Abstract
Background
With the development of more rapid and sensitive detection methods based on PCR techniques, the contributions of respiratory viral infections to community-acquired pneumonia (CAP) in adult patients are being more and more recognized. Yet, up to now, there has been a lack of synthetic data that clearly demonstrates the incidence of respiratory viral infections in adult patients with CAP.
Objectives
We intended to demonstrate the incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with CAP.
Methods
We searched PubMed and Embase for studies providing the incidence of respiratory viral infections in adult patients with CAP. We investigated potential sources of heterogeneity by a univariant metaregression analysis and calculated the combined incidence of viral infections, viral infections mixed with other pathogens and individual respiratory virus species.
Results
We eventually identified 23 eligible reports with a total number of 6,404 patients. Incidences ranged from 8.6 to 56.2% for overall respiratory viral infections. We noted significant heterogeneity in incidence estimates for the incidence of viral infections (Cochran's χ2 = 269.9, p < 0.0001, I2 = 91.8%). The combined incidence of viral infections was 22.4% (95% CI = 19.0-25.7). Incidences of viral coinfections with other pathogens ranged from 3 to 28%. A high level of heterogeneity was identified as well during the estimates for incidences of coinfections (χ2 = 200.9, p < 0.0001, I2 = 91.5%). The combined incidence of viral coinfections with other pathogens was 12.4% (95% CI = 9.7-15.0). Our heterogeneity analyses suggested that a lower respiratory tract sample was associated with higher overall viral incidence. Moreover, the influenza virus, rhinovirus and coronavirus were the 3 most frequently detected viral pathogens in adult patients with CAP according to our study.
Conclusions
Respiratory viruses are probably crucial pathogens of adult patients with CAP, with the influenza virus being the most frequent viral pathogen identified. More than half of the viral infections are characterized as mixed infections with other pathogens.
Key Words: Community-acquired pneumonia, Adults, Respiratory virus, Incidence of viral infections, Meta-analysis
Introduction
Community-acquired pneumonia (CAP) as the most common cause of death due to infection in adults brings a great clinical and economic burden to the health care systems worldwide [1, 2]. The definition of the etiology directly affects the decision concerning the clinical treatment of patients with CAP, which will finally influence the outcome for patients with CAP [3, 4]. The bacterial causes of CAP have been well characterized, with Streptococcus pneumoniae being the most important bacterial pathogen [5, 6]. In contrast to bacterial agents, the involvement of respiratory viral infections in adult patients with CAP has yet not been well defined.
With the development of virological diagnostic techniques, an increasing number of studies evaluating the incidence of respiratory viral infections in adult patients with CAP indicates the important contributions of respiratory viruses to CAP [7, 8]. However, up to now, no synthetic data has clearly demonstrated the incidence of overall respiratory viral infections in adult patients with CAP and the discrepancy in the contributions to the pathogenesis of CAP across respiratory viral species.
The aim of this meta-analysis is to determine the incidence of viral infections in adult patients with CAP and demonstrate the incidence discrepancy among respiratory viral species according to previous epidemiology researches based on PCR-related techniques.
Methods
Search Strategy
In July 2013, we conducted a systematic search utilizing PubMed and Embase search engines for citations published from January 1, 2000 to July 19, 2013. The initial search was undertaken using free-word, keyword and MeSH searches for ‘community-acquired pneumonia', 'virus', 'adult’ and 'PCR'. Two researchers (X.W. and Q.W.) independently searched the titles and abstracts identified initially on screen for the selection of the potential studies. We also searched the reference lists of the included studies. When needed, we contacted the authors to obtain missing or additional information. We settled any discrepancies through discussion with a third researcher (M.W.).
Inclusion/Exclusion Criteria
Studies had to meet the following criteria for inclusion: (1) patients were ≥16 years old; (2) studies clearly stated the definition of CAP; (3) studies provided the incidence of respiratory viral infections in CAP patients; (4) respiratory viruses were detected by highly sensitive techniques (PCR and real-time PCR); (5) at least 90% of the samples collected were used for viral detection; (6) the full paper was available in the English literature.
Studies were excluded if they focused on patients in an immunosuppressed state, such as in the case of patients with solid organ or bone marrow transplantations, AIDS or receiving chemotherapy or taking other immunosuppressive drugs etc.
Quality Assessment and Data Extraction
Two reviewers independently assessed the quality of all included studies using score quality criteria based on the principles of the quality assessment of observational studies (as shown in online suppl. table 1; see www.karger.com/doi/10.1159/000369561 for all online suppl. material). The relevant trail characteristics were independently extracted by 2 reviewers (X.W. and Q.W.), and any discrepancies were solved with complete agreement through rechecking the source papers and discussion. Data extraction for each study was done according to a standardized form designed by us to capture and record all relevant information required for analysis. For each study, 2 reviewers extracted the data independently, cross-checked with each other and resolved disagreements through discussion. For all included studies, the following information was recorded: author, year of publication, country, number of patients, number of respiratory virus species detected, viral detection method, specimen collected for viral detection, number of patients with viral infections and number of patients with mixed infections by viruses and other pathogens.
