Doxycycline, an FDA-approved tetracycline, is used in tuberculosis in vivo models for the temporal control of mycobacterial gene expression. In these models, animals are infected with recombinant Mycobacterium tuberculosis carrying genes of interest under transcriptional control of the doxycycline-responsive TetR-tetO unit. To minimize fluctuations of plasma levels, doxycycline is usually administered in the diet. However, tissue penetration studies to identify the minimum doxycycline content in food achieving complete repression of TetR-controlled genes in tuberculosis (TB)-infected organs and lesions have not been conducted.
KEYWORDS: doxycycline, TetR, tuberculosis, animal models, tissue penetration, genetic regulation in vivo
ABSTRACT
Doxycycline, an FDA-approved tetracycline, is used in tuberculosis in vivo models for the temporal control of mycobacterial gene expression. In these models, animals are infected with recombinant Mycobacterium tuberculosis carrying genes of interest under transcriptional control of the doxycycline-responsive TetR-tetO unit. To minimize fluctuations of plasma levels, doxycycline is usually administered in the diet. However, tissue penetration studies to identify the minimum doxycycline content in food achieving complete repression of TetR-controlled genes in tuberculosis (TB)-infected organs and lesions have not been conducted. Here, we first determined the tetracycline concentrations required to achieve silencing of M. tuberculosis target genes in vitro. Next, we measured doxycycline concentrations in plasma, major organs, and lung lesions in TB-infected mice and rabbits and compared these values to silencing concentrations measured in vitro. We found that 2,000 ppm doxycycline supplemented in mouse and rabbit feed is sufficient to reach target concentrations in TB lesions. In rabbit chow, the calcium content had to be reduced 5-fold to minimize chelation of doxycycline and deliver adequate oral bioavailability. Clearance kinetics from major organs and lung lesions revealed that doxycycline levels fall below concentrations that repress tet promoters within 7 to 14 days after doxycycline is removed from the diet. In summary, we have shown that 2,000 ppm doxycycline supplemented in standard mouse diet and in low-calcium rabbit diet delivers concentrations adequate to achieve full repression of tet promoters in infected tissues of mice and rabbits.
TEXT
Transcriptional regulators derived from the tetracycline repressor (TetR) of Escherichia coli have been exploited for more than 2 decades to chemically regulate gene expression with tetracyclines in eukaryotic cells (1–3). The specificity of the Tet repressor-operator-effector interaction and the favorable in vivo pharmacokinetics of tetracyclines make this regulatory system well suited for the temporal control of gene expression in vivo, such as in transgenic animals (4) and animals infected with recombinant pathogenic bacteria carrying genes of interest under the TetR-tetO transcriptional control unit (5–7).
Promoters that can be controlled by TetR and tetracyclines were initially developed in vitro in Gram-positive and Gram-negative bacteria (8). More recently, such systems have also been developed for medically relevant acid-fast bacteria, including M. tuberculosis (9, 10). Owing to its excellent oral bioavailability and pharmacokinetic properties in mice and its relative lack of activity against M. tuberculosis with an MIC of 8 to 16 μg/ml (11), doxycycline (DOX) has frequently been used in tuberculosis (TB) mouse models to induce or repress M. tuberculosis genes (12–15). In general, the affinity of DOX for TetR is several orders of magnitude higher than its affinity for the ribosome, providing a safe range within which genetic regulation can be achieved without affecting bacterial growth (16).
To regulate M. tuberculosis gene expression during infection, DOX is administered either in the drinking water at up to 2 mg/ml or in commercially available modified diets at 200 to 2,000 ppm, where it was reported to be stable for at least 7 days and 6 months, respectively (17, 18). Supplementation of DOX in mouse feed is considered the most convenient method to achieve adequate and consistent plasma levels (18).
Since DOX is commonly used as a broad-spectrum antibiotic in veterinary medicine and the food industry, tissue distribution studies have been conducted in animals such as horses and broiler chickens to assess its penetration into various organs and cell types. DOX shows favorable tissue partitioning and equilibration of free drug concentrations (with a free fraction in plasma of 20%) between plasma and interstitial fluid at steady state. Accumulation in specific cell types, such as polymorphonuclear leukocytes (19) and fibroblasts (20, 21), has been observed. However, no tissue penetration study has been conducted to determine whether adequate DOX levels are achieved in TB-infected organs and immune cell types in which the pathogen resides.
This work was undertaken to (i) identify the range of DOX concentrations that achieve full repression of Tet-OFF promoters in vitro, (ii) measure DOX concentrations in major organs and in lung lesions in TB-infected mice and rabbits in order to determine the lowest DOX feed content required to achieve the target concentrations identified in vitro, and (iii) explore the clearance kinetics of DOX from lung tissue and lesions to determine the number of days required for DOX levels to fall below concentrations sufficient to induce or repress tet promoters after DOX is removed from the diet.
RESULTS
Concentrations required for control of Tet-OFF constructs by tetracyclines in vitro.
Anhydrotetracycline (ATc) is conventionally used as the in vitro inducer or repressor of TetR-tetO constructs due to its lack of growth inhibitory activity against M. tuberculosis. DOX, on the other hand, is the preferred transcriptional regulator in vivo because it is orally bioavailable and inhibits M. tuberculosis growth only at high concentrations (11). To determine the concentrations of ATc and DOX required to achieve complete silencing of target genes in M. tuberculosis in vitro, we resorted to genetically engineered strains in which a dual-control switch combining repression of transcription and controlled proteolysis had been chromosomally integrated to silence gene and protein activities (14, 22–24). Three M. tuberculosis strains, each with a dual-control switch targeting either fum, rho, or trxB2 (three essential M. tuberculosis genes), were exposed to increasing concentrations of ATc or DOX. ATc concentrations ranging from 1 to 2 μg/ml were required to achieve 90% growth inhibition resulting from effective silencing in all three strains (Fig. 1A). When DOX was used as the TetR ligand in vitro, 50% growth inhibition was achieved at 80, 120, and 200 ng/ml for trxB2, rho, and fum, respectively; 90% growth inhibition was achieved between 230 and 350 ng/ml (Fig. 1B). Thus, lower DOX than ATc concentrations are required to control TetR-tetO constructs in M. tuberculosis. To ensure that these concentrations do not interfere with the growth of wild-type M. tuberculosis and to confirm published MICs, full dose-response curves were obtained with M. tuberculosis H37Rv exposed to both tetracyclines. M. tuberculosis growth remained unaffected by ATc up to the highest concentration tested (4 μg/ml), while DOX achieved approximately 30% growth inhibition at 4 μg/ml (Fig. 1C), consistent with the published MICs of 8 to 16 μg/ml (11). These results indicate that the concentrations required to achieve maximum control of TetR-tetO constructs do not affect growth of M. tuberculosis in vitro.
