Summary
Between 1989 and 2004, the prevalence of gestational diabetes mellitus (GDM) in the United States increased by 122%. Glycated haemoglobin, as measured by haemoglobin A1C (A1C), can potentially identify pregnant women at high risk for adverse outcomes associated with GDM including macrosomia and post-partum glucose intolerance. Our objective was to systematically review the literature with respect to A1C levels during pregnancy and associated maternal and offspring outcomes. We used MEDLINE to identify relevant publications from 1975 to 2009. We included articles if they met the following criteria: original full text articles in English; primary exposure of antepartum A1C; women with GDM at baseline or who developed GDM during the study; primary outcome of GDM, insulin use, post-partum abnormal glucose or type 2 diabetes (T2DM), birthweight, macrosomia or large for gestational age. Case series and case reports were excluded. Twenty studies met our criteria. A1C at GDM diagnosis was positively associated with post-partum abnormal glucose. Women with post-partum T2DM or impaired glucose tolerance had mean A1C at GDM diagnosis higher than those with normal post-partum glucose (P ≤ 0.002) and a 1% increase in A1C at GDM diagnosis was associated with 2.36 times higher odds of post-partum abnormal glucose 6 weeks after delivery [95% confidence interval 1.19, 4.68]. The association of A1C and birthweight varied substantially between studies, with correlation coefficients ranging from 0.11 to 0.51. A1C, a less burdensome and costly measure than an oral glucose tolerance test, appears to be an attractive measure for identifying women at high risk of adverse outcomes associated with GDM.
Keywords: gestational diabetes, haemoglobin A1C, birthweight, type 2 diabetes, macrosomia
Introduction
In the United States, prevalence of gestational diabetes (GDM) may range from 1 to 14% of pregnancies.1 GDM constitutes a significant health risk for mother and offspring during pregnancy, delivery and throughout the life course. Up to 60% of women with a pregnancy affected by GDM will develop type 2 diabetes mellitus (T2DM) within 5–10 years.2–5 Their offspring are at increased risk for neonatal complications, in particular macrosomia and large for gestational age (LGA).2 These offspring are also at high risk for obesity, insulin resistance and T2DM over their life course.6,7 Thus, the diagnosis and appropriate management of GDM has the potential to greatly reduce neonatal and maternal morbidity and the burden of T2DM.
Gestational diabetes is defined as glucose intolerance that is first evident during pregnancy.8 O’Sullivan and Mahan originally defined GDM based on the oral glucose challenge values that were associated with increased maternal risk of post-partum T2DM.9 Inconsistent definitions and diagnostic criteria, all using the oral glucose tolerance test (OGTT) and plasma glucose, are promulgated by at least three national and international organisations as illustrated in Figure 1. Beginning in 1979, the National Diabetes Data Group (NDDG) endorsed glucose thresholds which are slightly higher than those proposed by O’Sullivan and Mahan.10 In 1997, the American Diabetes Association (ADA) endorsed the thresholds proposed by Carpenter and Coustan,11 which are lower than those used by the NDDG. The World Health Organization (WHO) defined their diagnostic thresholds for GDM based on a 2 h 75 g OGTT rather than the 3 h 100 g OGTT utilised by the other organisations and applied the same thresholds used to diagnose T2DM in nonpregnant women.12 Following the publication of results from the Hyperglycemia and Pregnancy Outcomes study, new diagnostic criteria were recommended by the International Association of Diabetes and Pregnancy Study Groups and are currently under consideration.13
Figure 1.
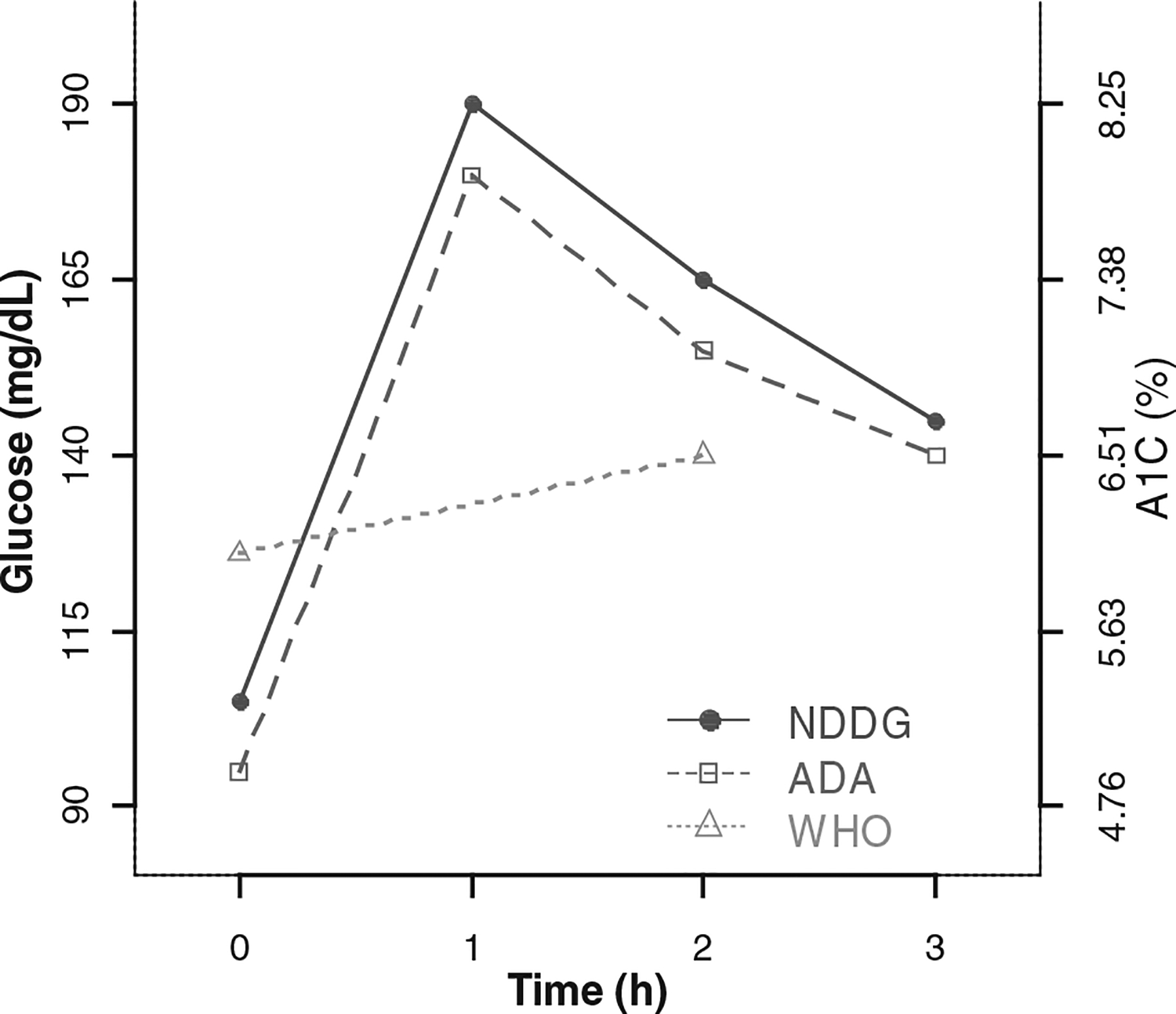
Glucose thresholds and equivalent A1C valuesa for diagnosis of gestational diabetes (GDM) by the National Diabetes Data Group (NDDG),b American Diabetes Association (ADA)b and the World Health Organization (WHO).c
a Conversion formula from Nathan et al.59 glucose (mg/dL) = 1.59*(A1C) −2.59.
