Abstract
Objectives
To construct a one-plasmid expression system of the armored RNA containing long chimeric RNA byincreasing the number and affinity of the pac site.
Methods
The plasmid pET-MS2-pac was constructedwith one C-variant pac site, and then the plasmid pM-CR-2C containing 1,891-bp chimeric sequences and two C-variant pac sites was produced. Meanwhile, three plasmids (pM-CR-C, pM-CR-2W and pM-CR-W) were obtained as parallel controls with a different number and affinity of the pac site. Finally, the armored RNA was expressed and purified.
Results
The armored RNA with 1,891 bases target RNA was expressed successfully by the one-plasmid expression system with two C-variant pac sites, while for one pac site, no matter whether the affinity was changed or not, only the 1,200 bases target RNA was packaged. It was also found that the C-variant pac site could increase the expression efficiency of the armored RNA. The armored RNA with 1,891-bp exogenous RNA in our study showed the characterization of ribonuclease resistance and stability at different time points and temperature conditions.
Conclusions
The armored RNA with 1,891 bases exogenous RNA was constructed and the expression system can be used as a platform for preparation of the armored RNA containing long RNA sequences.
Key Words: Package, Long chimeric RNA, Pac site, Armored RNA
Introduction
The armored RNA is a noninfectious recombinant pseudoviral particle, which is used as a calibration standard or internal assay control, because the packaged RNA is resistant to RNase digestion. The template RNA can be easily extracted from the armored RNA standard particles by using common RNA extraction methods, which make the RNA available as an RNA standard for quantification, RNA size standards and a transient gene expression system in vitro or in vivo [1, 2, 3, 4, 5].
A preferred strategy for producing the armored RNA is by self-assembly of coat protein of the MS2 bacteriophage. Package of the RNA genome by coat protein is initiated by the binding of the coat protein dimer to a specific 19-nucleotide stem-loop region (pac site, see fig. 1a) located at the 5′ terminus of the MS2 bacteriophage replicase gene [6].
Fig. 1.
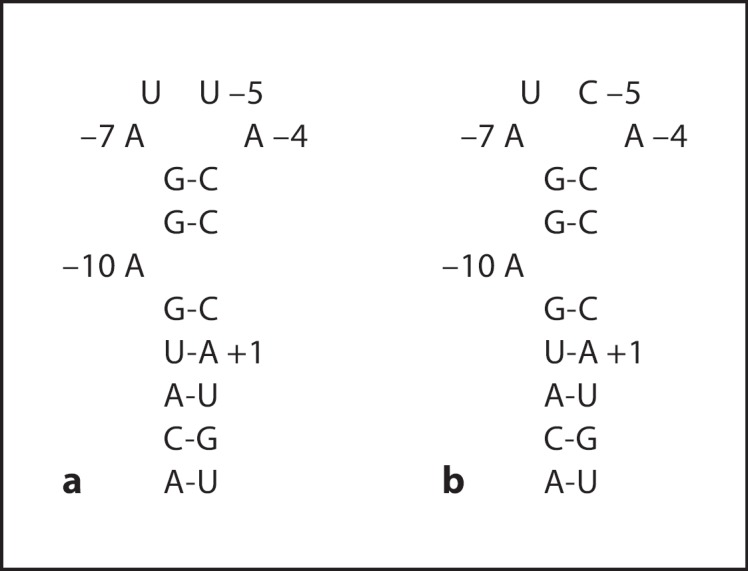
The sequence and secondary structure of the 19-nucleotide stem-loop region (pac site). Base numbering is relative to the start of the MS2 replicase initiation codon AUG; A is +1. a The wild-type pac site. b The C-variant pac site.
Theoretically, at least 2,000 bases of exogenous RNA sequence might be packaged into coat protein shell; however, the efficiency of packaging decreased quickly as the size of the RNA increased beyond 500 bases [4, 7, 8]. So far, the largest size of RNA packaged was 1,200 bases by utilizing one wild-type pac site [9], but it could not meet the needs of detecting a variety of viral genomes and the comparison of different clinical laboratory data [7].
