The comparative efficacy of ceftazidime-avibactam and meropenem-vaborbactam for treatment of carbapenem-resistant Enterobacteriaceae (CRE) infections remains unknown. This was a multicenter, retrospective cohort study of adults with CRE infections who received ceftazidime-avibactam or meropenem-vaborbactam for ≥72 hours from February 2015 to October 2018. Patients with a localized urinary tract infection and repeat study drug exposures after the first episode were excluded.
KEYWORDS: carbapenem-resistant Enterobacteriaceae, meropenem-vaborbactam, ceftazidime-avibactam, Gram-negative resistance
ABSTRACT
The comparative efficacy of ceftazidime-avibactam and meropenem-vaborbactam for treatment of carbapenem-resistant Enterobacteriaceae (CRE) infections remains unknown. This was a multicenter, retrospective cohort study of adults with CRE infections who received ceftazidime-avibactam or meropenem-vaborbactam for ≥72 hours from February 2015 to October 2018. Patients with a localized urinary tract infection and repeat study drug exposures after the first episode were excluded. The primary endpoint was clinical success compared between treatment groups. Secondary endpoints included 30- and 90-day mortality, adverse events (AE), 90-day CRE infection recurrence, and development of resistance in patients with recurrent infection. A post hoc subgroup analysis was completed comparing patients who received ceftazidime-avibactam monotherapy, ceftazidime-avibactam combination therapy, and meropenem-vaborbactam monotherapy. A total of 131 patients were included (ceftazidime-avibactam, n = 105; meropenem-vaborbactam, n = 26), 40% of whom had bacteremia. No significant difference in clinical success was observed between groups (62% versus 69%; P = 0.49). Patients in the ceftazidime-avibactam arm received combination therapy more often than patients in the meropenem-vaborbactam arm (61% versus 15%; P < 0.01). No difference in 30- and 90-day mortality resulted, and rates of AE were similar between groups. In patients with recurrent infection, development of resistance occurred in three patients that received ceftazidime-avibactam monotherapy and in no patients in the meropenem-vaborbactam arm. Clinical success was similar between patients receiving ceftazidime-avibactam and meropenem-vaborbactam for treatment of CRE infections, despite ceftazidime-avibactam being used more often as a combination therapy. Development of resistance was more common with ceftazidime-avibactam monotherapy.
INTRODUCTION
According to the most recent report from the Centers for Disease Control and Prevention (CDC), carbapenem-resistant Enterobacteriaceae (CRE) infections represent an urgent threat with an estimated 13,100 infections annually nationwide and associated mortality rates of up to 50% (1, 2). Of particular concern in the United States is Klebsiella pneumoniae carbapenemase (KPC)-producing CRE (3). Prior to 2015, management of CRE infections often required use of a combination of antibiotics with limited efficacy and significant toxicities (4, 5). Poor clinical outcomes were common despite combination therapy and dose optimization (6, 7). Since then, two new beta-lactam combination agents with activity against KPC-producing CRE have been approved by the Food and Drug Administration (FDA), ceftazidime-avibactam (CZA) in February 2015 and meropenem-vaborbactam (MVB) in October 2017. Avibactam and vaborbactam are both novel beta-lactamase inhibitors that have potent activity against Ambler class A carbapenemases, such as KPC (8, 9).
Currently, no published studies compare the treatment of CRE infections with CZA versus MVB. Some suggest that MVB is the preferred agent for treatment of KPC-producing CRE based on theoretical and in vitro data, as direct clinical comparison data of these agents is lacking (10). The objective of this study was to compare clinical outcomes in patients who received CZA versus MVB for CRE infections.
RESULTS
Baseline characteristics.
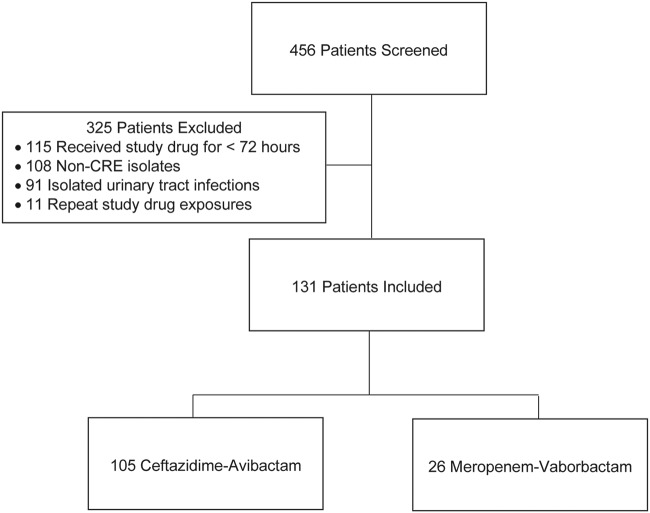
During the study period, 131 patients met the study criteria, with 105 patients treated with CZA and 26 patients treated with MVB (Fig. 1). Baseline characteristics were generally well balanced in terms of demographics, comorbidities, and severity of illness (Table 1). The majority of the patients (57.3%) were critically ill, with a median acute physiology and chronic health evaluation II (APACHE II) score of 26 in the CZA group and 27 in the MVB group. A total of 53 patients (40.5%) had bacteremia. The most common sources of bacteremia were the urinary tract (35.1%) in the CZA group and intra-abdominal (37.5%) sources in the MVB group. The most common nonbloodstream infection in both groups was respiratory. Klebsiella pneumoniae was the primary causative CRE organism in both groups, with most cases involving polymicrobial infections (Table 2). Candida species were the most common additional pathogen, isolated in 33 cultures (primarily from intra-abdominal and respiratory sources). Methicillin-resistant Staphylococcus aureus (MRSA) was more commonly isolated in the CZA group, but this was nonsignificant (14 total, primarily from soft tissue and respiratory sources). In the CZA group, 32 available isolates were tested for a CRE genetic mechanism, 23 (71.9%) of which were positive for KPC. Thirteen available isolates were tested in the MVB group, with ten (76.9%) testing positive for KPC. No other CRE genetic mechanisms were identified.