b NDDG and ADA use a 3 h 100 g oral glucose tolerance test, and require two out of four values to exceed the thresholds to diagnose GDM.
c WHO uses a 2 h 75 g oral glucose tolerance test, and requires one out of the two values to exceed the thresholds to diagnose GDM.
Glycated haemoglobin, as measured by haemoglobin A1C (A1C), is used as a marker of glycaemic control in people with type 1 diabetes (T1DM) and T2DM.14 Notably, the ADA recently recommended that A1C ≥ 6.5% be used as a diagnostic measure for T2DM and that women with A1C ≥ 6.5% at their first prenatal visit be diagnosed with T2DM rather than GDM.15 In the non-pregnant diabetic population sustained high A1C is associated with increased diabetes complications.16,17 A1C measures average glucose concentration over time rather than an acute response to a glucose challenge and the majority of women with GDM do not exhibit A1C levels equal to those shown in Figure 1.18 Therefore, optimal A1C levels in pregnancy should be defined by level of increased risk for adverse pregnancy outcomes. Elevated A1C may be an attractive option for identifying pregnant women at high risk for GDM, postpartum T2DM and macrosomia or delivering an LGA infant.
A1C has two technical advantages over plasma, blood or serum glucose measurements, which make it particularly attractive as a candidate for diagnosing and monitoring GDM. First, A1C measurement does not require the fasting or multiple timed measurements of the OGTT, and thus the burden on pregnant women (physical discomfort, fasting, and ingesting the concentrated glucose beverage) and staff (to administer the beverage and draw repeated blood samples) is minimised. Second, unlike glucose, A1C remains relatively stable after collection19 and has less intraindividual variation compared with fasting plasma glucose.20
However, there are also limitations to the interpretation of A1C during pregnancy. Changes in preprandial and postprandial blood glucose21 and in the concentration of young erythrocytes22 result in a decrease in A1C associated with pregnancy, necessitating the use of trimester specific thresholds which are lower than those used in non-pregnant women with diabetes. Additionally, in the pregnant and nonpregnant population, the presence of haemoglobin variants, such as HbS (haemoglobin S), or medical conditions, such as uraemia or thalassaemia that affect red blood cell turnover, may result in spuriously high or low measurements. In non-pregnant women, race/ethnicity is associated with differences in A1C. After adjustment for covariates Hispanics and Blacks have higher average A1C compared with Caucasians.23,24 These racial/ethnic disparities may persist in non-obese pregnant women.25 Therefore, we conducted an epidemiological review of the literature on A1C levels during pregnancy to examine the associations of antepartum A1C and maternal glucose intolerance and associations of A1C with offspring birthweight, macrosomia and LGA.
Methods
Literature search
We systematically reviewed the literature with regard to A1C and associated risk of outcomes of: GDM, postpartum glucose intolerance or T2DM, birthweight, macrosomia and LGA from 1975 to 2009. Relevant publications for both searches were identified from MEDLINE using the search term ‘(gestational diabetes OR pregnancy) AND A1C’. We also reviewed reference lists of original and review articles to identify additional studies. The search identified 984 articles, 49 were identified for full review, and of these 20 met our eligibility criteria.
Inclusion and exclusion criteria
To be included studies had to meet the following criteria: original articles; full text available in English; primary exposure of antepartum A1C; inclusion of women with GDM at baseline or who developed GDM over the course of the study; primary outcome of GDM, use of insulin, post-partum abnormal glucose or T2DM, birthweight, macrosomia, or LGA. Case series and case reports were excluded.
