Abstract
Objectives
The 2017 Workshop of the Society for Hematopathology/European Association for Haematopathology aimed to review clinical cases with germline predisposition to hematolymphoid neoplasms.
Methods
The Workshop Panel reviewed 51 cases with germline mutations and rendered consensus diagnoses. Of these, six cases were presented at the meeting by the submitting pathologists.
Results
The cases submitted to the session covering germline predisposition included 16 cases with germline GATA2 mutations, 10 cases with germline RUNX1 mutations, two cases with germline CEBPA mutations, two germline TP53 mutations, and one case of germline DDX41 mutation. The most common diagnoses were acute myeloid leukemia (15 cases) and myelodysplastic syndrome (MDS, 14 cases).
Conclusions
The majority of the submitted neoplasms occurring in patients with germline predisposition were myeloid neoplasms with germline mutations in GATA2 and RUNX1. The presence of a germline predisposition mutation is not sufficient for a diagnosis of a neoplasm until the appearance of standard diagnostic features of a hematolymphoid malignancy manifest: in general, the diagnostic criteria for neoplasms associated with germline predisposition disorders are the same as those for sporadic cases.
Keywords: Germline predisposition, GATA2, RUNX1, CEBPA, Molecular genetics, Targeted therapy, Hematolymphoid neoplasia, MDS, AML
Myeloid neoplasms generally present sporadically in older adult populations. With increasing use of molecular profiling, an evolving number of inherited cancer predisposition syndromes have now been described. The diagnosis of a myeloid neoplasm with genetic predisposition dictates a different approach for clinical management of the affected patient, impacting donor selection for stem cell transplantation, genetic counseling, and disease surveillance. The revised fourth edition (2016) World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues1 has included myeloid neoplasms with germline predisposition syndromes as a provisional category. The 2017 Workshop of the Society for Hematopathology/European Association for Haematopathology (SH/EAHP) included a session dedicated to these germline predisposition syndromes (session 1) and a total of 51 cases were submitted to this session. The diagnoses and genetic features of the cases from session 1 are summarized in Table 1, Table 2, Table 3, and Table 4 and presenting symptoms, clinical history, and family history in Table 5. The majority of these cases (42 cases) comprised myeloid neoplasms, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), with fewer cases of mixed myelodysplastic/myeloproliferative neoplasms (MDS/MPN) such as chronic myelomonocytic leukemia (CMML), and myeloproliferative neoplasm (MPN) such as essential thrombocythemia (ET). Lymphoid neoplasms with germline mutations submitted to this session included three cases of B-lymphoblastic leukemia (B-ALL) as well as one case each of Burkitt lymphoma, follicular lymphoma, and T-cell large granular lymphocytic leukemia. The remaining cases involved patients presenting with thrombocytopenia (six cases), bone marrow and immunodeficiency disorder in patients with GATA2 deficiency (two cases), and thrombocytosis with germline MPL mutation. Overall, the most common cases submitted were those involving germline mutations in GATA2 (16 cases) and RUNX1 (10 cases). Other germline mutations present in the submitted cases were CEBPA, DDX41, TP53, AKRD26, and PTPN11. The 2016 WHO classification1 subdivides myeloid neoplasms with germline predisposition into three main categories: those associated with preexisting platelet disorders (including RUNX1, ANKRD26, and ETV6 mutations); those associated with other organ dysfunction (including GATA2 mutation, bone marrow failure syndromes, Noonan syndrome, neurofibromatosis, and telomere biology disorders such as dyskeratosis congenita); and those without a preexisting platelet disorder or organ dysfunction (including DDX41 and CEBPA mutations). Because germline mutated GATA2 cases represented the largest number of submitted cases to this workshop, this entity will be discussed first, followed by other mutations associated with germline predisposition to MDS and AML, germline mutations associated with platelet disorders, and finally germline mutations associated with MPN and lymphomas.
Table 1 .
Features of Cases Submitted With Germline GATA2 Mutation
| GATA2 Mutation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Age, y | Sex | Genomic | Protein | Type | Familial or Sporadic | Infections | Other | Prior Disease | PB Cytopenias | Bone Marrow Diagnosis | Karyotype | NGS Additional Mutations |
| 20 | 18 | M | c.821delC | p.274fs | FS PS | F | NR | Family history of solid tumors | No | P, blasts | AML-MRC | –7, +8, add | NR |
| 40 | 17 | M | c.1081C>T | p.R361C | M ZF2 | NR | NR | Presented post MVA | No | Monocytosis | CMML | –7 | ASXL1 R693X, KRAS G12V, STAT3 S614R |
| 48 | 6 | M | c.610C>T | p.Arg204* | PS | F | NR | Megakaryoblasts | No | P | AML-MRC | –7 | WT1 |
| 52 | 22 | F | c.1114G>A | p.A372T | M ZF2 | S | PNA, cellulitis, cervical HPV | Lymphedema | Yes | P | MDS-EB2 | –7 | NR |
| 87 | 5 | M | c.1143 + 200_1198del | Lg Del | NR | cellulitis, warts | Lymphedema | Yes | N, B-NK | RCC | N | ASXL1 | |
| 105 | 57 | M | c.1085G>A | p.Arg362Gln | M ZF2 | F | NR | Patient was donor for brother with AML 20 y prior | Yes | P | MDS-MLD | N | NR |
| 138 | 13 | F | c.16delG | p.6fs | FS | S | NR | 2-y prior history of anemia | Yes | A, N | MDS-MLD | N | NR |
| 157 | 45 | F | c.1045T>C | p.C349R | M ZF2 | NR | HPV-carcinomas | Onset age 16, prior diagnosis of AA | Yes | P | MDS-MLD | +21 | NR |
| 176 | 10 | F | c.154_179del, | p.H51fs* | FS PS | NR | NR | Lymphedema, onset age 6 | Yes | N | MDS-EB1 | –7 | ASXL1, NRAS |
| 236 | 30 | M | c.706A>G | p.Met236Val | M VUS | F | NR | BT, pure erythroid leukemia | Yes, BT | P | AML-MRC | Complex | NR |
| 258 | 62 | F | c.1193G>A | p.R398Q | M ZF2 | NR | MAI | 0nset 52 y cytopenias | Yes | A, Mo, L | HLHBMID | +8 | NR |
| 266 | 16 | F | c.521del | p.174fs | FS | NR | NR | h/o MDS 1 y prior | Yes | P | AML-MRC | N | FANC |
| 275 | 44 | F | c.1192C>T | p.R398W | M ZF2 | F | Pulmonary aspergillosis, MAI, cervical HPV | Mother died colon cancer 31 y; sister died 36 y infection; AFB + marrow | Yes | T, Mo, L | BMID | N | STAG2 p.R614X |
| 307 | 12 | M | 3.1-3.3 Mb deletion; nt 128198265-128212030 | Lg Del | S | PNA | No | A,N,Mo, rare Blasts | RCC | –7 | CRLF2 p.N63S | ||
| 337 | 31 | F | c.1017 + 572C>T | R/I | F | Pannicullitis, PNA, HPV, MAI | Granulomata in marrow | Yes | P, Mo, B, NK | MDS-MLD | +8 | NR | |
| 381 | 17 | F | c.1084C>T; | p.R362* | PS ZF2 | NR | NR | No | A, N | RCC | Der(1;7), +8 | NR | |
A, anemia; AA, aplastic anemia; AFB, acid-fast bacilli; AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; B, B-lymphopenia; BMID, bone marrow and immunodeficiency disorder; BT, β-thalassemia; cellu, cellulitis; cerv, cervical; CMML, chronic myelomonocytic leukemia; F, familial; FS, frameshift; HLH, hemophagocytic lymphohistiocytosis; HPV, human papillomavirus; L, lymphopenia; Lg Del, large deletion; M, missense; Mo, monocytopenia; MAI, Mycobacterium avium-intracellulare; MDS-EB1, myelodysplastic syndrome with excess blasts I; MDS-EB2, myelodysplastic syndrome with excess blasts II; MDS-MLD, myelodysplastic syndrome with multilineage dysplasia; MVA, motor vehicle accident; N, neutropenia; NGS, next-generation sequencing; NK, NK-lymphopenia; NR, not reported; P, pancytopenia; PNA, pneumonia; PNA, pneumonia; PS, premature stop; RCC, refractory cytopenia of childhood; R/I, regulatory/intronic; S, sporadic; T, thrombocytopenia; VUS, variant of unknown significance; ZF2, zinc finger 2.
Table 2 .