Statistical Analysis
According to the expected heterogeneity across studies, the combined incidence of respiratory viral infections with 95% was calculated with a DerSimonian-Laird random-effects model [9]. Statistical heterogeneity was tested with Cochran's χ2 and quantified with the I2 statistic (30-60% for moderate heterogeneity; 50-90% for substantial heterogeneity; 75-100% for considerable heterogeneity) [10]. In order to reduce the heterogeneity across studies and perform further analysis, a subgroup analysis was required. We further investigated potential sources of heterogeneity by metaregression analysis. Factors examined individually in univariable models were the sample type used for viral detection (by comparing a lower respiratory tract specimen with an upper respiratory tract specimen), time span of patient enrollment, geographical region, number of viral species detected and number of viral detection methods. We did all analyses in Stata (version 12.1, StataCorp LP, College Station, Tex., USA) with the commands ‘metan’ (for random-effects meta-analysis), ‘metareg’ (for metaregression) and ‘metainf’ (for sensitivity analysis).
Results
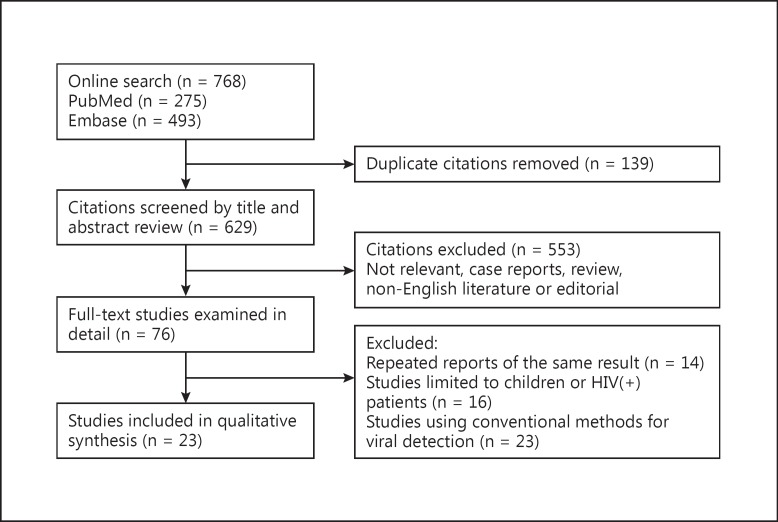
A total of 768 records were returned during our searches. After initial screening and removal of duplicates, we reviewed 76 papers in full text. Finally, 23 studies (n = 6,404) published between February 2001 and July 19, 2013 were included as eligible reports for our further analysis (fig. 1, table 1). The quality score of those 23 reports is listed in table 1. Ten of the 23 reports achieved a score ≥7, and the other 13 reports scored between 4 and 6. Of the 23 reports, 2 were conducted in adult outpatients with CAP [11, 12], 17 in adult inpatients with CAP [7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28] and 4 in both outpatients and inpatients with CAP [29, 30, 31, 32]. Nine reports were carried out in Europe (4 in Spain, 3 in the Netherlands, 1 in the UK and 1 in Sweden) [7, 14, 17, 18, 20, 24, 28, 29, 30], 5 in Southeast Asia (2 in China, 1 in Japan, 1 in Korea and 1 in Vietnam) [11, 12, 15, 19, 32], 3 in Australia [22, 26, 27], 4 in America (2 in the USA, 1 in Canada and 1 in Chile) [13, 16, 25, 31] and 2 in Israel [21, 23]. Moreover, respiratory viral detection in 12 studies was solely based on PRC/real-time PCR [7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 23, 27, 29], and viral detection in the other studies was based on a combination of PCR/real-time PCR and conventional methods [20, 21, 22, 24, 25, 26, 28, 30, 31, 32].
Fig. 1.
Flowchart of the selection of the studies.
Table 1.