It has been identified that the nucleotides at only a few positions of the pac site, the loop residues A-4, U-5, and A-7 and bulge A-4 position, are important for package [10, 11], and even these key recognition sites can be varied. For example, substitution of U-5 with C (C-variant, fig. 1b) yields a significantly higher affinity for coat protein than wild-type pac site in vitro [12, 13, 14, 15, 16]. It has also been confirmed that the presence of a second pac site permits the cooperative binding of the coat protein dimer to the RNA and results in a higher affinity compared with a single pac site [17, 18]. Recently, Wei et al. [19] successfully used armored RNA technology to package 2,248 bases exogenous RNA sequences by a two-plasmid coexpression system with the C-variant pac site.
In order to explore whether the armored RNA could package exogenous RNA longer than 1,200 bases by a one-plasmid expression system by increasing the number and affinity of the pac site, we constructed 1,891 bases chimeric RNA sequences that included three fragments of SARS-CoV, one fragment of HCV, one C-variant pac site and one fragment of H5N1, and then inserted them into the plasmid pET-MS2-pac containing one C-variant pac site to form a two pac sites expression system, namely, pM-CR-2C. Meanwhile, three expression plasmids (pM-CR-C, pM-CR-W and pM-CR-2W) with a different number and affinity of the pac site were constructed as parallel controls.
Material and Methods
Construction of pET-MS2-pac
The fragment encoding MS2 maturase and coat protein was obtained from the plasmid pMS27 (kindly provided by D.S. Peabody) by PCR using the following primer pairs: 5′-CGGGATCCTGGCTATCGCTGTAGGTAGCC-3′and5′-AAGGAAAAAAGCGGCCGCACATGGGTGATCCTCATGTATGGCCGGCGTCTATTAGTAG-3′. The underlined sequences were BamH and Notl restriction enzyme sites, respectively, and the bold was the C-variant pac site. The amplified products were inserted into pET28(b) vector (Novagen, USA) to generate the recombinant plasmid pET-MS2-pac. The positive constructions were verified by sequencing.
Construction of pM-CR-2C
The chimeric sequences with one C-variant pac site containing five fragments (totally 1,891 bp) were composed of SARS-CoV1 (nt 15224-15618, GenBank accession No. AY864806), SARS-CoV2 (nt 18038-18340, GenBank accession No. AY864806), SARS-CoV3 (nt 28110-28692, GenBank accession No. AY864806), HCV-5′-UTR (nt 18-310, GenBank accession No. AF139594) and H5N1 (nt 295-611, HA300, GenBank accession No. DQ864720) and were obtained as follows [20]. First, SARS-CoV1 and SARS-CoV3 fragment was amplified from the template plasmid pBSSR-V6 and pBSSR7-8 (provided by Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College) using the primers S-SARS1 and LAP-SARS1, LAP-SARS3 and A-SARS3, respectively. SARS-CoV2 fragment was amplified from the template plasmid pNCCL-SARS (constructed by our laboratory) using the primers LAP-SARS2 and A-SARS2. HCV fragment was produced from the template plasmid pNCCL-HCV (constructed by our laboratory) using the primers S-HCV (containing one C-variant pac site) and HCV-LAP1. HA300 fragment was amplified from the template plasmid pNCCL-H5N1 (constructed by our laboratory) using the primers HA300LAP and A-HA300. Then, the gel-purified PCR products were used in the following overlapping extension PCR reaction. SARS-CoV1+SARS-CoV2 fragments and HCV+HA300 fragments were amplified using the primers S-SARS1 and LAP-SARS2+, FIVELAP2 and 3V-FIVE-07-A, respectively. Then SARS-CoV1+SARS-CoV2+SARS-CoV3 fragments were amplified using primers FIVELAP1 and 3V-FIVE-07-S. Finally, the full-length chimeric sequences SARS-CoV1+SARS-CoV2+SARS-CoV3+HCV+HA300 with one C-variant pac site were amplified using the primers 3V-FIVE-07-S and 3V-FIVE-07-A. The primers described above were shown in table 1. The purified PCR products and the pET-MS2-pac plasmid were then digested with the Not I restriction enzyme, respectively. The resulting fragments were ligated to produce the recombinant expression plasmid pM-CR-2C. The positive constructions were verified by sequencing.