FIG 1.
Patient selection. CRE, carbapenem-resistant Enterobacteriaceae.
TABLE 1.
Baseline characteristics of study patientsa
| Ceftazidime-avibactam group (n = 105) | Meropenem-vaborbactam group (n = 26) | P value | |
|---|---|---|---|
| No. of males (%) | 58 (55.2) | 12 (46.2) | 0.41 |
| Median age (yrs) (IQR) | 62.0 (51.0–69.0) | 57.5 (50.0–70.0) | 0.68 |
| Median weight (kg) (IQR) | 75.9 (64.0–95.7) | 82.5 (70.4–91.0) | 0.50 |
| Race | |||
| No. white (%) | 77 (73.3) | 19 (73.1) | 0.58 |
| No. African American (%) | 24 (22.9) | 5 (19.2) | 0.58 |
| No. Asian (%) | 1 (0.9) | 0 | 0.58 |
| No. other (%) | 3 (2.9) | 2 (7.7) | 0.58 |
| No. of hospital-acquired infections (%) | 65 (61.9) | 13 (50.0) | 0.27 |
| No. of community-acquired infections (%) | 40 (38.1) | 13 (50.0) | 0.27 |
| No. with history of CRE infection or colonization (%) | 24 (22.9) | 7 (26.9) | 0.66 |
| Median Charlson comorbidity index (IQR) | 5.0 (3.0–6.0) | 5.0 (3.0–8.0) | 0.65 |
| No. of comorbidities (%) | |||
| Diabetes mellitus | 49 (46.7) | 13 (50.0) | 0.76 |
| Chronic kidney disease | 33 (31.4) | 9 (34.6) | 0.76 |
| Chronic respiratory disease | 33 (31.4) | 8 (30.8) | 0.95 |
| History of malignancy | 23 (21.9) | 5 (19.2) | 0.77 |
| Chronic liver disease | 16 (15.2) | 6 (23.1) | 0.38 |
| Human immunodeficiency virus | 0 | 1 (3.9) | 0.20 |
| No. immunocompromised (%) | 12 (11.4) | 4 (15.4) | 0.52 |
| Severity of illness | |||
| No. with ICU status at time of positive culture (%) | 58 (55.2) | 17 (65.4) | 0.35 |
| Median APACHE II score (IQR) | 26.0 (22.0–30.0) | 27.0 (24.0–34.0) | 0.19 |
| No. with primary bacteremia (%) | 7 (6.7) | 1 (3.8) | 0.75 |
| No. with secondary bacteremia (%) | 37 (35.2) | 8 (30.8) | 0.75 |
| Urinary tract | 13 (35.1) | 1 (12.5) | 0.43 |
| Intra-abdominal | 6 (16.2) | 3 (37.5) | 0.43 |
| Respiratory | 7 (18.9) | 2 (25.0) | 0.43 |
| Catheter-associated | 5 (13.5) | 0 | 0.43 |
| Soft tissue | 2 (5.4) | 1 (12.5) | 0.43 |
| Other | 4 (10.8) | 1 (12.5) | 0.43 |
| No. with nonbloodstream infections (%) | 61 (58.1) | 17 (65.4) | 0.75 |
| Respiratory | 30 (49.2) | 10 (58.8) | 0.47 |
| Soft tissue | 18 (29.5) | 2 (11.8) | 0.47 |
| Intra-abdominal | 12 (19.7) | 5 (29.4) | 0.47 |
| Other | 1 (1.6) | 0 | 0.47 |
CRE, carbapenem-resistant Enterobacteriaceae; ICU, intensive care unit; IQR, interquartile range; KPC, Klebsiella pneumoniae carbapenemase.
TABLE 2.
