients had slight elevated concentrations of C-reactive protein (>4 mg/L). The concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), lactate dehydrogenase, D-dimer and complement were normal during hospitalization in our hospital.
All of the ten patients showed lung abnormalities by chest CT images after delivery, six patients underwent CT scan before delivery on admission (one was normal, five were abnormal), and all patients underwent CT scan for postpartum fever after delivery. Four cases showed single lobe and six cases showed bilateral multilobe lesions. Two of them showed peripheral distribution, five of them showed random distribution and two showed diffuse distribution of pulmonary lesions. According to the image manifestation of those cases, there were six cases showing patch, two cases showing strip, four cases showing ground glass opacities (GGO), five cases showing pleural effusion and one cases showing pleural thickening. Five of ten patients showed pleural effusion simultaneously, one of whom only showed pleural effusion on admission.
All of the enrolled patients showed lung abnormalities by pulmonary CT images, six patients underwent CT before delivery and one of them was normal in CT result. All of the patients were reexamined by chest CT scan 3-4 days after the first CT examination during hospitalization period in our hospital
From the chest CT images, six cases showed aggravation, one case showed no change and three cases showed dissipation ( Table 3 ).
Table 3.
CT manifestations of ten COVID-19 patients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT (admission) | abnormal | abnormal | abnormal | abnormal | - | - | abnormal | normal | - | - | 6 |
| CT (after fever) | - | - | - | - | abnormal | abnormal | - | abnormal | abnormal | abnormal | 5 |
| Involvement of the lesions | |||||||||||
| Single lobe | - | Yes | Yes | - | - | - | Yes | - | - | Yes | 4 |
| Bilateral multilobe | Yes | - | - | Yes | Yes | Yes | - | Yes | Yes | - | 6 |
| Distribution of pulmonary lesions | |||||||||||
| Periphera | - | - | Yes | - | Yes | - | - | - | - | - | 2 |
| Random | Yes | Yes | - | Yes | - | Yes | - | - | - | Yes | 5 |
| Diffuse | - | - | Yes | - | - | - | - | - | Yes | - | 2 |
| Image manifestation | |||||||||||
| Patch | - | - | Yes | Yes | Yes | Yes | - | - | Yes | Yes | 6 |
| Strip | Yes | - | - | - | - | - | Yes | - | - | - | 2 |
| GGO | - | Yes | - | - | - | - | Yes | - | Yes | Yes | 4 |
| Pleural effusion | Yes | - | - | - | Yes | Yes | - | Yes | Yes | - | 5 |
| Pleural thickening | - | - | - | Yes | - | - | - | - | - | - | 1 |
| Variation trend (after 3-4 days) | dissipate | Aggravate | Aggravate | dissipate | dissipate | Aggravate | maintain | Aggravate | Aggravate | Aggravate |
Four newborns were premature, two of them had a birthweight lower than 2500 g. All eleven live births had a 1-min Apgar score of 8–9 and a 5-min Apgar score of 10 (Table 4 ). 5 newborns (including twins) underwent throat swab test for COVID-19 after birth and the results were negative. From birth to February 25, 2020, which was a 14-day period, no neonatal death or neonatal asphyxia was observed, and no one presented with fever, cough, or diarrhea.
Table 4.
