Infections with nontuberculous mycobacteria (NTM) have a poor prognosis in patients with underlying respiratory diseases. Clofazimine (CFZ) showed both experimental and clinical promising results against clinically relevant NTM. However, there are no data on CFZ in combination with the current recommended treatment; therefore, we aimed to study its in vivo activity in an aerosol mouse model of Mycobacterium avium. In an aerosol infection BALB/c mouse model using M. avium strain Chester, we treated 58 mice with four combinations of rifampin (RIF) at 10 mg/kg, CFZ at 25 mg/kg, and clarithromycin (CLR) and ethambutol (EMB) at 100 mg/kg.
KEYWORDS: Mycobacterium avium, clofazimine, mouse model, treatment
ABSTRACT
Infections with nontuberculous mycobacteria (NTM) have a poor prognosis in patients with underlying respiratory diseases. Clofazimine (CFZ) showed both experimental and clinical promising results against clinically relevant NTM. However, there are no data on CFZ in combination with the current recommended treatment; therefore, we aimed to study its in vivo activity in an aerosol mouse model of Mycobacterium avium. In an aerosol infection BALB/c mouse model using M. avium strain Chester, we treated 58 mice with four combinations of rifampin (RIF) at 10 mg/kg, CFZ at 25 mg/kg, and clarithromycin (CLR) and ethambutol (EMB) at 100 mg/kg. Treatment efficacy was assessed on the basis of lung CFU counts after 2 (M2) and 4 (M4) months of treatment. At M2, CLR-RIF-EMB was slightly but significantly more efficient than CFZ-RIF-EMB (3.02 ± 0.12 versus 3.55 ± 0.28, respectively, P < 0.01), whereas CLR-CFZ-EMB and CLR-CFZ-RIF-EMB dramatically decreased lung CFU counts by 4.32 and 4.47 log10, respectively, compared to untreated group. At M4, CLR-RIF-EMB was significantly more efficient than CFZ-RIF-EMB (2 ± 0.53 versus 2.66 ± 0.22, respectively, P = 0.01). The addition of CLZ to CLR dramatically decreased the lung CFU count, with CFU counts 5.41 and 5.79 log10 lower in the CLR-CFZ-EMB and CLR-CFZ-RIF-EMB groups, respectively, than in the untreated group. The addition of CFZ to CLR seems to improve the efficacy of CLR as early as M2 and was confirmed at M4. CFZ, in addition to RIF and EMB, on the other hand, is less effective than CLR-RIF-EMB. These results need to be confirmed by similar studies along with CFZ potential for shortening treatment.
INTRODUCTION
Infections with nontuberculous mycobacteria (NTM) have a poor prognosis in patients with underlying respiratory diseases. Patients face relapses, side effects, and status deterioration: their quality of life is greatly reduced, both because of the infection and also because of the drug toxicity (1). The current treatment for M. avium pulmonary disease (MAC-PD) (the most frequent NTM pulmonary disease) consists in at least three antibiotics in combination during 12 months after sputum conversion of the samples (i.e., 15 to 18 months) (1). The need for new, more effective, and better tolerated treatments is therefore crucial to improving the quality of life of these patients.
Clofazimine (CFZ), a riminophenazine dye antileprosy drug, showed both experimental and clinical high activity against tuberculosis (2, 3). Similarly, CFZ showed some activity against M. avium in vitro. An in vitro synergism has been described between CFZ and clarithromycin (CLR) and between CFZ and amikacin (AMK) (4, 5). It seems to prevent regrowth in MAC strains exposed to AMK and CLR. Some data are available on its potential efficacy in refractory patients and on its potential better efficacy on MAC-PD compared to rifampin (RIF) (1, 4–6). However, there are no data on CFZ in combination with the current recommended treatment as a potential shortening regimen or to check the synergism activity of CFZ with CLR in vivo. Therefore, we studied the in vivo activity of CFZ in combination in an aerosol mouse model of M. avium pulmonary disease.
