Abstract
Background:
Thymic malignancies are rare and there are limited contemporary population-based epidemiological studies for this uncommon cancer.
Methods:
Adults aged 20 years and older diagnosed with thymic malignancies between 1988-2015 were identified from the California Cancer Registry (n=1,588). Trends in age-adjusted incidence rates were examined overall and by race/ethnicity, and the proportion diagnosed by stage was evaluated over time. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for overall survival (OS), and Fine and Gray competing risks regression for cause-specific survival (CSS).
Results:
Age-adjusted incidence increased on average 2.08% per year over the study period (95% CI: 1.30%, 2.86%; p < 0.0001), with an incidence of 0.277 cases per 100,000 in 2015. Incidence was highest among Asians/Pacific Islanders and non-Hispanic blacks. The proportion of unknown stage at diagnosis declined as localized diagnoses increased over time. Compared to patients with thymoma, those with thymic carcinoma had significantly worse OS (HR=1.63, 95% CI:1.33-2.01, p<0.0001) and CSS (Sub-distribution HR=2.99, 95% CI: 2.29-3.91, p<0.0001). Advanced stage at diagnosis was also associated with worse survival. Surgical intervention was associated with better prognosis for patients with localized (HR = 0.08, 95% CI: 0.02-0.30, p = 0.0002) or regional disease (HR = 0.14, 95% CI: 0.06-0.34, p < 0.0001).
Conclusion:
Thymic malignancy incidence is increasing in California. There was incidence variation across race/ethnicity, which warrants future study. These findings provide contemporary insight into the incidence and prognostic factors of thymic malignancies.
Micro Abstract:
Due to limited population-based epidemiological studies on thymic malignancies in the current literature, we sought to evaluate incidence and survival trends in thymic malignancies in the California Cancer Registry. Among 1,588 adult cases of thymic malignancy diagnosed between 1988 and 2015, we found that thymic malignancy incidence is rising and variation in incidence between race/ethnicity. As in prior studies, advanced stage and thymic carcinoma were found to be associated with worsened survival. There also appears to be a trend towards detecting more localized stage disease over time, possibly due to the increased use of thoracic imaging studies. Treatment with surgery was associated with improved OS in all stages of disease and improved CSS in local and regional disease. Further research is required to evaluate and better understand contemporary incidence and prognostic factors in thymic malignancies.
Introduction
The thymus is a primary lymphoid organ located in the superior anterior mediastinum within which T cells mature.1 Tumors of the thymus are rare, and only represent 0.2% to 1.5% of all malignancies.2 Nonetheless, thymic malignancies are the most common primary anterior mediastinal tumor as they account for up to 50% of all such tumors.3 Up to 50% of patients with thymoma may present with a paraneoplastic syndrome such as myasthenia gravis.4 Thymic carcinoma is a more aggressive thymic epithelial tumor with higher potential for local and distant spread, often presenting with metastatic (bone, lungs, pleura, or liver) or lymphatic disease.5
Previous published population-based epidemiological studies in the United States on thymic malignancies are limited. In 2003, Engels and Pfeiffer described demographic patterns of thymic malignancy incidence in the United States and studied nine states and metropolitan regions: Connecticut, Hawaii, Iowa, New Mexico, Utah, as well as metropolitan Atlanta, Detroit, Seattle and San Francisco/Oakland.6 Their study of 849 cases diagnosed between 1973 and 1998 found that the incidence of thymomas is 0.15 per 100,000 person-years in the United States based off data collected by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. In addition, the study also found that thymoma incidence is higher in blacks and Asian/Pacific Islanders than in whites or Hispanics. A follow-up study in 2010 by Engels suggested a decrease in the incidence of thymoma, particularly from 1998 to 2006. 7 Using data from the CCR between 1988-2015, we sought to provide a contemporary update on incidence and trends in thymic malignancies in the state of California, the largest state by population in the U.S. In addition, we aimed to evaluate prognostic factors for thymic malignancies and assess how treatment impacts survival across stages at diagnosis.