Table 1.
PCR primers
| Primer name | Primer sequence (5′ to 3′) |
|---|---|
| MS2-07-S | 5′CGGGATCCTGGCTATCGCTGTAGGTAGCC3′ |
| MS2-07-A | 5′AAGGAAAAAAGCGGCCGCACATGGGTGATCCTCATGTATGGCCGGCGTCTATTAGTAG3′ |
| S-SARS1 | 5′TATCCAAAATGTGACAGAGCCATG3′ |
| LAP-SARS1 | 5′ACGCTGAGGTGTGTAGGT GCAGGTAAGCGTAAAACTCATCCAC3′ |
| A-SARS2 | 5′TAACCAGTCGGTACAGCTACTAAG3′ |
| LAP-SARS2 | 5′AGTTTTACGCTTACCTGC ACCTACACACCTCAGCGTTGATATAAAG3′ |
| A-SARS3 | 5′ACTACGTGATGAGGAGCGAGAAGAG3′ |
| LAP-SARS3 | 5′AGCTGTACCGACTGGTTA ACAAATTAAAATGTCTGATAATGGACCCC3′ |
| LAP-SARS2+ | 5′ATCAGACATTTTAATTTGT TAACCAGTCGGTACAGCTACTAAG3′ |
| S-HCV | 5′ACATGAGGATCACCCATGTGGCGACACTCCACCATAGATCACTC3′ |
| HCV-LAP1 | 5′ATGTAAGACCATTCCGGC TCGCAAGCACCCTATCAGGCAGTAC3′ |
| A-HA300 | 5′GAATCCGTCTTCCATCTTTCCCCCACAGTACCAAAAGATCTTC3′ |
| HA300LAP | 5′CTGATAGGGTGCTTGCGA GCCGGAATGGTCTTACATAGTGGAG3′ |
| 3V-FIVE-07-S | 5′AAGGAAAAAAGCGGCCGC TATCCAAAATGTGACAGAGCCATG3′ |
| 3V-FIVE-07-A | 5′AAGGAAAAAAGCGGCCGC TCCCCCACAGTACCAAAAGATCTTC3′ |
| FIVELAP1 | 5′CATGGGTGATCCTCATGT ACTACGTGATGAGGAGCGAGAAGAG3′ |
| FIVELAP2 | 5′CGCTCCTCATCACGTAGT ACATGAGGATCACCCATGTGGC3′ |
| Overlap-A | 5′CCCACAGTACCAAAAGATCTTCTTG3′ |
Underlined sequences are BamH I or Not I restriction enzyme sites; bold typeface is the C-variant pac site.
Construction of the Control Plasmids
We also constructed three parallel control recombinant expression plasmids: pM-CR-C, pM-CR-W and pM-CR-2W. These plasmids had the full-length chimeric RNA sequences, but the pac site was different to each other. The plasmid pM-CR-C contained one C-variant pac site and the plasmid pM-CR-W contained one wild-type pac site, in which the pac site was located between the MS2 coat protein and the chimeric sequences. The plasmid pM-CR-2W contained two wild-type pac sites, in which one pac site was located between the MS2 coat protein and the chimeric sequences and another was at the middle of the chimeric sequences.
Production and Purification of the Armored RNA Particles
The four kinds of recombinant plasmid were transformed into the competent Escherichia coli BL21 (DE3) strain separately and protein expression was induced with 1 mM isopropyl-β-D-thiogalactoside at 37° for 16 h. The cells were then harvested by centrifugation and lysed by ultrasonic disruption (Branson Sonifier 350). The supernatant was incubated with 25 U DNase I and 100 U RNase A at 37° for 60 min. Finally, the products were detected by agarose gel electrophoresis (1%), with ethidium bromide staining. The armored RNA particles were further purified by CsCl density gradient centrifugation (Beckman Ti90 rotor) according to the conventional protein purification steps [2, 21]. The main fractions were then pooled and purified by dialysis against sonication buffer (100 mM NaCl, 5 mM MgSO4, 50 mM Tris-Cl, pH 8.0).