The neonatal reports of the ten COVID-19 patients after delivery
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gestational age | 34 + 6 | 40 + 3 | 38 + 1 | 33 + 6 | 40 + 0 | 38 + 3 | 35 + 0 | 39 + 2 | 39 + 1 | 40 + 6 |
| Birthweight (g) | 2650 | 3250 | 3180 | 2050 | 3400 | 2900 | 2310/2510 | 3750 | 3800 | 3235 |
| Apgar score (1, 5 min) | 8,10 | 9,10 | 8,10 | 8,10 | 9,10 | 9,10 | 8,10 | 9,10 | 9,10 | 9,10 |
| amniotic fluid | clear | turbidity | clear | clear | turbidity | clear | clear | clear | clear | clear |
| Premature | Yes | No | No | Yes | No | No | Yes | No | No | No |
| neonatal asphyxia | No | No | No | No | No | No | No | No | No | No |
| Neonatal death | No | No | No | No | No | No | No | No | No | No |
| Neonatal confirmatory test (COVID-19 qRT-PCR) | negative | - | - | negative | negative | - | negative | - | - | - |
| follow up after 14 days of birth | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal |
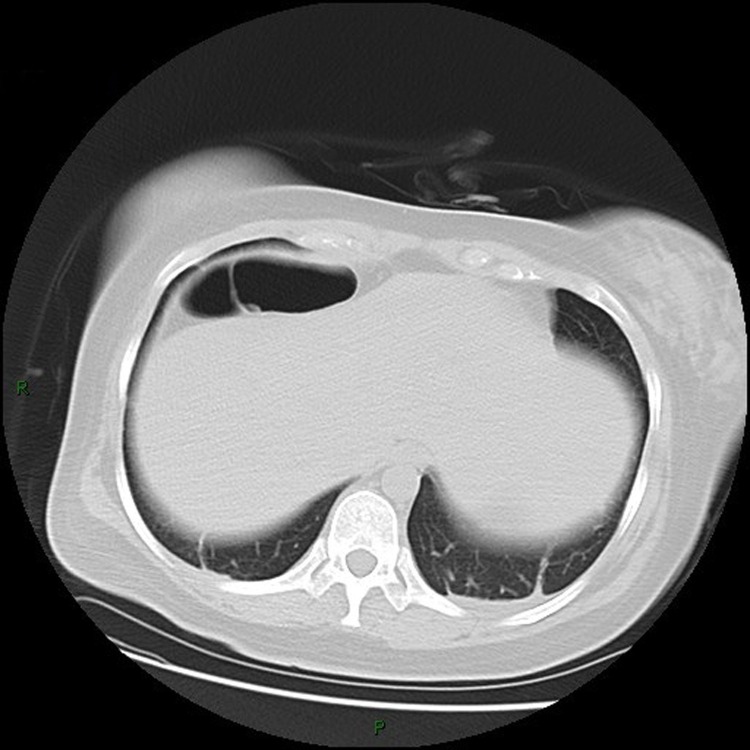
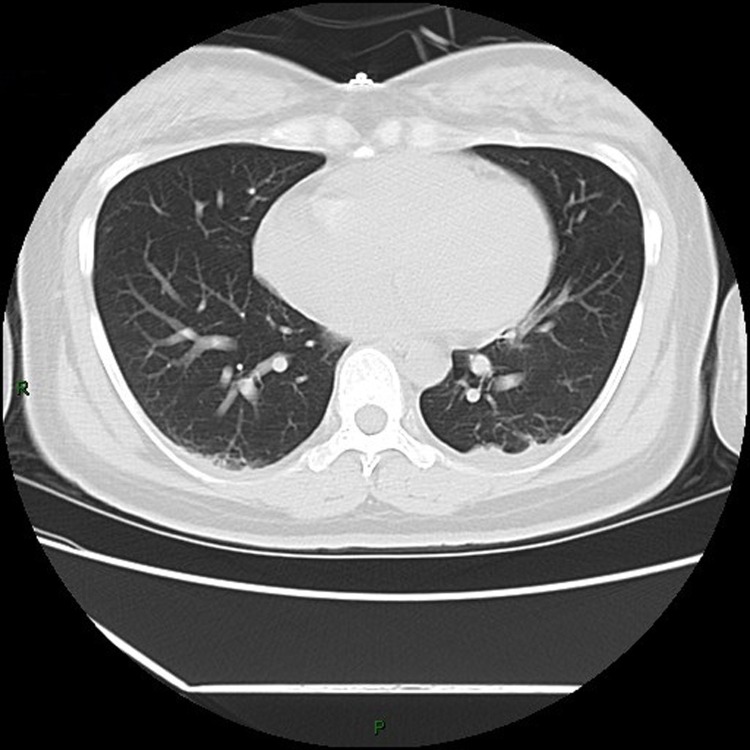
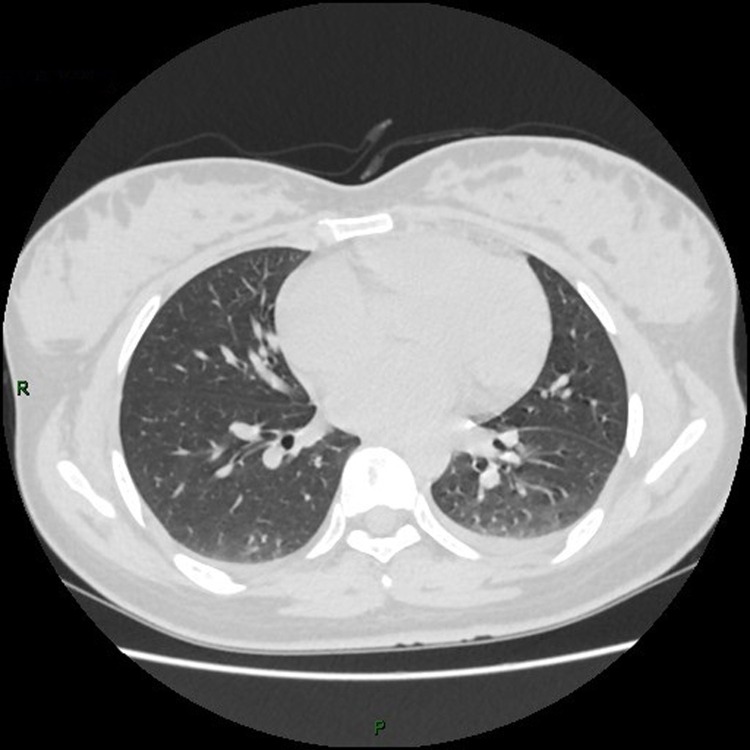
3.1. The chest CT images
(Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10 )
Fig. 1.