Methods
Data in this study were obtained from the California Cancer Registry (CCR), the largest population-based state cancer registry in the U.S. The CCR contains demographic, diagnostic, initial treatment, and outcome information on all reportable cancers diagnosed in California residents since January 1988. The registry was queried to identify patients who were at least 20 years old when diagnosed with a first primary invasive tumor of the thymus (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] site codes C37.9 and histology codes 8580-8586) in California between 1988 and 2015. Autopsy and death certificate only diagnoses were excluded.
Age at diagnosis was categorized into 20-49 years, 50-64 years, and 65+ years. Race/ethnicity was categorized as non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, or Asian/Pacific Islander (API). Neighborhood socioeconomic status (nSES) was based on U.S. Census data on neighborhood characteristics of the patient address at the time of diagnosis, including educational attainment, occupation type, employment rate, median household income, poverty level, median rent, and house values. For patients diagnosed 1988-2005, nSES was estimated using census-block group data from the Census 2000 Summary File. For cases diagnosed 2006-2015, the American Community Survey was used to compute nSES. These two sources were combined to form quintiles at the block group level across the state. To ensure stable estimates for stratified analysis, the 28 years in the study period were collapsed into seven four-year periods.
Stage at diagnosis was based on Surveillance, Epidemiology, and End Results (SEER) summary staging and was categorized as localized (non-invasive or invasive disease confined to the thymus), regional (extension to adjacent tissues or the organs/structures in mediastinum), and remote (involvement of distant lymph nodes or metastasis). Both thymoma (ICD-O-3 codes 8580-8585) and thymic carcinoma (code 8586) were included. First course of treatment was defined as cancer-directed therapy documented in a patient’s medical record and given before disease progression, recurrence, or treatment failure. Treatment was categorized as receipt of: 1) chemotherapy and/or radiation (no surgery), 2) surgery only, 3) surgery plus single modality treatment (chemotherapy or radiation), 4) surgery, chemotherapy, and radiation, and 5) no treatment.
Age-adjusted incidence rates were calculated overall and by race/ethnicity in SEER*Stat version 8.3.5, and trends were analyzed with Joinpoint Regression Program 4.6.0. The proportion of cases diagnosed by stage at diagnosis was assessed over time. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for overall survival (OS), and Fine and Gray competing risks regression was used to calculate sub-distribution hazard ratios (SHRs) for thymic malignancy cause-specific survival (CSS). Models were adjusted for sex, age at diagnosis, nSES, stage at diagnosis, histology, and first course of treatment. To better account for treatment differences by stage, an additional analysis was conducted stratified by stage at diagnosis. Survival analysis was conducted using SAS version 9.4.
The CCR database is linked annually to the National Death Index, hospital discharge data, Medicare files, the Department of Motor Vehicles, and other administrative databases to ensure accurate vital status and cause of death information. SEER’s cause-specific death classification was used to determine if the patient died of their cancer or another cause. We have included this discussion in the Methods section of the manuscript.
Results
Between 1988 and 2015, a total of 1,588 adult cases of thymic malignancy were identified in California (Table 1). Males represented 57.8% of these cases, whereas females represented 42.2%. Just over half (51.8%) of patients were NHW, while 23.9% were API. As nSES quintile increased, so did the number of patients—individuals from the highest nSES quintile constituted the largest proportion of cases (26.0%) while the lowest nSES constituted the smallest proportion (12.1%). Most cases were diagnosed with thymoma (87.1%) and nearly three-quarters (72.5%) of patients received surgery as part of the first course of treatment.
Table 1.
Characteristics of adults aged 20 and older diagnosed with invasive thymus cancer in California, 1988-2015.