RESULTS
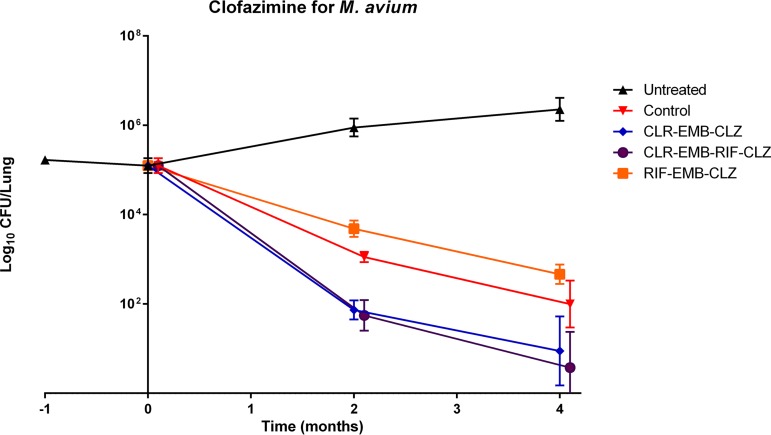
Results of lung CFU counts are presented in Table 1 and Fig. 1. One day after M. avium infection (M-1), the mean CFU count of M. avium in the lungs ± the standard deviation (SD) was 5.22 ± 0.03 log10. By day 0 (D0), 1 month later, this had grown to 5.12 ± 0.18. It steadily grew in untreated mice thereafter: 5.89 ± 0.22 at month 2 (M2) and 6.34 ± 0.28 at M4.
TABLE 1.
Mean lung CFU counts in BALB/c mice (log10/lung)a
| Regimen | Mean CFU ± SD (n) |
|||
|---|---|---|---|---|
| M-1 | D0 | M2 | M4 | |
| Untreated | 5.22 ± 0.03 (6) | 5.12 ± 0.18 (6) | 5.89 ± 0.22 (6) | 6.36 ± 0.26 (6) |
| CLR-RIF-EMB | 3.02 ± 0.12 (6) | 2.0 ± 0.53 (6) | ||
| CFZ-RIF EMB | 3.55 ± 0.28 (6) | 2.66 ± 0.22 (6) | ||
| CLR-CFZ-EMB | 1.57 ± 0.17 (6) | 0.95 ± 0.77 (6) | ||
| CLR-CFZ-RIF-EMB | 1.42 ± 0.42 (6) | 0.57 ± 0.8 (6) | ||
| Total of mice (n = 72) | 6 | 6 | 30 | 30 |
CLR, clarithromycin; RIF, rifampin; EMB, ethambutol; CFZ, clofazimine. M-1 = 1 month before start of treatment; D0 = start of treatment; M2 and M4 = 2 and 4 months after the start of treatment, respectively. n, number of mice used at each time point.
FIG 1.
Lung CFU counts in BALB/c mice.
After 2 months of treatment, CLR reduced lung CFU counts 0.5 log10 more than CFZ, rendering the CLR-RIF-ethambutol (EMB) combination significantly more efficient than the CFZ-RIF-EMB combination (3.02 ± 0.12 versus 3.55 ± 0.28, respectively, P < 0.01), whereas the CLR-CFZ-EMB and CLR-CFZ-RIF-EMB combinations decreased lung CFU counts by 4.32 and 4.47 log10, respectively, compared to untreated group (or by 1.45 and 1.6 log10 compared to the CLR-RIF-EMB group).
After 4 months of treatment, CLR reduced lung CFU counts 0.66 log10 more than CFZ (2 ± 0.53 for the CLR-RIF-EMB combination versus 2.66 ± 0.22 for the CFZ-RIF-EMB combination). This difference was again statistically significant (P = 0.01). The addition of CLZ to CLR decreased the lung CFU counts, yielding CFU counts 5.41 and 5.79 log10 lower in the CLR-CFZ-EMB and CLR-CFZ-RIF-EMB groups, respectively, compared to the untreated group and 1.05 and 1.43 log10 lower compared to the CLR-RIF-EMB group. The addition of RIF to the CLR-CFZ combination improved its efficacy by 0.38 log10, but the difference was not statistically significant (P > 0.05).
DISCUSSION
M. avium is the most common NTM in the world (7) and, except for clarithromycin, which is the cornerstone of the treatment, effective and safe drugs to combine with clarithromycin are not available. Of the most effective and safest drugs, we decided to focus on clofazimine. To our knowledge, this is the first study to suggest in vivo synergistic activity between CLR and CFZ. In vitro activity of Clofazimine against M. avium has been reported but inconsistently. Indeed, Huang et al. found little effect of CFZ against clinical isolates of M. avium using the MIC (5), whereas Ferro et al. found growth inhibition using time-kill assays but a regrowth appeared 48 to 168 h after (depending on the CFZ concentration) (4). The MICs of CFZ against M. avium complex vary between 0.06 and 0.25 μg/ml (4, 8). Synergistic in vitro activity has been shown between CFZ and amikacin (8), CFZ, and bedaquiline (9) and between CFZ and CLR, but only with delay and for lower concentrations (0.25× and 0.5× the MIC) (4). As CFZ concentrates in macrophages, it is possible that synergistic activity of CFZ could not be fully demonstrated in vitro but rather in vivo.