FIG 1.
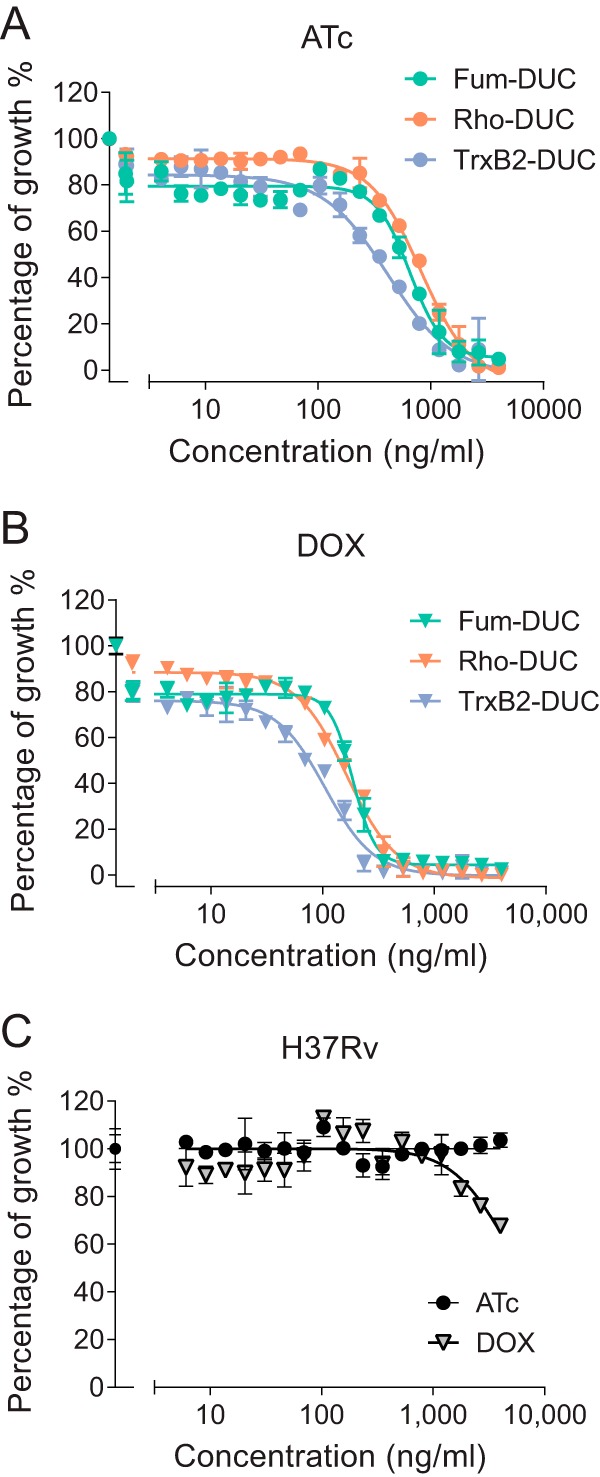
Effect of tetracyclines on in vitro growth of M. tuberculosis H37Rv harboring essential genes under TetR control compared to wild type. (A and B) Growth inhibitory dose response of anhydrotetracycline (ATc) and doxycycline (DOX) in M. tuberculosis strains that harbor a TetR-dependent dual-control genetic switch (14) driving expression of Fum (fumarate hydratase, green symbols), Rho (transcription termination factor, orange symbols), or TrxB2 (thioredoxin reductase, blue symbols). (C) Growth inhibitory dose response of ATc and DOX against wild-type M. tuberculosis H37Rv.
Pharmacokinetics and tissue distribution of DOX in mice.
To compare the DOX concentrations achieved in mice relative to DOX MICs and concentrations required to regulate TetR-controlled promoters, we first measured DOX concentrations in the plasma of TB-infected mice receiving 2,000 ppm DOX in the diet. Plasma was collected on days 1, 2, 3, and 7 following the switch from DOX-free to DOX-medicated chow, 3 weeks postinfection with M. tuberculosis strain H37Rv. Plasma levels of DOX ranged from 300 to 1,190 ng/ml (average, 637 ng/ml; standard deviation [SD], 411 ng/ml) on day 1 and reached an average of 1,081 ng/ml (SD, 386 ng/ml; range, 757 to 1,850 ng/ml) on day 7 (Fig. 2A), thus achieving the concentrations required to inhibit 90% of the growth of strains harboring representative Tet-OFF-controlled essential genes in vitro (Fig. 1B). To assess potential lot-to-lot variability in DOX chow, groups of 3 mice were fed three different lots, and plasma DOX concentrations were measured in serial blood samples from 0 to 5 h on day 1 (see Fig. S1 in the supplemental material), showing consistent plasma levels across lots. Actual DOX concentrations were measured in each lot and found to be within 25% of the target content of 2,000 ppm (Table S1).
FIG 2.