Microbiological characteristicsa
| Ceftazidime-avibactam group (n = 105) | Meropenem-vaborbactam group (n = 26) | P value | |
|---|---|---|---|
| No. of CRE organisms (%) | |||
| Klebsiella spp. | 76 (72.4) | 15 (57.7) | 0.15 |
| Enterobacter spp. | 20 (19.1) | 8 (30.8) | 0.19 |
| Escherichia coli | 9 (8.6) | 3 (11.5) | 0.70 |
| Citrobacter spp. | 2 (1.9) | 2 (7.7) | 0.18 |
| Serratia spp. | 0 | 1 (3.9) | 0.20 |
| No. of polymicrobial infections %) | 62 (59.1) | 15 (57.7) | 0.90 |
| Candida spp. | 26 (24.8) | 5 (19.2) | 0.55 |
| Enterobacteriaceae (non-CRE) | 21 (20.0) | 7 (26.9) | 0.44 |
| Enterococcus spp. | 20 (19.1) | 6 (23.1) | 0.64 |
| Pseudomonas spp. | 16 (15.2) | 2 (7.7) | 0.52 |
| Methicillin-resistant Staphylococcus aureus | 13 (12.4) | 1 (3.9) | 0.30 |
| Methicillin-susceptible Staphylococcus aureus | 3 (2.9) | 0 | 1.0 |
| Other Gram-negative organism | 9 (8.6) | 3 (11.5) | 0.70 |
| Other Gram-positive organism | 2 (1.9) | 2 (7.7) | 0.18 |
| Acinetobacter spp. | 3 (2.9) | 1 (3.9) | 1.0 |
| Streptococcus spp. | 1 (0.9) | 0 | 1.0 |
| No. of bloodstream infections (%) | 44 (41.9) | 9 (34.6) | 0.75 |
| Median time to negative blood culture (days) (IQR) | 2.9 (1.1–4.7) | 2.2 (2.1–2.2) | 0.34 |
| Presence of blaKPC gene (no. positive/no. tested) (%) | 23/32 (71.9) | 10/13 (76.9) | 1.0 |
CRE, carbapenem-resistant Enterobacteriaceae; IQR, interquartile range; KPC, Klebsiella pneumoniae carbapenemase.
Antimicrobial treatment.
In the CZA group, all 90 isolates tested susceptible to CZA. MVB susceptibility was tested on 14 isolates, and all were susceptible except for a KPC-producing Klebsiella pneumoniae isolate from blood with an intermediate MIC of 8 mg/liter. This patient had received 7 days of meropenem at the beginning of admission but had never received MVB before. The time to study drug initiation from positive culture was significantly longer in the CZA group than the MVB group (48.7 versus 19.9 hours; P = 0.02). However, the time to active in vitro therapy from positive culture and duration of active in vitro therapy were similar between groups (Table 3). CZA was used in combination with other agents in 61.0% of patients compared to 15.4% of patients treated with MVB (P < 0.01). For the agents used as combination therapy for the CRE isolate, 89.7% displayed in vitro activity. A total of five patients received inhaled products, four colistin and one tobramycin. The median duration of combination therapy was 8.8 days with CZA and 3.1 days with MVB (P = 0.08).
TABLE 3.
Antimicrobial dataa
| Ceftazidime-avibactam group (n = 105) | Meropenem-vaborbactam group (n = 26) | P value | |
|---|---|---|---|
| Median time from positive culture to active in vitro therapy (h) (IQR) | 25.0 (2.7–56.7) | 15.4 (0.5–64.2) | 0.62 |
| Median time from positive culture to study drug initiation (h) (IQR) | 48.7 (10.2–96.6) | 19.9 (2.3–64.2) | 0.02 |
| Median duration of active in vitro therapy (days) (IQR) | 13.0 (8.3–17.7) | 15.2 (9.0–18.1) | 0.36 |
| Median duration of study drug (days) (IQR) | 10.8 (7.4–14.4) | 12.3 (7.7–16.6) | 0.51 |
| Susceptibility of study drug (no. susceptible/no. tested) (%) | 90/90 (100) | 13/14 (92.9) | 0.13 |
| No. of changes of therapy (%) | 6 (5.7) | 5 (19.2) | 0.04 |
| Clinical failure/escalation | 2 (33.3) | 4 (80.0) | 0.52 |
| Adverse event | 2 (33.3) | 0 | 0.52 |
| Deescalation | 1 (16.7) | 0 | 0.52 |
| Unknown reason | 1 (16.7) | 1 (20.0) | 0.52 |
| No. of combination therapies () | 64 (61.0) | 4 (15.4) | <0.01 |
| Carbapenem | 1 (0.9) | 0 | 1.0 |
| Aminoglycoside | 15 (14.3) | 0 | 0.04 |
| Polymyxin B | 10 (9.5) | 1 (3.9) | 0.69 |
| Colistin | 20 (19.1) | 2 (7.7) | 0.24 |
| Tigecycline | 18 (17.1) | 1 (3.9) | 0.12 |
| Fluoroquinolone | 8 (7.6) | 0 | 0.36 |
| Sulfamethoxazole-trimethoprim | 4 (3.8) | 0 | 0.58 |
| Median duration of combination therapy (days) (IQR) | 8.8 (4.2–13.0) | 3.1 (1.2–6.8) | 0.08 |
| No. with therapy continued as outpatient (%) | 20 (19.1) | 6 (23.8) | 0.64 |
IQR, interquartile range.
Efficacy.