Myeloid Neoplasms With Germline Predisposition Syndrome
| Case No. | Panel Diagnosis | Pathogenic Genetic Alterations |
|---|---|---|
| Acute Myeloid Leukemia (AML) | ||
| 230 | AML with biallelic mutations of CEBPA (one germline, one somatic) | CEBPA p.Q41fs (G) |
| CEBPA p.K313dup | ||
| WT1 p.V379fs*72 | ||
| 283 | AML with biallelic mutations of CEBPA (one germline, one somatic) | CEBPA p.E57 (G)* |
| 234 | AML with inv(3)(p21:3q26.2); GATA2, MECOM [with germline constitutional t(8;21)] | RUNX1 p.L219* |
| FLT3-ITD | ||
| ASXL1 p.G646fs | ||
| inv(3)(p21:3q26.2) | ||
| 38 | AML with mutated RUNX1 (germline) | RUNX1 p.Y260*(G) |
| 284 | AML with mutated RUNX1 (possibly germline) | RUNX1 c.351 + 1G>C (G) |
| NRAS p.G12D | ||
| BCOR p.K839fs | ||
| t(2;21)(q23;q22) | ||
| 264 | AML MRC (in a patient with Maffucci syndrome with mosaic IDH1 mutation) | IDH1 p.R132C |
| NRAS p.G13A | ||
| WT1 p.R445Q | ||
| 253 | AML MRC with germline BLM mutation | BLM p.Y736Lfs (G) |
| NF1 p.I679Dfs | ||
| WT1 c.616 + 1dup | ||
| 20 | AML MRC with germline GATA2 mutation | GATA2 c.821del(G) |
| 48 | AML MRC with germline GATA2 mutation | GATA2 p.R204* (G) |
| WT1 p.P376fs* | ||
| WT1 p.A314fs*4 | ||
| JAK2 p.V617F | ||
| CSF3R p.Y814* | ||
| KRAS p.G12D | ||
| 236 | AML MRC with germline GATA2 mutation | GATA2 p.M236V (G) |
| 266 | AML MRC with germline GATA2 mutation | GATA2 p.P174fs (G) |
| 318 | AML, NOS with germline DDX41 mutation | DDX41 p.M1? (G) |
| 55 | AML, NOS with germline NF1 mutation | NF1 p.L380R (G) |
| 225 | Therapy-related AML [myeloid sarcoma, with t(8;16)(p11:2;p13.3);KAT6A-CREBBP] with germline 9q22.32-9q31.1 microdeletion of unknown significance | KAT6A-CREBBP |
| PTCH TGRB1 microdeletion (G) | ||
| 167 | Therapy-related AML with germline TP53 mutation (Li-Fraumeni syndrome) | TP53 p.E204Sfs*43 |
| Myelodysplastic Syndrome (MDS) | ||
| 176 | MDS-EB1 with germline GATA2 mutation | GATA2 p.H51fs* (G) |
| PTPN11 p.A72T | ||
| ASXL1 p.D879fs* | ||
| NRAS p.G12D | ||
| NRAS p.G13D | ||
| 52 | MDS EB-2 with germline GATA2 mutation | GATA2 p.A372T (G) |
| 170 | MDS EB-2 with germline RBM8A mutation | RBM8A mutation (G) |
| 196 | MDS MLD with germline G6P3 mutation | G6PC3 c.218 + 1G>A (G) |
| 105 | MDS MLD with germline GATA2 mutation | GATA2 p.R362Q (G) |
| 138 | MDS MLD with germline GATA2 mutation | GATA2 p.E6fs (G) |
| 337 | MDS MLD with germline GATA2 mutation | GATA2 c.1017 + 572C>T (G) |
| 157 | MDS MLD with germline GATA2 mutation | GATA2 p.C349R (G) |
| 39 | MDS MLD with germline RUNX1 mutation | RUNX1 p.Y189* (G) |
| 339 | MDS MLD with germline RUNX1 mutation | RUNX1 p.K194N (G) |
| 87 | RCC with germline GATA2 mutation | GATA2 c.365del (G) |
| ASXL1 p.T880Qfs*2 | ||
| 307 | RCC with germline GATA2 mutation | GATA2 3.1-3.3 Mb deletion (G) |
| 381 | RCC with germline GATA2 mutation | GATA2 p.R362*(G) |
| 80 | RCC with germline SAMD9 mutation | SAMD9 p.E1136Q (G) |
| Other Myeloid Neoplasms | ||
| 40 | CMML-1 with germline GATA2 mutation | GATA2 p.R361C (G) |
| KRAS p.G12V | ||
| ASXL1 p.R693* | ||
| KRAS p.K117N | ||
| NF1 p.I679fs*21 | ||
| SETBP1 p.I871T | ||
| STAT3 p.S614R | ||
| WT1 p.A382fs*4 | ||
| WT1 p.S381* | ||
| 273 | MDS/MPN, unclassifiable with germline SAMD9 mutation | SAMD9 p.R685Q (G) |
| SAMD9 p.C835R (G) | ||
| 42 | ET with germline SH2B3 mutation | SH2B3 p.D231fs (G) |
| FANCE p.K475R | ||
| EPHB1 p.S690N | ||
| AXIN2 p.R834Q | ||
| 292 | JMML in a patient with Noonan syndrome | PTPN11 p.T73I (G) |
CMML, chronic myelomonocytic leukemia; EB, excess blasts; ET, essential thrombocythemia; G, germline; JMML, juvenile myelomonocytic leukemia; MLD, multilineage dysplasia; MPN, myeloproliferative neoplasm; MRC, myelodysplasia related changes; NOS, not otherwise specified; RCC, refractory cytopenia of childhood.
Table 3 .
Lymphoid Neoplasms With Germline Predisposition Syndrome
| Case No. | Panel Diagnosis | Pathogenic Genetic Alterations |
|---|---|---|
| B-ALL | ||
| 194 | B-ALL with germline ELA2 mutation (congenital neutropenia) | ELANE (G) |
| 101 | B-ALL with germline PAX5 mutation | PAX5 p.G183S (G) |
| 99 | B-ALL with hypodiploidy in a patient with Noonan syndrome (germline SHOC2 mutation) | SHOC2 p.S2G (G) |
| Lymphoid neoplasms | ||
| 209 | Follicular lymphoma grade 1-2 of 3 in a patient with germline TP53 mutation (Li-Fraumeni syndrome) | TP53 p.R158H (G) |
| 106 | Burkitt lymphoma in a patient with X-linked lymphoproliferative syndrome (germline SH2D1A mutation) | SH2D1A c.163C>T (G) |
| 342 | T-cell large granular lymphocytic leukemia with pure red cell aplasia and with germline CTLA4 mutation | CTLA4 p.R51* (G) |
B-ALL, B-lymphoblastic leukemia; G, germline.
Table 4 .
Other Disorders With Germline Predisposition Syndromes
| Case No. | Panel Diagnosis | Pathogenic Alterations |
|---|---|---|
| 268 | Thrombocytopenia with germline ANKRD26 mutation | ANKRD26 c.-119C>G |
| 271 | Thrombocytopenia with germline RUNX1 mutation | RUNX1 exon 6 deletion |
| ATM K2253T | ||
| 309 | Thrombocytopenia with germline RUNX1 mutation | RUNX1 p.I366_G367dup |
| TET2 p.M533T | ||
| 364 | Thrombocytopenia with germline RUNX1 mutation | RUNX1 c.967 + 2_967 + 5delTAAG |
| 219 | Thrombocytopenia with germline RUNX1 variant of unknown significance | RUNX1 p.R207W |
| 275 | Bone marrow and immunodeficiency disorder (monoMAC syndrome) with germline GATA2 mutation | GATA2 p.R398W |
| STAG2 p.R614* | ||
| PALB2 p.?splice | ||
| 258 | Hemophagocytic lymphohistiocytosis in a patient with bone marrow and immunodeficiency disorder (MonoMAC syndrome) with germline GATA2 mutation | GATA2 p.R398Q (G) |
| 97 | Thrombocytosis with germline MPL mutation | MPL p.P106L (G) |
| 346 | 1: Classical Hodgkin lymphoma2: Congenital neutropenia (germline CSF3R variant of unknown significance) | CSF3Rp.R583C (G) |
| 320 | Transient myeloproliferative disorder in a patient with germline PTPN11 mutation (Noonan syndrome) | PTPN11 p.S502L |
| 200 | TAFRO (thrombocytopenia, ascites, fibrosis, renal dysfunction, organomegaly), a variant of multicentric Castleman disease | RUNX1 p.G87C |
| 333 | Constitutional t(12;18) of undetermined significance | t(12;18) |
G, germline.
Table 5 .