Definitions of outcomes
Gestational diabetes, T2DM, impaired fasting glucose and impaired glucose tolerance were defined by thresholds for the OGTT established by national10,26 or international organisations.12 Due to inconsistencies in the published literature we used multiple definitions of macrosomia and LGA that were not mutually exclusive.27 Macrosomia was defined as birthweight greater than 4000 g or 4500 g, regardless of gestational age or as birthweight greater than the 90th or 95th percentile for gestational age. LGA was defined as birthweight greater than the 90th or 95th percentile for sex and gestational age. Fetal macrosomia was defined as abdominal circumference >90th percentile for gestational age as measured by ultrasound.28
Results
Study characteristics
Our initial search identified 984 articles. Of these 174 were not available in English, 524 did not pertain to either GDM or A1C, and 237 did not include women with GDM. The remaining 49 articles were reviewed: 29 were subsequently excluded as they did not include our pre-specified maternal and offspring outcomes of interest. In total, 20 studies meeting our criteria were identified. The majority of these studies were retrospective cohort studies using medical records. Nine studies addressed the association of maternal outcomes with antepartum A1C measured either before,29,30 at the time of31–35 or after GDM diagnosis.36,37 Eleven studies addressed the association of antepartum A1C measured at the time of38–45 or after GDM diagnosis,46–48 and birthweight, macrosomia or LGA. The majority of studies used the WHO thresholds for diagnosis of GDM30,34,35,41,42,44,45,47,48 and diagnosis of post-partum abnormal glucose.34,35,37 Only one study reported the racial/ethnic make-up of their study population and examined the impact of race/ethnicity on their findings.34
We first discuss the findings from studies of A1C and maternal outcomes and then the findings from studies of A1C and LGA or macrosomia. As interpretation of study results reflects the context and timing of measurement of A1C with respect to GDM diagnosis, the results are further subdivided by time of A1C measurement.
Associations of antepartum A1C and maternal outcomes
A1C before GDM screening and risk of GDM High A1C in the first trimester appears to be associated with increased risk of GDM (Table 1). Leipold et al. reported higher mean A1C at 11–14 weeks gestation among women who developed GDM compared with those who maintained normal glucose tolerance throughout their pregnancy (4.95 vs. 5.38, P < 0.001).29 These findings were corroborated by Balaji et al.30 who, in their study of 255 women at high risk for GDM, reported that high (>6%) and intermediate (5.3%–6%) A1C values in the first trimester were associated with subsequent elevated GDM risk. In this study, 100% of the women who had normal glucose tolerance but A1C >6% in the first trimester subsequently developed GDM later in pregnancy. Furthermore, 23% of the women who developed GDM in the second or third trimester had normal glucose tolerance in the first trimester and A1C between 5.3% and 6%.30 Taken together these findings indicate that early pregnancy A1C can identify women at high risk for GDM.
Table 1.
Studies of A1C, risk of GDM, and severity of GDM by year of publication
| First author | Study design (n) | Inclusion/exclusion | GDM criteria & prevalence n (%) | Time of antepartum A1C measurement and GDM diagnosis | GDM development or antenatal insulin use, prevalence n (%), and findings |
|---|---|---|---|---|---|
| Leipold29 | Cohort (n = 464) | Inclusion: | ADA 135 (29) | Before screening for GDM (11–14 weeks gestation) | GDM 135 (29) |
| Singleton, | Women with GDM had higher mean A1C (P < 0.001) | ||||
| Delivered Jan-Aug 2003 | |||||
| Exclusion: | Normal glycaemia: 4.95 ± 0.4 | ||||
| Chronic or infectious disease, | GDM: 5.38 ± 0.6 | ||||
| Previous pathological OGTT, | |||||
| Already being treated for | |||||
| GDM | |||||
| Balaji30 | Cohort (n = 507) | Inclusion: | WHO 155 (31), 69 (14) Diagnosed second or third trimester | At first screening for GDM (first trimester) | GDM 155 (31) |
| Attending referral centre | 23% of women who subsequently developed GDM had first trimester A1C 5.3–6.0% | ||||
| Exclusion: | |||||
| T2DM or history of GDM | |||||
| Gonzalez-Quintero31 | Cohort (n = 2365) | Inclusion: | 3 h OGTT (criteria not otherwise specified) 2365 (100) | Time of GDM diagnosis (gestational age not specified) | Antenatal insulin use 597 (25) |
| Managed for GDM ≥ 7 days, | A1C ≥6% positively associated with antenatal insulin use | ||||
| Documented lab results, | |||||
| Given option of diet only | HR: 1.61 [95% Cl 1.29, 2.00] | ||||
| Exclusion: | |||||
| Oral agents following diagnosis | |||||
| Sapienza32 | Cohort (n = 294) | Inclusion: | ADA 294 (100) | Time of GDM diagnosis (gestational age not specified) | Antenatal insulin use 117 (40) |
| 100 g OGTT | Higher A1C associated with antenatal insulin use | ||||
| Prenatal care July 2002-June 2008 | |||||
| OR: 2.63 [95% Cl 1.66, 4.17] |
HR, hazard ratio. For other abbreviations see text.
A1C at time of GDM diagnosis and severity of GDM
A1C at time of GDM diagnosis is associated with the severity of GDM, as measured by the use of insulin (Table 1). Gonzalez-Quintero et al.31 found that A1C ≥ 6% at GDM diagnosis was associated with a 61% increase in the odds of insulin use during pregnancy after controlling for OGTT results and gestational age at diagnosis [95% confidence interval (CI) 1.29, 2.00]. Similarly, Sapienza et al.32 in a study of 294 women diagnosed with GDM between 24 and 34 weeks gestation, found that a 1% increase in A1C was associated with 2.63 [95% CI 1.66, 4.17] times greater odds of insulin use.
A1C at time of GDM diagnosis and post-partum abnormal glucose or T2DM
Table 2 shows the studies of A1C and maternal postpartum abnormal glucose or T2DM by timing of A1C measurement with respect to GDM diagnosis. All three studies that analysed the association of A1C at time of GDM diagnosis and post-partum abnormal glucose reported a positive association.33–35 In a cohort study of 189 women diagnosed with GDM using the WHO criteria, Oldfield et al.34 reported an increase in odds of post-partum T2DM within 8 years of the affected pregnancy associated with a 1% increase in A1C of 9.15 [95% CI 1.91, 43.87] among Caucasians and 4.95 among South Asians [95% CI 1.35, 12.40]. This study excluded women diagnosed with GDM very early in pregnancy who may have had undiagnosed pre-pregnancy diabetes. Similarly, in a case–control study of 318 women with GDM diagnosed by the ADA criteria, Ogonowski et al. found that a 1% increase in A1C was associated with a 2.36 [95% CI 1.19, 4.68] increase in odds of abnormal post-partum glucose tolerance.35 These findings consistently indicate that higher A1C at GDM diagnosis is associated with increased risk of post-partum abnormal glucose.