| N | % | |
|---|---|---|
| Total | 1588 | 100.0 |
| Sex | ||
| Male | 918 | 57.8 |
| Female | 670 | 42.2 |
| Age at Diagnosis | ||
| 20-49 years | 516 | 32.5 |
| 50-64 years | 539 | 33.9 |
| 65+ years | 533 | 33.6 |
| Race/Ethnicity | ||
| Non-Hispanic White | 823 | 51.8 |
| Non-Hispanic Black | 146 | 9.2 |
| Hispanic | 229 | 14.4 |
| Asian/Pacific Islander | 379 | 23.9 |
| Other/Unknown | 11 | 0.7 |
| Neighborhood Socioeconomic Status | ||
| Lowest | 192 | 12.1 |
| Lower-Middle | 254 | 16.0 |
| Middle | 350 | 22.0 |
| Upper-Middle | 350 | 22.0 |
| Highest | 413 | 26.0 |
| Unknown | 29 | 1.8 |
| Year of Diagnosis | ||
| 1988-1991 | 123 | 7.7 |
| 1992-1995 | 180 | 11.3 |
| 1996-1999 | 157 | 9.9 |
| 2000-2003 | 232 | 14.6 |
| 2004-2007 | 266 | 16.8 |
| 2008-2011 | 308 | 19.4 |
| 2012-2015 | 322 | 20.3 |
| Stage at Diagnosis | ||
| Localized | 367 | 23.1 |
| Regional | 705 | 44.4 |
| Remote | 394 | 24.8 |
| Unknown | 122 | 7.7 |
| Histology | ||
| Thymoma | 1383 | 87.1 |
| Thymic Carcinoma | 205 | 12.9 |
| Treatment | ||
| No Treatment | 109 | 6.9 |
| Chemotherapy and/or Radiation | 300 | 18.9 |
| Surgery Only | 434 | 27.3 |
| Surgery and Single Modality | 508 | 32.0 |
| Surgery, Chemotherapy, and Radiation | 209 | 13.2 |
| Unknown | 28 | 1.8 |
The age-adjusted incidence rate of thymus malignancies significantly increased from 0.191 cases per 100,000 in 1988 to 0.277 cases per 100,000 in 2015 (Table 2), with an estimated annual percent change (APC) of 2.08% per year over the study period (95% CI: 1.30-2.87, p<0.0001). As shown in Table 3, incidence varies by race/ethnicity, with rates highest among APIs. Between 2012 and 2015, the incidence rate among APIs was 0.514 per 100,000 (95% CI 0.414-0.632), compared to 0.381 per 100,000 in NHBs (95% CI 0.251-0.556), 0.241 per 100,000 in NHWs (95% CI 0.201-0.286), and 0.188 per 100,000 in Hispanics (95% CI 0.14-0.248). Though incidence appeared to increase for each race/ethnicity group over the study period, the yearly increase only reached statistical significance for NHWs (APC= 1.65%, 95% CI: 0.23-3.10, p=0.0306).
Table 2.
Age-adjusted incidence rates* of thymus cancer among adults aged 20 years and older in California, 1988-2015 (n=1,588)
| Year of Diagnosis | Age-Adjusted Rate (95% CI) | Number of Cases |
|---|---|---|
| 1988 | 0.191 (0.131, 0.268) | 34 |
| 1989 | 0.160 (0.106, 0.231) | 29 |
| 1990 | 0.157 (0.104, 0.225) | 29 |
| 1991 | 0.157 (0.106, 0.224) | 31 |
| 1992 | 0.233 (0.168, 0.314) | 44 |
| 1993 | 0.179 (0.124, 0.250) | 35 |
| 1994 | 0.217 (0.158, 0.292) | 45 |
| 1995 | 0.280 (0.212, 0.365) | 56 |
| 1996 | 0.184 (0.130, 0.254) | 38 |
| 1997 | 0.203 (0.146, 0.275) | 42 |
| 1998 | 0.211 (0.154, 0.283) | 45 |
| 1999 | 0.147 (0.100, 0.208) | 32 |
| 2000 | 0.279 (0.214, 0.358) | 62 |
| 2001 | 0.289 (0.224, 0.368) | 66 |
| 2002 | 0.214 (0.158, 0.283) | 49 |
| 2003 | 0.234 (0.176, 0.305) | 55 |
| 2004 | 0.245 (0.186, 0.317) | 58 |
| 2005 | 0.265 (0.204, 0.338) | 65 |
| 2006 | 0.311 (0.244, 0.389) | 76 |
| 2007 | 0.264 (0.204, 0.336) | 67 |
| 2008 | 0.312 (0.247, 0.389) | 81 |
| 2009 | 0.304 (0.240, 0.380) | 79 |
| 2010 | 0.285 (0.224, 0.357) | 77 |
| 2011 | 0.255 (0.198, 0.323) | 71 |
| 2012 | 0.238 (0.184, 0.304) | 67 |
| 2013 | 0.281 (0.222, 0.351) | 80 |
| 2014 | 0.301 (0.240, 0.372) | 88 |
| 2015 | 0.277 (0.221, 0.343) | 87 |
Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard.