Myeloid Neoplasia Highlighting Key Clinical, Family History, and Morphologic Findings Associated With the Most Common Genetic Mutations in This Study
| Case No. | Diagnosis | Clinical History | Family History | Morphologic Findings |
|---|---|---|---|---|
| 230 | AML with biallelic mutations of CEBPA (one germline, one somatic) | Two-month history of increasing dyspnea on exertion, associated with a cough, fever, easy bruising, and weight loss | Daughter and younger brother with AML | Medium to large-sized blasts with irregular nuclear contour, fine chromatin, and prominent nucleoli |
| 283 | AML with biallelic mutations of CEBPA (1 germline, 1 somatic) | One-month history of bruising, chest pain, night sweats | None | Blasts with mostly round nuclei, occasionally indented nuclear contours, fine chromatin, large prominent nucleoli, and moderate amounts of cytoplasm containing occasional granules and Auer rods |
| 38 | AML with mutated RUNX1 (germline) | Easy bruising, with platelets in the low to normal range, but no significant excessive bleeding; presented with high fevers, hypotension, diffuse lymphadenopathy, and splenomegaly | Family history of platelet function defect; affected family members include her 2 older sisters, father, paternal aunt, paternal uncle, cousins, and paternal great-grandfather | Medium to large-sized blasts with high nuclear-to-cytoplasmic ratios, irregular nuclear contours, smooth chromatin, prominent nucleoli, and mild amounts of basophilic cytoplasm with Auer rods |
| 284 | AML with mutated RUNX1 (possibly germline) | History of thrombocytopenia since the age of 4 y, treated with steroids for presumptive ITP, without significant response | None | Predominance of myeloid blasts and small, hypolobated megakaryocytes |
| 39 | MDS MLD with germline RUNX1 mutation | Thrombocytopenia (dating back to the newborn period) and neutropenia in the setting of chronic idiopathic elevation in creatine kinase and myopathy of unclear etiology | Unknown | Hypercellular marrow with trilineage dysplasia and prominent dysmegakaryopoiesis |
| 339 | MDS MLD with germline RUNX1 mutation | Progressive thrombocytopenia and anemia and a life-long history of bruising and mild thrombocytopenia | Father with long-standing history of easy bruising and asymptomatic 4-y-old sister with mild thrombocytopenia | Hypercellular marrow with trilineage dysplasia, decreased megakaryocytes with frequent small hypolobated forms and 5% blasts |
| 20 | AML MRC with germline GATA2 mutation | Fatigue and weight loss | Family history of both solid tumors and hematologic malignancies, including colon cancer in his father and 5 paternal uncles, breast cancer in 3 paternal aunts, and leukemia in his sister and a paternal aunt | Intermediate to large in size blasts with open chromatin, round to slightly irregular nuclear contours, occasional prominent nucleoli, and scant cytoplasm; no cytoplasmic granules or Auer rods were seen |
| 48 | AML MRC with germline GATA2 mutation | Pancytopenia | None | Numerous blasts with abundant dysplastic megakaryocytes |
| 236 | AML MRC with germline GATA2 mutation | Pancytopenia, lymphadenopathy, and splenomegaly | Unknown | Hypercellular bone marrow with increased and slightly dysplastic erythroids, moderate reticulin fibrosis, and rare hemophagocytic histiocytes |
| 266 | AML MRC with germline GATA2 mutation | Pancytopenia with macrocytosis | Unknown | Large blasts with round to irregular nuclear contours, variably prominent nucleoli, and large amounts of cytoplasm; a subset of the blasts had cytoplasmic granules |
| 176 | MDS-EB1 with germline GATA2 mutation | Hemihypertrophy developed right lower extremity lymphedema over several years now presented with high WBC count, anemia, and thrombocytosis. | None | Hypercellular marrow with increased megakaryocytes showing prominently separated nuclear lobes; erythroid elements exhibited megaloblastic features with irregularly shaped nuclei; myeloid cells exhibited left-shifted maturation with abnormal nuclear lobation |
| 52 | MDS EB-2 with germline GATA2 mutation | Pancytopenia and recent multiple infections including pneumonia, bladder and sinus infections, and cellulitis; medical history notable for occasional left lower extremity edema and human papillomavirus of the cervix | None | Hypocellular bone marrow with 16% blasts, rare dysplastic erythroid forms, and left-shifted myeloid elements with hypolobation and hypogranulation and absent megakaryocytes |
| 105 | MDS MLD with germline GATA2 mutation | Cytopenias | Brother with AML | Hypercellular bone marrow with trilineage dysplasia along with <15% ring sideroblasts and 2.6% marrow blasts without identifiable Auer rods |
| 138 | MDS MLD with germline GATA2 mutation | 2-year history of anemia of unknown origin | None | Bone marrow aspirate showed trilineage dysplasia and <5% blasts |
| 337 | MDS MLD with germline GATA2 mutation | Sudden onset of fatigue at 21 and was found to be pancytopenic | Maternal family history is significant for MDS and warts; patient's sister has mild cytopenias | Hypocellular bone marrow with trilineage dysplasia and <5% blasts; granulomas seen at later time point |
| 157 | MDS MLD with germline GATA2 mutation | History of worsening cytopenias diagnosed at age 16, ultimately progressing to transfusion dependence and aplastic anemia | None | Hypercellular marrow with increased ratio of myeloid cells to erythroid cells, abnormal erythroid maturation including atypical forms; left-shifted and dysplastic myelopoiesis, rare hypolobated megakaryocytes; markedly increased iron stores with rare ringed sideroblasts |
| 87 | RCC with germline GATA2 mutation | Recurrent group A streptococcal lymphangitis/cellulitis, streptococcal bacteremias, lymphangiectasis in the right thigh region; HPV-driven warts, mainly involving the fingers and the toes; recurrent scrotal and penile lymphedema | None | Hypocellular bone marrow with relative erythroid predominance, left-shifted granulocytes with toxic appearance, no increase in blasts or hemophagocytosis, occasional abnormal megakaryocytes (small, monolobated and widely separated nuclei) |
| 307 | RCC with germline GATA2 mutation | 3 days of fever, cough, and a diagnosis of pneumonia; CBC showed macrocytic anemia, leukopenia with neutropenia, monocytopenia, and rare circulating blasts | None | Hypocellular marrow with dysplasia |
| 381 | RCC with germline GATA2 mutation | May-Thurner syndrome and pancytopenia; initially felt to have megaloblastic anemia due to folate deficiency | None | Hypocellular marrow with megaloblastic changes and dyserythropoiesis |
| 40 | CMML-1 with germline GATA2 mutation | Motor vehicle accident resulting in postoperative course complications | None | Hypercellular marrow with dysplasia and monocytosis |
AML, acute myeloid leukemia; HPV, human papillomavirus; ITP, immune thrombocytopenia; MDS, myelodysplastic syndrome; MLD, multilineage dysplasia; MRC, myelodysplasia related changes; RCC, refractory cytopenia of childhood.
Germline Predisposition Associated With Mutated GATA2
GATA2, located on chromosome 3q21, encodes a zinc finger transcription factor that is critical for hematopoiesis,2 and maintenance of hematopoietic stem cells.3 Depending on the isoform, it consists of six or seven exons and its transcription is initiated from the first two exons. GATA2 protein contains two highly conserved zinc finger domains (C-ZnF and N-ZnF) responsible for its DNA-binding ability and interaction with other proteins.3 Additionally, other nonfinger domains are distinguished: two transactivation domains, a nuclear localization signal, and a negative regulatory domain.4 Germline heterozygous autosomal dominant and sporadic mutations in GATA2 result in haploinsufficiency4 and are the underlying genetic cause of a number of previously described syndromes with overlapping features, including monocytopenia and Mycobacterium avium complex (monoMAC)5,6 MDS EB2; dendritic cell, monocyte, B and NK lymphoid deficiency (DCML)7; Emberger syndrome8; and familial MDS/AML.9 More recently, it has been shown that germline mutations in GATA2 are present in up to 7% of primary pediatric MDS and 37% of pediatric MDS with monosomy 7.10 Patients with germline GATA2 mutations may also present with chronic neutropenia11 or with features overlapping between aplastic anemia and hypoplastic MDS.12 Overall disease penetrance is high, and it has been estimated that individuals harboring germline GATA2 mutations have a 90% risk of clinical complications by age 60,13 with an estimated 50% to 75% developing myeloid neoplasia.14,15 The phenotype and age at presentation are variable. In one large study, the median age at presentation was 20 years with a broad range of 4 to 78 years.14 However, the most common feature of all reported cohorts is the high propensity to develop myeloid neoplasms, particularly MDS, AML, and CMML. For this reason, the syndromes and entities involving germline mutations in GATA2 are now referred to as a single protean disorder termed GATA2 deficiency.13-16
A total of 16 cases with germline GATA2 mutations were submitted to the 2017 SH workshop. The age at the time of initial presentation, family history, clinical history of immunodeficiency, infections, cytopenias, and type of myeloid neoplasm varied among the cases (Table 1). The age range was 5 to 62 years (median 17.5 years), with seven pediatric cases (under the age of 18 years). The male:female ratio was 7:9. There were four cases of AML, two cases of MDS with excess blasts (MDS-EB), four cases of MDS with multilineage dysplasias (MDS-MLD), three cases of refractory cytopenia of childhood (RCC), and one case of CMML. Two cases (cases 258 and 275) displayed abnormal bone marrow pathology but did not meet formal WHO criteria for a diagnosis of MDS or any other myeloid neoplasm and were diagnosed as bone marrow and immunodeficiency disorder with germline GATA2 mutation. In nine cases, patients presented with a history of infections and/or cytopenias preceding the development of myeloid neoplasm (MDS/AML, [case 52], RCC [cases 87 and 381], AML [case 266], RCC [case 381], and other MDS subtypes [cases 105, 138, 157, 176, and 337]). In five cases, patients presented with a myeloid neoplasm without any recognized preceding period of cytopenias or infections (AML [cases 20 and 48], CMML [case 40], RCC [case 307], and MDS with hemophagocytic syndrome [case 236]).