Table 2.
Studies of the association of antepartum A1C and maternal post-partum abnormal glucose or type 2 diabetes (T2DM), by time of A1C measurement and year of publication
| First author | Study design (n) | Inclusion/exclusion | GDM criteria & prevalence n (%) | Time of antepartum A1C measurement and GDM diagnosis | Post-partum abnormal glucose criteria, prevalence n (%), and findings |
|---|---|---|---|---|---|
| Greenberg33 | Case-control (n = 94) | Inclusion: | NDDG 94 (100) | Time of GDM diagnosis (gestational age not specified) | NDDG 32 (34) |
| GDM, | Diabetes 15 (16) | ||||
| 6 week post-partum | IGT 17 (18) | ||||
| 75 g OGTT | Women with diabetes or IGT had higher average A1C (P ≤ 0.002) | ||||
| Normal glucose: 5.7 ± 1.2 | |||||
| T2DM: 7.2 ± 1.1 | |||||
| IGT: 6.0 ± 1.0 | |||||
| Oldfield34 | Cohort (n = 73) | Inclusion: | WHO 73 (100) | Time of GDM diagnosis (gestational age not specified) | WHO 43 (59) |
| GDM | T2DM 27 (34) | ||||
| Exclusion: | IFG 2 (3) | ||||
| Diagnosed < 20 weeks gestation | IGT 14 (19) | ||||
| Higher A1C associated with increased odds of post-partum T2DM | |||||
| Caucasian OR: 9.15 [95% Cl 1.91, 43.87] | |||||
| South Asian OR: 4.95 [95%CI 1.35, 12.40] | |||||
| Ogonowski35 | Case-control (n = 318) | Inclusion: | WHO 318 (100) | Time of GDM diagnosis (gestational age not specified) | WHO 43 (14) |
| GDM, | Higher A1C associated with increased odds of post-partum abnormal glucose | ||||
| 6 week post-partum 75 g OGTT | |||||
| Exclusion: | OR: 2.36 [95% Cl 1.19, 4.68] | ||||
| Pre-pregnancy diabetes, | |||||
| Non-singleton pregnancy | |||||
| Pallardo36 | Case-control (n = 788) | Inclusion: | NDDG 788 (100) | After GDM diagnosis (third trimester) | ADA 200 (25) |
| GDM, | T2DM 43 (6) | ||||
| post-partum 75 g OGTT | Women with T2DM had higher average A1C (P < 0.001) | ||||
| Normal glucose: 5.07 ± 0.52 | |||||
| T2DM: 5.52 ± 0.66 | |||||
| Dalfra37 | Cohort (n = 65) | Inclusion: | ADA 65 (100) | After GDM diagnosis (third trimester) | WHO 16 (25) |
| GDM | T2DM 10 (15) | ||||
| Exclusion: | IGT 6 (9) | ||||
| Developed T1DM (n = 5) | Women with abnormal glucose did not have higher average A1C (P > 0.05) | ||||
| Normal glucose: 5.0 ± 0.6 | |||||
| T2DM: 4.9 ± 0.4 | |||||
| IGT: 5.2 ± 1.0 |
IGT, impaired glucose tolerance; IFG, impaired fasting glucose. See text for all other abbreviations.
A1C after GDM diagnosis and post-partum abnormal glucose or T2DM
In contrast, when A1C was measured after diagnosis of GDM, findings were not as uniform. In a study of 788 women diagnosed with GDM using NDDG criteria, Pallardo et al.36 found that women with post-partum T2DM had higher mean A1C in the third trimester compared with women without post-partum T2DM (5.52 vs. 5.07, P < 0.0001). However, in a cohort study of 70 women with GDM, Dalfra et al.37 measured A1C in the third trimester after treatment for GDM was initiated and found no significant difference in mean A1C between those who developed post-partum T2DM and those who did not.37
Associations of A1C with birthweight, macrosomia, LGA and fetal macrosomia
A1C at time of GDM diagnosis, birthweight, macrosomia, LGA and fetal macrosomia
While A1C at diagnosis of GDM may be associated with LGA, it is not necessarily associated with fetal macrosomia at the time of GDM diagnosis (Table 3). Schaefer-Graf et al.41 did not find a statistically significant difference in maternal A1C at the time of GDM diagnosis between mothers with fetal macrosomia at their initial ultrasound and those without. Furthermore, the association between A1C and LGA may not be present or may be attenuated among pregnant women without diabetes. In a study of 611 women, which included 101 women diagnosed with GDM using the ADA criteria, Lapolla et al.43 found that a 1% increase in A1C, at GDM screening, was associated with 2.76 times higher odds of delivery of an LGA infant after adjusting for maternal glucose status [95% CI 0.83, 9.22] although the odds ratio did not reach statistical significance.
Table 3.