Characterization of the Armored RNA Particles
The total RNA of the armored RNA particles was isolated using QIAamp® viral RNA mini kit (Qiagen, Germany) according to the manufacturer's instructions. Reverse transcription (RT) reactions were performed in a total volume of 50 μl containing 5 μl of total RNA, 10 pmol primer Overlap-A (table 1), 4 μl of first strand buffer, 0.2 mM dNTP, 2.5 mM MgCl2, and 200 U of superscript III reverse transcriptase (Invitrogen, USA). The mixture was incubated at 55° for 50 min, 70° for 15 min to denature the reverse transcriptase, and cooled at 4°.
PCR was performed in a 50-μl reaction volume at 94° for 5 min, followed by 40 cycles of 30 s at 95°, 30 s at 56° and 140 s at 72°, and finally a 10-min elongation at 72°. The PCR products were purified and ligated with pEGM®-T Easy vector (Promega, USA) and then transformed into the competent E. coli DH5α. The recombinant plasmid was verified by sequencing.
Expression Efficiency of Different Armored RNA Particles
The optical density (OD260) of the armored RNA particles was measured at 260 nm. The expression efficiency was then estimated based on the relationship that an optical density of 1.0 corresponded to a concentration of 0.125 mg/ml of the armored RNA.
Analysis of the Stability of the Armored RNA Particles
The armored RNA particles were 10-fold serially diluted with the newborn calf serum to obtain 10,000 and 1,000,000 IU/ml. A single batch of the armored RNA particles was aliquoted into single time point samples of 100 μl for the stability study. Samples were then incubated at 4°, 37° and room temperature, respectively. After reservation for 3 days, 1 week, 2 weeks, 4 weeks and 8 weeks, samples were removed at each time point and stored at −80° for 8 weeks. Finally, all samples were quantified using HCV RNA PCR-Fluorescence Quantitative Diagnostic Kit (Kehua, China) according to the manufacturer's instructions. The data were then analyzed with LightCycler software (Roche, USA).
Results
Production of the Armored RNA
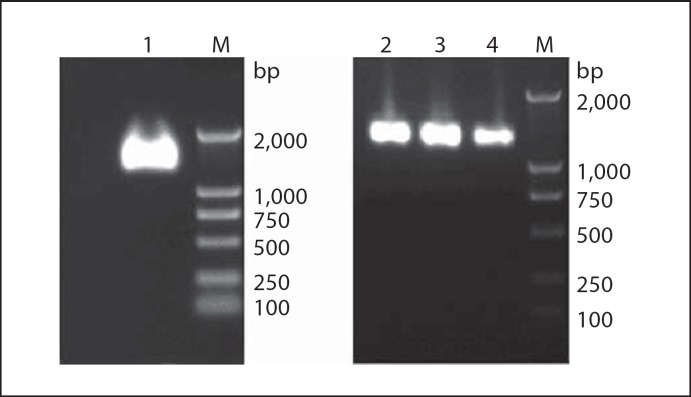
The armored RNA was expressed in E. coli BL21 (DE3), digested by DNase I and RNase A at 37° for 60 min, and purified with CsCl gradient centrifugation. A single band of about 1,500 bp of DNA corresponding to the armored RNA is shown in figure 2.
Fig. 2.
The results of purified armored RNA by CsCl gradient centrifugation (1% agarose gel). Lane 1 = pM-CR-2C armored RNA; lane 2 = pM-CR-2W armored RNA; lane 3 = pM-CR-C armored RNA; lane 4 = pM-CR-W armored RNA.