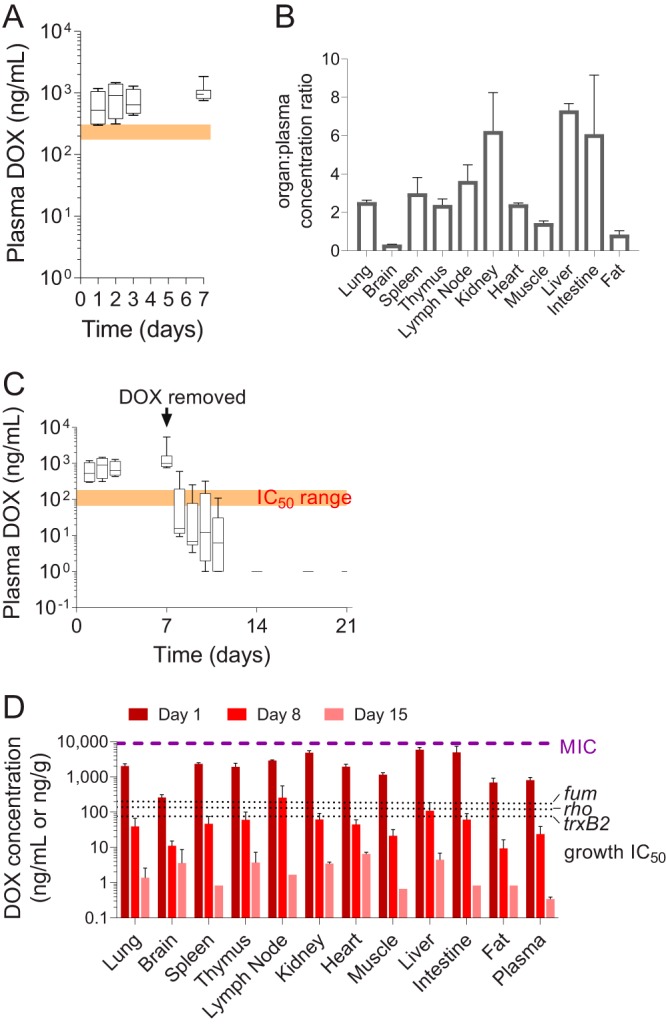
Plasma and tissue pharmacokinetics of DOX in mice receiving DOX-supplemented diet. (A) Box and whisker plot of DOX plasma concentrations in mice receiving 2,000 ppm DOX in standard diet for 7 days. Mean values (bar), minimum and maximum (box) and standard deviations (error bars) are shown for n = 4 to 11 mice per group. The orange shaded window indicates the range of in vitro DOX concentration that inhibits 90% of bacterial growth (IC90) for the three reporter strains shown in Fig. 1. (B) DOX concentrations in mouse organs relative to plasma after 7 days on the DOX diet. Mean values and standard deviations (error bars) are shown (n = 3). (C) Kinetics of DOX clearance in plasma following removal of DOX from the diet for 14 days. Mean values (bar) and minimum and maximum (box) and standard deviations (error bars) are shown (n = 3 to 12). The orange shaded window indicates the range of in vitro DOX concentrations that inhibit 50% of bacterial growth (IC50) for the three reporter strains shown in Fig. 1. (D) Kinetics of DOX clearance from major organs of TB-infected mice 7 and 14 days after removal of DOX from the diet. Day 1 corresponds to DOX levels in mice that had received a DOX-supplemented diet for 1 week, after which it was replaced by a DOX-free diet for the duration of the experiment. The purple dotted line indicates the lower boundary of published MIC ranges against wild-type M. tuberculosis (11), and the black dotted lines show individual DOX IC50s for the three reporter strains (Fig. 1). Mean values and standard deviations (error bars) are shown (n = 3).
To quantify penetration into tissues, we measured DOX concentrations in all major organs in the TB-infected mice that had received DOX-medicated chow for 7 days (Fig. 2B), showing favorable penetration in all organs except for brain tissue. In infected lung, spleen, and lymph node homogenates, total DOX concentrations were 2- to 4-fold higher than in plasma, thus exceeding levels required to achieve 90% growth inhibition, while not reaching the MIC of 8 to 16 μg/ml (Fig. 2B). To determine the time required for DOX to clear plasma and infected organs below concentrations sufficient to induce or repress Tet-ON and Tet-OFF promoters, mice were switched to a DOX-free diet after 7 days on DOX chow, and DOX concentrations in plasma were measured at 1, 2, 3, 4, 7, 11, and 14 days post-DOX removal (Fig. 2C). To determine how this translated within tissue, we quantified DOX in homogenized organs from 3 mice per group at 1, 8, and 15 days post-DOX removal, showing that concentrations fell below the levels required to achieve 50% growth inhibition in all organs except lymph nodes within 8 days post-DOX removal (Fig. 2D).
Dose finding pharmacokinetics of DOX in rabbits.
The rabbit model of TB disease is well suited to assess the essentiality and vulnerability of bacterial targets in mature cellular and necrotic lesions resembling those found in human TB (25, 26). However, DOX pharmacokinetics (PK) and distribution in pulmonary lesions have not been characterized in rabbits. Preliminary PK studies in rabbits that received a modified diet with 200 and 400 ppm DOX showed unexpectedly low plasma levels (Fig. S2A). When the DOX content of the rabbit diet was increased to 2,000 ppm (corresponding approximately to 100 mg/kg), plasma levels increased proportionally, but remained lower than in mice, at 100 to 200 ng/ml throughout the 10-day PK study (Fig. 3A).
FIG 3.
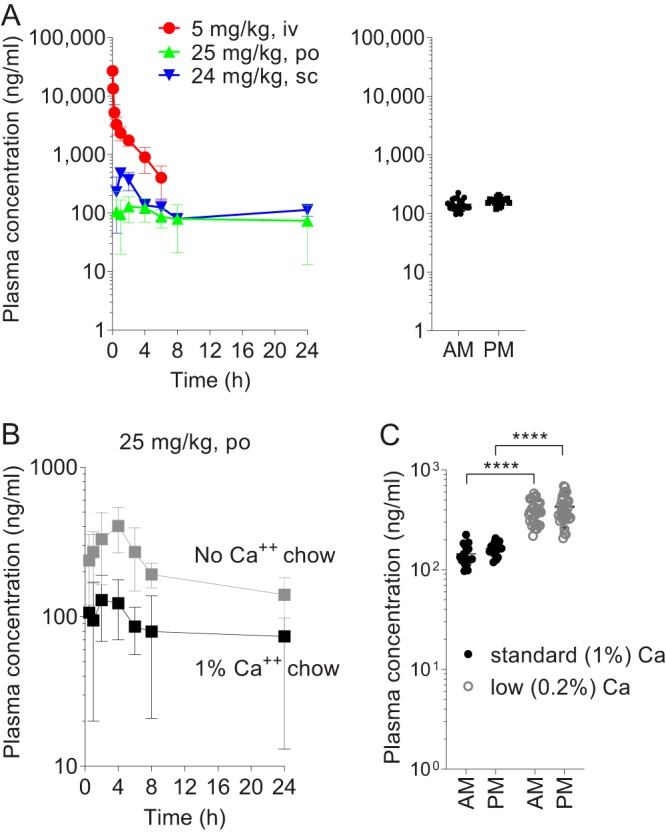
Plasma and tissue pharmacokinetics of DOX in rabbits receiving DOX-supplemented chow. (A) Left panel: DOX concentration-time profile in rabbit plasma following a single DOX dose administered intravenously (i.v.), by oral gavage (p.o.), and subcutaneously (s.c.), as indicated. Right panel: DOX plasma concentrations in rabbits receiving 2,000 ppm DOX in standard chow for 7 days. AM, immediately prior to receiving daily DOX-supplemented feed in the morning; PM, 6 h after receiving DOX feed. (B) Effect of dietary calcium removal on the plasma pharmacokinetics of DOX in rabbits following a single oral dose of 25 mg/kg. (C) Effect of dietary calcium lowering (0.2% total calcium content, or 5-fold lower than in standard chow) on DOX plasma levels in rabbits that received 2,000 ppm DOX in chow for 5 to 7 days. AM and PM time points are as described for right panel A. Data are represented as scatter dot plots of individual animal values and are pooled from two separate experiments with 4 and 6 rabbits, respectively (horizontal bar indicates mean, n = 20 to 34 blood samples from 10 rabbits).