Table 3:
Age-adjusted incidence rates* of thymus cancer among adults aged 20 years and older in California by race/ethnicity, 1988-2015.
| Year of Diagnosis |
Rate in NHW | Rate in NHB | Rate in Hispanic |
Rate in API |
|---|---|---|---|---|
| 1988-1991 | 0.176 (95% CI 0.141-0.217) | ^ | ^ | 0.286 (95% CI 0.16-0.474) |
| 1992-1995 | 0.191 (95% CI 0.155-0.233) | 0.351 (95% 0.202-0.568) | 0.17 (95% CI 0.105-0.263) | 0.537 (95% CI 0.386-0.731) |
| 1996-1999 | 0.159 (95% 0.127-0.197) | 0.302 (95% CI 0.175-0.49) | 0.138 (95% CI 0.036-0.077) | 0.368 (95% CI 0.253-0.517) |
| 2000-2003 | 0.243 (95% CI 0.203-0.289) | 0.419 (95% CI 0.262-0.634) | 0.116 (95% CI 0.07-0.182) | 0.499 (95% CI 0.373-0.653) |
| 2004-2007 | 0.250 (95% CI 0.209-0.297) | 0.44 (95% CI 0.286-0.648) | 0.204 (95% CI 0.144-0.28) | 0.453 (95% CI 0.343-0.586) |
| 2008-2011 | 0.251 (95% CI 0.21-0.298) | 0.452 (95% CI 0.301-0.654) | 0.244 (95% CI 0.14-0.248) | 0.491 (95% CI 0.387-0.615) |
| 2012-2015 | 0.241 (95% CI 0.201-0.286) | 0.381 (95% CI 0.251-0.556) | 0.188 (CI 0.14-0.248) | 0.514 (95% CI 0.414-0.632) |
NHW, Non-Hispanic White; NWB, Non-Hispanic Black; API, Asian/Pacific Islander
Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups – Census P25-1130) standard
Statistic not displayed due to fewer than 15 cases
The proportion of patients diagnosed with localized disease increased, from 10.6% between 1988-1991 to 32.6% between 2012-2015 (Table 4). This rise in early-stage diagnoses appeared to coincide with a notable decline in diagnoses of unknown stage, from 19.5% between 1988-1991 to 3.4% between 2012-2015. The proportion of regional diagnoses also decreased, though these still constituted the highest percentage of diagnoses in the population. There was no clear pattern in the proportion of remote diagnoses over time; about one-fifth to one-quarter of patients were diagnosed with advanced disease throughout the study period. Of note, the proportion of disease staging at the time of diagnosis was not different by SES.
Table 4.
Proportion of adults aged 20 years and older diagnosed with thymic malignancies in California by stage at diagnosis, 1988-2015.