Summarized characteristics of the studies included in the meta-analysis
| Author | Year of publication | Viral identification method | Specimens | Viral species, n | Patients, n | Quality score1 |
|---|---|---|---|---|---|---|
| Takahashi et al. [15] | 2013 | PCR | Nasopharyngeal swabs | 13 | 167 | 9 |
| Luchsinger et al. [31] | 2013 | Culture, IFA, serology, PCR | Serum, sputum, nasopharyngeal aspirate | 10 | 356 | 6 |
| Wiemken et al. [13] | 2013 | PCR | Nasopharyngeal swabs | 12 | 393 | 7 |
| Viasus et al. [14] | 2013 | RT-PCR | Nasopharyngeal swabs or BALF | 13 | 747 | 8 |
| Musher et al. [16] | 2013 | PCR | Nasopharyngeal swabs | 7 | 259 | 6 |
| Huijskens et al. [17] | 2013 | RT-PCR | Throat swabs, sputum | 14 | 408 | 8 |
| Yin et al. [11] | 2012 | RT-PCR | Throat swabs, sputum | 13 | 215 | 5 |
| Sangil et al. [18] | 2012 | PCR | Nasopharyngeal swabs, sputum | 12 | 131 | 6 |
| Choi et al. [19] | 2012 | RT-PCR | Nasopharyngeal aspirates, BALF | 16 | 64 | 6 |
| Johansson et al. [7] | 2010 | RT-PCR | Nasopharyngeal samples | 16 | 184 | 5 |
| Cilloniz et al. [20] | 2011 | PCR, serology | Nasopharyngeal swabs, serum | 14 | 362 | 4 |
| Shibli et al. [21] | 2010 | PCR, serology | Nasopharyngeal swabs, serum | 8 | 126 | 5 |
| Mermond et al. [22] | 2010 | serology, PCR, IFA | Serum, nasopharyngeal swabs, TBA, BALF or PSB samples | 7 | 137 | 5 |
| Lieberman et al. [23] | 2010 | RT-PCR | Oropharyngeal swabs, nasopharyngeal swabs and nasopharyngeal washing | 12 | 183 | 9 |
| Cao et al. [12] | 2010 | RT-PCR | Sputum and throat swabs | 16 | 197 | 6 |
| Diederen et al. [24] | 2009 | PCR, serology | Throat swabs, sputum | 10 | 242 | 5 |
| Johnstone et al. [25] | 2008 | direct fluorescent antigen tests, RT-PCR | Nasopharyngeal swabs | 13 | 193 | 7 |
| Jennings et al. [26] | 2008 | PCR, serology, IFA, culture | Nasopharyngeal swabs, serum | 11 | 304 | 9 |
| Charles et al. [27] | 2008 | PCR | Nose and throat swabs | 8 | 885 | 5 |
| Saito et al. [32] | 2006 | PCR, serology | Sputum and serum | 5 | 232 | 7 |
| Angeles et al. [28] | 2006 | RT-PCR, IFA, culture | Nasopharyngeal swabs | 12 | 198 | 6 |
| Templeton et al. [29] | 2005 | RT-PCR | Throat washes and throat swab specimens | 12 | 105 | 8 |
| Macfarlane et al. [30] | 2001 | PCR, serology, culture | Throat swabs, serum | 7 | 316 | 7 |
BALF = Bronchial alveolar lavage fluid; IFA = indirect immunofluorescence assay; PSB = protected specimen brush; RT-PCR = real-time polymerase chain reaction; TBA = tracheobronchial aspirate.
Maximum score = 9.
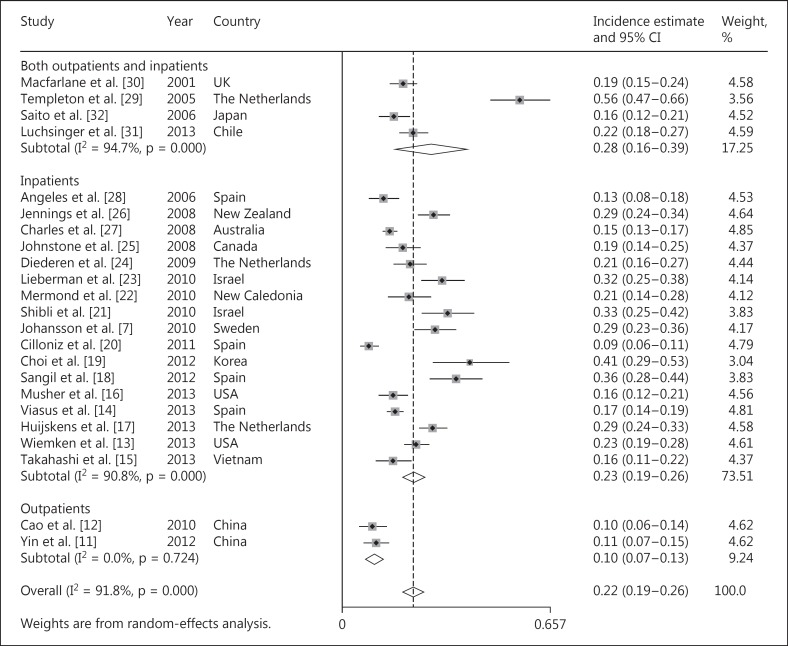
Incidence estimates of respiratory viruses in patients with CAP ranged from 8.6 to 56.2% (fig. 2); heterogeneity was considerable (χ2 = 269.9, p < 0.0001, I2 = 91.8%). The random-effects combined incidence was 22.4% (95% CI = 19.0-25.7, I2 = 91.8%). Considering the high level of heterogeneity, we further conducted a subgroup analysis for the separate calculation of combined incidences according to the types of patients (inpatients, outpatients and mixed patients), time span of patient enrollment (1 year vs. >1 year), economic level of the countries (developed vs. developing countries), PCR methods (routine PCR vs. real-time PCR) or according to the region where each report was conducted (Europe, Southeast Asia, Australia, America and the Middle East), as shown in table 2. The combined incidence of respiratory viruses was 10.2% (95% CI = 7.3-13.1, I2 = 0%) in outpatients [11, 12], 22.7% (95% = 19.0-26.4, I2 = 90.8%) in inpatients [7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 31, 32] and 27.7% (95% = 16.4-39.0, I2 = 94.7%) in mixed patients [29, 30]. In the subgroup analysis according to regions, the combined incidence was highest in the Middle East (32%, 95% CI = 27-38, I2 = 0%) [21, 23] and lowest in Southeast Asia (16.6%, 95% CI = 10.5-22.8, I2 = 85.1%) [11, 12, 15, 19, 32]. The combined incidence was 24.3% (95% CI = 20.3-28.3, I2 = 92.7%) and 15.8% (95% CI = 10.4-21.3, I2 = 84.2%) in developed countries and developing countries, respectively. In the subgroup analysis stratified by the time span of the studies, the combined incidence of respiratory viruses in the studies covering 1 year and >1 year was 23.6% (95% CI = 22.1-25.1, I2 = 92.6%) and 24.3% (95% CI = 22.5-26.2, I2 = 96.9%), respectively. In addition, considering that real-time PCR is more sensitive than routine PCR, which might lead to the significant heterogeneity in our study, we further performed a subgroup analysis stratified by PCR methods (routine PCR vs. real-time PCR). The combined incidence of respiratory viruses was 20.9% (95% CI = 17.1-24.6, I2 = 89.2%) in studies using routine PCR and 24.7% (95% CI = 18.1-31.3, I2 = 94.3%) in studies using real-time PCR.