Analysis of the Armored RNA Content
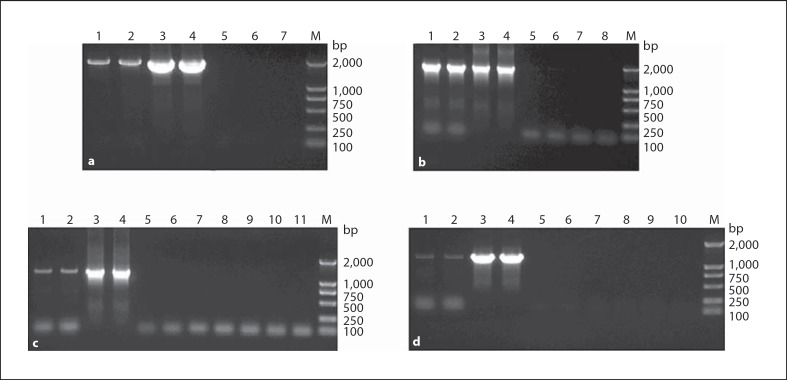
After purification by CsCl gradient centrifugation, total RNA was isolated and RT-PCR was performed. PCR was also performed to check for DNA contaminants. The results of RT-PCR showed that the full-length chimeric RNA was packaged into the armored RNA corresponding to the plasmid pM-CR-2C and pM-CR-2W and sequencing results verified that they were the chimeric sequences desired. However, only 1,200-bp RNA was packaged into the armored RNA corresponding to the plasmid pM-CR-C and pM-CR-W. These results indicated the use of a two pac sites expression system could increase the length of packaged RNA. The results of RT-PCR and PCR are shown in figure 3.
Fig. 3.
The results of RT-PCR and PCR (1% agarose gel). a RT-PCR and PCR of the pM-CR-2C armored RNA. Lanes 1 and 2 = amplification products of the full length of the chimeric RNA by RT-PCR about 2,000 bp; lanes 3 and 4 = positive control; lanes 5 and 6 = negative amplification results of the full length of the chimeric RNA by PCR; lane 7 = negative control. b RT-PCR and PCR of the pM-CR-2W armored RNA. Lanes 1 and 2 = amplification products of the full length of the chimeric RNA by RT-PCR about 2,000 bp; lanes 3 and 4 = positive control; lanes 5 and 6 = negative amplification results of the full length of the chimeric RNA by PCR; lanes 7 and 8 = negative control. c RT-PCR and PCR of the pM-CR-C armored RNA. Lanes 1 and 2 = amplification products of SARS-CoV1+SARS-CoV2+SARS-CoV3 by RT-PCR about 1,200 bp; lanes 3 and 4 = positive control; lane 5 = negative control; lanes 6 and 7 = negative amplification results of SARS-CoV1+SARS-CoV2+SARS-CoV3 by PCR; lanes 8 and 9 = negative amplification results of SARS-CoV1+SARS-CoV2+SARS-CoV3+HCV by RT-PCR; lanes 10 and 11 = negative amplification results of the full length of the chimeric RNA by RT-PCR. d RT-PCR and PCR of the pM-CR-W armored RNA. Lanes 1 and 2 = amplification products of SARS-CoV1+SARS-CoV2+SARS-CoV3 by RT-PCR about 1,200 bp; lanes 3 and 4 = positive control; lanes 5 and 6 = negative amplification results of SARS-CoV1+SARS-CoV2+SARS-CoV3 by PCR; lanes 7 and 8 = negative amplification results of SARS-CoV1+SARS-CoV2+SARS-CoV3+HCV by RT-PCR; lanes 9 and 10 = negative amplification of the full length of the chimeric RNA by RT-PCR.
Analysis of the Expression Efficiency
The optical density at 260 nm was measured to calculate the expression efficiency of the armored RNA. The expression efficiency of the plasmid pM-CR-2C, pM-CR-2W, pM-CR-C, and pM-CR-W was 0.51, 0.35, 0.35 and 0.23 mg/ml, respectively. The expression efficiency of the plasmid pM-CR-2C was 38% higher than that of the plasmid pM-CR-2W, and that of the plasmid pM-CR-C was 52% higher than that of the plasmid pM-CR-W. Thus, the C-variant pac site could enhance the expression efficiency of the armored RNA and the two C-variant pac sites plasmid, i.e. pM-CR-2C had the highest expression efficiency.