Clinical success was observed in 61.9% of patients in the CZA group and 69.2% in the MVB group (P = 0.49). When assessing the subgroup of isolates with documented susceptibility, there was also no difference in clinical success (CZA: n = 90, 61.1% versus MVB: n = 13, 84.6%; P = 0.13). Mortality at 30 and 90 days and hospital and ICU lengths of stay were similar between the groups. Within 90 days of the index infection, 14.3% of patients who received CZA and 11.5% of patients who received MVB had a recurrence of their CRE infection. Emergence of MVB resistance was not observed within the study period; however, three of the 15 (20%) patients in the CZA group developed resistance within 90 days. Clinical outcomes are summarized in Table 4.
TABLE 4.
Clinical outcomesa
| Ceftazidime-avibactam group (n = 105) | Meropenem-vaborbactam group (n = 26) | P value | |
|---|---|---|---|
| No. of clinical successesb (%) | 65 (61.9) | 18 (69.2) | 0.49 |
| No. of failures to resolve signs and symptoms of infection (%) | 4 (3.8) | 1 (3.8) | 1.0 |
| Failure to sterilize blood cultures within 7 days of treatment initiation [no. of failures/no. of bacteremias (%)] | 1/44 (2.3) | 1/9 (11.1) | 0.31 |
| No. of 30-day mortalities (%) | 20 (19.1) | 3 (11.5) | 0.57 |
| No. of 90-day mortalities (%) | 30 (28.6) | 7 (26.9) | 0.48 |
| Median length of hospital stayc (days) (IQR) | 15.3 (9.3–28.5) | 15.6 (9.5–33.1) | 0.99 |
| Median length of ICU stay (days) (IQR) | 15.0 (5.0–32.0) | 12.0 (5.0–22.0) | 0.53 |
| No. of recurrences of CRE infection (%) | 15 (14.3) | 3 (11.5) | 1.0 |
| No. of increases in study drug MIC in mg/liter (%) | 6 (40.0) | 0 | 0.51 |
| No. of emergences of study drug resistance (%) | 3 (20.0) | 0 | 1.0 |
CRE, carbapenem-resistant Enterobacteriaceae; ICU, intensive care unit; IQR, interquartile range.
Clinical success was defined as survival at 30 days, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation in patients with bacteremia, and absence of recurrent infections within 90 days of the index infection.
Length of stay was calculated from onset of CRE infection.
A total of five patients received both CZA and MVB during the study period, two of whom received agents over a year apart. Two patients experienced treatment failure after receiving approximately 2 weeks of CZA monotherapy but achieved clinical success after a course of MVB; recurrent infections occurred 17 days (polymicrobial intra-abdominal infection) and 49 days (polymicrobial pneumonia) after the end of CZA therapy, with the latter case resulting with a CZA MIC increase from 4 to 12 mg/liter. The last patient received 13 days of MVB for a monomicrobial KPC-confirmed K. pneumoniae pneumonia and experienced a recurrent infection within 7 days from the MVB end of therapy that was successfully treated with CZA.
Safety.
Rates of adverse events were similar between the CZA group and the MVB group (34.3% versus 23.1%, respectively; P = 0.27). Nephrotoxicity was the most frequent adverse event, with a rate of 29.2% in the CZA group and 14.3% in the MVB group (P = 0.16). Of the 26 patients who experienced nephrotoxicity in the CZA group, 38.5% received monotherapy, and the remaining patients were on combination therapy with the following: colistin (23.1%), polymyxin B (15.4%), tigecycline (15.4%), fluoroquinolone (11.5%), and aminoglycoside (3.8%). In the MVB group, one of three patients received combination therapy with colistin, and the other two patients received monotherapy. A total of four patients required renal replacement therapy (RRT) after initiation of the study drug (Table 5).
TABLE 5.
Adverse patient eventsa
| Ceftazidime-avibactam group (n = 105) | Meropenem-vaborbactam group (n = 26) | P value | |
|---|---|---|---|
| No. of all adverse events (%) | 36 (34.3) | 6 (23.1) | 0.27 |
| No. with nephrotoxicity/no. without baseline RRT (%) | 26/89 (29.2) | 3/21 (14.3) | 0.16 |
| AKIN stage 1 | 12 (13.5) | 1 (4.8) | 0.43 |
| AKIN stage 2 | 7 (7.9) | 0 | 0.43 |
| AKIN stage 3 | 7 (7.9) | 2 (9.5) | 0.43 |
| No. of initiations of RRT (%) | 3 (2.9) | 1 (4.8) | 1.0 |
| Median time to start RRT from study drug initiation (days) (IQR) | 9.7 (4.7–13.6) | 1.5 | |
| No. with leukopeniab (%) | 11 (10.5) | 2 (7.7) | 1.0 |
| No. with rash (%) | 4 (3.8) | 1 (3.9) | 1.0 |
| No. with neurotoxicity (%) | 1 (0.9) | 0 | 1.0 |
AKIN, Acute Kidney Injury Network; IQR, interquartile range; RRT, renal replacement therapy.