Fig. 2.
The combined incidence of viral infections in adult patients with CAP.
Table 2.
Combined incidence estimates of viral infections in adult patients with CAP by geographical region
| Region | Incidence of respiratory viral infections, % | 95% CI | I2, % | X2 | p |
|---|---|---|---|---|---|
| Europe | 24.7 | 18.0-31.5 | 95.1 | 162.6 | 0.000 |
| Southeast Asia | 16.6 | 10.5-22.8 | 85.1 | 26.9 | 0.000 |
| Australia | 21.5 | 12.2-30.8 | 91.9 | 24.6 | 0.000 |
| America | 20.4 | 17.1-23.8 | 52.9 | 6.4 | 0.095 |
| Middle East | 32.4 | 27.1-37.6 | 0.0 | 0.1 | 0.763 |
Considering that the subgroup analysis cannot explain all sources of heterogeneity among the incidence of respiratory viruses in patients with CAP, we further carried out a univariate metaregression analysis (table 3) and found that the viral incidence was higher in studies in which lower respiratory tract specimens were collected for viral detection (p = 0.048) than in those in which only upper respiratory tract specimens were collected for viral detection. Additionally, we did a sensitivity analysis and found that the study by Cilloniz et al. [20] might have contributed to the heterogeneity. However, the recombined incidence of respiratory viruses after omission of that study was 23.0% (95% CI = 19.7-26.3, I2 = 90.5%), which was similar to the combined incidence before omission.
Table 3.
Univariate metaregression for the incidence of viral infections and viral infections mixed with other pathogens in adult patients with CAP
| Metaregression coefficient | 95% CI | p | |
|---|---|---|---|
| Incidence of respiratory viral infections in patients with CAP | |||
| LRI specimens | 0.091 | 0.001 to 0.18 | 0.048 |
| Region | 0.002 | -0.04 to 0.04 | 0.904 |
| Number of viral species detected | 0.008 | -0.01 to 0.02 | 0.273 |
| Number of viral detection methods | -0.019 | -0.10 to 0.06 | 0.620 |
| Time span | -0.017 | -0.05 to 0.02 | 0.350 |
| Incidence of respiratory viral coinfections with other pathogens in patients with CAP | |||
| LRI specimens | 0.049 | -0.03 to 0.13 | 0.198 |
| Region | -0.0004 | -0.03 to 0.03 | 0.975 |
| Viral species detected, n | 0.006 | -0.01 to 0.02 | 0.314 |
| Viral detection methods, n | -0.004 | -0.05 to 0.04 | 0.843 |
| Time span, year | 0.0008 | -0.10 to 0.10 | 0.986 |
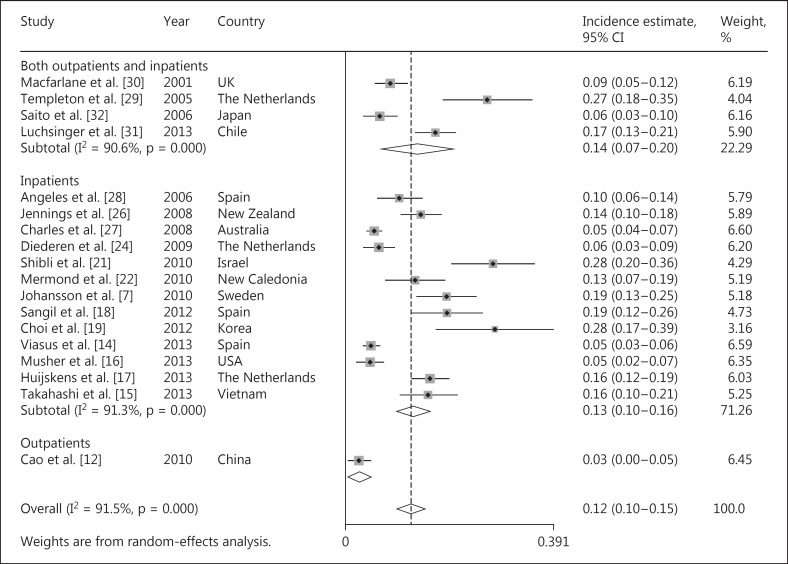
Of the 23 reports, 18 studies [7, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 24, 26, 27, 28, 29, 30, 31, 32] provided data for the calculation of the coinfection incidences of respiratory viruses with other pathogens (n = 5,058). As shown in figure 3, the incidence estimates of coinfections ranged from 3 to 28%; heterogeneity was considerable (χ2 = 200.9, p < 0.0001, I2 = 91.5%). The combined overall incidence of coinfections of respiratory viruses with other pathogens (mainly bacteria) was 12.4% (95% CI = 9.7-15.0, I2 = 91.5%). Due to the high level of heterogeneity, the subgroup analysis was performed according to the patient types, economic level of the countries and regions where the studies were performed, as mentioned above. The combined incidence of coinfections was 12.9% (95% CI = 9.7-16.1, I2 = 91.3%) in