Stability of the Armored RNA
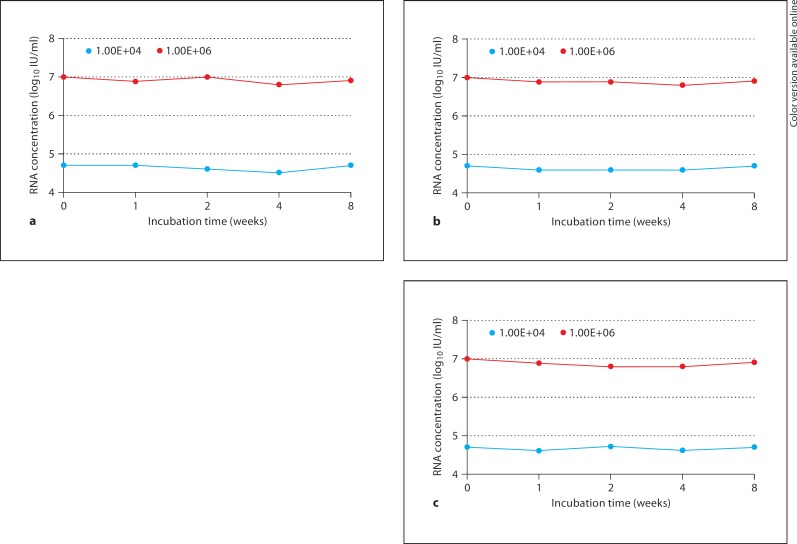
The armored RNA samples were incubated at 4°, 37° and room temperature, respectively, for the stability analysis. Samples taken at different time points (3 days, 1 week, 3 weeks, 4 weeks and 8 weeks) were stored at −80° for 8 weeks. Subsequent real-time PCR results showed that the armored RNA was rather stable at different time points and temperature conditions (fig. 4).
Fig. 4.
Analysis of stability of the 104 and 106 IU/ml armored RNA containing long chimeric RNA sequences at 4° (a), 37° (b) and room temperature (c) for 0, 1, 2, 4 and 8 weeks.
Discussion
The armored RNA containing about 1,700 bases MS2 bacteriophage RNA genome was obtained by utilizing a one-plasmid expression system [22], which indicated that the maximal length of the packaged RNA would be approximately 2,000 bases. However, so far, only 1,200 bases exogenous RNA was packaged into armored RNA by the one-plasmid expression system with one wild-type pac site [9]. In the present study, we constructed a new one-plasmid expression system with two C-variant pac sites to demonstrate the possibility of forming the armored RNA containing exogenous RNA about 2,000 bases in length.
The C-variant pac site could increase the affinity between the coat protein and RNA to 6-fold or even as high as 50-fold [23, 24]. Nevertheless, we utilized the C-variant pac site to construct the two pac sites armored RNA expression system. Our study revealed that the expression efficiency of the plasmid pM-CR-2C and pM-CR-C with the C-variant pac site(s) was higher than the plasmid pM-CR-2W and pM-CR-W with the wild-type pac site(s), respectively.
In addition, because the pac site plays an important role in the package processes, the expression efficiency and package capacity might be further enhanced if inserting a second pac site [25, 26]. We tried to use two pac sites to package the 1,819 bases chimeric RNA. Our study demonstrated that the plasmid pM-CR-2C and pM-CR-2W with two C-variant or wild-type pac sites could package 1,891 bases RNA, which was close to the theoretical length 2,000 bases, while only 1,200 bases RNA was packaged into the plasmid pM-CR-C and pM-CR-W with one C-variant or wild-type pac site.
The armored RNA containing long chimeric RNA, including three SARS-CoV fragments, one HCV fragment, and one H5N1 fragment, can be used as a control or calibrator for qualitative or quantitative detection of SARS-CoV, HCV, and H5N1 by RT-PCR. Simultaneously, our study indicated that the multiple target viral RNAs can be packaged into a single armored RNA to serve as a common calibrator for detection of different RNA virus.