Chest CT images, showing bilateral lower lung cord from patient 1.
Fig. 2.
Chest CT images, showing ground-glass nodule on the right upper lobe from patient 2.
Fig. 3.
Chest CT images, showing spotted slightly denser shadows scattered on the right lower lobe from patient 3.
Fig. 4.
Chest CT images, showing patchy shadows of bilateral lungs, thickened dorsal pleura from patient 4.
Fig. 5.
Chest CT images, showing a little patchy shadow on the dorsal side of bilateral lower lungs and a little pleural effusion from patient 5.
Fig. 6.
Chest CT images, showing a few patchy shadows on bilateral lungs and a small amount of pleural effusion on both sides from patient 6.
Fig. 7.
Chest CT images, showing ground-glass density nodules on the left from patient 7.
Fig. 8.
Chest CT images, showing patches near the pleura, bilateral pleural effusion from patient 8.
Fig. 9.
Chest CT images, showing ground glass and patch shadows scattered on the bilateral lungs, especially obvious on the right, the bronchi dilation in the right lobe, a little pleural effusion on both sides from patient 9.
Fig. 10.
Chest CT images, showing patchy ground glass shadow of the left upper lobe with high density of some lesions from patient 10.
4. Discussion
Coronaviruses (CoVs) are spherical, enveloped non-segmented positive-strand RNA viruses. As of December 2019, seven coronavirus species have produced human infection (Huang et al., 2020, Yu et al., 2008, Aasiyh Chafekar and Fielding, 2018), including HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, MERS-CoV, SARS-CoV and SARS-CoV-2. The pandemic of SARS, MERS and COVID-19 demonstrate that coronaviruses are a significant public health threat causing significant loss of lives (Hui, 2017, Song et al., 2019). When the SARS-CoV and MERS-CoV infected pregnant women, poor obstetric outcomes can be resulted, including maternal morbidity and death (Schwartz and Graham, 2020, Lam et al., 2004, WHO, in press, Wong et al., 2004), but there is limited experience with coronavirus infections during pregnancy, and it now appears certain that pregnant women have become infected during the present COVID-19 epidemic (Schwartz and Graham, 2020). The COVID-19 has at least 70% similarity in genetic sequence to SARS-CoV (Hui et al., 2020). However, the effect of COVID-19 on pregnant women remains unknown at present. Due to the normal maternal physiologic changes and immune suppression during the pregnant period, these women are particularly susceptible to respiratory pathogens. During the Asian flu epidemic in 1957–1958, 10% of mortality occurred in pregnant women, and the fatality rate of pregnant women was twice as high as that of infected women who were non-pregnant (Eickhoff et al., 1961). A case-control study (Lam et al., 2004) on the effect of SARS among pregnant women and non-pregnant women revealed that maternal mortality rate, renal failure, disseminated intravascular coagulopathy (DIC), the intensive care unit (ICU) admission rate were higher in pregnant SARS patients. Huang C et al (Huang et al., 2020) reported that the COVID-19 infection of non-pregnant people caused clusters of severe respiratory illness similar to SARS, of which common symptoms were fever (98%), cough (76%), and myalgia or fatigue (44%), less common symptoms were sputum production (28%), headache (8%), haemoptysis (5%), and diarrhoea (3%), and the complications were acute respiratory distress syndrome (29%), RNA anaemia (15%), acute cardiac injury (12%) and secondary infection (10%). However, in our study, none of the ten pregnant women developed or died of severe COVID-19 pneumonia but only showed similar clinical symptoms to non-pregnant adult patients such as fever and cough, whereas others common symptoms such as myalgia, sore throat and dyspnea were not observed. This is probably due to the fact our hospital is not a designated hospital for COVID-19 and lacks serious patients with typical symptoms, only a small number of cases were enrolled in our observation and the patients’ length of stay was short. Therefore, we should also be alert to the possibility that the disease course and prognosis of COVID-19 could follow the same trend as SARS in pregnant women.