inpatients with CAP [7, 14, 15, 16, 17, 18, 19, 21, 22, 24, 26, 27, 28] and 13.7% (95% CI = 7.1-20.3, I2 = 90.6%) in mixed patients with CAP [29, 30, 31, 32]. Since only 1 study provided the incidence of coinfections in outpatients with CAP [12], a combined estimate was not performed. In the subgroup analysis stratified by the time span of the studies, the combined incidence of respiratory viruses in the studies covering 1 year and >1 year was 12.5% (95% CI = 9.2-15.7, I2 = 91.3%) and 12.4% (95% CI = 9.7-15.0, I2 = 94.1%), respectively. Additionally, the combined incidence of coinfections was 12.3% (95% CI = 9.3-15.3, I2 = 91.1%) and 12.4% (95% CI = 9.7-15.0, I2 = 93.6%) in developed countries and developing countries, respectively. Moreover, the combined incidence of coinfections was similar between Europe [7, 14, 17, 18, 24, 28, 29, 30], Southeast Asia [12, 15, 19, 32] and Australia [22, 26, 27] (12.9, 11.4 and 10.6%, respectively) as shown in table 4. In the individual variable metaregression analysis, none of the factors we explored further was significantly associated with heterogeneity (table 3). Moreover, in the sensitivity analysis, none of the studies were identified as contributing to the heterogeneity.
Fig. 3.
The combined incidence of viral infections mixed with other pathogens in adult patients with CAP.
Table 4.
Combined incidence estimates of viral infections mixed with other pathogens in adult patients with CAP by geographical region
| Region | Incidence of viral infections mixed with other pathogens, % | 95% CI | I2, % | χ2 | p |
|---|---|---|---|---|---|
| Europe | 12.9 | 8.6-17.3 | 91.4 | 81.7 | 0.000 |
| Southeast Asia | 11.4 | 4.2-18.6 | 91.0 | 22.2 | 0.000 |
| Australia | 10.6 | 3.8-17.3 | 91.0 | 22.2 | 0.000 |
| America | 10.7 | 0.0-22.6 | 96.2 | 26.5 | 0.000 |
| Middle East | 27.8 | 20.0-35.6 | – | - | - |
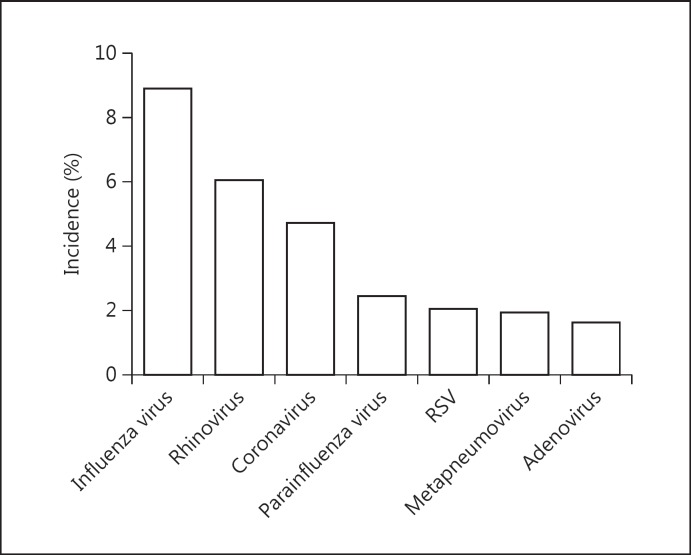
Taking into account the discrepancy of incidence among respiratory virus species, we extracted the incidence of the 7 most common respiratory viral species for an individual combined incidence estimate. As shown in figure 4 and table 5, the 3 most frequently detected viruses were the influenza virus (8.9%, 95% CI = 7-11, I2 = 79.7%), rhinovirus (6.0%, 95% CI = 4-8, I2 = 87.4%) and coronavirus (4.7%, 95% CI = 3-7, I2 = 77.4%). However, the respiratory syncytial virus, metapneumovirus and adenovirus were less commonly detected.
Fig. 4.
Discrepancies of the combined incidence across the common respiratory viral species. RSV = Respiratory syncytial virus.
Table 5.
Discrepancies of the combined incidence across the common respiratory viral species
| Viral species | Incidence, % | 95% CI | I2, % | χ2 | p |
|---|---|---|---|---|---|
| Influenza virus | 8.9 | 7.1-10.6 | 79.7 | 83.6 | 0.000 |
| Rhinovirus | 6.0 | 4.3-7.7 | 87.4 | 111.2 | 0.000 |
| Coronavirus | 4.7 | 2.9-6.6 | 77.4 | 39.8 | 0.000 |
| Parainfluenza virus | 2.4 | 1.4-3.4 | 75.9 | 49.7 | 0.000 |
| RSV | 2.0 | 1.3-2.7 | 74.4 | 62.4 | 0.000 |
| Metapneumovirus | 1.9 | 1.0-2.8 | 48.5 | 15.5 | 0.049 |
| Adenovirus | 1.6 | 0.9-2.4 | 65.9 | 29.4 | 0.001 |
RSV = Respiratory syncytial virus.