Leukopenia = white blood cell count of <4,000 cells/mm3.
Post hoc subgroup analyses.
In a post hoc analysis, primary and secondary endpoints were compared in patients who received CZA monotherapy, CZA combination therapy, and MVB monotherapy (Table 6); clinical success remained similar between the three groups (63.4% versus 60.9% versus 68.2%; P = 0.83). An increase in recurrence of CRE infection was observed in the CZA monotherapy group. Five of the nine CRE isolates in this group demonstrated an MIC increase for CZA, and three developed resistance. The average duration of CZA in the isolates that developed resistance was 9.3 days. No isolates developed resistance in the CZA combination therapy or MVB monotherapy groups, with all of the isolates from recurrent infection in the MVB group having susceptibility data available. Interestingly, nephrotoxicity rates were similar between CZA monotherapy and combination therapy groups (24.4% versus 25.0%). Although not statistically significant, there were signals suggesting increased nephrotoxicity in both CZA subgroups compared to the MVB monotherapy group (9.1%; P = 0.27).
TABLE 6.
Post hoc subgroup analysisa
| CZA monotherapy group (n = 41) | CZA combination therapy group (n = 64) | MVB monotherapy group (n = 22) | P value | |
|---|---|---|---|---|
| No. of clinical successes (%) | 26 (63.4) | 39 (60.9) | 15 (68.2) | 0.83 |
| No. of 90-day mortalities (%) | 9 (22.0) | 20 (31.2) | 6 (27.3) | 0.58 |
| No. of recurrences of CRE infection (%) | 9 (22.0) | 6 (9.4) | 3 (13.6) | 0.20 |
| No. of increases in study drug MIC in mg/liter (%) | 5 (12.2) | 1 (1.6) | 0 | 0.03 |
| No. of emergences of study drug resistance (%) | 3 (7.3) | 0 | 0 | 0.07 |
| No. of all adverse events (%) | 14 (34.2) | 22 (34.4) | 5 (22.7) | 0.57 |
| No. with nephrotoxicity (%) | 10 (24.4) | 16 (25.0)b | 2 (9.1) | 0.27 |
| No. of initiations of RRT (%) | 1 (2.4) | 2 (3.1) | 0 | 1.0 |
| Median time to start RRT from study drug initiation (days) (IQR) | 13.6 | 7.2 (4.7–9.7) | 0 | |
| No. with leukopenia (%) | 4 (9.8) | 7 (10.9) | 2 (9.1) | 1.0 |
| No. with rash (%) | 3 (7.3) | 1 (1.6) | 1 (4.6) | 0.31 |
| No. with neurotoxicity (%) | 0 | 1 (1.6) | 0 | 1.0 |
CRE, carbapenem-resistant Enterobacteriaceae; ICU, intensive care unit; IQR, interquartile range; RRT, renal replacement therapy.
Combination therapy use with CZA: colistin (37.5%), polymyxin B (25%), tigecycline (25%), fluoroquinolone (18.8%), aminoglycoside (6.3%).
DISCUSSION
To our knowledge, this was the first study that compared outcomes of treatment with CZA to treatment with MVB for CRE infections. No differences in clinical success were observed, while combination therapy was used more frequently in the CZA group. To assess the impact of the study drugs versus adjunctive therapies on clinical outcomes and adverse effects, we conducted a post hoc analysis of patients receiving CZA monotherapy, CZA combination therapy, and MVB monotherapy. MVB combination therapy was not evaluated due to low use.
Our rates of clinical success for CZA and MVB were generally similar to those reported in previously published reports (11–13). Various success rates with CZA treatment in other studies are likely accounted for due to differing populations and outcome measures compared to our study. In a retrospective review of patients with KPC-producing K. pneumoniae bacteremia, CZA was associated with higher rates of clinical success of 85% versus a clinical success rate of 62% observed in our study, which could be in part due to increased severity of illness in the patients included (median APACHE II score of 26 versus 20) and the inclusion of polymicrobial infections (11). Compared to the CRACKLE study, which evaluated outcomes between CZA- and colistin-based regimens, higher 30-day mortality was observed in patients that received CZA in our study (19% versus 8%), but it should be noted that only in-hospital 30-day mortality was evaluated in their study, and death was not assessed in over 20% of discharged patients who received CZA (n = 38) (12). For MVB, the efficacy observed in our study can be compared with results from the TANGO II trial of 47 patients, which evaluated MVB versus best available therapy. In patients receiving MVB, the reported clinical success of 64% closely matched the 69% seen in our cohort (13).
In our study, there were no differences in rates of adverse events observed between the CZA group and the MVB group. Past studies have found that rates of acute kidney injury were significantly higher in those receiving aminoglycoside or colistin combinations (11, 12). Surprisingly, in the post hoc analysis, nephrotoxicity was not significantly different between the CZA monotherapy and combination therapy arms.