To determine whether poor oral bioavailability or species-specific metabolism was the reason for the low DOX concentration in rabbits, we dosed uninfected rabbits with DOX via the intravenous (i.v.), oral (p.o.), and subcutaneous (s.c.) routes. Following bolus i.v. dosing, a biphasic concentration-time profile was observed in plasma, with a distribution and elimination phase, consistent with a two-compartment PK model (Fig. 3A, Table 1). The volume of distribution at steady state (steady-state volume of distribution [Vdss] or volume of distribution [Vd] area) was high, reaching a maximum of approximately 1 liter/kg, corresponding to total body volume and similar to the Vd measured in domestic rabbits (27) and in pigs (28). The clearance was low at 26 ml/min or 15% of liver blood flow (Table 1). The i.v. concentration time profile and PK parameters were consistent with published data that used a bioassay for DOX quantitation following i.v. injection in domestic rabbits (27). DOX administration by oral gavage resulted in 3.2% bioavailability (Fig. 3A, Table 1), indicating that poor absorption rather than fast metabolism may partially account for the low oral bioavailability. To determine whether pharmaceutical grade formulation might improve oral absorption, we dosed rabbits with DOX tablets at approximately 15 and 30 mg/kg, which did not result in detectable exposure improvement (Fig. S2B). Lastly, subcutaneous injection resulted in a 1.5-fold increase in the area under the concentration-time curve (AUC) and average concentration throughout the dosing interval (Fig. 3A, Table 1).
TABLE 1.
Pharmacokinetic parameters of DOX in rabbitsa
| Route | Dose (mg/kg) | AUC0–24 (ng/h/ml) | Cave (ng/ml) | Elim T1/2 (h) | kel (h−1) (liters/kg) | Vdss/V area (liters/kg)b | CL (ml/min) | F (%) |
|---|---|---|---|---|---|---|---|---|
| Intravenous bolus | 5 | 12,478 (4,058) | NA | 1.95 (0.49) | 0.35 (0.08) | 1,056/1,138 | 26.2 (5.6) | NA |
| Oral gavage | 25 | 2,046 (973) | 85 (40) | 3.2 | ||||
| Subcutaneous | 24 | 3,175 (356) | 132 (15) | 5.2 | ||||
| Oral gavage (standard 1% calcium chow) | 25 | 2,317 (275) | 97 (11) | 3.6 | ||||
| Oral gavage (calcium-free chow) | 25 | 5,014 (950) | 209 (40) | 7.9 | ||||
| Oral gavage (TB-infected) | 20 | 360 | 15 | 0.7 |
AUC0–24, area under the concentration-time curve; Elim T1/2, elimination half-life; Cave, average plasma concentration over 24 h; kel, elimination rate constant; CL, clearance; F, bioavailability.
Vdss = dose · [(A/alphâ2 + B/betâ2)/AUĈ2]; V area = dose/beta kel · AUC (45).
Calcium found in dairy products interferes with tetracycline oral absorption because it forms insoluble chelates resulting in decreased bioavailability (29). While most mammals tightly regulate blood calcium, rabbits absorb nearly all dietary calcium and excrete the excess through the kidneys. This results in a surge of blood calcium following food intake (30), proportional to calcium levels in the diet. We therefore hypothesized that DOX absorption and plasma concentrations may increase in rabbits receiving a calcium-free or low-calcium diet. Rabbits were fed a calcium-free or 1% calcium diet for a minimum of 2 days, at which point they received a single 25-mg/kg dose of DOX via oral gavage. As hypothesized, exposure (AUC) after a single dose was twice as high in rabbits on a calcium-free diet (Fig. 3B, Table 1).
To determine whether a similar improvement in DOX exposure would be seen when DOX is provided in the chow, under dietary conditions compatible with long-term experiments (i.e., not calcium-free), we placed rabbits on either standard (1% calcium) chow with DOX at 2,000 ppm or 0.2% calcium chow with DOX at 2,000 ppm, as described in Materials and Methods. Blood samples were collected twice a day (a.m. and p.m.) after 5 to 7 days on DOX chow as indicated. The respective mean a.m. and p.m. plasma DOX concentrations were 144 and 163 ng/ml in the standard-calcium group and 385 and 429 ng/ml in the low-calcium group (Fig. 3C), showing a 2 to 3-fold increase in the low-calcium group and reinforcing the trend observed when DOX was received as a single oral dose. Having optimized constant DOX exposure when provided in the diet, we next studied its distribution in the cellular and necrotic compartments of mature rabbit lesions in order to determine the DOX concentrations to which M. tuberculosis bacilli are exposed in tissues.
In vitro lesion pharmacokinetics.
Previous work by our group indicated that drug distribution and partitioning within lesions is a function of uptake into macrophages and binding to caseum macromolecules (31, 32). To predict the penetration of DOX in cellular lesions and its behavior at the interface between the cellular rim and necrotic core of TB lesions, we measured DOX uptake into macrophages and caseum binding. DOX uptake was quantified in THP-1 macrophages, where it reached an intracellular to extracellular concentration ratio of 4.1 (SD, 0.6). This favorable cellular uptake predicted good penetration into cellular lesions. The average unbound drug fraction in caseum (fu-caseum) was moderate to high at 12 to 14% (SD 3%) of total drug. Together with the moderate uptake in macrophages, the DOX fraction unbound predicted favorable partitioning at the cellular/caseum interface and good passive diffusion through nonvascularized caseum. Taken together, these results suggest that DOX should reach higher concentrations in both cellular and necrotic lesions than plasma.
Distribution of DOX in cellular and necrotic lesion compartments.