Discussion
Our meta-analysis of the incidence of respiratory viral infections in adult patients with CAP identified 23 studies of 6,404 individuals. At present, there is a lack of quantitative syntheses of studies regarding the incidence of respiratory viral infections in adult patients with CAP worldwide. Our main finding is that respiratory viruses contribute to approximately 1/5 infections in adult patients presenting with CAP, highlighting their role in the pathogenesis of CAP. Furthermore, the influenza virus is the most frequent respiratory viral etiology in adult patients with CAP.
The incidence of viral infections in adult outpatients with CAP is lower than that in adult inpatients (10 vs. 22%) as demonstrated in the subgroup analysis, implying that viral infections could aggravate the condition of patients presenting with pneumonia. Moreover, the combined incidence of viral infections mixed with other pathogens (mainly bacteria) is 12% in our study, constituting more than half of the total viral infections in adult patients with CAP. Evidence from several studies illustrated that viral infections made hosts susceptible to secondary bacterial infections because mucosal barriers in the respiratory tracts were impaired during previous viral infections [33, 34], which led to the bacterial invasions becoming more facile. There is evidence as well, although sparse, suggesting that mixed viral-bacterial infections would generate a severer inflammatory status and clinical presentation than individual bacterial or viral infections [24, 26, 35]. Severe fatal pneumonia can be caused by concomitant infection with the influenza virus and Staphylococcus aureus in both children and adults [36, 37, 38]. Moreover, in 1 pneumonia study focused on adults, rhinovirus-pneumococcal coinfections correlated with severe pneumonia and raised mortality [24, 26, 35]. Therefore, our results would be helpful for clinicians to better recognize the burden of viral infections and mixed viral-bacterial infections on adult patients presenting with CAP.
The influenza virus is the most frequent viral pathogen detected in adult patients with CAP as our study demonstrates, which is inconsistent with the fact that the respiratory syncytial virus is the leading viral pathogen in pediatric patients with CAP [39, 40, 41, 42]. Influenza vaccination has been previously proven to be efficient in preventing influenza viral infections especially during a pandemic influenza period [43]. Furthermore, several studies which enrolled patients with a chronic lung disease indicated that an influenza vaccination significantly reduced the incidence of pneumonia or acute exacerbations in those patients [44]. Therefore, a regular and routine influenza vaccination of patients with chronic lung disease would be needed to lower the burden of pneumonia caused by the influenza virus. Moreover, newly developed anti-influenza virus drugs (neuraminidase inhibitors, such as oseltamivir and zanamivir), which have established roles in the early treatment of influenza infections through reducing the median time to the resolution of the symptoms and the risk of pneumonia [45], provided an efficient weapon for clinicians to manage patients with viral pneumonia caused by the influenza virus.
Respiratory viruses have been realized as a common etiology in patients with CAP, especially children with CAP. However, conventional virological diagnostic techniques such as serology, immunofluorescence and culture have been underestimating the contribution of viruses to the pathogenesis of CAP [46, 47], especially in adult patients, due to their low sensitivity and narrow application. Owing to the development of more rapid and sensitive detection methods (PCR and real-time PCR), the viral diagnosis is largely improved, particularly through the combined application of these methods with conventional detection techniques [7]. Lots of previous studies evaluating the role of respiratory viruses in adult patients with CAP have been reported; nevertheless, there are discrepancies in the detection methods used for viral diagnosis across those studies. Therefore, we only included studies applying sensitive detection techniques (PCR and real-time PCR) for viral diagnosis in our further meta-analysis to reduce possible heterogeneity along with obtaining quantitative syntheses of original data.
Nevertheless, significant heterogeneity was still found during the meta-analysis and subgroup analysis. Our following heterogeneity analyses generated 1 potentially important finding, namely that lower respiratory tract specimens were associated with higher overall viral incidence. This may be because the molecular detection of the virus in lower respiratory tract specimens was more sensitive than the one in upper respiratory tract specimens. The potential implication of this result is that the presence of viral nucleic acids, detected by PCR of lower respiratory tract samples, implies stronger evidence of the infection by virus. However, no relation was identified between the incidence of coinfection and the lower respiratory tract specimens, which might be explainable. As we could speculate, discrepancies of both bacterial diagnostic and viral detection would lead to a significant heterogeneity of the incidence of coinfection. Caution is necessary when combined incidence estimates are used due to the significant heterogeneity, and further surveys are required for the standardization of the specimen collection, specimen quality, PCR primers and PCR reaction conditions during respiratory viral etiology diagnosis.
Our study had several limitations. First, we excluded many studies in the non-English literature and thus lost the raw data of those reports. Second, studies included in our meta-analysis were predominantly performed in high-income and middle-income regions. Therefore, fundamental incidence data for low-income countries are missing. Third, due to the lack of associated original data we were unable to explore the relationship between clinical severity of pneumonia and causative viral pathogens.