Studies of the association of antepartum A1C and birthweight, macrosomia, large for gestational age (LGA) and fetal macrosomia, by time of A1C measurement and year of publication
| First author | Study design (n) | Inclusion/exclusion | GDM criteria & prevalence n (%) | Time of antepartum A1C measurement and GDM diagnosis | Outcome, prevalence n (%), and findings |
|---|---|---|---|---|---|
| Baxi38 | Cohort (n = 180) | Inclusion: | 100 g OGTT (thresholds not specified) 32 (18) | Time of GDM diagnosis (gestational age not specified) | Macrosomia 10 (31) |
| High risk of GDM | Higher prevalence of macrosomia among GDM patients with A1C ≥ 6.78 | ||||
| Exclusion: | A1C ≥ 6.78: 10 (50) | ||||
| Variant haemoglobin | A1C < 6.78: 0 (0) | ||||
| Morris39 | Cohort (n = 69) | Inclusion: | NDDG 21 (30) | Time of GDM diagnosis (<17 weeks gestation) | LGA 29 (42) |
| Screened at 10–15 weeks gestation and >23 weeks | Higher prevalence of LGA among non-diabetic women with highest A1C | ||||
| A1C <6%: | |||||
| Non-diabetic 4 (10) | |||||
| A1C 6–6.9%: | |||||
| Non-diabetic 6 (75) | |||||
| GDM 4 (40) | |||||
| A1C > 7%: | |||||
| GDM 4 (36) | |||||
| Morris40 | Cohort (n = 64) | Inclusion: | NDDG 15 (23) | Time of GDM diagnosis (10–15 weeks gestation) | LGA 15 (23) |
| Prenatal care Apr 1983-Mar 1984, | Higher prevalence of LGA among women with A1C ≥ 6.3 | ||||
| GDM screen at 10–15 weeks gestation | A1C ≥ 6.3: 9 (44) | ||||
| A1C < 6.3: 6 (14) | |||||
| Exclusion: | |||||
| T2DM | |||||
| Schaefer-Graf41 | Cohort (n = 403) | Inclusion: | WHO 306 (76) | Time of GDM screening (0–40 weeks gestation) | Fetal macrosomia 75 (19) |
| Singleton, | No difference in mean A1C between those with and without fetal macrosomia (P = 0.09) | ||||
| GDM or impaired glucose tolerance, | Fetal macrosomia: 6.08 ± 1.3 | ||||
| Ultrasound at <20 weeks | Normal: 6.2 ± 1.0 | ||||
| Exclusion: | |||||
| Vascular disease, | |||||
| Fetal anomalies | |||||
| Pietryga42 | Cohort (n = 146) | Inclusion: | WHO 146 (100) | Time of GDM diagnosis (24–28 weeks gestation) | LGA 33 (23) |
| GDM, | No difference in mean A1C between those with and without LGA | ||||
| Delivery at 24–40 weeks gestation | LGA: 5.8 ± 1.2 | ||||
| AGA: 5.7 ± 1.0 | |||||
| Lapolla43 | Cohort (n = 611) | Inclusion: | ADA 101 (17) | Time of GDM screening (24–27 weeks gestation) | LGA 85 (14) |
| Screened at 24–27 weeks gestation | Higher A1C associated with increased odds of LGA | ||||
| Exclusion: | OR: 2.76 [95% Cl 0.83, 9.22] | ||||
| Smoking, | |||||
| Chronic hypertension or specific condition effecting metabolism | |||||
| Zawiejska44 | Cohort (n = 357) | Inclusion: | WHO 357 (100) | Time of GDM diagnosis (5–40 weeks gestation) | Birthweight |
| GDM, | A1C and birthweight positively correlated (P < 0.05) | ||||
| Singleton livebirth | r = 0.11 | ||||
| Exclusion: | |||||
| Fetal malformation | |||||
| Seshiah45 | Cohort (n = 207) | Inclusion: | WHO 87 (42) | Time of GDM diagnosis (gestational age not specified) | Birthweight |
| 75 g OGTT | Women diagnosed with GDM at ≤12 weeks gestation compared with women with GDM at >30 weeks had higher A1C mean (SD) 6.93 (1.6) vs. 6.20 (0.3) | ||||
| Exclusion: | Women diagnosed with GDM at ≤12 weeks gestation compared women with GDM at >30 weeks had lower birthweights mean (SD) | ||||
| Pre-gestational diabetes | 3.15 (0.5) vs. 3.51 (0.6) kg | ||||
| Miller46 | Cohort (n = 56) | Inclusion: | NDDG 27 (48) | After GDM diagnosis (third trimester) | Relative birthweight |
| Attending obstetric services | A1C and birthweight not significantly correlated (P ≥ 0.05) | ||||
| r = 0.176 | |||||
| Djelmis47 | Cohort (n = 290) | Inclusion: | WHO 43 (15) | After GDM diagnosis (4 weeks preceding delivery or miscarriage) | Birthweight |
| Treated and delivered 1990–1995 | A1C and birthweight positively correlated (P < 0.05) | ||||
| r = 0.51 | |||||
| Gandhi48 | Cohort (n = 94) | Inclusion: | WHO 67 (71) | After GDM diagnosis (second and third trimester) | ′Birthweight centile’ |
| Diabetes | Higher ‘birthweight centile’ among women with elevated A1C (P = 0.02) | ||||
| Exclusion: | |||||
| Delivery at <36 weeks gestation | A1C < 6.5: 78.9% ± 29.2 | ||||
| A1C > 6.5: 90.2% ± 28.6 |
See text for abbreviations.
Among women with diabetes in pregnancy, A1C at time of GDM diagnosis does appear to be associated with birthweight,44,47,48 macrosomia,38 and LGA.39,40 In a retrospective cohort study of 357 women with GDM using the WHO criteria, Zawiejska et al.44 found that A1C at GDM diagnosis was positively correlated with birthweight (r = 0.11, P < 0.05). In this study, A1C was measured at GDM diagnosis and the mean gestational age at GDM diagnosis was 28 weeks (range 5–40 weeks).
The association between A1C at GDM diagnosis and birthweight may also be modified by the gestational week of diagnosis of GDM. Seshiah et al.45 stratified women with GDM by gestational week at diagnosis [≤12 weeks (n = 36), 13–23 weeks (n = 18), 24–30 weeks (n = 15) and >30 weeks (n = 18)] and then compared birthweight from these groups with each other and to that of women without GDM (n = 120).45 Comparing women diagnosed at >30 weeks gestation with women diagnosed at ≤12 weeks gestation the former had higher A1C at diagnosis, but lower mean birthweight. Thus, among women diagnosed with GDM early in pregnancy an association between A1C and birthweight may not be present, possibly due to longer time on treatment.
A1C after GDM diagnosis and birthweight
The association of A1C and birthweight was stronger when antepartum A1C was measured after GDM diagnosis. Djelmis et al.47 reported a correlation coefficient of 0.51 (P < 0.05) between antepartum A1C measured after GDM diagnosis and birthweight. This study included women diagnosed with GDM using the WHO criteria, and measured A1C 4 weeks before delivery. Supporting these findings, a post hoc analysis in a study by Gandhi et al.48 measured A1C in the second and third trimester, and found that when A1C was dichotomised into high (>6.5%) and low (<6.5%) categories, mean ‘birthweight centile’ was highest among those with high A1C.