Uninvolved lung, cellular, and necrotic lesions were dissected from the lungs of TB-infected rabbits receiving 2,000 ppm DOX in low-calcium (20% of standard or 0.2% final) chow for 7 days. DOX was also quantified in spleen, lymph nodes, liver, fat, and brain tissue. We found higher DOX levels in cellular and necrotic lesions than in plasma (Fig. 4A), indicating that DOX accumulates in infected tissues relative to plasma, as predicted by the macrophage uptake assay and consistent with the reported accumulation in polymorphonuclear leukocytes (19). Favorable penetration was also measured in spleen, liver, and lymph nodes. All tissue concentrations were above the levels required to achieve 90% growth control, with the exception of one animal with borderline DOX concentrations in some of the tissues. These lower concentrations were explained by low food consumption (Table S2). DOX levels were below the M. tuberculosis MIC in all tissues (Fig. 4A). To quantify the partitioning of DOX at the cellular/caseum interface, we used laser-capture microdissection in thin sections of large necrotic lesions (33) from the same rabbits and found caseum-to-cellular DOX concentration ratios ranging from 0.3 to 3.2 with a mean value of around 1.0 (Fig. 4B). Overall, we found (i) higher DOX concentrations in fully cellular lesions than in plasma in line with the macrophage uptake data and (ii) comparable concentrations in caseum and cellular rims, confirming the favorable passive diffusion of DOX through avascular caseum predicted by the caseum binding assay. Collectively, these results indicate that in rabbits on low-calcium chow supplemented with DOX at 2,000 ppm, DOX concentrations are sufficient to achieve complete repression of Tet-OFF genetic constructs but not high enough to affect growth and viability of wild-type M. tuberculosis.
FIG 4.
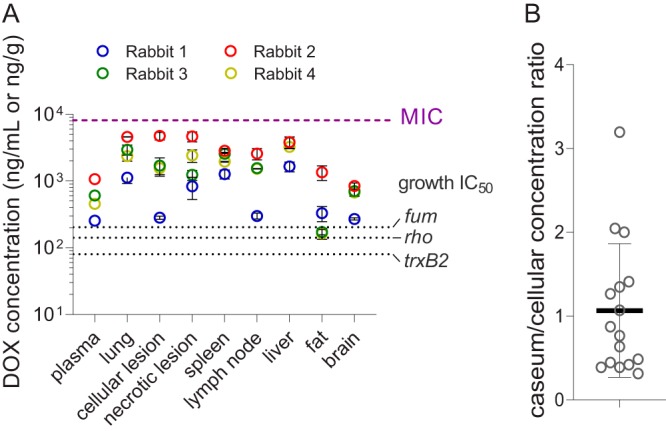
DOX penetration in major organs, lung lesions, and necrotic caseum of TB-infected rabbits. (A) DOX concentrations in plasma, uninvolved lung tissue, lung lesions, and major organs in 4 rabbits receiving a low-calcium 2,000 ppm DOX diet for 7 days. Mean values and standard deviations (error bars) are shown (n = 3). (B) Scatter dot plots of caseum-to-cellular DOX concentration ratios in necrotic granulomas collected from TB-infected lungs. Ratios obtained in 10 lesions from 5 infected rabbits are shown.
DISCUSSION
We have shown that 2,000 ppm DOX supplemented in a standard mouse diet and in a low-calcium rabbit diet deliver DOX concentrations adequate to achieve full repression of representative Tet-OFF promoters in infected tissues of both mice and rabbits. These concentrations remain below the DOX MIC in all tissues and are thus likely to primarily affect M. tuberculosis growth and viability by regulation of genes under the control of TetR. The clearance kinetics of DOX from lung lesions and other infected organs indicate that DOX levels fall below concentrations sufficient to repress tet promoters within 7 days after DOX is removed from the diet.
DOX bioavailability is species specific and subject to food effect. It is very poor in rats (34) and moderate to low (20%) in pigs (28) but almost complete in calves (35) and humans (36). In nonfasted rabbits that received 150 mg/kg tetracycline by oral gavage (27), bioavailability was estimated at 4% (Data Set S1), in line with the present findings. Interestingly, DOX exposure was also very low in rabbits that received subcutaneous injections as part of this work, indicating that low bioavailability is not exclusively due to poor oral absorption. Tetracyclines are known to localize in bones and teeth, where 99% of the body calcium is found and where they form calcium-bound depots. They also accumulate in liver and spleen, where they are more highly bound than in plasma (http://www.cyto.purdue.edu/cdroms/cyto2/17/chmrx/tetra.htm). Such trapping in selected tissues could contribute to species-specific plasma levels. In early studies, the distribution of DOX in tissues was described in rats (37) and in patients undergoing biopsies during surgery (38). DOX concentrations were measured in plasma/serum and in major organs using a bioassay and indicated lung-to-serum concentration ratios between 2 and 3, consistent with our findings. Penetration was highest in kidneys and intestines and lowest in fat tissue (37, 38), again in line with our results (Fig. 2B).
Rabbits possess a unique calcium metabolism. Most mammals regulate blood calcium to maintain levels within a narrow range by controlling the absorption of calcium from the diet according to need. Rabbits have adopted the different strategy of absorbing nearly all dietary calcium ingested and renally excreting the excess. As a result, blood concentrations transiently reflect calcium levels in the diet, and calcium carbonate is excreted in large amounts in the urine (30). It has been determined that 0.2% calcium in rabbit chow is sufficient to sustain normal growth and weight gain (30), whereas standard diets contain 1% calcium. Reducing calcium content to 0.2% in rabbit chow supplemented with 2,000 ppm DOX led to improved oral bioavailability and at least 2-fold higher plasma levels throughout the day. DOX concentrations in all relevant organs, in lung lesions, and in plasma were higher than the minimum required to regulate tet promoters and did not reach the lower threshold of published MICs in broth (8 to 16 mg/liter [11]). Similar MICs have been measured against intracellular M. tuberculosis in macrophages, and bactericidal activity was observed at 40 mg/liter (39). DOX showed favorable partitioning into the necrotic foci of caseous lesions, with an average caseum/cellular concentration ratio of 1/1. Since caseum M. tuberculosis is nonreplicating and overall phenotypically resistant to most TB drugs, DOX is not expected to affect M. tuberculosis viability in this lesion compartment. Given the similar penetration of DOX in uninvolved lung, cellular, and caseous lesions (Fig. 4A), partial lesion resolution over time due to effective gene silencing by DOX and reduction of bacterial burden is unlikely to impact DOX penetration. Slow tissue clearance has been reported for DOX (40), consistent with our observation that levels fall below effective concentrations after approximately 7 days on a DOX-free diet. These findings have implications as to the kinetics of tet regulation following DOX removal and should be taken into consideration when interpreting related data.