The stability experiment and subsequent real-time PCR results revealed that the available armored RNA was stable at different time points and temperature conditions. All of these showed that the armored RNA constructed in this study met the criteria as a calibration standard or an internal assay control.
In conclusion, we have demonstrated that the chimeric RNA with a length of 1,891 bases can be packaged into MS2 coat protein to produce the armored RNA by utilizing a one-plasmid expression system with two C-varaint pac sites, which exhibited the highest expression efficiency. The expression system can be used as a platform for preparation of the ribonuclease-resistant armored RNA containing long RNA sequences, which could act as a calibration standard or an internal assay control in clinical laboratories. Additionally, an approximately 2,248 bases chimeric armored RNA was also successfully expressed by a two-plasmid coexpression system with the C-variant pac site [19]. So it is possible to produce an armored RNA packaging of about 3,000 bases exogenous RNA by a one- or two-plasmid expression system by increasing the number and affinity of the pac site.
Acknowledgement
This work was supported in part by the SEPSDA Project of the European Commission (under No. Sp22-CT-2004-003831), the National Natural Science Foundation of China (30371365 and 30571776), and the Capital Medicine Development Foundation of Beijing (2002-3041).
References
- 1.Walkerpeach CR, Winkler M, DuBois DB, Pasloske BL. Ribonuclease- resistant RNA controls (armored RNA) for reverse transcription-PCR, branched DNA, and genotyping assays for hepatitis C virus. Clin Chem. 1999;45:2079–2085. [PubMed] [Google Scholar]
- 2.DuBois DB, Winkler MM, Pasloske BL. Ribonuclease-resistant viral RNA standards. US Patent No. 5677124. 1997 doi: 10.1128/jcm.36.12.3590-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C, Seifried E, Roth WK. Taqman 5′-nuclease human immunodeficiency virus type 1 PCR assay with phage-packaged competitive internal control for high-throughput blood donor screening. J Clin Microbiol. 2001;39:4302–4308. doi: 10.1128/JCM.39.12.4302-4308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hietala SK, Crossley BM. Armored RNA as virus surrogate in a real-time reverse transcriptase PCR assay proficiency panel. J Clin Microbiol. 2006;44:67–70. doi: 10.1128/JCM.44.1.67-70.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Yang CM, Wang LN, Meng S, Deng W, Li JM. A novel real-time multiplex reverse transcriptase-polymerase chain reaction for the detection of HIV-1 RNA by using dual-specific armored RNA as internal control. Intervirology. 2008;51:42–49. doi: 10.1159/000119119. [DOI] [PubMed] [Google Scholar]
- 6.Legendre D, Fastrez J. Production in Saccharomyces cerevisiae of MS2 virus-like particles packaging functional heterologous mRNAs. J Biotechnol. 2005;117:183–194. doi: 10.1016/j.jbiotec.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Pasloske BL, Walkerpeach CR, Obermoeller RD, Winkler M, DuBios DB. Armored RNA technology for production of ribonuclease-resistant viral RNA controls and standards. J Clin Microbiol. 1998;36:3590–3594. doi: 10.1128/jcm.36.12.3590-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beld M, Minnaar R, Weel J, Sol C, Damen M, van der Avoort H, Wertheim-van Dillen P, van Breda A, Boom R. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J Clin Microbiol. 2004;42:3059–3064. doi: 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q, Chen Y, Guo Q, Li Q. Preparation of a chimeric armored RNA as a versatile calibrator for multiple virus assays. Clin Chem. 2006;52:1446–1458. doi: 10.1373/clinchem.2006.069971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parrott AM, Lago H, Adams CJ, Aschcroft AE, Stonehouse NJ, Stockley PG. RNA aptamers for the MS2 bacteriophage coat protein and the wild-type RNA operator have similar solution behaviour. Nucleic Acids Res. 2000;28:489–497. doi: 10.1093/nar/28.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grahn E, Moss T, Helgstrand C, Fridborg K, Sundaram M, Tars K, Lago H, Stonehouse NJ, Davis DR, Stockley PG, Liljas L. Structural basis of pyrimidine specificity in the MS2 RNA hairpin-coat-protein complex. RNA. 2001;7:1616–1627. [PMC free article] [PubMed] [Google Scholar]