Cellular depletion and immunodeficiency with loss of monocytes, B cells, and NK cells is a feature often seen in GATA2 deficiency prior to the development of overt myeloid neoplasia.14,16 The presenting phenotypic of immunodeficiency and severe infections was reported in six submitted cases. There were three cases with infections involving Mycobacterium avium complex (cases 258, 275, and 337), with granulomata in the bone marrow and acid-fast bacilli-positive organisms in case 275 Image 1A and Image 1B. Warts and severe human papillomavirus (HPV) infection, as previously noted in monoMAC, DCML, and Emberger syndromes,14,17 were reported in four submitted cases, including HPV-related vulvar and/or anal carcinoma (cases 157 and 175), cervical HPV resulting in hysterectomy (case 275), epidermal dysplasia verruciformis (case 157), and warts (case 337). Cellulitis and panniculitis are disease manifestations of GATA2 deficiency18 and were reported in at least three submitted cases (cases 52, 87, and 337). Pulmonary infections were present in four cases (case 275 [pulmonary aspergillosis], 157 [Klebsiella], 307, and 337). Lymphedema was noted in cases 52 and 87. Scattered hemophagocytic cells are often seen in the bone marrow of patients with GATA2 deficiency19 without clinical evidence of hemophagocytic lymphohistiocytosis (HLH). However, severe secondary HLH can be a serious complication in the setting of GATA2 deficiency,20 as demonstrated in cases 258, 236, and 275 Image 1C. Other manifestations of GATA2 deficiency reported in the literature that were not represented in the cases submitted include pulmonary alveolar proteinosis21 and complications of Epstein-Barr virus (EBV) infection.22 While monocytopenia is a common feature of the monoMAC and DCML phenotypes, GATA2 deficiency patients can develop monocytosis with disease progression to CMML or MDS/MPN,23 or may present initially with monocytosis due to CMML, as reported in case 40.
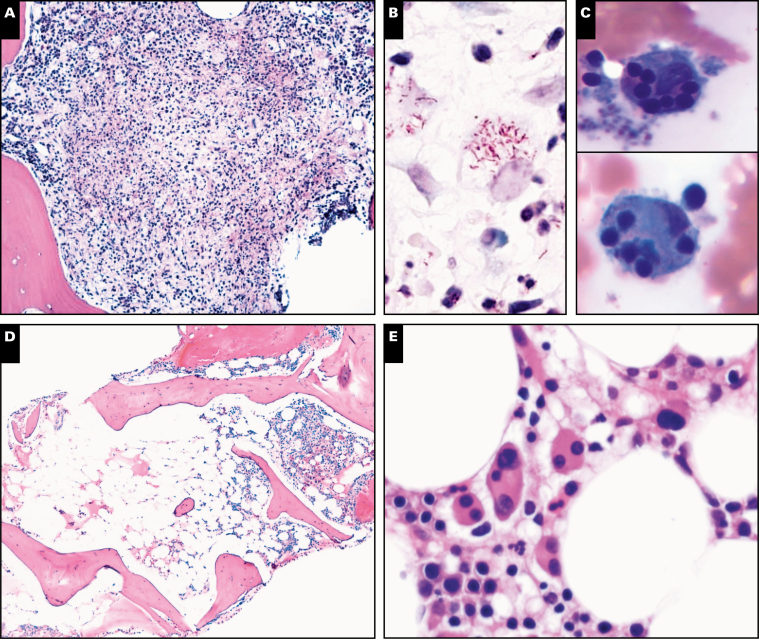
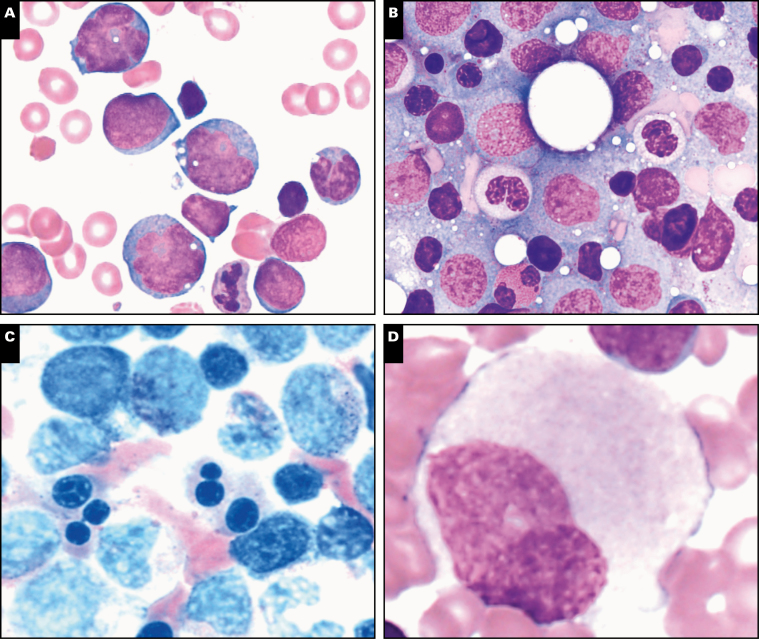
Image 1 .
Spectrum of bone marrow features in GATA2 deficiency, part 1. A subset of patients have bone marrows with granulomatous inflammation (A) and acid-fast bacilli-positive organisms (B), as in this patient with Mycobacterium avium-intracellulare infection (case 275). Secondary hemophagocytic lymphohistiocytosis with hemophagocytic macrophages (C) is a severe complication of GATA2 deficiency and was reported in case 258. Myelodysplasia in the setting of germline GATA2 mutation can present with hypocellular/hypoplastic bone marrow, as reported in half of submitted GATA2 cases (D, case 52). Megakaryocytic atypia and dysplasia is a common feature seen on core biopsies and aspirates of GATA2 marrows, as demonstrated in case 307 (E). Cases 307 (F and G) and 20 (H).
Myelodysplasia in GATA2 deficiency is often hypoplastic, as seen in half of the submitted cases Image 1D. Atypical/dysplastic megakaryocytes with separated nuclear lobes and micromegakaryocytes are a common feature in the bone marrow from patients with GATA2 deficiency and may precede the development of overt MDS.19 Atypical/dysplastic megakaryocytes were present in 12 of 16 (75%) of the submitted cases Image 1E, Image 1F, Image 1G, and Image 1H, including some cases with very large giant megakaryocytes with abnormal detached nuclei Image 2A. Dysplastic features were also common in erythroid Image 2B and Image 2C and myeloid lineages Image 2D and Image 2E. A subset of cases showed normocellular or hypercellular marrows, particularly those with advanced myeloid neoplasms such as MDS-EB Image 2F and Image 2G, AML Image 2H, CMML, or those with granulomatous inflammation (Image 1A). In the setting of pancytopenia, some GATA2 marrows are markedly hypocellular (less than 10% cellularity) and these cases may meet criteria for a diagnosis of aplastic anemia (AA), as indicated in the clinical history for case 157. The distinction between idiopathic AA and GATA2 deficiency is important for proper patient management, as GATA2 deficiency patients may have a poor outcome when treated with immunosuppression. Bone marrow flow cytometry analysis12 can be helpful in identifying patients with pancytopenia and hypocellular marrows that may harbor GATA2 mutations, while awaiting genetic sequencing results: patients with GATA2 deficiency often show a disproportionate loss of, or even absent, bone marrow B cells and B-cell precursors,24 NK-cells, plasmacytoid dendritic cells, and monocytes in comparison to AA and control patients.12 Those presenting with hypocellular marrows without overt dysplasia may progress to overt MDS as demonstrated in case 157, which showed progression to MDS in a GATA2-deficient patient previously diagnosed with AA. Patients with GATA2 deficiency who develop MDS may subsequently progress to overt AML, as demonstrated in cases 236 and 266. The majority of cases with increased blasts reported blasts with a myeloid phenotype expressing CD34, CD117, and MPO, with or without aberrant expression of CD7. Two notable exceptions included case 48, AML with myelodysplasia-related changes (MRC) with megakaryoblasts that were positive for CD61 and negative for CD34; and case 236, pure erythroid leukemia with blasts that expressed E-cadherin and glycophorin A, and were negative for CD34 and CD117.
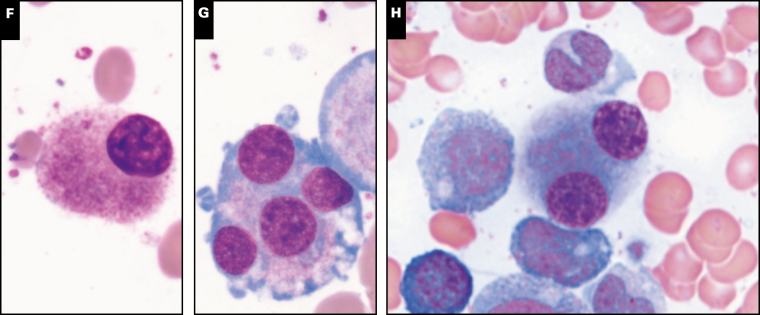
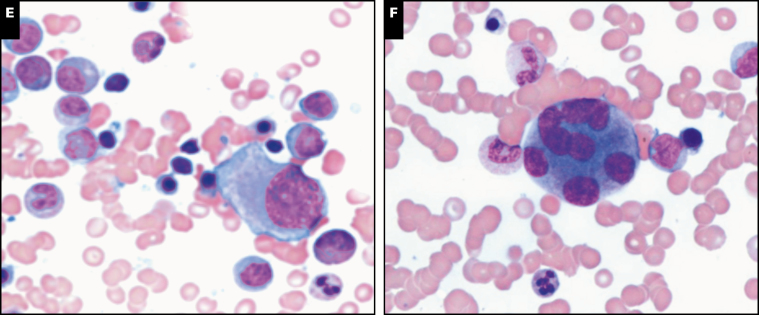
Image 2 .