Thus, our study suggests that approximately one fifth of adult patients with CAP are infected by respiratory viruses, more than half of those viral infections are characterized as mixed infections with other pathogens, and influenza virus is the leading viral pathogen in adult patients with CAP, although there is significant heterogeneity during the quantitative synthesis of the raw data. Further surveys are required to establish a standardization of specimen management and detection processes for viral diagnostics.
Financial Disclosure and Conflicts of Interest
The authors have no support or funding to report. They declare that there are no competing interests.
References
- 1.WHO: The top 10 causes of death. 2013 http://who.int/mediacentre/factsheets/fs310/en/.
- 2.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, Wani M, Woodhead MA. BTS guidelines for the management of community-acquired pneumonia in adults: update 2009. Thorax. 2009;64((suppl 3)):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44((suppl 2)):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durrington HJ, Summers C. Recent changes in the management of community-acquired pneumonia in adults. BMJ. 2008;336:1429–1433. doi: 10.1136/bmj.a285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis. 2011;52((suppl 4)):S296–S304. doi: 10.1093/cid/cir045. [DOI] [PubMed] [Google Scholar]
- 6.Moran GJ, Rothman RE, Volturo GA. Emergency management of community-acquired bacterial pneumonia: what is new since the 2007 Infectious Diseases Society of America/American Thoracic Society guidelines. Am J Emerg Med. 2013;31:602–612. doi: 10.1016/j.ajem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiveljung-Lindell A, Rotzen-Ostlund M, Gupta S, Ullstrand R, Grillner L, Zweygberg-Wirgart B, Allander T. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol. 2009;81:167–175. doi: 10.1002/jmv.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin YD, Zhao F, Ren LL, Song SF, Liu YM, Zhang JZ, Cao B. Evaluation of the Japanese Respiratory Society guidelines for the identification of Mycoplasma pneumoniae pneumonia. Respirology. 2012;17:1131–1136. doi: 10.1111/j.1440-1843.2012.02227.x. [DOI] [PubMed] [Google Scholar]
- 12.Cao B, Ren LL, Zhao F, Gonzalez R, Song SF, Bai L, Yin YD, Zhang YY, Liu YM, Guo P, Zhang JZ, Wang JW, Wang C. Viral and Mycoplasma pneumoniae community-acquired pneumonia and novel clinical outcome evaluation in ambulatory adult patients in China. Eur J Clin Microbiol Infect Dis. 2010;29:1443–1448. doi: 10.1007/s10096-010-1003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiemken T, Peyrani P, Bryant K, Kelley RR, Summersgill J, Arnold F, Carrico R, McKinney WP, Jonsson C, Carrico K, Ramirez J. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the Severe Influenza Pneumonia Surveillance (SIPS) project. Eur J Clin Microbiol Infect Dis. 2013;32:705–710. doi: 10.1007/s10096-012-1802-8. [DOI] [PubMed] [Google Scholar]
- 14.Viasus D, Marinescu C, Villoslada A, Cordero E, Galvez-Acebal J, Farinas MC, Gracia-Ahufinger I, Fernandez-Navarro A, Niubo J, Ortega L, Munez-Rubio E, Romero-Gomez MP, Carratala J. Community-acquired pneumonia during the first post-pandemic influenza season: a prospective, multicentre cohort study. J Infect. 2013;67:185–193. doi: 10.1016/j.jinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Suzuki M, Minh le N, Anh NH, Huong LT, Son TV, Long PT, Ai NT, Tho le H, Morimoto K, Kilgore PE, Anh DD, Ariyoshi K, Yoshida LM. The incidence and aetiology of hospitalised community-acquired pneumonia among Vietnamese adults: a prospective surveillance in central Vietnam. BMC Infect Dis. 2013;13:296. doi: 10.1186/1471-2334-13-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect. 2013;67:11–18. doi: 10.1016/j.jinf.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huijskens EG, van Erkel AJ, Palmen FM, Buiting AG, Kluytmans JA, Rossen JW. Viral and bacterial aetiology of community-acquired pneumonia in adults. Influenza Other Respir Viruses. 2013;7:567–573. doi: 10.1111/j.1750-2659.2012.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangil A, Calbo E, Robles A, Benet S, Viladot ME, Pascual V, Cuchi E, Perez J, Barreiro B, Sanchez B, Torres J, Canales L, De Marcos JA, Garau J. Aetiology of community-acquired pneumonia among adults in an H1N1 pandemic year: the role of respiratory viruses. Eur J Clin Microbiol Infect Dis. 2012;31:2765–2772. doi: 10.1007/s10096-012-1626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SH, Hong SB, Ko GB, Lee Y, Park HJ, Park SY, Moon SM, Cho OH, Park KH, Chong YP, Kim SH, Huh JW, Sung H, Do KH, Lee SO, Kim MN, Jeong JY, Lim CM, Kim YS, Woo JH, Koh Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 20.Cilloniz C, Ewig S, Ferrer M, Polverino E, Gabarrus A, Puig de la Bellacasa J, Mensa J, Torres A. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care. 2011;15:R209. doi: 10.1186/cc10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibli F, Chazan B, Nitzan O, Flatau E, Edelstein H, Blondheim O, Raz R, Colodner R. Etiology of community-acquired pneumonia in hospitalized patients in northern Israel. Isr Med Assoc J. 2010;12:477–482. [PubMed] [Google Scholar]