Many tetracyclines, including DOX, have anti-inflammatory properties (41) mediated by suppression of tumor necrosis factor-α (TNF-α) and matrix metalloproteases (MMP). In vitro, DOX inhibits secretion of MMPs induced by TB infection at 5 mg/liter and higher (39). Thus, fine-tuning the DOX dose in both mouse and rabbit diets to reach but not exceed effective concentrations is important to minimize anti-inflammatory side effects and potential gastrointestinal disturbances.
MATERIALS AND METHODS
In vitro repression of Tet promoters.
The construction of the ATc/DOX-controlled M. tuberculosis mutants (Fum-DUC, Rho-DUC, and TrxB2-DUC) has been described (22–24). Mutant strains were grown to mid-log phase and diluted to an optical density (OD) of 0.03 in 7H9 medium. Bacteria were then exposed to 1.5-fold serial dilution of ATc or DOX. Optical density was recorded after 14 days and normalized to the corresponding strains without drug treatment.
Animal infection and DOX administration.
All animal studies were performed in biosafety level 2 (BSL2) and BSL3 facilities and approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School, Rutgers University, Newark, NJ. All samples collected from M. tuberculosis-infected animals were handled and processed in the BSL3 facility in compliance with protocols approved by the Institutional Biosafety Committee of the New Jersey Medical School, Rutgers University, Newark, NJ.
For pharmacokinetic studies in uninfected mice, 4- to 6-week-old CD-1 female mice received a modified rodent diet containing 2,000 ppm DOX for the duration indicated in each experiment. Several diet lots from two different manufacturers were tested in pilot experiments. Laboratory rodent diet 5001 with DOX 2,000 ppm (DOX chow) from Purina (catalog number c113-200i) provided the highest and most consistent plasma levels and was selected for all experiments reported here. Blood was collected at various time points for up to 3 days while mice were receiving DOX chow, kept on ice, centrifuged at approximately 2,350 × g (5,000 rpm in a table-top centrifuge) for 5 min, and stored at –80°C until analyzed for DOX content.
For plasma and tissue pharmacokinetic studies in infected mice, 8- to 10-week-old female BALB/c mice were infected with a low dose of 100 to 200 CFU M. tuberculosis H37Rv (ATCC 27294) using a full-body inhalation exposure system (Glas-Col) as previously described (42). Mice received DOX chow for 7 days 4 weeks postinfection. For the determination of DOX content in major organs relative to plasma, one group of 3 mice was euthanized on day 7 and organs were collected, weighed, and stored at –80°C until analyzed for DOX content. Blood was collected at the time of euthanasia and processed as described above. To study the time to clearance of DOX from plasma and tissues after DOX chow (received for 7 days) was replaced with a DOX-free diet, groups of 3 mice were euthanized just prior to and then 1, 2, 4, and 7 h and 1, 2, 3, 4, 7, 10, and 14 days after replacement of DOX chow with standard rodent diet. Plasma and lungs were collected, processed, and stored as described above.
For pharmacokinetic studies in rabbits, female New Zealand white (NZW) rabbits (Millbrook Farm, Concord, MA), weighing 2.2 to 2.6 kg, were maintained under specific pathogen-free conditions and fed water and chow ad libitum. For dose-finding pharmacokinetic studies in uninfected animals, rabbits received DOX at the following doses/routes: 5 mg/kg intravenously formulated in saline, 20 mg/kg by oral gavage and formulated in deionized water, 24 mg/kg subcutaneously formulated in deionized water, or 15 and 30 mg/kg administered as DOX pills (either half a pill or one full pill of 100 mg doxycycline hyclate; Henry Schein Animal Health, Covetrus, OH) by oral gavage. Rabbit weights were approximately 3.0 to 3.5 kg at the time of dosing.
For plasma and tissue pharmacokinetics in TB-infected NZW rabbits, animals were infected with M. tuberculosis HN878 using a nose-only aerosol exposure system as described (43). At 12 to 20 weeks postinfection, once mature cellular and necrotic lesions had developed, rabbits received LabDiet 5001 supplemented with DOX at 200 ppm, 400 ppm, or 2,000 ppm (Research Diets, Inc., New Brunswick, NJ) for the duration indicated in each experiment.
For pharmacokinetic studies of DOX in a modified diet containing low or no calcium, uninfected rabbits received a gradually phased diet as follows. To test DOX PK in a calcium-free diet, rabbits received 25/75% calcium-free chow mixed with standard high-fiber rabbit chow (Laboratory Rabbit Diet HF 5326; LabDiet)—containing 10,000 ppm or 1% calcium—for 2 days, followed by 50/50% calcium-free chow mixed with standard chow for 2 days, then 75/25% calcium-free chow mixed with standard chow for 2 days, and finally, 100% calcium-free chow for 2 days, at which point they received a single oral dose of 25 mg/kg DOX formulated in deionized water (data shown in Fig. 3B). To test DOX PK in a low-calcium diet (20% of standard calcium content or 2,000 ppm), rabbits received 20/80% calcium-free chow mixed with standard high-fiber rabbit chow for 2 days, followed by 50% calcium-free chow mixed with 50% standard chow for 2 days, and finally, 80/20% calcium-free chow mixed with standard chow for 2 days, at which point DOX was added to the chow at 2,000 ppm final content (data shown in Fig. 3C). Customized calcium-free chow was manufactured with purified ingredients aiming to resemble the dietary parameters of standard high-fiber rabbit chow (18.2% protein, 56.2% carbohydrate, 3.6% fat; per kg of chow: 175 g casein, 390 g corn starch, 25 g maltodextrin, 125 g sucrose, 150 g cellulose, 25 g inulin, 35 g soybean oil, 38.3 g calcium-free mineral mix, 10 g vitamin mix, and 2 g choline bitartrate). Modified rabbit chow was obtained from Research Diets, New Brunswick, NJ.
In vitro pharmacokinetic assays and analytical methods.
Caseum binding and macrophage uptake of DOX were measured as described previously (31, 44).