| Localized (n=367) | Regional (n=705) | Remote (n=394) | Unknown (n=122) | |||||
|---|---|---|---|---|---|---|---|---|
| Year of Diagnosis | N | Row% | N | Row% | N | Row% | N | Row% |
| 1988-1991 | 13 | 10.6% | 60 | 48.8% | 26 | 21.1% | 24 | 19.5% |
| 1992-1995 | 34 | 18.9% | 84 | 46.7% | 29 | 16.1% | 33 | 18.3% |
| 1996-1999 | 30 | 19.1% | 83 | 52.9% | 29 | 18.5% | 15 | 9.6% |
| 2000-2003 | 35 | 15.1% | 109 | 47.0% | 68 | 29.3% | 20 | 8.6% |
| 2004-2007 | 66 | 24.8% | 124 | 46.6% | 66 | 24.8% | 10 | 3.8% |
| 2008-2011 | 84 | 27.3% | 125 | 40.6% | 90 | 29.2% | 9 | 2.9% |
| 2012-2015 | 105 | 32.6% | 120 | 37.3% | 86 | 26.7% | 11 | 3.4% |
The predictors of OS and CSS are displayed in Table 5. Women had significantly better survival than men with respect to OS, but this effect became insignificant for CSS. Similarly, older age at diagnosis was associated with worse OS, but age was not associated with CSS. There did not appear to be any significant differences in OS by race/ethnicity, but Hispanic patients had significantly worse CSS than NHWs (SHR=1.70, 95% CI:1.24-2.32, p=0.0009). Lower nSES appears to be associated with worse OS compared to the highest nSES group, but nSES was not significantly associated with death due to thymic malignancies. For both OS and CSS, prognosis was worse with later stage at diagnosis, with patients diagnosed at the remote stage having nearly 3 times worse survival from any cause (HR=2.83, 95% CI: 2.18-3.66, p<0.0001) and over 5 times the hazard of death from thymic malignancies (SHR=5.44, 95% CI: 3.24-9.15) compared to patients with localized disease. Compared to patients with thymoma, those with thymic carcinoma had significantly worse OS (HR=1.63, 95% CI:1.33-2.01, p<0.0001) and CSS (SHR=2.99, 95% CI: 2.29-3.91, p<0.0001) consistent with previous studies.8,9
Table 5:
Overall and cause-specific survival of adults aged 20 years and older diagnosed with thymus cancer in California, 1988-2015.
| Overall Survival | Cause-Specific Survival | |||
|---|---|---|---|---|
| HR (95% CI) | P-Value | SHR (95% CI) | P-Value | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.85 (0.73, 0.99) | 0.0419* | 0.90 (0.71, 1.14) | 0.3825 |
| Age at Diagnosis | ||||
| 20-49 years | Reference | Reference | ||
| 50-64 years | 1.22 (1.01, 1.48) | 0.0448* | 1.04 (0.79, 1.36) | 0.7824 |
| 65+ years | 2.13 (1.76, 2.58) | <0.0001* | 1.13 (0.84, 1.50) | 0.4232 |
| Race/Ethnicity | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 1.08 (0.84, 1.40) | 0.5428 | 1.15 (0.78, 1.70) | 0.4740 |
| Hispanic | 1.06 (0.84, 1.34) | 0.6224 | 1.70 (1.24, 2.32) | 0.0009* |
| Asian/Pacific Islander | 0.89 (0.73, 1.07) | 0.2150 | 0.82 (0.60, 1.12) | 0.2132 |
| Neighborhood Socioeconomic Status | ||||
| Lowest | 1.25 (0.95, 1.63) | 0.1107 | 0.72 (0.47, 1.11) | 0.1356 |
| Lower-Middle | 1.51 (1.19, 1.91) | 0.0008* | 1.41 (1.00, 1.99) | 0.0530 |
| Middle | 1.13 (0.90, 1.41) | 0.2844 | 0.98 (0.71, 1.37) | 0.9120 |
| Upper-Middle | 1.16 (0.93, 1.45) | 0.1786 | 0.96 (0.69, 1.34) | 0.8096 |
| Highest | Reference | Reference | ||
| Stage at Diagnosis | ||||
| Localized | Reference | Reference | ||
| Regional | 1.70 (1.34, 2.15) | <0.0001* | 2.55 (1.56, 4.15) | 0.0002* |
| Remote | 2.83 (2.18, 3.66) | <0.0001* | 5.44 (3.24, 9.15) | <0.0001* |
| Histology | ||||
| Thymoma | Reference | Reference | ||
| Thymic Carcinoma | 1.63 (1.33, 2.01) | <0.0001* | 2.99 (2.29, 3.91) | <0.0001* |
| First Course of Treatment | ||||
| No treatment | Reference | Reference | ||
| Chemotherapy and/or Radiation | 0.80 (0.60, 1.08) | 0.1498 | 0.94 (0.59, 1.51) | 0.8035 |
| Surgery Only | 0.37 (0.27, 0.51) | <0.0001* | 0.32 (0.18, 0.58) | 0.0001* |
| Surgery and Single Modality | 0.40 (0.29, 0.54) | <0.0001* | 0.45 (0.27, 0.73) | 0.0011* |
| Surgery, Chemotherapy, and Radiation | 0.45 (0.32, 0.63) | <0.0001* | 0.59 (0.36, 0.98) | 0.0401* |
Statistically significant
Abbreviations: HR=hazard ratio, SHR=sub-distribution hazard ratio, CI=confidence interval Estimates are adjusted for all variables listed in the table.