Discussion
This study summarises the associations of A1C with outcomes of both mother and offspring in pregnancies complicated by GDM. A1C is a potentially powerful tool for management of GDM to guide treatment and reduce the risk of LGA, macrosomia and post-partum abnormal glucose. Our epidemiological review of the literature suggests that A1C may be useful for identifying women in early pregnancy who are at high risk for GDM and who develop the most severe GDM. Higher A1C at the time of GDM diagnosis was associated with increased risk of post-partum abnormal glucose or T2DM. Finally, among women with GDM, A1C after GDM diagnosis may also be useful for identifying women at highest risk for delivering an LGA or macrosomic infant.
Higher A1C in early pregnancy, even within normal levels is associated with increased risk of GDM. Identifying women in early pregnancy at highest risk of GDM provides an opportunity for disease prevention, and currently at least three randomised trials are seeking to determine the efficacy of physical activity interventions for prevention of GDM.49–51 All studies measuring A1C in the first trimester prior to diagnosis of GDM were conducted among women with a higher perceived risk of GDM, and therefore A1C was indicative of the overall increased risk of diabetes among study subjects. Thus, it is not clear whether these findings are generalisable beyond populations already at high risk for diabetes.
The majority of studies we reviewed identified a positive association between A1C and abnormal postpartum glucose tolerance. However, Dalfra et al.37 did not detect a difference in mean A1C between those with abnormal post-partum glucose tolerance and those with normal post-partum glucose. It is important to consider the context and timing of measurement of A1C when interpreting these conflicting findings. Use of different criteria for diagnosing GDM leads to identification of populations with different risk profiles and degrees of hyperglycaemia in pregnancy (Figure 1). For example, Pallardo et al.36 used the more restrictive NDDG criteria for GDM diagnosis, identifying a population with more severe glucose intolerance, and found that A1C after diagnosis was associated with postpartum abnormal glucose. In contrast, Dalfra et al.37 used the lower ADA thresholds and failed to detect such an association.
Additionally, Dalfra et al.37 measured A1C after commencement of treatment, whereas Pallardo et al. measured A1C at GDM diagnosis. A1C is a measure of average glucose over the preceding 2–3 months; however, this is a weighted average with glucose exposure in the most recent month contributing more than earlier months.52 Thus, in the study by Pallardo et al.36 A1C combined the subjects’ underlying risk of GDM and the severity of disease prior to detection, but in the study by Dalfra et al. A1C reflected patient adherence to treatment and time on treatment. Women with the highest A1C values at diagnosis are most likely to receive intensive treatment and as a consequence have reduced A1C later in pregnancy. If these same women were also at greatest risk of post-partum abnormal glucose, this could explain the lack of association between A1C and post-partum abnormal glucose in the study by Dalfra and colleagues.
A1C at the time of GDM diagnosis was only weakly correlated with birthweight,44 but A1C measured after GDM diagnosis was strongly correlated with birthweight.47,48 This finding highlights the importance of the timing of A1C measurement with respect to GDM diagnosis and the positive impact of appropriate mangement of GDM on neonatal outcomes. Women with the highest A1C values after GDM diagnosis were most likely to be the ones with the poorest glycaemic control during the third trimester when fetal growth may be most affected by maternal hyperglycaemia.53 In contrast, when A1C was measured at the time of GDM diagnosis, many of the women with the highest A1C may have benefited from GDM management and good glucose control, weakening the correlation of A1C and birthweight.
The association between A1C and birthweight may be modified by gestational week at GDM diagnosis. Seshiah et al.45 reported that compared with women diagnosed at >30 weeks gestation, women diagnosed at ≤12 weeks gestation had higher A1C at diagnosis, but lower mean birthweight. It has long been postulated that maternal hyperglycaemia results in fetal hyperinsulinaemia, leading to macrosomia.53 Among women with T1DM, third trimester A1C is positively correlated with birthweight and macrosomia,54,55 but A1C in the first trimester is negatively correlated with birthweight, possibly due to limited placental development.55,56 Many women with GDM only develop hyperglycaemia in their second and third trimester; therefore, the association of A1C and birthweight would be expected to be stronger among women with GDM compared with those with T1DM or T2DM. However, a proportion of women with GDM have undiagnosed pre-gestational T2DM or are diagnosed at <24 weeks gestation due to perceived risk factors. Therefore, the association of A1C and LGA or macrosomia may differ between those diagnosed at <24 weeks gestation and those diagnosed at ≥24 weeks gestation. It is also difficult in this setting to distinguish between intrauterine growth restriction resulting from maternal pre-pregnancy or first trimester hyperglycaemia56 and the effects of early and aggressive treatment.
The timing of fetal growth measurement may also contribute to observed variations in associations between A1C and macrosomia. Schaefer-Graf et al.41 did not detect a statistically significant difference in maternal A1C at the time of GDM diagnosis between mothers with fetal macrosomia at their initial ultrasound and those without. However, if maternal hyperglycaemia, as reflected by A1C, is most strongly associated with fetal growth during the third trimester of pregnancy, then the failure to detect an association does not conflict with the positive findings previously described.
Strengths of this review include summary of associations of A1C with both maternal and offspring outcomes, and synthesis of study findings in the context of timing of A1C measurement. A key limitation to consider is that publication bias is possible, in particular whether studies with null findings remain unpublished. While it is difficult to assess potential publication bias, our search did identify multiple studies with null findings.37,41,42,46
A1C is an accepted measure of glycaemic control for those with T2DM or T1DM14 and has been recommended for the diagnosis of T2DM,57 yet controversy persists with respect to its use among pregnant women with and without GDM. Currently, the American College of Obstetrics and Gynecology (ACOG) recommends maintaining A1C of <6% in pregnancies with established diabetes. There are no recommendations with respect to GDM despite the fact that GDM accounts for 90% of the pregnancies affected by diabetes in the United States.58 Additionally, while it is known that A1C is higher in certain racial and ethnic groups independent of glycaemic status,24 the potential impact of these differences on maternal and neonatal outcomes in GDM pregnancies is unknown.