To improve CLR efficacy, the addition of CFZ would be a good candidate. In our study, it increased the killing rate at month 2 and was confirmed at month 4. CFZ in combination with RIF and EMB is, on the other hand, less effective than CLR-RIF-EMB. Thus, CLR should not be replaced by CFZ in cases of CLR-susceptible MAC disease. The role of CLR-CFZ synergy on treatment duration is still unclear, and experiments are under way to determine whether this combination can decrease treatment duration. Because the dosing of CFZ by inhalation suspension allows for approximately four times higher concentrations in lung tissue than does oral dosing (10), this route of treatment should be considered in future research to improve the efficacy of the CFZ-CLR combination.
Our data confirmed that the model of M. avium aerosol infection is a good tool for evaluating NTM treatment, since the SD in CFU count of untreated mice is very low. More importantly, our data are consistent with the published use of CFZ in patients with MAC pulmonary disease (6, 11, 12), although no study has prospectively compared a CLR regimen to a CLR-CFZ regimen. The growing literature regarding CFZ use and safety pleads for trials on CFZ use in first line regimen.
The main limitation to our study is the use of a non-mouse-passaged M. avium strain. This has possibly diminished the virulence of the strain and could explain our lower lung CFU counts at M2 and M4 than previously published (13). This can overestimate our results. Another limitation is the choice of a mouse model without pulmonary lung disease, but the choice of healthy BALB/c mice was made based on a previous study on different mouse models wherein nude mice and BALB/c mice were the most appropriate mouse model for drug susceptibility testing against M. avium (13).
This study shows a great improvement in efficacy when CFZ is associated with CLR, which could correspond in vivo to the synergistic activity found in vitro between CLR and CFZ. These data need to be confirmed by similar studies, along with its potential for shortening treatment. Thus, a sterilizing activity study, evaluating three-month relapse, is warranted.
MATERIALS AND METHODS
Mouse strain.
The BALB/c female 6-week-old mice were used for this study were purchased from Charles River, Saint-Germain Nuelles, France.
Mycobacterial strain.
M. avium strain Chester (MAC 101, ATCC 700898) was used after being grown to a log-phase culture in Middlebrook 7H9 broth (Difco, Detroit, MI) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose (OADC; Becton Dickinson, Le Pont de Claix, France) and 0.05% (vol/vol) Tween 80 (Sigma, St. Louis, MO). M. avium cultures were incubated for 4 weeks before use in an experiment or infection. This strain has previously been demonstrated to multiply to high numbers in beige mice, allowing for assessment of treatment (14).
Drugs.
Rifampin (RIF) was purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France), prepared in distilled water at 10 mg/kg, and administered by gavage 1 h before other drugs in 0.1 ml. Clarithromycin (CLR) was purchased from Sigma-Aldrich or Arrow Génériques (Lyon, France), solubilized in 10% absolute ethanol and then in distilled water. Clofazimine (CFZ) was purchased from Sigma-Aldrich and prepared in 0.05% agarose. Ethambutol (EMB) was purchased from Sigma-Aldrich and prepared in distilled water. All stock solutions were stored at 4°C for up to 1 week.
Aerosol infection with M. avium.
All animal procedures were approved by the Animal Care and Use Committee of Amiens Picardy Jules Verne University. Mice were aerosol infected using an inhalation exposure system (Glas-Col, Terre Haute, IN) using 10 ml of pure culture of M. avium strain Chester. One day after infection, six mice were humanely killed to determine the number of bacteria implanted in the lungs. This murine model of MAC infection has been validated previously (13).
Study design.
Sixty BALB/c mice were simultaneously aerosol-infected with M. avium using a 7H9 broth containing 9.08 log10 CFU/ml using the inhalation exposure system. After infection, the mice from each strain were randomized into five subgroups, and at 28 days postinfection treatment was initiated with one of the following drug combinations: CLR-RIF-EMB (n = 12), CFZ-RIF-EMB (n = 12), CLR-CFZ-EMB (n = 12), or CLR-CFZ-RIF-EMB (n = 12) with a 4-month period of treatment. A total of 24 mice were left untreated. Daily drug doses were 10 mg/kg for rifampin, 25 mg/kg for clofazimine, and 100 mg/kg for clarithromycin and ethambutol (2, 15, 16). All drugs were administered in a total volume of 0.1 ml by esophageal cannula; rifampin was given 1 h before administration of the other drugs to avoid possible adverse pharmacokinetic interactions (17–19). Six animals from the untreated group were sacrificed the day after infection and on the day of treatment initiation to determine the CFU counts implanted and pretreatment, respectively. Six animals from both treated and untreated groups were humanely sacrificed at 8 and 16 weeks after the beginning of treatment to assess treatment efficacy.
Assessment of treatment efficacy.