While both CZA and MVB appear to be superior over traditional CRE therapies in both safety and efficacy, concern has emerged regarding the development of resistance to both CZA and MVB. In the CZA monotherapy group of this study, three patients developed CZA-resistant bacterial isolates. CZA resistance has been described previously both in isolates with no history of drug exposure and developing after CZA therapy (14–17). In past case series, development of CZA resistance was more common in patients treated with monotherapy as in our cohort. Based on a recent study by Shields et al., pneumonia and RRT were found to be independent risk factors for CZA treatment failure, hypothesized to be due to inadequate CZA exposure (18). Additionally, RRT was found to be an independent risk factor for the development of CZA resistance in their study. Interestingly, among the three patients who developed CZA resistance in our cohort, all were found to have respiratory sources and received RRT (Table 7). CZA was dosed per the prescribing information at 0.94 g every 48 hours in patients receiving intermittent hemodialysis. It should be noted that two different dosing recommendations are provided in the package insert for dialysis, 0.94 g every 24 hours or 0.94 g every 48 hours (19). It is unclear if use of the every 48 hours frequency drove the development of CZA resistance in this study due to underdosing.
TABLE 7.
CZA monotherapy MIC increase and emergence of resistancea
| Initial infection MIC (mg/liter) | Recurrent infection MIC (mg/liter) | Emergence of resistance? | Duration of CZA (days) | Source of infection | Baseline RRT? |
|---|---|---|---|---|---|
| 0.25 | 0.75 | No | 10.6 | Intra-abdominal | No |
| 0.75 | 1.5 | No | 7.6 | Respiratory | No |
| 0.75 | 12 | Yes | 10.3 | Respiratory | Yes |
| 4 | 12 | Yes | 13.2 | Respiratory | Yes |
| 2 | 32 | Yes | 4.4 | Respiratory | Yes |
CZA, ceftazidime-avibactam; RRT, renal replacement therapy.
In vitro models have suggested that MVB has a higher barrier to resistance for KPC-producing Enterobacteriaceae than CZA (20, 21). This may be because vaborbactam is able to overcome D179Y mutations at the KPC binding site, which confers resistance to CZA (22). However, recent treatment-emergent MVB nonsusceptibility has also been described (23). In our cohort of patients with recurrent CRE infections, there were no instances of developed resistance in the MVB group. We observed MVB resistance in a patient who had received four prior courses of MVB, but this episode occurred outside the study period. Interestingly, this patient was treated with MVB for bacteremia and clinically responded.
Our study has several limitations, including its retrospective design. A power analysis was not performed; therefore, the study may not have been adequately powered to detect a true difference between groups. However, with a total of 131 patients included, this is one of the largest studies evaluating the treatment of CRE infections and is the first comparing CZA and MVB for CRE infections to date.
There were several practice changes that occurred during the study period. Increased use of CZA in combination therapy occurred due to emerging evidence of CZA resistance development when used as monotherapy. MVB was added to our hospital formularies, and institutional guidelines replaced CZA with MVB as the empirical agent of choice for most CRE infections (with the exception of cystitis) due to the possible higher barrier to resistance. Finally, the introduction of rapid diagnostic testing during study periods, particularly for blood cultures at Atrium Health, allowed for earlier detection of the KPC enzyme and streamlined therapy for positive blood cultures. This may also partially explain the difference in study drug initiation, but no difference in time from positive culture to in vitro active therapy was observed between groups, so it is plausible that the difference in time to study drug had less of an impact.
There was limited study drug susceptibility and resistance mechanism testing available during the entire study period. Some of these data were obtained retrospectively from banked frozen CRE isolates. However, this study represents real-world use of these agents. Of note, based on CRE surveillance testing from 2015 through 2018 at Atrium Health, including infection and colonization, rates of NDM and OXA isolation were low (1.9% and 1.6%, respectively); KPC remained the dominant carbapenemase identified in cultures (66.1%). When tested, KPC was the only carbapenemase identified at AdventHealth during the study period. For CRE isolates that did not test positive for a carbapenemase via molecular testing, one hypothesis regarding the mechanism of resistance is the presence of ampC beta-lactamases with simultaneous porin mutations (24), and if this is the cause, both CZA and MVB would most likely still be active against these isolates (8, 9). Notably, 28 (21.4%) of our CRE isolates were Enterobacter species known to harbor ampC beta-lactamases. There were more patients in the MVB group with Enterobacter or Citrobacter spp. than in the CZA group, which may have been driven by the drop in carbapenem breakpoints used during the latter part of the study period (during which more MVB was used). Some of these isolates had discrepant carbapenem susceptibility results (susceptible to meropenem but resistant to ertapenem). In theory, this may have impacted the time to active in vitro therapy in the MVB group if meropenem was received as empirical therapy; however, none of the patients in the MVB group with a meropenem-susceptible isolate received meropenem as initial therapy. It is also important to note that only Etest susceptibility results were available for polymyxins during the study period, which have been associated with high error rates, and no established breakpoints for Enterobacteriaceae by disk diffusion are available from the CLSI or the European Committee on Antimicrobial Susceptibility Testing (25). Because of this, the time to active therapy and definitions for combination therapy may have been impacted. Finally, the majority (58%) of patients had polymicrobial infection with an additional pathogen cultured from the same site as the CRE infection, most commonly from respiratory and intra-abdominal sites. Although the rates of polymicrobial infection were equivalent between the CZA and MVB groups (59% versus 58%), the adequacy of treatment of the non-CRE pathogens was not assessed and may have impacted our results.