Spectrum of bone marrow features in GATA2 deficiency, part 2. Very large giant abnormal megakaryocytes with detached nuclei can often be seen on aspirate smears as shown in case 138 (A). Multilineage dysplasia, with dyserythropoiesis (B, C, case 105) and dysmyelopoiesis (D, case 52; and E, case 105) is common in GATA2 deficiency-associated myelodysplastic syndrome (MDS). High-grade MDS and acute myeloid leukemia (AML) are often associated with hypercellularity as demonstrated in case 176 diagnosed as MDS-EB2 with a hypercellular marrow with marked megakaryocytic dysplasia (F). Increased CD34-positive blasts by immunohistochemistry (G), and case 20 diagnosed as AML with 80% cellularity on the core biopsy (not shown) and sheets of myeloblasts on the aspirate smear (H).
Germline mutations in GATA2 comprise pathogenic variants in exonic and in intronic/regulatory regions of the gene.10,14 Exonic missense mutations frequently involve the second zinc finger domain and were present in six cases (Table 1). Frameshift mutations and/or mutations resulting in premature stop codons were reported in six cases. There was one mutation involving the regulatory region in intron 5, and two cases with large deletions. One case reported a GATA2 variant of unknown significance involving a missense change upstream of the zinc finger domains. Germline mutations in GATA2 result in haploinsufficiency and all appear to confer predisposition to myeloid malignancy; however, a greater incidence of viral infections and lymphedema is reported in individuals with large deletions, premature stop codons, or frameshift mutations.13 However, the age of disease onset, severity, and phenotype can vary widely in patients with germline GATA2 mutations, even among family members harboring the same mutation, and the phenotype is not a reliable predictor of mutation status. Ideally, it is important to confirm the germline nature of the GATA2 mutation, particularly in cases without family history of disease. Sequencing of cultured skin fibroblasts (as performed in case 157) is preferable to buccal swabs that may be contaminated with circulating peripheral blood cells harboring somatic mutations (which can be seen in the GATA2 gene in sporadic myeloid neoplasms).
Many patients with germline GATA2 mutations have family history of disease and mutations that are inherited. However, a significant number of GATA2 deficiency patients (up to 71%) harbor de novo rather than inherited germline mutations, which are not detected in family members.6,10 Cases 52, 138, and 307 documented de novo/sporadic GATA2 mutations, detected in the patient and not in the parents (including cases 52, 138, and 307). An increased incidence of solid tumors is also associated with GATA2 deficiency, with most related to underlying HPV or EBV infections; however, breast cancer, other solid tumors, and melanoma are also reported.14,25 Cases 20 and 275 reported a family history of solid tumors, some of which were diagnosed in the third decade.
GATA2 is highly expressed in immature hematopoietic cells and declines with blood cell maturation. It is essential for the maintenance and proliferation of hematopoietic stem cells by the complex interactions with a network of other hematopoietic transcription factors, including RUNX1, SCL/TAL1, MYB, GFI1, FLI1, LYL1, or PU.1.25 Balanced expression of GATA2 is essential for proper hematopoiesis and the disruption of its structure and/or activity can contribute to leukemogenesis. GATA2 overexpression is associated with development of AML and correlates with poor prognosis in this setting.26
Targeted next-generation sequencing myeloid sequencing panels were performed for many of the submitted cases (Table 1). Somatic acquired ASXL1 mutations have been previously reported in myeloid neoplasms with germline GATA2 mutations23,26 and were detected in several cases (cases 40, 87, and 176). Other genes with detected somatic variants/mutations included STAG2 (case 44), CRLF2 (case 307), WT1 (case 48), NRAS (case 176), and KRAS (case 40).
Hematopoietic stem cell transplantation is the only definitive cure for GATA2 deficiency-associated immunodeficiency and myeloid neoplasms (MDS/AML/CMML).27-30
However, care must be taken in selecting the donor, as illustrated in case 105 that describes a patient diagnosed with MDS-MLD with germline GATA2 mutation at the age of 57 years. This patient had been a healthy marrow donor for his brother who was diagnosed with AML 20 years prior. The brother also harbored the germline GATA2 mutation (assessed by sequencing archived bone marrow) and ultimately died posttransplant after receiving the GATA2 mutation-positive donor marrow. Further details are not provided, but this case shows two siblings with the same mutation that develop MDS/AML over 20 years apart, and underscores the need for screening all potential related donors for the patient’s GATA2 mutation prior to stem cell transplantation regardless of the state of health of the related donor.31
Noonan Syndrome
Noonan syndrome is a relatively common developmental disorder that is found in one of 2000 births. Patients with Noonan syndrome have characteristic dysmorphic features such as low-set, posteriorly rotated ears, down-slanting palpebral fissures, widely spaced nipples, and increased nuchal tissue.32 These patients have an increased risk of hematologic malignancy, including juvenile myelomonocytic leukemia (JMML) and acute lymphoblastic leukemia (ALL) among others.32 JMML is a myeloproliferative disorder of early childhood that originates from the multipotent hematopoietic stem cell and is characterized by overproduction of myeloid cells. Hyperactive RAS signaling is assumed to be the main driving event in JMML and is thought to be caused by somatic mutations in KRAS, NRAS, or PTPN11 in about 50% of patients.32 Case 292 reported a 49-day-old infant with Noonan syndrome who presented with mild hepatosplenomegaly and persistent leukocytosis (50-97 × 109/L) after treatment for an abscess. Peripheral blood showed neutrophilia and a monocytosis with 6% circulating blasts. Next generation sequencing identified a pathogenic heterozygous PTPN11 mutation, which in this setting is diagnostic of JMML associated with Noonan syndrome. Case 99 presented a 19-month-old child with Noonan syndrome with a more rare SHOC2 mutation who developed B-ALL.
Up to 10% of Noonan syndrome patients develop a JMML-like transient myeloproliferative disorder during the neonatal or early infantile period.32 This is morphologically indistinguishable from JMML but usually regresses spontaneously over months or years, as was seen in case 320. The treatment recommendation for transient myeloproliferative disorder in this setting is to monitor closely with consideration for only mild cytoreductive therapy in severe cases. The patient in case 320 was closely monitored without treatment and had clinically stable disease with resolving leukocytosis and monocytosis at 1 year.
Other Myeloid Neoplasms With Germline Predisposition Associated With Other Organ Dysfunction
Five AML cases that developed in individuals with other congenital disorders were submitted to the workshop, and these patients ranged in age from 4 months to 53 years (Table 2). Most of these cases were AML-MRC or therapy-related AML. Case 55 described a 4-month-old child who presented with splenomegaly, hepatomegaly, leukocytosis, and anemia and was found to have 29% myeloid blasts in bone marrow Image 3A. Next-generation sequencing targeting JMML-associated genes revealed NF1 mutation c.1139T>G p.Leu380Arg with an allele burden of 90% in bone marrow and 49% in buccal sampling, suggesting unrecognized neurofibromatosis type I (NF1) due to germline mutation in the NF1 that encodes neurofibromin.33 AML cases were submitted describing patients with other cancer predisposition syndromes including Bloom syndrome (case 253) and Maffucci syndrome (case 264). Cancer risk is generally high in patients with Bloom syndrome and is a leading cause of death; AML accounts for about 12.5% of all tumors.34 Maffucci syndrome is a rare nonhereditary disorder caused by somatic mosaic isocitrate dehydrogenase 1 or 2 (IDH1 or IDH2) mutations characterized by enchondromatosis with an increased risk for skeletal deformity and sarcomatous transformation, but also with rare AML cases reported.35
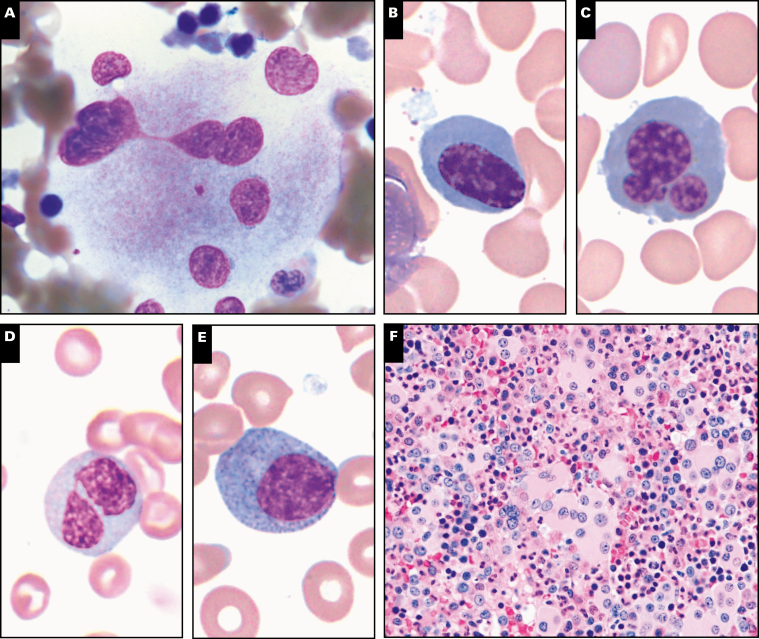
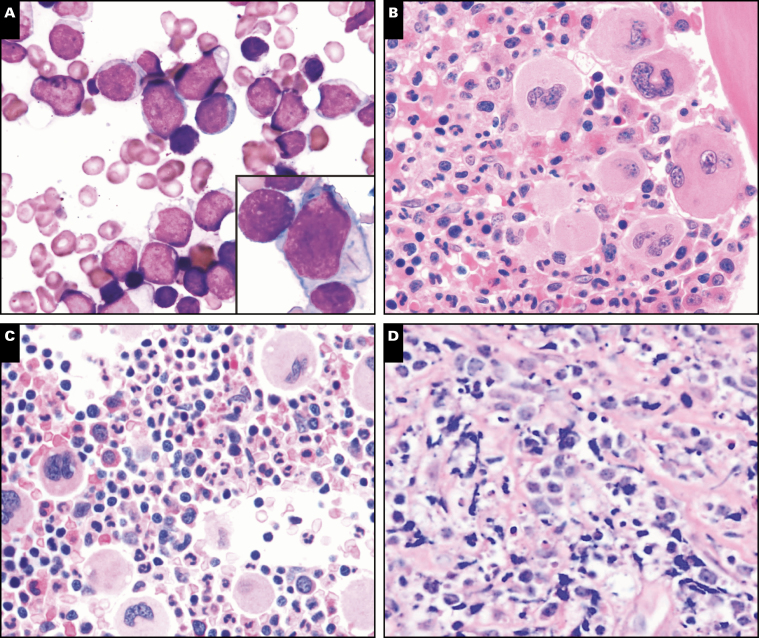
Image 3 .