- 22.Mermond S, Berlioz-Arthaud A, Estivals M, Baumann F, Levenes H, Martin PM. Aetiology of community-acquired pneumonia in hospitalized adult patients in New Caledonia. Trop Med Int Health. 2010;15:1517–1524. doi: 10.1111/j.1365-3156.2010.02653.x. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diederen BM, Van Der Eerden MM, Vlaspolder F, Boersma WG, Kluytmans JA, Peeters MF. Detection of respiratory viruses and Legionella spp by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand J Infect Dis. 2009;41:45–50. doi: 10.1080/00365540802448799. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134:1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, Young SA, Chambers ST, Murdoch DR. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 27.Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, Holmes PW, Christiansen KJ, Waterer GW, Pierce RJ, Mayall BC, Armstrong JG, Catton MG, Nimmo GR, Johnson B, Hooy M, Grayson ML. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 28.Angeles Marcos M, Camps M, Pumarola T, Antonio Martinez J, Martinez E, Mensa J, Garcia E, Penarroja G, Dambrava P, Casas I, Jimenez de Anta MT, Torres A. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11:351–359. [PubMed] [Google Scholar]
- 29.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macfarlane J, Holmes W, Gard P, Macfarlane R, Rose D, Weston V, Leinonen M, Saikku P, Myint S. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax. 2001;56:109–114. doi: 10.1136/thorax.56.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luchsinger V, Ruiz M, Zunino E, Martinez MA, Machado C, Piedra PA, Fasce R, Ulloa MT, Fink MC, Lara P, Gebauer M, Chavez F, Avendano LF. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68:1000–1006. doi: 10.1136/thoraxjnl-2013-203551. [DOI] [PubMed] [Google Scholar]
- 32.Saito A, Kohno S, Matsushima T, Watanabe A, Oizumi K, Yamaguchi K, Oda H. Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother. 2006;12:63–69. doi: 10.1007/s10156-005-0425-8. [DOI] [PubMed] [Google Scholar]
- 33.Van der Sluijs KF, van der Poll T, Lutter R, Juffermans NP, Schultz MJ. Bench-to-bedside review: bacterial pneumonia with influenza - pathogenesis and clinical implications. Crit Care. 2010;14:219. doi: 10.1186/cc8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wunderink RG. Influenza and bacterial pneumonia - constant companions. Crit Care. 2010;14:150. doi: 10.1186/cc8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, Uyeki T. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122:805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 37.Reed C, Kallen AJ, Patton M, Arnold KE, Farley MM, Hageman J, Finelli L. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. 2009;28:572–576. doi: 10.1097/INF.0b013e31819d8b71. [DOI] [PubMed] [Google Scholar]
- 38.Seki M, Kosai K, Yanagihara K, Higashiyama Y, Kurihara S, Izumikawa K, Miyazaki Y, Hirakata Y, Tashiro T, Kohno S. Disease severity in patients with simultaneous influenza and bacterial pneumonia. Intern Med. 2007;46:953–958. doi: 10.2169/internalmedicine.46.6364. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento-Carvalho CM, Ribeiro CT, Cardoso MR, Barral A, Araujo-Neto CA, Oliveira JR, Sobral LS, Viriato D, Souza AL, Saukkoriipi A, Paldanius M, Vainionpaa R, Leinonen M, Ruuskanen O. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatr Infect Dis J. 2008;27:939–941. doi: 10.1097/INF.0b013e3181723751. [DOI] [PubMed] [Google Scholar]
- 40.Cevey-Macherel M, Galetto-Lacour A, Gervaix A, Siegrist CA, Bille J, Bescher-Ninet B, Kaiser L, Krahenbuhl JD, Gehri M. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168:1429–1436. doi: 10.1007/s00431-009-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Garcia ML, Calvo C, Pozo F, Villadangos PA, Perez-Brena P, Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 42.Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, Fossali E, Pelucchi C, Principi N. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses. 2013;7:18–26. doi: 10.1111/j.1750-2659.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 44.Furumoto A, Ohkusa Y, Chen M, Kawakami K, Masaki H, Sueyasu Y, Iwanaga T, Aizawa H, Nagatake T, Oishi K. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine. 2008;26:4284–4289. doi: 10.1016/j.vaccine.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Liao Q, Yuan Y, Zhou L, Xiang N, Huai Y, Guo X, Zheng Y, van Doorn HR, Farrar J, Gao Z, Feng Z, Wang Y, Yang W. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. doi: 10.1136/bmj.c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Kleemola M, Koskela M, Leinonen M, Ronnberg PR, Saikku P, Sten M, Tarkiainen A, Tukiainen H, Pyorala K, Makela PH. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis. 2001;32:1141–1154. doi: 10.1086/319746. [DOI] [PubMed] [Google Scholar]
- 47.Ishida T, Hashimoto T, Arita M, Ito I, Osawa M. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest. 1998;114:1588–1593. doi: 10.1378/chest.114.6.1588. [DOI] [PubMed] [Google Scholar]