In summary, similar rates of clinical success between CZA and MVB were observed for treatment of KPC-producing CRE infections. Our study contributes to the growing body of evidence associating CZA monotherapy with the development of resistance. Further investigation of the use of combination therapy to prevent resistance is warranted. Our study supports the use of MVB as a monotherapy; therefore, MVB may be the preferred agent for KPC-producing CRE infections.
MATERIALS AND METHODS
This was a multicenter, retrospective cohort study involving 18 academic and community-based hospitals comprising over 5,000 beds from the Atrium Health and AdventHealth hospital systems in the greater Charlotte, North Carolina, and Orlando, Florida, areas, respectively. The study was approved by the Atrium Health and AdventHealth institutional review boards. Electronic hospital databases were used to identify adult inpatients (≥18 years of age) admitted between February 2015 and October 2018 who received CZA or MVB for the first time for at least 72 hours for treatment of a documented CRE infection as determined by an infectious disease (ID) physician. A formal ID consultation was required for use of ≥24 hours of either study drug. Use of combination therapy, defined as two or more antibiotics, including inhaled products, regardless of in vitro activity, was noted. If a recurrent infection was treated with a different study drug than the initial infection, both occurrences were included. Any repeat study drug exposure for the treatment of a subsequent CRE infection was evaluated for development of resistance but excluded from the primary outcome analysis. Polymicrobial infections, defined as additional pathogens from the same culture as the CRE, were included. Patients were excluded if the CRE infection was localized to a urinary source (bloodstream infections from a urinary source were included). Study data were collected and managed using a REDCap database stored on a secure server at Atrium Health.
Patients and clinical data.
The primary endpoint was clinical success, defined as survival at 30 days, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation in patients with bacteremia, and absence of recurrent infections within 90 days of the index infection. Resolution of signs and symptoms of infection was defined as no fever or hypothermia, cough, chest pain, dyspnea, malaise, leukocytosis, or leukopenia; this was assessed in the provider’s progress note on the day that antibiotics were discontinued. Antibiotics either changed or added due to perceived nonresponse were considered clinical failures and did not meet the criteria for clinical success. Recurrent infections were defined as the same organism at the same site. In patients with recurrent CRE infections, the proportion with an increase in the MIC or development of resistance per FDA-approved breakpoints for CZA and MVB were evaluated. Other secondary endpoints included 30-day mortality, 90-day mortality, adverse events occurring while on a study drug, hospital length of stay, and intensive care unit (ICU) length of stay.
Primary bacteremia was defined as a bloodstream infection without a documented primary source of infection, and secondary bacteremia was defined as a bloodstream infection secondary to a localized focus of infection, such as respiratory, intra-abdominal, soft tissue, catheter-associated, and urinary tract sources. The Charlson comorbidity index was determined at hospital admission, and the APACHE II score was determined with available values closest to Gram stain positivity. Nephrotoxicity was defined using the Acute Kidney Injury Network (AKIN) classification and/or the initiation of RRT while receiving CZA or MVB (26). Patients receiving RRT at baseline were not included when assessing nephrotoxicity rates; only those starting RRT while on study drug were included.
Microbiology.
The MicroScan WalkAway system (Beckman Coulter, Pasadena, CA) at Atrium Health and the Vitek 2 system (bioMérieux, Durham, NC) at AdventHealth were automated systems used for susceptibility testing of CRE isolates for all drugs, except MVB, CZA, and polymyxin B, which were tested with the gradient diffusion method. CZA and polymyxin B were tested with Epsilometer tests (Etests, bioMérieux, Durham, NC), and MVB was tested using MIC test strips (MTS, Liofilchem, Waltham, MA). In accordance with the CDC definition, an Enterobacteriaceae isolate was classified as CRE if it was resistant to any carbapenem per the Clinical and Laboratory Standards Institute (CLSI) breakpoints (27). Of note, older carbapenem breakpoints were used for part of the study period; however, updated carbapenem breakpoints were implemented by the microbiology laboratories in July 2016. Due to the lack of CLSI recommendations during the study period, tigecycline, CZA, and MVB breakpoints were based on their FDA-approved labeling (19, 28, 29). The Xpert Carba-R PCR assay (Cepheid, Sunnyvale, CA) was used to determine the presence of the carbapenemase-producing genes, blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48.
At AdventHealth, study drug susceptibility was conducted in real time during the patient’s admission. Rapid diagnostic testing for blood cultures (Verigene; Luminex, Austin, TX) was implemented in 2015. The Xpert Carba-R PCR assay was implemented in March 2018. At Atrium Health, MVB susceptibility testing was implemented a year after MVB was added to the formulary. The Xpert Carba-R PCR assay was not available. The introduction of rapid diagnostic testing for blood cultures (FilmArray; BioFire, Salt Lake City, UT) occurred in January 2017. Before the study was conducted, Atrium Health’s microbiology laboratory banked several CRE isolates for possible future testing. Once patient selection was completed, the MVB patients who met the study criteria were assessed to see if they had a frozen CRE isolate available for testing. Ten isolates were identified and shipped to an outside lab for testing.