Bone marrow morphology findings in germline predisposition syndromes. A, Acute myeloid leukemia not otherwise specified with germline NF1 mutation shows prominent monocytic differentiation (case 55). B, Prominent background dysplasia is seen in a case of therapy-related acute myeloid leukemia with germline TP53 mutation (Li-Fraumeni syndrome, case 167) C, Myelodysplastic/myeloproliferative neoplasm, unclassifiable with germline SAMD9 mutation (MIRAGE syndrome [myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy]). D, Small hypolobated megakaryocytes seen in case of thrombocytopenia with germline RUNX1 mutation (case 271). E, F, Abnormal megakaryocytes seen in a case of myelodysplastic syndrome with multilineage dysplasia with germline RUNX1 mutation including small size, hypolobation and separated nuclear lobes (case 39).
Case 167 described a 44-year-old woman with multiple malignancies who presented with AML with complex karyotype and germline TP53 mutation, representing a new diagnosis of Li-Fraumeni syndrome. Morphology showed numerous blasts in a background of prominent dysplastic erythroid cells and megakaryocytes Image 3B. Li-Fraumeni syndrome is a rare autosomal dominant syndrome that is characterized by a high incidence of a variety of malignant neoplasms (usually solid tumors, less frequently acute leukemias) within affected families.36,37 Case 170 described an 11 year old with thrombocytopenia absent radius (TAR) syndrome, an extremely rare congenital disorder involving biallelic loss of function of RBM8A (RNA binding motif protein 8A) gene, who developed MDS-EB while being treated for Langerhans cell histiocytosis. TAR syndrome is not generally associated with bone marrow failure or malignancy and development of myeloid neoplasms are only rarely reported in this syndrome.38
Two cases (case 80 and 273) were submitted that involved SAMD9 mutations, which have been recently associated with a clinical spectrum of disorders including the MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy) syndrome, ataxia-pancytopenia syndrome, and myelodysplasia and leukemia with monosomy 7 syndrome (Table 2). MIRAGE syndrome is a multisystem disorder associated with MDS and monosomy 7.39 Germline mutations in the related gene SAMD9L have also been reported in ataxia pancytopenia syndrome and in families with immune deficiencies, neurologic abnormalities, and MDS.40 Case 80 was RCC and case 273 was MDS/MPN, unclassifiable, both showing monosomy 7 on karyotype Image 3C.
Myeloid Neoplasms With Germline Predisposition and Preexisting Platelet Disorders
Runt-related transcription factor 1 (RUNX1) is a protein-coding gene located in 21q22.12, which encodes the DNA-binding subunit of the core-binding factor transcription complex that is essential for normal hematopoiesis.41RUNX1 point mutations were first identified in leukemia in 1999 in six families.41 Many subsequent studies documented frequent somatic mutations in RUNX1 in MDS, AML, ALL, and other myeloid neoplasms. Germline RUNX1 mutations are associated with familial platelet disorders with an increased risk of myeloid malignancy, and these patients have variable thrombocytopenia from birth, with or without bleeding tendencies.42 Decreased platelet aggregation to collagen and epinephrine may also be seen. A total of four cases submitted to the workshop showed germline RUNX1 mutation and thrombocytopenia (Table 2). These patients ranged in age from 3 to 37 years and one of these patients had an extensive family history of AML. Most cases showed some degree of morphologic dysplasia, which was overall insufficient for a diagnosis of MDS. In case 309, the RUNX1 mutation was an incidental finding discovered by targeted sequencing that eventually led to germline confirmation. Prior to the recognition of germline RUNX1 mutation familial platelet disorders, most of these patients were considered to have immune thrombocytopenia. Thus, it is important to consider the possibility of germline mutations in patients presenting with isolated thrombocytopenia, particularly if there is evidence of megakaryocytic atypia, such as seen in case 271 Image 3D. Patients with germline RUNX1 mutation have a lifetime risk of 35% to 44% of developing AML or MDS, although the risk depends on the specific RUNX1 mutation43,44 and likely requires acquisition of additional mutations. Three cases submitted to the workshop were AML and MDS arising in the setting of an underlying germline RUNX1 mutation and one of these cases (case 284) showed additional somatic NRAS and BCOR mutations (Table 2). Clinical findings and relevant family history are listed in Table 5. Prominent megakaryocytic dysplasia, including hypolobated megakaryocytes with eccentric nuclei and megakaryocytes with separated nuclear lobes, are often seen in cases of MDS with germline RUNX1 mutation Image 3E and Image 3F
A variety of RUNX1 mutations have been described throughout the gene, including frameshift or nonsense mutations. Germline RUNX1 mutations generally cluster in the N-terminal region, resulting in disruption of DNA binding, and are less frequent in the C-terminal region, which maintains DNA binding but lacks a functional transactivation domain.44 Often novel mutations are seen, such as in case 219, which showed a 37-year-old man with persistent isolated thrombocytopenia and a RUNX1 mutation that resulted in p.Arg207Trp amino acid change that had not been previously reported (Table 4).
Making a definitive diagnosis in patients with inherited thrombocytopenia is important because different forms of this disorder differ with respect to prognosis and treatment. Thrombocytopenia 2 is characterized by germline mutations in ANKRD26, located on chromosome band 10p12.1, and is an autosomal dominant disorder characterized by moderate thrombocytopenia and increased risk of developing MDS or AML.45 Case 268 is of a 43-year-old woman who presented with history of thrombocytopenia. Genetic testing on a skin culture specimen showed a sequence change in the 5′ untranslated region ANKRD26 c.-119C>G (Table 4). Of note, this is an overall exceedingly rare form of inherited thrombocytopenia.45
Myeloid Neoplasms With Germline Predisposition Without a Preexisting Disorder or Organ Dysfunction: CEPBA and DDX41 Mutations
Two cases (cases 283 and 230) were AML with germline CEBPA mutations, both with classic germline N-terminal CEBPA frameshift mutation and somatic C-terminal insertion mutation (Table 2 and Table 5).46 Both cases had CD7 expression on myeloblasts, often observed in these patients. Blasts in AML with germline CEBPA mutations often show Auer rods Image 4A. Both patients were in remission at the latest follow-up, consistent with reported favorable clinical outcomes seen in this entity. One submitted case of AML with germline DDX41 mutation (case 138) was in a 67-year-old man who had slowly decreasing peripheral blood counts and an extensive family history of leukemias. Unlike many other germline mutations, patients with germline mutations in DDX41 tend to present with myeloid neoplasms in adulthood, but with similar clinical course to other myeloid neoplasm presenting with similar genetics.47
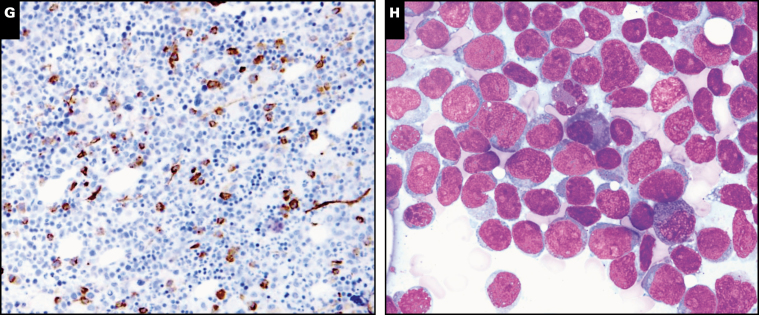
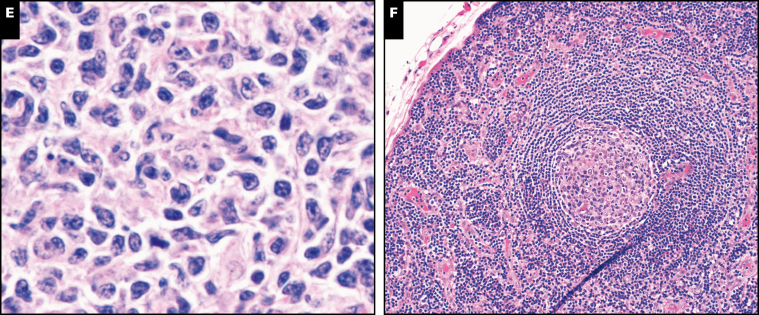
Image 4 .