Improved accuracy and standardisation of measurement techniques, and the recent recommendation for use in the diagnosis of T2DM will continue to raise interest in A1C. As a result, clinicians may be encouraged to use A1C more frequently for the management of GDM. A1C may be a particularly useful tool for identifying women during their first trimester at high risk of GDM, for guiding aggressive treatment of GDM to prevent adverse neonatal outcomes, and to identify and motivate behaviour change among women at highest risk for post-partum abnormal glucose. Future research should include determination of optimal levels of A1C in GDM pregnancies, the associations of A1C at different time points in pregnancy with maternal abnormal glucose and LGA or macrosomia, and the impact of race/ethnicity and pre-pregnancy body mass index on these associations.
Acknowledgements
Jodie Katon is supported by the Reproductive, Perinatal and Pediatric Training Grant number T32 HD052462 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Health (NIH) and by a grant from the Seattle chapter of Achievement Rewards for College Scientists (ARCS).
References
- 1.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004; 27 (Suppl. 1):S88–S90. [DOI] [PubMed] [Google Scholar]
- 2.Vohr BR, Boney CM. Gestational diabetes: The forerunner for the development of maternal and childhood obesity and metabolic syndrome? The Journal of Maternal-Fetal and Neonatal Outcomes 2008; 21:149–157. [DOI] [PubMed] [Google Scholar]
- 3.Hunger-Dathe W, Mosebach N, Samann A, Wolf G, Muller UA. Prevalence of impaired glucose tolerance 6 years after gestational diabetes. Experimental and Clinical Endocrinology & Diabetes 2006; 114:11–17. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes. Diabetes Care 2007; 30:878–883. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002; 25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 6.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 1991; 40 (Suppl. 2):121–125. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003; 111:e221–e226. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstetrics and Gynecology 2001; 98:525–538. [PubMed] [Google Scholar]
- 9.O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964; 13:278–285. [PubMed] [Google Scholar]
- 10.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28:1039–1057. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American Journal of Obstetrics and Gynecology 1982; 144:768–773. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine 1998; 15:539–553. [DOI] [PubMed] [Google Scholar]
- 13.Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2010; 202:654.e1–654.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DE, Nathan D, Little RR, Peterson CM, Lorenz RA, Sacks DB, et al. Tests of glycemia in diabetes. Diabetes Care 2004; 27:1761–1773. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33 (Suppl. 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Cleary PA, Backlund J-YC, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine 2005; 353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton I, Adler AI, Neil AW, Mathews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. British Medical Journal 2000; 321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosca A, Paleari R, Dalfra MG, Di Cianni G, Cuccuru I, Pellegrini G, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clinical Chemistry 2006; 52:1138–1143. [DOI] [PubMed] [Google Scholar]
- 19.Little RR, Rohlfing CL, Tennill AL, Connolly S, Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technology & Therapeutics 2007; 9:36–42. [DOI] [PubMed] [Google Scholar]
- 20.Petersen PH, Jorgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and hba1c for the diagnosis and prognosis of diabetes mellitus. Scandinavian Journal of Clinical and Laboratory Investigation 2005; 65 (Suppl. 240):51–60. [DOI] [PubMed] [Google Scholar]
- 21.Cousins L, Rigg L, Hollingsworth D, Brink G, Aurand J, Yen SS. The 24-hour excursion and diurnal rhythm of glucose, insulin, and C-peptide in normal pregnancy. American Journal of Obstetrics and Gynecology 1980; 136:483–488. [DOI] [PubMed] [Google Scholar]
- 22.Radder JK, van Roosmalen J. HbA1c in healthy, pregant women. Netherlands Journal of Medicine 2005; 63:256–259. [PubMed] [Google Scholar]
- 23.Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC Jr. et al. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. The Annals of Pharmacotherapy 2005; 39:1489–1501. [DOI] [PubMed] [Google Scholar]
- 24.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007; 30:2453–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holcomb JWR, Mostello DJ, Leguizamon GF. African-American women have higher initial HbA1c levels in diabetic pregnancy. Diabetes Care 2001; 24:280–283. [DOI] [PubMed] [Google Scholar]
- 26.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen T The macrosomic fetus: a challenge in current obstetrics. Acta Obstetrica et Gynecologica Scandinavica 2008; 87:134–145. [DOI] [PubMed] [Google Scholar]
- 28.Bochner CJ, Medearis AL, Williams J 3rd, Castro L, Hobel CJ, Wade ME. Early third-trimester ultrasound screening in gestational diabetes to determine the risk of macrosomia and labor dystocia at term. American Journal of Obstetrics and Gynecology 1987; 157:703–708. [DOI] [PubMed] [Google Scholar]
- 29.Leipold H, Worda C, Ozbal A, Husslein P, Krampl E. First-trimester nuchal translucency screening in pregnant women who subsequently developed gestational diabetes. Journal of the Society for Gynecologic Investigation 2005; 12:529–532. [DOI] [PubMed] [Google Scholar]
- 30.Balaji V, Madhuri BS, Ashalatha S, Sheela S, Suresh S, Seshiah V. A1C in gestational diabetes mellitus in Asian Indian women. Diabetes Care 2007; 30:1865–1867. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Quintero VH, Istwan NB, Rhea DJ, Tudela CM, Flick AA, de la Torre L, et al. Antenatal factors predicting subsequent need for insulin treatment in women with gestational diabetes. Journal of Women’s Health 2008; 17:1183–1187. [DOI] [PubMed] [Google Scholar]