In our study, ten pregnant women were tested positive for COVID-19, while only one patient had lymphopenia on admission, and six cases exhibited lymphopenia after delivery. Three cases did not have any discomfort. In contrast, all patients showed lung abnormalities in chest CT images. However, among them five patients showed pleural effusion, which was different from the typical images like patch, strip, or GGO (NHC, in press). The produce of pleural effusion probably due to postpartum physiological changes in pregnant women. Therefore, non-specific manifestations such as pleural effusion should also be attached with great attention in diagnosis of COVID-19 infection if there is postpartum fever. In Wuhan where the incidence of COVID-19 is exceptionally high, it is necessary to do pulmonary CT screening on admission, which will help to detect asymptomatic infection and latent period patients, thus reducing the transmission of virus between people and nosocomial infection.
Chen H et al (Chen et al., 2020) observed that all nine pregnant women underwent cesarean delivery (CD), and the indications for CD were severe pre-eclampsia, a history of CD and fetal distress. In our study, two patients underwent vaginal delivery successfully, two patients underwent intrapartum CD for fetal distress, and the remaining six pregnant women underwent selected CD directly for previous CD history, pre-eclampsia, placenta abruption, twins pregnancy. Although COVID-19 infection is not one of the indications for CD, given the uncertainties of a novel disease, we may be more inclined to choose CD.
Previous studies (Lam et al., 2004, WHO, in press) have shown that pregnant women with SARS is associated with a high incidence of adverse neonatal complications, such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction. Zhu et al (Zhu et al., 2020b) studied 9 new babies delivered by COVID-19-confirmed women and found 6 were born premature, 2 were small-for-gestational infants and 1 was large-for-gestational infant and 6 had a pediatric critical illness score less than 90, which indicates that perinatal COVID-19 infection may have adverse effects on newborns. But in our study, only four babies were treated for premature delivery and the other seven babies were observed in an isolated NICU suite. Moreover, we followed up the eleven newborns and found that they did not exhibit respiratory distress, fever, feeding intolerance, vomiting or death up to the present time. The difference of results reported here from other publications may be simply due to the small number or other relative risk factors. Although all ten pregnant women tested COVID-19 positive, 5 of these newborns tested negative. This result is consistent with the latest research (Chen et al., 2020, Zhu et al., 2020b) and past studies of SARS (Lam et al., 2004, Wong et al., 2004). These data do not support the possibility of vertical transmission of CoVs infection but the small sample size suggests caution in making this assumption.
5. Limitation
There are several limitations in our study. First, the sample size was small, collected from non-designated hospital of COVID-19. Second, we did not take throat swab samples from the two newborns delivered vaginally to exclude the possibility of spreading COVID-19 via vaginal delivery. It should be noted also that the diagnosis of COVID-19 in puerpera were 2 days after natural delivery. Third, the samples such as placenta, amniotic fluid and cord blood were not collected for COVID-19 test.
6. Conclusion
During the outbreak period of COVID-19 in Wuhan, a series of cases of COVID-19 were reported in pregnant women at non-designated hospitals. They were reported here as having mild symptoms or no symptom. Therefore, it is important to undertake chest CT to detect asymptomatic during latent period so as to reduce the transmission of COVID-19 infection among pregnant women. Also COVID-19 infection is not necessarily an indication for CD.
7. Declarations
7.1. Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province. (Record number: [2020]IEC(XM002)). All parturient women with COVID-19 had signed informed consent to publish.
7.2. Availbility of data and materials
All data are publicly available or listed in the results of the paper
7.3. Funding this research
No funding is associated with this report.