High-pressure liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) methods for plasma and whole tissue analysis DOX-HCl and Verapamil were purchased from Sigma-Aldrich. DOX-d3 internal standard was purchased from Toronto Research Chemicals. Drug-free K2EDTA plasma and lungs from CD1 mice and NZW rabbits were obtained from BioIVT for use as blank matrices to build standard curves. Neat 1 mg/ml dimethyl sulfoxide (DMSO) stocks DOX were serial diluted in 50/50 acetonitrile (ACN)/Milli-Q water to create neat standards. Control tissue homogenate and study sample homogenate were created by adding 9 parts phosphate-buffered saline (PBS) buffer to 1 part tissue (10× dilution) and shaking the samples using a Fisher bead mill for 1 min at 6,000 × g with zirconia beads. Standard, quality control, and study samples for DOX were extracted by adding 10 μl of tissue homogenate or plasma, 10 μl of DOX-d3 internal standard, and 100 μl of extract solvent containing 33% trichloroacetic acid. Extracts were vortexed for 5 min and centrifuged at 2,350 × g for 5 min. Then, 100 μl of supernatant was transferred to a 96-well plate for LC-MS/MS analysis. LC-MS/MS analysis was performed on a Sciex Applied Biosystems Qtrap 6500+ triple-quadrupole mass spectrometer coupled to a Shimadzu Nextera X2 ultrahigh-performance liquid chromatography (UHPLC) system to quantify each drug in plasma. Chromatography was performed on an Agilent Zorbax SB-C8 column (2.1 × 30 mm; particle size, 3.5 μm) using a reverse-phase gradient elution with aqueous mobile phase. Milli-Q deionized water with 0.1% formic acid (FA) and 0.1% heptafluorobutyric acid (HFBA) was used for the aqueous mobile phase, and 0.1% FA and 0.1% HFBA in ACN was used for the organic mobile phase. Multiple-reaction monitoring (MRM) of precursor/fragment transitions in electrospray positive-ionization mode was used to quantify the analytes. MRM transitions of 445.20/428.20 and 449.00/432.00 were used for DOX and DOX-d3, respectively. Sample analysis was accepted if the concentrations of the quality control samples were within 20% of the nominal concentration. Data processing was performed using Analyst software version 1.6.2 (Applied Biosystems Sciex).
Laser-capture microdissection coupled to LC-MS/MS.
Laser-capture microdissection (LCM) sample quantification was carried out according to a previously published protocol (33). Briefly, 1 mg/ml neat DMSO stocks of DOX were serial diluted in 50/50 ACN/Milli-Q water to create neat standards. Control tissue homogenate was created by adding 25.6 parts PBS buffer to 1 part tissue (26.7× dilution) and shaking the samples using a Fisher bead mill for 1 min at 6,000 rpm with zirconia beads. Standard, quality control, and control samples were extracted by adding 2 μl of blank homogenate, 10 μl of neat standard, 5 μl of DOX-d3 internal standard, and 50 μl of extract solvent containing 33% trichloroacetic acid. LCM study samples were extracted that were identical to standards using 2 μl of PBS in place of tissue homogenate. Extracts were bath sonicated for 10 min and centrifuged at 2,350 × g for 5 min. Then, 50 μl of supernatant was transferred to a 96-well plate for HPLC-MS/MS analysis. HPLC-MS/MS analysis was performed as described above using the whole-tissue analysis methods.
Supplementary Material
ACKNOWLEDGMENTS
This work was carried out with funding from grants OPP1182859 (D.S. and V.D.) and OPP1180610 (V.D.) from the Bill and Melinda Gates Foundation and NIH shared instrumentation grant S10OD023524 to V.D.
We thank the technical teams from the International Center of Public Health, Rutgers University, and the Center for Discovery and Innovation of Hackensack Meridian Health for their excellent support with all animal-related work.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gossen M, Bujard H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A 89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 3.Berens C, Hillen W. 2003. Gene regulation by tetracyclines. Constraints of resistance regulation in bacteria shape TetR for application in eukaryotes. Eur J Biochem 270:3109–3121. doi: 10.1046/j.1432-1033.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. 2002. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol 13:121–128. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 5.Ehrt S, Guo XV, Hickey CM, Ryou M, Monteleone M, Riley LW, Schnappinger D. 2005. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz R, Bujard H. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissendorfer M, Hillen W. 1990. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl Microbiol Biotechnol 33:657–663. doi: 10.1007/bf00604933. [DOI] [PubMed] [Google Scholar]
- 8.Bertram R, Hillen W. 2008. The application of Tet repressor in prokaryotic gene regulation and expression. Microb Biotechnol 1:2–16. doi: 10.1111/j.1751-7915.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnappinger D, Ehrt S. 2014. Regulated expression systems for mycobacteria and their applications. Microbiol Spectr 2MGM2-0018-2013. doi: 10.1128/microbiolspec.MGM2-0018-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalo X, Casali N, Broda A, Pardieu C, Drobniewski F. 2015. Combination of amikacin and doxycycline against multidrug-resistant and extensively drug-resistant tuberculosis. Int J Antimicrob Agents 45:406–412. doi: 10.1016/j.ijantimicag.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. 2007. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med 13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenoehrl EJ, Rae Sajorda D, Berney-Meyer L, Johnson S, Tufariello JM, Fuhrer T, Cook GM, Jacobs WR Jr, Berney M. 2019. Derailing the aspartate pathway of Mycobacterium tuberculosis to eradicate persistent infection. Nat Commun 10:4215. doi: 10.1038/s41467-019-12224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-H, O’Brien KM, Sharma R, Boshoff HIM, Rehren G, Chakraborty S, Wallach JB, Monteleone M, Wilson DJ, Aldrich CC, Barry CE, Rhee KY, Ehrt S, Schnappinger D. 2013. A genetic strategy to identify targets for the development of drugs that prevent bacterial persistence. Proc Natl Acad Sci U S A 110:19095–19100. doi: 10.1073/pnas.1315860110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S. 2010. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci U S A 107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A 97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redelsperger IM, Taldone T, Riedel ER, Lepherd ML, Lipman NS, Wolf FR. 2016. Stability of doxycycline in feed and water and minimal effective doses in tetracycline-inducible systems. J Am Assoc Lab Anim Sci 55:467–474. [PMC free article] [PubMed] [Google Scholar]