Statistical methods.
The data were divided into CZA and MVB groups; for each group, counts and percentages were calculated for categorical variables, and medians and interquartile ranges (IQR) were calculated for continuous variables. Median and IQR, rather than mean and standard deviation, were reported, and nonparametric tests were employed, due to a lack of normality in the distributions of continuous variables. The primary analysis was a chi-square test comparing the proportions of patients achieving clinical success in the CZA and MVB groups. Other outcomes and baseline variables were compared between the CZA and MVB groups using the chi-square test or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. For the post hoc comparisons of three groups (CZA monotherapy, CZA combination therapy, and MVB monotherapy), the chi-square test or Fisher’s exact test was used for categorical variables, and the Kruskal-Wallis test was used for continuous variables. For all tests, a P value of 0.05 was considered statistically significant. No adjustment was made for multiple testing. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, NC, USA).
ACKNOWLEDGMENTS
This work was supported by Melinta Therapeutics, Inc., via an investigator-initiated research grant (3000301634). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Amanda Elchynski for assistance with data collection and Ryan Shields for assistance with susceptibility testing for select isolates.
REFERENCES
- 1.Centers for Disease Control and Prevention (C DC). 2019. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 2.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. 2017. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 61:e02349-16. doi: 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Duin D, Perez F, Rudin SD, Cober E, Hanrahan J, Ziegler J, Webber R, Fox J, Mason P, Richter SS, Cline M, Hall GS, Kaye KS, Jacobs MR, Kalayjian RC, Salata RA, Segre JA, Conlan S, Evans S, Fowler VG Jr, Bonomo RA. 2014. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 58:4035–4041. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Duin D, Kaye KS, Neuner EA, Bonomo RA. 2013. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Messina JA, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Bonomo RA, Fowler VG, van Duin D, Antibacterial Resistance Leadership Group. 2016. Hospital readmissions in patients with carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 37:281–288. doi: 10.1017/ice.2015.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livermore DM, Meunier D, Hopkins KL, Doumith M, Hill R, Pike R, Staves P, Woodford N. 2018. Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015–16. J Antimicrob Chemother 73:648–657. doi: 10.1093/jac/dkx438. [DOI] [PubMed] [Google Scholar]
- 9.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogue JM, Bonomo RA, Kaye KS. 2019. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 68:519–524. doi: 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 11.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG Jr, Paterson DL, Bonomo RA, Evans S, Antibacterial Resistance Leadership Group. 2018. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. 2018. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries RM, Hemarajata P. 2017. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 61:e00537-17. doi: 10.1128/AAC.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athans V, Neuner EA, Hassouna H, Richter SS, Keller G, Castanheira M, Brizendine KD, Mathers AJ. 2019. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-resistant Klebsiella pneumoniae bacteremia and abscess in a liver transplant recipient. Antimicrob Agents Chemother 63:e01551-18. doi: 10.1128/AAC.01551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. 2018. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 62:e02497-17. doi: 10.1128/AAC.02497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allergan USA, Inc. 2019. Avycaz (ceftazidime/avibactam). Prescribing information Allergan USA Inc., Irvine, CA. [Google Scholar]
- 20.Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. 2017. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 61:e01694-17. doi: 10.1128/AAC.01694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabet M, Tarazi Z, Rubio-Aparicio D, Nolan TG, Parkinson J, Lomovskaya O, Dudley MN, Griffith DC. 2018. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant Enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob Agents Chemother 62:e01969-17. doi: 10.1128/AAC.01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noval M, Banoub M, Claeys KC, Heil E. 2020. The battle is on: new beta-lactams for the treatment of multidrug-resistant Gram-negative organisms. Curr Infect Dis Rep 22:1. doi: 10.1007/s11908-020-0710-9. [DOI] [PubMed] [Google Scholar]
- 23.Shields RK, McCreary EK, Marini RV, Kline EG, Jones CE, Hao B, Chen L, Kreiswirth BN, Doi Y, Clancy CJ, Nguyen MH. 2019. Early experience with meropenem-vaborbactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis doi: 10.1093/cid/ciz1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Boxtel R, Wattel AA, Arenas J, Goessens WH, Tommassen J. 2017. Acquisition of carbapenem resistance by plasmid-encoded-AmpC-expressing Escherichia coli. Antimicrob Agents Chemother 61:e01413-16. doi: 10.1128/AAC.01413-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasoo S. 2017. Susceptibility testing for the polymyxins: two steps back, three steps forward? J Clin Microbiol 55:2573–2582. doi: 10.1128/JCM.00888-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed (M100S). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer, Inc. 2020. Tygacil (tigecycline). Prescribing information. Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc., Philadelphia, PA. [Google Scholar]
- 29.Melinta Therapeutics. 2018. Vabomere (meropenem and vaborbactam). Prescribing information Melinta Therapeutics, Lincolnshire, IL. [Google Scholar]