7.4. Dislaimer
No funding agencies had role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; review, or approval of the manuscript; or decision to submit the manuscript for publication.
7.5. Conflict of Interests
The authors declare that they have no competing interests.
Authors’ Contributions
Conceptualization: Dongmei Cao, Heng Yin, Jun Chen, Yun Zhao, Guoqiang Sun. Date collection: Dongmei Cao, Heng Yin, Fei Tang, Min Peng, Ruobing Li, Xiaoying Wei. Date analysis:Dongmei Cao, Heng Yin, Hui Xie, Visualization: Heng Yin, Yun Zhao. Writing-original draft, Dongmei Cao, Heng Yin, Jun Chen, Writing -review and editing: Yun Zhao, Guoqiang Sun.
Contributor Information
Dongmei Cao, Email: 237544804@qq.com.
Heng Yin, Email: yinheng@hbfy.com.
Jun Chen, Email: 953208528@qq.com.
Fei Tang, Email: tangfei87169226@163.com.
Min Peng, Email: pm19751023@163.com.
Ruobing Li, Email: 1175978442@qq.com.
Hui Xie, Email: 1165557196@qq.com.
Xiaoying Wei, Email: rosewxy1986@163.com.
Yun Zhao, Email: zhao020060@163.com.
Guoqiang Sun, Email: sunguoqiang@hbfy.com.
References
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. PMID: 31978945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. PMID: 32134861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes A.K., Heim A. Rapid random access detection of the novel SARS-coronavirus-2 (SARS-CoV-2, previously 2019-nCoV) using an open access protocol for the Panther Fusion. J Clin Virol. 2020;125:104305. doi: 10.1016/j.jcv.2020.104305. MID: 32143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China . 2020. The latest situation of novel coronavirus pneumonia as of 24:00 on 23 February. http://www.nhc.gov.cn/xcs/yqtb/202004/bbaa7ee15b214fef9b6d2c90d75e8547.shtml. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. PMID: 31986264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. PMID: 32151335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. PMID: 32154135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr Pathol. 2020;2:1–5. doi: 10.1080/15513815.2020.1747120. PMID: 32238084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122. PMID: 32244166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China. The National Health Commission of China. New coronavirus pneumonia prevention and control program (4th edition). February 07, http://www.nhc.gov.cn/jkj/s3577/202002/573340613ab243b3a7f61df260551dd4.shtml.
- WHO . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. Jan 17. https://www.who.int/publication-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. [Google Scholar]
- Health Commission of Hubei Province . 2020. Public Announcement on Designated Hospitals for Treatment of Pneumonia Infected by Special Population of COVID-19 in Hubei Province. Feb 02. http://www.hubei.gov.cn/zhuanti/2020/xgfyyqfkzszq/zclxx/msbz/202003/t20200305_2173022.shtml. [Google Scholar]
- Yu M., Stevens V., Berry J.D., Crameri G., McEachern J., Tu c. Determination and application of immunodominant regions of SARS coronavirus spike and mucleocapsid proteins recognized by sera from different animal species. J Immunol Methods. 2008;331:1–12. doi: 10.1016/j.jim.2007.11.009. PMID: 18191140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasiyh Chafekar, Fielding Burtram C. Mers-cov: understanding the latest human coronavirus threat. Viruses. 2018;10:93. doi: 10.3390/v10020093. PMID: 29495250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S. Epidemic and Emerging Coronaviruses (Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome) Clin Chest Med. 2017;38:71–86. doi: 10.1016/j.ccm.2016.11.007. PMID: 28159163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019:11. doi: 10.3390/v11010059. pii: E59. MID: 30646565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.A., Graham A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses. 2020:12. doi: 10.3390/v12020194. Pii: E194.PMID: 32050635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C.M., Wong S.F., Leung T.N., Chow K.M., Yu W.C., Wong T.Y. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774. doi: 10.1111/j.1471-0528.2004.00199.x. PMID: 15270922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS)Available online: https://www.who.int/csr/sars/en/WHOconsensus.pdf.
- Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. PMID: 15295381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar I., Madani E., Ntoumi T.A., Kock F., Dar R.O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J of Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. PMID: 31953166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T.C., Sherman I.L., Serfling R.E. Observations on excess mortality associated with epidemic influenza. JAMA. 1961;176:776–782. doi: 10.1001/jama.1961.03040220024005. PMID:13726091. [DOI] [PubMed] [Google Scholar]