Receiving surgery, chemotherapy, radiation, or a combination of these modalities was associated with significantly better prognosis for both OS and CSS compared to forgoing these treatments. Table 6 indicates treatment associated with OS and CSS for each stage at diagnosis. With respect to OS, receiving surgery alone or in combination with chemotherapy and/or radiation was associated with a significantly reduced hazard of death, regardless of stage at diagnosis. For CSS, receiving surgery alone or with chemotherapy and/or radiation was associated with significantly better prognosis for patients with localized or regional disease, but not for individuals diagnosed at the remote stage.
Table 6:
Overall and cause-specific survival of adults aged 20 years and older diagnosed with thymus cancer in California by stage at diagnosis, 1988-2015.
| Stage at Diagnosis |
Overall Survival | Cause-Specific Survival | |||
|---|---|---|---|---|---|
| First Course of Treatment | HR (95% CI) | P-Value | SHR (95% CI) | P-Value | |
| Localized | No Treatment | Reference | Reference | ||
| Chemotherapy and/or Radiation | 1.62 (0.61, 4.26) | 0.3303 | 0.80 (0.15, 4.27) | 0.7903 | |
| Surgery Only | 0.28 (0.15, 0.53) | 0.0001* | 0.08 (0.02, 0.30) | 0.0002* | |
| Surgery and Single Modality | 0.37 (0.19, 0.72) | 0.0038* | 0.08 (0.02, 0.34) | 0.0008* | |
| Surgery, Chemotherapy, and Radiation | 0.48 (0.18, 1.32) | 0.1559 | 0.97 (0.23, 4.12) | 0.9713 | |
| Regional | No Treatment | Reference | Reference | ||
| Chemotherapy and/or Radiation | 0.58 (0.33, 1.03) | 0.0613 | 0.38 (0.17, 0.89) | 0.0260* | |
| Surgery Only | 0.34 (0.19, 0.58) | <0.0001* | 0.14 (0.06, 0.34) | <0.0001* | |
| Surgery and Single Modality | 0.34 (0.20, 0.58) | <0.0001* | 0.24 (0.11, 0.53) | 0.0004* | |
| Surgery, Chemotherapy, and Radiation | 0.42 (0.24, 0.74) | 0.0027* | 0.30 (0.13, 0.68) | 0.0041* | |
| Remote | No Treatment | Reference | Reference | ||
| Chemotherapy and/or Radiation | 0.94 (0.62, 1.42) | 0.7666 | 1.40 (0.74, 2.67) | 0.2994 | |
| Surgery Only | 0.50 (0.27, 0.93) | 0.0278* | 1.01 (0.41, 2.48) | 0.9917 | |
| Surgery and Single Modality | 0.43 (0.26, 0.69) | 0.0006* | 0.65 (0.32, 1.31) | 0.2243 | |
| Surgery, Chemotherapy, and Radiation | 0.40 (0.24, 0.67) | 0.0004* | 0.75 (0.37, 1.52) | 0.4256 | |
Statistically significant
Abbreviations: HR=hazard ratio, SHR=sub-distribution hazard ratio, CI=confidence interval Estimates are adjusted for sex, age at diagnosis, race/ethnicity, neighborhood socioeconomic status, and histology type.