- 32.Sapienza AD, Francisco RP, Trindade TC, Zugaib M. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Research and Clinical Practice 2010; 88:81–86. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg LR, Moore TR, Murphy H. Gestational diabetes mellitus: antenatal variables as predictors of post-partum glucose intolerance. Obstetrics and Gynecology 1995; 86:97–101. [DOI] [PubMed] [Google Scholar]
- 34.Oldfield MD, Donley P, Walwyn L, Scudamore I, Gregory R. Long term prognosis of women with gestational diabetes in a multiethnic population. Postgraduate Medical Journal 2007; 83:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogonowski J, Miazgowski T. The prevalence of 6 weeks post-partum abnormal glucose tolerance in Caucasian women with gestational diabetes. Diabetes Research and Clinical Practice 2009; 84:239–244. [DOI] [PubMed] [Google Scholar]
- 36.Pallardo F, Martin-Vaquero P, Herranz L, Janez M, Garcia-Ingelmo T, Gonzalez A, et al. Early post-partum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999; 22:1053–1058. [DOI] [PubMed] [Google Scholar]
- 37.Dalfra MG, Lapolla A, Masin M, Giglia G, Dalla Barba B, Toniato R, et al. Antepartum and early post-partum predictors of type 2 diabetes development in women with gestational diabetes mellitus. Diabetes Metabolism 2001; 27:675–680. [PubMed] [Google Scholar]
- 38.Baxi L, Barad D, Reece EA, Farber R. Use of glycosylated hemoglobin as a screen for macrosomia in gestational diabetes. Obstetrics and Gynecology 1984; 64:347–350. [PubMed] [Google Scholar]
- 39.Morris MA, Grandis AS, Litton JC. Glycosylated hemoglobin concentration in early gestation associated with neonatal outcome. American Journal of Obstetrics and Gynecology 1985; 153:651–654. [DOI] [PubMed] [Google Scholar]
- 40.Morris MA, Grandis AS, Litton J. Glycosylated hemoglobin: a sensitive indicator of gestational diabetes. Obstetrics and Gynecology 1986; 68:357–361. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer-Graf UM, Heuer R, Kilavuz O, Pandura A, Henrich W, Vetter K. Maternal obesity not maternal glucose values correlates best with high rates of fetal macrosomia in pregnancies complicated by gestational diabetes. Journal of Perinatal Medicine 2002; 30:313–321. [DOI] [PubMed] [Google Scholar]
- 42.Pietryga M, Brazert J, Wender-Ozegowska E, Dubiel M, Gudmundsson S. Placental Doppler velocimetry in gestational diabetes mellitus. Journal of Perinatal Medicine 2006; 34:108–110. [DOI] [PubMed] [Google Scholar]
- 43.Lapolla A, Dalfra MG, Bonomo M, Castiglione MT, Di Cianni G, Masin M, et al. Can plasma glucose and HbA1c predict fetal growth in mothers with different glucose tolerance levels? Diabetes Research and Clinical Practice 2007; 77:465–470. [DOI] [PubMed] [Google Scholar]
- 44.Zawiejska A, Wender-Ozegowska E, Brazert J, Sodowski K. Components of metabolic syndrome and their impact on fetal growth in women with gestational diabetes mellitus. Journal of Physiology and Pharmacology 2008; 59 (Suppl. 4): 5–18. [PubMed] [Google Scholar]
- 45.Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, et al. Detection and care of women with gestational diabetes mellitus from early weeks of pregnancy results in birth weight of newborn babies appropriate for gestational age. Diabetes Research and Clinical Practice 2008; 80:199–202. [DOI] [PubMed] [Google Scholar]
- 46.Miller JM, Crenshaw MC Jr, Welt SI. Hemoglobin A1c in normal and diabetic pregnancy. Journal of the American Medical Association 1979; 242:2785–2787. [PubMed] [Google Scholar]
- 47.Djelmis J, Blajic J, Bukovic D, Pfeifer D, Ivanisevic M, Kendic S, et al. Glycosylated hemoglobin and fetal growth in normal, gestational and insulin dependent diabetes mellitus pregnancies. Collegium Antropologicum 1997; 21:621–629. [PubMed] [Google Scholar]
- 48.Gandhi RA, Brown J, Simm A, Page RC, Idris I. HbA1c during pregnancy: its relationship to meal related glycaemia and neonatal birth weight in patients with diabetes. European Journal of Obstetrics, Gynecology and Reproductive Biology 2008; 138:45–48. [DOI] [PubMed] [Google Scholar]
- 49.Chasan-Taber L, Marcus BH, Stanek E 3rd, Ciccolo JT, Marquez DX, Solomon CG, et al. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design and methods. Journal of Womens Health 2009; 18:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luoto RM, Kinnunen TI, Aittasalo M, Ojala K, Mansikkamaki K, Toropainen E, et al. Prevention of gestational diabetes: design of a cluster-randomized controlled trial and one-year follow-up. BMC Pregnancy Childbirth 2010; 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oostdam N, van Poppel MN, Eekhoff EM, Wouters MG, van Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy Childbirth 2009; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995; 18:440–447. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinologica (Copenh) 1954; 16:330–342. [DOI] [PubMed] [Google Scholar]
- 54.Penney GC, Mair G, Pearson DW. The relationship between birth weight and maternal glycated haemoglobin (HbA1c) concentration in pregnancies complicated by Type 1 diabetes. Diabetic Medicine 2003; 20:162–166. [DOI] [PubMed] [Google Scholar]
- 55.Kerssen A, de Valk HW, Visser GH. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes Care 2007; 30:1069–1074. [DOI] [PubMed] [Google Scholar]
- 56.Johnstone FD, Mao JH, Steel JM, Prescott RJ, Hume R. Factors affecting fetal weight distribution in women with type I diabetes. British Journal of Obstetrics and Gynaecology 2000; 107:1001–1006. [DOI] [PubMed] [Google Scholar]
- 57.International Expert Committee. Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: temporal trends 1989 through 2004. American Journal of Obstetrics and Gynecology 2008; 198:525.e1–525.e5. [DOI] [PubMed] [Google Scholar]
- 59.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]