Other neoplasms with germline predisposition syndromes. A, Blasts in acute myeloid leukemia with germline CEBPA mutations often show Auer rods (case 230). Inset, prominent Auer rods. B, Proliferation of large atypical megakaryocytes in essential thrombocythemia with germline SH2B3 mutation (case 42). C, Increased number of mostly normal-appearing megakaryocytes in thrombocytosis with MPL mutation (case 97). D, Sheets of intermediate-sized lymphoid cells in Burkitt lymphoma (case 106) in a patient with X-linked lymphoproliferative disorder. E, Follicular lymphoma, grade 1 to 2 of 3, in a patient with germline TP53 mutation (Li-Fraumeni syndrome; case 209). F, TAFRO (thrombocytopenia, ascites, fibrosis, renal dysfunction, organomegaly), a variant of multicentric Castleman disease (case 200).
Other possible germline predisposition mutations without a prior phenotype included a therapy-related AML in a patient with germline PTCH TGRB1 microdeletion of unknown significance (case 225) and AML in a patient with a germline t(8;21) translocation encompassing the RUNX1 gene and somatic RUNX1 mutation (case 234). Case 234 is of a 53-year-old man who was found to have leukocytosis without other symptoms and no family history of hematologic disorders. The t(8;21) translocation encompassed the RUNX1 gene and was seen in all metaphases regardless of disease involvement, suggestive of a constitutional abnormality. Concurrent detection of a somatic RUNX1 loss-of-function mutation suggests a two-hit mechanism of RUNX1 loss.
Germline Predisposition to Myeloproliferative Neoplasms
The vast majority of myeloid neoplasms associated with the above germline predisposition conditions are MDS, MDS/MPN, or AML. However, rare examples of MPN can also be associated with germline mutations. Case 42 describes a 6-year-old girl with history of B-ALL, autoimmune hepatitis, and progressive splenomegaly since birth that was out of proportion to her liver disease. She underwent splenectomy; splenic infarcts were noted but otherwise histology was unremarkable. After splenectomy, the patient’s platelet count rose to 2,131 × 109/L within 9 days and remained elevated. Bone marrow biopsy showed atypical large megakaryocytes with hyperlobated nuclei, frequently occurring in clusters, features consistent with ET Image 4B. Testing confirmed the presence of a homozygous frameshift mutation in the SH2B3 gene. SH2B3 encodes an adaptor (LNK) that inhibits the JAK/STAT pathway and negatively modulates signaling of several cytokine receptors, including MPL. This mutation had been shown in vivo to increase JAK2/STAT3 phosphorylation and increase growth and proliferation, and SH2B3 knockout mice have been shown to have accelerated leukemogenesis.48 The germline loss-of-function variant in SH2B3 likely played a role in the development of ALL and MPN in this patient.
Case 97 was of a 6-year-old boy who presented for evaluation as a bone marrow donor candidate for his older sibling with relapsed classical Hodgkin lymphoma. He was noted to have thrombocytosis and bone marrow examination showed an increased number of megakaryocytes with normal morphology Image 4C. Whole-exome sequencing showed a germline homozygous MPL P106L activating mutation. This MPL variant, present in 6% of individuals of Arab ancestry, has been associated with thrombocytosis in the homozygous state and normal or mildly elevated platelet counts in the heterozygous state. Although definite myeloproliferative disorders have not yet developed in the families reported with this mutation, it may have an oncogenic potential, as the mutation appears to affect thrombopoietin binding to the MPL receptor.49,50 A 19 year old with longstanding neutropenia and recurrent multiple systemic infections since childhood, diagnosed with classical Hodgkin lymphoma and a germline CSF3R mutation, was identified (case 346). Although somatic CSF3R mutations are associated with the myeloid neoplasm chronic neutrophilic leukemia and leukocytosis, the germline mutations are associated with neutropenia, and the discovery of this mutation shed light on the etiology of the patient’s longstanding neutropenia and infections. Acquired somatic truncating mutations in CSF3R, analogous to those found in chronic neutrophilic leukemia, have been described in up to 30% to 40% of patients with severe congenital neutropenia concurrent with inherited, pathogenically relevant mutations such as ELANE or HAX1.51
Germline Predisposition to Lymphoid Malignancies
Lymphoid neoplasms can also develop in the setting of germline predisposition syndromes (Table 3). Case 106 reported a 6-year-old boy who presented with typical Burkitt lymphoma Image 4D and family history of lymphoma in siblings, which ultimately led to a diagnosis of X-linked lymphoproliferative disorder. X-linked lymphoproliferative disease type 1 (XLP1) is a rare primary immunodeficiency caused by the mutation in SH2D1A located on the long arm of the X chromosome. About one-third of XLP1 patients present with lymphoma, and of these up to 50% are Burkitt lymphoma.52 Case 101 and 194 were B-ALLs in young children arising in setting of germline PAX5 and ELANE mutations, respectively (Table 3). Case 342 described a 40 year old with recurrent respiratory and gastrointestinal infections who presented with T-cell large granular lymphocytic leukemia in association with a germline CTLA4 mutation. Morphologic evaluation of the bone marrow showed numerous lymphoid aggregates and increased large granular lymphocytes. Germline mutations in the CTLA4 gene have been recently discovered in patients with autoimmunity and immunodeficiency.53 Case 209 detailed a 38-year-old female patient with a documented history of Li-Fraumeni syndrome who had undergone a prophylactic bilateral mastectomy. Routine surveillance with annual magnetic resonance imaging disclosed an enlarging left inguinal lymph node that on excision showed follicular lymphoma, grade 1 to 2 Image 4E. Li-Fraumeni syndrome patients tend to develop tumors for which the sporadic counterparts also carry a high incidence of TP53 mutations: while 2% of these patients develop lymphomas, there are no previously reported cases of follicular lymphoma in the setting of Li-Fraumeni syndrome.
The syndrome of thrombocytopenia, ascites, fibrosis, renal dysfunction, organomegaly (TAFRO) is a variant of multicentric Castleman disease. Its etiology is still unknown but it likely represents a systemic inflammatory disorder mediated by redundant cytokines including interleukin-6 (IL-6), vascular endothelial growth factor, and IL-10.54 Case 200 described a 3-year-old boy with no known family history who presented with fever, generalized lymphadenopathy, hepatosplenomegaly, bilateral pleural effusions, ascites, thrombocytopenia, and anemia. An excised lymph node showed preserved nodal architecture with scattered reactive follicles, some with expanded mantle zones and regressed germinal centers Image 4F. The overall findings were diagnostic of TAFRO syndrome; an underlying germline RUNX1 mutation was identified, which had not been previously reported in TAFRO syndrome.
Conclusions and Future Directions: Testing and Recognition of Germline Predisposition in Clinical Practice
Although any single cancer predisposition syndrome is rare, recent studies have demonstrated that germline cancer predisposition mutations as a whole may not be as rare as previously presumed. A group from St Jude Children’s Research Hospital, Memphis, TN,55 analyzed a panel of 565 genes in 1,120 patients with pediatric cancer and identified germline mutations in 8.5% of patients, including in 4.4% of leukemia patients. Strikingly, only 23% of patients with an identified mutation had a family history that was suggestive of cancer predisposition.55 Similarly, a study that used a targeted panel of 28 genes identified germline mutations in 18% of adult patients.56 Inherited and acquired alterations of genes encoding hematopoietic transcription factors are central events in the pathogenesis of ALL. Recent studies have identified germline mutations in TP53, PAX5, ETV6, and IKZF1 in families with familial ALL.57 Forty-nine genes with a (presumed) causative role in lymphomagenesis have been described, and germline variants in diffuse large B-cell lymphoma have been identified.58 The cases submitted to this workshop appear to reflect the variety of myeloid neoplasms with germline predisposition that have been described in the literature. Although lymphoid neoplasms with germline predisposition are less frequent, they reflect the overall distribution of cases in this workshop.