Treatment efficacy was assessed on the basis of lung CFU counts after 2 and 4 months of treatment. Lungs were homogenized in sterile phosphate-buffered saline and diluted.
Serial dilutions of whole-lung homogenates were plated in duplicate on selective Middlebrook 7H11 agar plates enriched with 10% OADC plus antibiotics as follows: 5% cycloheximide, 5% carbenicillin, 2.5% polymyxin B, and 2% trimethoprim (all purchased from Sigma-Aldrich). Plates were incubated for 8 weeks at 37°C before determining final CFU counts.
Statistical analysis.
CFU counts (x) were log transformed as log10(x + 1) before analysis. To compare combination effects, we performed one-way analysis of variance assuming normal distribution. A P value of ≤0.05 was considered significant. The Bonferroni correction was used to adjust for multiple comparisons, using Prism version 5 (GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank AGIR team members for their help in the study and PlatAnN members for the care provided to the animals of the study.
J.-P.L. and C.A. designed the study. All authors contributed to the study. J.-P.L., C.A., C.J., and S.C. drafted the manuscript.
This study was funded by the Comité Départemental De Lutte Contre Les Maladies Respiratoires, an independent association fighting for patients’ health, and by the Conseil Régional de Picardie.
REFERENCES
- 1.Kwon YS, Koh WJ, Daley CL. 2019. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Respir Dis (Seoul) 82:15–26. doi: 10.4046/trd.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li SY, Ammerman NC, Bishai WR, Enarson D, Trebucq A. 2013. Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188:608–612. doi: 10.1164/rccm.201304-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyagi S, Ammerman NC, Li SY, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferro BE, Meletiadis J, Wattenberg M, de Jong A, van Soolingen D, Mouton JW, van Ingen J. 2016. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob Agents Chemother 60:1097–1105. doi: 10.1128/AAC.02615-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CC, Wu MF, Chen HC, Huang WC. 2018. In vitro activity of aminoglycosides, clofazimine, d-cycloserine and dapsone against 83 Mycobacterium avium complex clinical isolates. J Microbiol Immunol Infect 51:636–643. doi: 10.1016/j.jmii.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Field SK, Cowie RL. 2003. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 124:1482–1486. doi: 10.1378/chest.124.4.1482. [DOI] [PubMed] [Google Scholar]
- 7.Hoefsloot W, Nontuberculous Mycobacteria Network European Trials Group, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PNR, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gómez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh P-R, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh W-J, Lange B, Leao S, Macedo R, Mannsåker T, Marras TK, Maugein J, Milburn HJ, Mlinkó T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Peña M, Piersimoni C, Polanová M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabó N, Thomson R, Tórtola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 8.van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. 2012. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 56:6324–6327. doi: 10.1128/AAC.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruth MM, Sangen JJN, Remmers K, Pennings LJ, Svensson E, Aarnoutse RE, Zweijpfenning SMH, Hoefsloot W, Kuipers S, Magis-Escurra C, Wertheim HFL, van Ingen J. 2019. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 74:935–943. doi: 10.1093/jac/dky526. [DOI] [PubMed] [Google Scholar]
- 10.Banaschewski B, Verma D, Pennings LJ, Zimmerman M, Ye Q, Gadawa J, Dartois V, Ordway D, van Ingen J, Ufer S, Stapleton K, Hofmann T. 2019. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J Cyst Fibros 18:714–720. doi: 10.1016/j.jcf.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. 2016. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 149:1285–1293. doi: 10.1378/chest.15-0543. [DOI] [PubMed] [Google Scholar]
- 12.Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. 2017. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 152:800–809. doi: 10.1016/j.chest.2017.04.175. [DOI] [PubMed] [Google Scholar]
- 13.Andrejak C, Almeida DV, Tyagi S, Converse PJ, Ammerman NC, Grosset JH. 2015. Characterization of mouse models of Mycobacterium avium complex infection and evaluation of drug combinations. Antimicrob Agents Chemother 59:2129–2135. doi: 10.1128/AAC.04841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangadharam PR, Perumal VK, Farhi DC, LaBrecque J. 1989. The beige mouse model for Mycobacterium avium complex (MAC) disease: optimal conditions for the host and parasite. Tubercle 70:257–271. doi: 10.1016/0041-3879(89)90020-2. [DOI] [PubMed] [Google Scholar]
- 15.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother 53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosset J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc 53:5–12. [PubMed] [Google Scholar]
- 17.Dhillon J, Dickinson JM, Sole K, Mitchison DA. 1996. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother 40:552–555. doi: 10.1128/AAC.40.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson J, Guy A, Mitchison DA. 1992. Bioavailability of rifampin in experimental murine tuberculosis. Antimicrob Agents Chemother 36:2066–2067. doi: 10.1128/aac.36.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]