- 12.Lago H, Fonseca SA, Murray JB, Stonehouse NJ, Stockley PG. Dissecting the key recognition features of the MS2 bacteriophage translational repression complex. Nucleic Acids Res. 1998;26:1337–1344. doi: 10.1093/nar/26.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockley PG, Stonehouse NJ, Murray JB, Goodman ST, Talbot SJ, Adams CJ, Liljas L, Valegard K. Probing sequence-specific RNA recognition by the bacteriophage MS2 coat protein. Nucleic Acids Res. 1995;23:2512–2518. doi: 10.1093/nar/23.13.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romaniuk PJ, Uhlenbeck OC. Nucleoside and nucleotide inactivation of R17 coat protein: evidence for a transient covalent RNA-protein bond. Biochemistry. 1985;24:4239–4244. doi: 10.1021/bi00336a064. [DOI] [PubMed] [Google Scholar]
- 15.Sawata SY, Taira K. Development of an advanced polysome display system dependent on a specific protein-RNA motif interaction. Nucleic Acids Res Suppl. 2001:99–100. doi: 10.1093/nass/1.1.99. [DOI] [PubMed] [Google Scholar]
- 16.LeCuyer KA, Behlen LS, Uhlenbeck OC. Mutants of the bacteriophage MS2 coat protein that alter its cooperative binding to RNA. Biochemistry. 1995;34:10600–10606. doi: 10.1021/bi00033a035. [DOI] [PubMed] [Google Scholar]
- 17.Witherell GW, Wu HN, Uhlenbeck OC. Cooperative binding of R17 coat protein to RNA. Biochemistry. 1990;29:11051–11057. doi: 10.1021/bi00502a006. [DOI] [PubMed] [Google Scholar]
- 18.Pickett GG, Peabody DS. Encapsidation of heterologous RNAs by bacteriophage MS2 coat protein. Nucleic Acids Res. 1993;21:4621–4626. doi: 10.1093/nar/21.19.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei YX, Yang CM, Wei BJ, Huang J, Wang LN, Meng S, Zhang R, Li JM. Ribonuclease-resistant virus-like particles containing long chimeric RNA sequences produced by a two-plasmid coexpression system. J Clin Microbiol. 2008;46:1734–1740. doi: 10.1128/JCM.02248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 21.Pasloske BL, DuBois DB, Brown DM, Winkler MM. Methods of quantifying viral load in an animal with a ribonuclease-resistant RNA preparation. US Patent No. 6399307. 2002 [Google Scholar]
- 22.Pasloske BL, DuBois DB, Brown DM, Winkler MM. Ribonuclease-resistant RNA preparation and utilization. US Patent 6214982. 2001 [Google Scholar]
- 23.Talbot SJ, Goodman S, Bates SR, Fishwick CW, Stockley PG. Use of synthetic oligoribonucleotides to probe RNA-protein interactions in the MS2 translational operator complex. Nucleic Acids Res. 1990;18:3521–3528. doi: 10.1093/nar/18.12.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowary PT, Uhlenbeck OC. An RNA mutation that increases the affinity of an RNA-protein interaction. Nucleic Acids Res. 1987;15:10483–10493. doi: 10.1093/nar/15.24.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romaniuk PJ, Lowary P, Wu HN, Stormo G, Uhlenbeck OC. RNA binding site of R17 coat protein. Biochemistry. 1987;26:1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- 26.Rowsell S, Stonehouse NJ, Convery MA, Adams CJ, Ellington AD, Hirao I, Peabody DS, Stockley PG, Phillips SE. Crystal structures of a series of RNA aptamers complexed to the same protein target. Nat Struct Biol. 1998;5:970–975. doi: 10.1038/2946. [DOI] [PubMed] [Google Scholar]