Germline mutations in an increasing number of genes are known to be associated with increased risk for development of myeloid or lymphoid malignancies. With more frequent and widespread use of molecular testing in clinical diagnostics, an increasing number of patients and families will likely be recognized as having an inherited cancer predisposition syndrome. Clinical history that would prompt consideration for the presence of a germline predisposition syndrome includes a personal history of multiple cancers and early onset of malignancies, longstanding thrombocytopenia and/or platelet dysfunction, and multiple cases of myeloid malignancies or solid tumors occurring within two generations in the family.59,60 Detailed family histories should be obtained by physicians or genetic counselors knowledgeable in these syndromes.
When a clear clinical phenotype strongly suggests germline predisposition syndrome, targeted sequencing for mutation in the suspected single gene or a sequential series of genes based on the phenotype is a reasonable strategy.60 However, phenotypic features often overlap between multiple different syndromes, genetic disorders exhibit broad phenotypic variability and penetrance, and clinical or familial findings to inform diagnosis are often lacking. Hematologic malignancy gene panels used in the clinical setting are designed to target specific gene regions known to be somatically mutated and often omit regions of the gene that are mutated in germline hematologic malignancy disorders. Therefore, targeted gene panels that are designed for somatic mutation analysis of myeloid neoplasm should not be relied upon for diagnostic evaluation of germline mutations. Increasingly, gene panels specifically designed to evaluate genes associated with germline predisposition are becoming available. Whole-exome sequencing is also a powerful tool for evaluation, and the breadth of this coverage can be helpful when traditional targeted panels have not identified a causative gene. However, some genes can harbor mutations in noncoding or intronic regions that are not covered by whole-exome sequencing.
Acknowledgement
The authors acknowledge all of the contributors who submitted cases and contributed to the discussion of the cases discussed in this report.
References
- 1. Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC; 2017. [Google Scholar]
- 2. Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575-581. [PubMed] [Google Scholar]
- 3. Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636-3643. [PubMed] [Google Scholar]
- 4. Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to monomac syndrome. Blood. 2013;121:3830-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (monomac) syndrome. Blood. 2011;118:2653-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43:929-931. [DOI] [PubMed] [Google Scholar]
- 9. Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wlodarski MW, Hirabayashi S, Pastor V, et al. EWOG-MDS Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387-1397; quiz 1518. [DOI] [PubMed] [Google Scholar]
- 11. Pasquet M, Bellanné-Chantelot C, Tavitian S, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to monomac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganapathi KA, Townsley DM, Hsu AP, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169:173-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wlodarski MW, Collin M, Horwitz MS. GATA2 deficiency and related myeloid neoplasms. Semin Hematol. 2017;54:81-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickinson RE, Milne P, Jardine L, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. West ES, Kingsbery MY, Mintz EM, et al. Generalized verrucosis in a patient with GATA2 deficiency. Br J Dermatol. 2014;170:1182-1186. [DOI] [PubMed] [Google Scholar]
- 18. Polat A, Dinulescu M, Fraitag S, et al. Skin manifestations among GATA2-deficient patients. Br J Dermatol. 2018;178:781-785. [DOI] [PubMed] [Google Scholar]
- 19. Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96:1221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spinner MA, Ker JP, Stoudenmire CJ, et al. GATA2 deficiency underlying severe blastomycosis and fatal herpes simplex virus-associated hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2016;137:638-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griese M, Zarbock R, Costabel U, et al. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm Med. 2015;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen JI, Dropulic L, Hsu AP, et al. Association of GATA2 deficiency with severe primary Epstein-Barr virus (EBV) infection and EBV-associated cancers. Clin Infect Dis. 2016;63:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. West RR, Hsu AP, Holland SM, et al. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nováková M, Žaliová M, Suková M, et al. Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica. 2016;101:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen J, Alexander T, Jiang H, et al. Melanoma in patients with GATA2 deficiency. Pigment Cell Melanoma Res. 2018;31:337-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher KE, Hsu AP, Williams CL, et al. Somatic mutations in children with GATA2-associated myelodysplastic syndrome who lack other features of GATA2 deficiency. Blood Adv. 2017;1:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parta M, Shah NN, Baird K, et al. Allogeneic hematopoietic stem cell transplantation for GATA2 deficiency using a busulfan-based regimen. Biol Blood Marrow Transplant. 2018;24:1250-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tholouli E, Sturgess K, Dickinson RE, et al. In vivo T-depleted reduced-intensity transplantation for GATA2-related immune dysfunction. Blood. 2018;131:1383-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hickstein D. HSCT for GATA2 deficiency across the pond. Blood. 2018;131:1272-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galera P, Hsu AP, Wang W, et al. Donor-derived MDS/AML in families with germline GATA2 mutation. Blood. 2018;132:1994-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lipka DB, Witte T, Toth R, et al. RAS-pathway mutation patterns define epigenetic subclasses in juvenile myelomonocytic leukemia. Nat Commun. 2017;8:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). J Med Genet. 1996;33:2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flanagan M, Cunniff C Bloom’s syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews Seattle, WA: University of Washington, Seattle; 2019. https://www.ncbi.nlm.nih.gov/books/NBK1398. Accessed May 11, 2019.
- 35. Akiyama M, Yamaoka M, Mikami-Terao Y, et al. Somatic mosaic mutations of IDH1 and NPM1 associated with cup-like acute myeloid leukemia in a patient with maffucci syndrome. Int J Hematol. 2015;102:723-728. [DOI] [PubMed] [Google Scholar]
- 36. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233-1238. [DOI] [PubMed] [Google Scholar]
- 37. Talwalkar SS, Yin CC, Naeem RC, et al. Myelodysplastic syndromes arising in patients with germline TP53 mutation and Li-Fraumeni syndrome. Arch Pathol Lab Med. 2010;134:1010-1015. [DOI] [PubMed] [Google Scholar]
- 38. Jameson-Lee M, Chen K, Ritchie E, et al. Acute myeloid leukemia in a patient with thrombocytopenia with absent radii: a case report and review of the literature. Hematol Oncol Stem Cell Ther. 2018;11:245-247. [DOI] [PubMed] [Google Scholar]
- 39. Narumi S, Amano N, Ishii T, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48:792-797. [DOI] [PubMed] [Google Scholar]
- 40. Davidsson J, Puschmann A, Tedgård U, et al. SAMD9 and SAMD9L in inherited predisposition to ataxia, pancytopenia, and myeloid malignancies. Leukemia. 2018;32:1106-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liew E, Owen C. Familial myelodysplastic syndromes: a review of the literature. Haematologica. 2011;96:1536-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood. 2017;129:2070-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Owen CJ, Toze CL, Koochin A, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood. 2008;112:4639-4645. [DOI] [PubMed] [Google Scholar]
- 44. Godley LA. Inherited predisposition to acute myeloid leukemia. Semin Hematol. 2014;51:306-321. [DOI] [PubMed] [Google Scholar]
- 45. Noris P, Perrotta S, Seri M, et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011;117:6673-6680. [DOI] [PubMed] [Google Scholar]
- 46. Tawana K, Rio-Machin A, Preudhomme C, et al. Familial CEBPA-mutated acute myeloid leukemia. Semin Hematol. 2017;54:87-93. [DOI] [PubMed] [Google Scholar]
- 47. Polprasert C, Schulze I, Sekeres MA, et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 2015;27:658-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perez-Garcia A, Ambesi-Impiombato A, Hadler M, et al. Genetic loss of SH2B3 in acute lymphoblastic leukemia. Blood. 2013;122:2425-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stockklausner C, Klotter AC, Dickemann N, et al. The thrombopoietin receptor P106L mutation functionally separates receptor signaling activity from thrombopoietin homeostasis. Blood. 2015;125:1159-1169. [DOI] [PubMed] [Google Scholar]
- 50. He X, Chen Z, Jiang Y, et al. Different mutations of the human c-mpl gene indicate distinct haematopoietic diseases. J Hematol Oncol. 2013;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szuber N, Tefferi A. Chronic neutrophilic leukemia: new science and new diagnostic criteria. Blood Cancer J. 2018;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rezaei N, Mahmoudi E, Aghamohammadi A, et al. X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol. 2011;152:13-30. [DOI] [PubMed] [Google Scholar]
- 53. Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurose N, Mizutani KI, Kumagai M, et al. An extranodal histopathological analysis of idiopathic multicentric Castleman disease with and without TAFRO syndrome. Pathol Res Pract. 2019;215:410-413. [DOI] [PubMed] [Google Scholar]
- 55. Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373:2336-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the hereditary hematologic malignancy clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Churchman ML, Qian M, Te Kronnie G, et al. Germline genetic IKZF1 variation and predisposition to childhood acute lymphoblastic leukemia. Cancer Cell. 2018;33:937-948.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leeksma OC, de Miranda NF, Veelken H. Germline mutations predisposing to diffuse large B-cell lymphoma. Blood Cancer J. 2017;7:e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood. 2017;130:424-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Furutani E, Shimamura A. Germline genetic predisposition to hematologic malignancy. J Clin Oncol. 2017;35:1018-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]