Discussion:
This study is one of the largest known epidemiological studies of thymic malignancies with 1,588 total cases. In 2015, the incidence rate of thymic malignancies was 0.277 per 100,000, which is higher than the incidence rate of 0.15 per 100,000 reported by Engels & Pfeiffer in 2003 and 0.13 per 100,000 reported by Engels in 2010. 6,7 Of note, both prior studies only included cases of malignant thymomas, whereas approximately 13% of this study’s population included thymic carcinoma. The inclusion of thymic carcinoma in this study may partly explain the reported rise in incidence. In addition, the Engels study population included patients of all ages, whereas this study excluded the pediatric population between ages 0 to 20 due to the rarity of thymic malignancies in children. In fact, only 13 cases from ages 0 to 20 were identified in the study population between 1988 and 2015. Nevertheless, these findings indicate higher incidence rates of thymic malignancies than previously reported.6,7 Incidence appears to be increasing, which differs from some earlier published studies that suggested a decline in incidence.7
Similar to prior studies, we found that rates of thymic malignancies were higher among APIs than other races/ethnicities.6,7 Engels and Pfeiffer found that incidence rates were highest in Hawaii and San Francisco, reflecting the high incidence among APIs. 6 Less racially/ethnically diverse states, namely Iowa and Utah, had the lowest incidence rates of thymic malignancies in their study. California, a racially/ethnically diverse state, has a large and growing API population. According to census studies, the proportion of APIs in the state increased from 8% in 1988 to 12% in 2015.10 The racial/ethnic distribution of California may explain in part why incidence appears to be higher in this state than national estimates reported previously. It is unclear why APIs have higher incidence rates of thymic malignancies than other race/ethnic groups. Further studies are needed to understand this disparity in incidence rates.
The rising incidence of thymic malignancies in California may also be partly attributable to the increased use of imaging studies in the United States over the study period. A 2009 study by Smith-Bindman et al. examined the use of imaging studies in a large health plan group in Washington state between 1997 and 2006.11 During this time, CT imaging studies increased by 14% per year, and over the ten-year study period, doubled overall. More specifically, the number of CT chest imaging studies nearly tripled from approximately 30 per 1,000 enrollees in 1997 to approximately 90 per 1,000 enrollees in 2006. As demonstrated in our study, there appears to be a trend towards early stage disease at the time of diagnosis. The rising use of diagnostic imaging studies may have led to an increased number of incidental findings of thymic malignancies at early stage disease. Another possibility is classification bias with improved pathologic diagnosis of thymoma and thymic carcinoma over time. These associations are speculative but deserve to be examined further.
In our study, gender, age, and nSES were associated with OS; however, our findings suggest these are not significant prognostic factors for survival from thymic malignancies. Rather, cancer-specific variables already known to predict survival in thymic malignancy patients were confirmed, including histology and stage at diagnosis. 12,13,14
As thymomas and thymic carcinomas are rare malignancies, there have been no randomized studies comparing treatment regimens. Nonetheless, surgical resection is considered the gold standard treatment for resectable disease.15,16 We observed that surgery (alone or in combination with other therapies) was associated with improved OS regardless of stage and with improved CSS for localized and regional disease. The findings in this retrospective study likely reflect substantial patient selection bias, though these outcomes by treatment modality are congruent with the current standard of care for earlier stage resectable disease.
There are several additional limitations of this study including the lack of more detailed information regarding staging and treatment. Due to availability of data over the study period, SEER’s summary staging was used rather than the more often clinically used Masaoka-Koga staging system17,18 The CCR does not routinely collect information on cancer recurrence, the number of rounds of chemotherapy and radiation received, or subsequent treatments. In addition, CCR does not collect data on paraneoplastic autoimmune syndromes, which is a known complication of thymoma. Despite these limitations, this study uses the one of the largest known datasets of thymic malignancies to date to give insight into trends in incidence and predictors of survival in a large, diverse population.
Conclusion:
Thymic malignancy appears to be increasing over time in California, with higher rates observed in APIs and NHBs. There also appears to be a trend towards detecting more localized stage disease, possibly due to the increased use of thoracic imaging studies. Advanced stage and thymic carcinoma were found to be associated with worsened survival, consistent with previous studies. Treatment with surgery was associated with improved OS in all stages of disease and improved CSS in local and regional disease.