- 18.Cawthorne C, Swindell R, Stratford IJ, Dive C, Welman A. 2007. Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech 18:120–123. [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JL, Salmon JH, Papich MG. 2006. Pharmacokinetics and tissue distribution of doxycycline after oral administration of single and multiple doses in horses. Am J Vet Res 67:310–316. doi: 10.2460/ajvr.67.2.310. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Nakkula RJ, Walters JD. 2002. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J Dent Res 81:836–840. doi: 10.1177/154405910208101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavda M, Clausnitzer CE, Walters JD. 2004. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol 75:1663–1667. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botella L, Vaubourgeix J, Livny J, Schnappinger D. 2017. Depleting Mycobacterium tuberculosis of the transcription termination factor Rho causes pervasive transcription and rapid death. Nat Commun 8:14731. doi: 10.1038/ncomms14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin K, O’Brien KM, Trujillo C, Wang R, Wallach JB, Schnappinger D, Ehrt S. 2016. Mycobacterium tuberculosis thioredoxin reductase is essential for thiol redox homeostasis but plays a minor role in antioxidant defense. PLoS Pathog 12:e1005675. doi: 10.1371/journal.ppat.1005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruecker N, Jansen R, Trujillo C, Puckett S, Jayachandran P, Piroli GG, Frizzell N, Molina H, Rhee KY, Ehrt S. 2017. Fumarase deficiency causes protein and metabolite succination and intoxicates Mycobacterium tuberculosis. Cell Chem Biol 24:306–315. doi: 10.1016/j.chembiol.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan G, Tsenova L. 2011. Pulmonary tuberculosis in the rabbit, p 107–128. In Leong FJ, Dartois V, Dick T (ed), A color atlas of comparative pathology of pulmonary tuberculosis. CRC Press, Boca Raton, FL. [Google Scholar]
- 26.Blanc L, Sarathy JP, Alvarez Cabrera N, O’Brien P, Dias-Freedman I, Mina M, Sacchettini J, Savic RM, Gengenbacher M, Podell BK, Prideaux B, Ioerger T, Dick T, Dartois V. 2018. Impact of immunopathology on the antituberculous activity of pyrazinamide. J Exp Med 215:1975–1986. doi: 10.1084/jem.20180518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percy DH, Black WD. 1988. Pharmacokinetics of tetracycline in the domestic rabbit following intravenous or oral administration. Can J Vet Res 52:5–11. [PMC free article] [PubMed] [Google Scholar]
- 28.Baert K, Croubels S, Gasthuys F, De Busser J, De Backer P. 2000. Pharmacokinetics and oral bioavailability of a doxycycline formulation (DOXYCYCLINE 75%) in nonfasted young pigs. J Vet Pharmacol Ther 23:45–48. doi: 10.1046/j.1365-2885.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 29.Neuvonen PJ. 1976. Interactions with the absorption of tetracyclines. Drugs 11:45–54. doi: 10.2165/00003495-197611010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Redrobe S. 2002. Calcium metabolism in rabbits. Semin Avian Exotic Pet Med 11:94–101. doi: 10.1053/saep.2002.125100. [DOI] [Google Scholar]
- 31.Sarathy JP, Liang HH, Weiner D, Gonzales J, Via LE, Dartois V. 2017. An in vitro caseum binding assay that predicts drug penetration in tuberculosis lesions. J Vis Exp doi: 10.3791/55559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarathy JP, Zuccotto F, Hsinpin H, Sandberg L, Via LE, Marriner GA, Masquelin T, Wyatt P, Ray P, Dartois V. 2016. Prediction of drug penetration in tuberculosis lesions. ACS Infect Dis 2:552–563. doi: 10.1021/acsinfecdis.6b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman M, Blanc L, Chen PY, Dartois V, Prideaux B. 2018. Spatial quantification of drugs in pulmonary tuberculosis lesions by laser capture microdissection liquid chromatography mass spectrometry (LCM-LC/MS). J Vis Exp 134:e57402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter WP, Bitar YS, Strandberg JD, Charache PC. 1985. Absence of therapeutic blood concentrations of tetracycline in rats after administration in drinking water. Lab Anim Sci 35:71–75. [PubMed] [Google Scholar]
- 35.Meijer LA, Ceyssens KG, de Greve BI, de Bruijn W. 1993. Pharmacokinetics and bioavailability of doxycycline hyclate after oral administration in calves. Vet Q 15:1–5. doi: 10.1080/01652176.1993.9694358. [DOI] [PubMed] [Google Scholar]
- 36.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 37.Blanchard P, Rudhardt M, Fabre J. 1975. Behaviour of doxycycline in the tissues. Chemotherapy 21 Suppl 1:8–18. doi: 10.1159/000221886. [DOI] [PubMed] [Google Scholar]
- 38.Belli G, Ciaffi G, Ricci P. 1968. Blood and tissue levels of 2 antibiotics of the tetracycline group orally administered to man. Antibiotica 6:109–115. [PubMed] [Google Scholar]
- 39.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, D’Armiento JM, Meintjes G, Mauri FA, Williams A, Wilkinson RJ, Friedland JS, Elkington PT. 2012. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med 185:989–997. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anadon A, Martinez-Larranaga MR, Diaz MJ, Bringas P, Fernandez MC, Fernandez-Cruz ML, Iturbe J, Martinez MA. 1994. Pharmacokinetics of doxycycline in broiler chickens. Avian Pathol 23:79–90. doi: 10.1080/03079459408418976. [DOI] [PubMed] [Google Scholar]
- 41.Tilakaratne A, Soory M. 2014. Anti-inflammatory actions of adjunctive tetracyclines and other agents in periodontitis and associated comorbidities. Open Dent J 8:109–124. doi: 10.2174/1874210601408010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhuri S, Li L, Zimmerman M, Chen Y, Chen YX, Toosky MN, Gardner M, Pan M, Li YY, Kawaji Q, Zhu JH, Su HW, Martinot AJ, Rubin EJ, Dartois VA, Javid B. 2018. Kasugamycin potentiates rifampicin and limits emergence of resistance in Mycobacterium tuberculosis by specifically decreasing mycobacterial mistranslation. Elife 7:e36782. doi: 10.7554/eLife.36782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subbian S, Tsenova L, Yang G, O’Brien P, Parsons S, Peixoto B, Taylor L, Fallows D, Kaplan G. 2011. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol 1:110016. doi: 10.1098/rsob.110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman M, Lestner J, Prideaux B, O’Brien P, Dias-Freedman I, Chen C, Dietzold J, Daudelin I, Kaya F, Blanc L, Chen PY, Park S, Salgame P, Sarathy J, Dartois V. 2017. Ethambutol partitioning in tuberculous pulmonary lesions explains its clinical efficacy. Antimicrob Agents Chemother 61:e00924-17. doi: 10.1128/AAC.00924-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenblatt DJ, Abernethy DR, Divoll M. 1983. Is volume of distribution at steady state a meaningful kinetic variable? J Clin Pharmacol 23:391–400. doi: 10.1002/j.1552-4604.1983.tb02753.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