Figure 1.
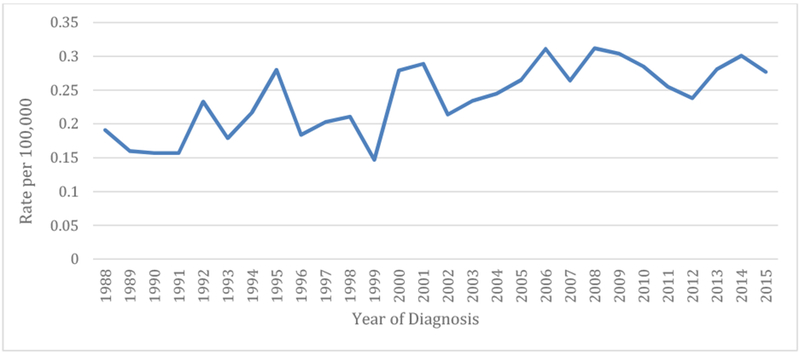
Age-adjusted incidence rates of thymus cancer among adults aged 20 years and older in California, 1988-2015 (n=1,588)
Clinical Practice Points.
Although some prior studies have suggested a decrease in incidence, our study shows that thymic malignancy incidence appears to be rising.
Incidence varies among race/ethnicity, with higher incidence among Asian Pacific Islanders and non-Hispanic blacks.
There is a trend towards localized stage disease possibly due to the increased use of imaging studies.
Thymic carcinoma and advanced stage were both associated with worsened survival, which is consistent with prior studies.
Treatment with surgery is associated with improved OS in all stages of disease, and improved CSS in local and regional disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller JF. The discovery of thymus function and of thymus derived lymphocytes. Immunol Rev, July 2002. (185): 7–14. [DOI] [PubMed] [Google Scholar]
- 2.Fornasiero A, Daniele O, Ghiotto C, et al. : Chemotherapy of invasive thymoma. J Clin Oncol 8 (8): 1419–23, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors: Part 1. Tumors of the anterior mediastinum. Chest. 1997;112:511–522. [DOI] [PubMed] [Google Scholar]
- 4.Riedel RF and Burfeind WR. Thymoma: Benign Appearance, Malignant Potential. The Oncologist. 2006;11(8):887–894. [DOI] [PubMed] [Google Scholar]
- 5.Venuta F, Rendina EA, Anile M, De Giacomo T, Vitolo D, Coloni GF. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg. 2012;60:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA and Pfeiffer RM. Malignant thymomas in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105(4):546–51. [DOI] [PubMed] [Google Scholar]
- 7.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(suppl 4):S260–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg D, et al. Thymic carcinoma: current staging does not predict prognosis. J Thorac Cardiovasc Surg. 1998;115:303–9. [DOI] [PubMed] [Google Scholar]
- 9.Kondo K and Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–85. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(suppl 4):S260–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Race/Ethnic Population with Age and Sex Detail, 1970 to 2040. State of California: Department of Finance. [Google Scholar]
- 12.Smith-Bindman Rebecca, Miglioretti DL Larson EB. Rising Use Of Diagnostic Medical Imaging In A Large Integrated Health System. Health Aff (Millwood). 2008. Nov-Dec; 27(6): 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger DS, et al. Thymic malignancies. J Natl Compr Canc Netw. 2010;8(10):1302–15. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymomas. J Thorac Cardiovasc Surg. 2009;138:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng TY, Fuller CD, Jagirdar J, et al. : Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 59 (3): 654–64, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, Zucali P, Gatta G, Garassino MC. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol.2016. March;99:332–50. doi: 10.1016/j.critrevonc.2016.01.012. Epub 2016 Jan 19. [DOI] [PubMed] [Google Scholar]
- 17.Gubens MA. Treatment Updates in Advanced Thymoma and Thymic Carcinoma. Current Treatment Options in Oncology. 2012;13:527–34. [DOI] [PubMed] [Google Scholar]
- 18.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. [DOI] [PubMed] [Google Scholar]
- 19.Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359–367. [DOI] [PubMed] [Google Scholar]


