The effects of multiple-dose administration of tenofovir disoproxil fumarate (TDF) on the pharmacokinetics of morinidazole (MOR) were compared in healthy subjects. MOR exposure was similar, with an area under the curve from 0 h to infinity (AUC0-∞) treatment ratio for MOR+TDF/MOR of 1.01 (90% confidence interval, 0.97 to 1.06). No relevant differences were observed regarding plasma exposure of metabolites. Renal clearances of MOR and its metabolites were not affected by TDF.
KEYWORDS: morinidazole, tenofovir, clinical pharmacokinetics, drug-drug interaction, organic anion transporter
ABSTRACT
The effects of multiple-dose administration of tenofovir disoproxil fumarate (TDF) on the pharmacokinetics of morinidazole (MOR) were compared in healthy subjects. MOR exposure was similar, with an area under the curve from 0 h to infinity (AUC0-∞) treatment ratio for MOR+TDF/MOR of 1.01 (90% confidence interval, 0.97 to 1.06). No relevant differences were observed regarding plasma exposure of metabolites. Renal clearances of MOR and its metabolites were not affected by TDF. No unexpected safety or tolerability issues were observed.
TEXT
Morinidazole is a new type of broad-spectrum nitroimidazole antimicrobial that exhibits greater activity and less toxicity than metronidazole (1, 2). It has been reported that morinidazole undergoes extensive metabolism, primarily via N-glucuronidation (yielding the N-glucuronide of S-morinidazole [M8-1] and of R-morinidazole [M8-2]) and o-sulfation (yielding the sulfate conjugate of morinidazole [M7]) (3). Morinidazole and its conjugates (M7, M8-1, and M8-2) are mainly excreted through urine (4). The main transporters involved in the renal secretion of drugs in humans include organic anion transporter (OAT) 1, OAT 3, and organic cation transporter (OCT) 2 (5–7). Morinidazole is not a substrate for OAT 1/3 or OCT 2, whereas M7, M8-1, and M8-2 are OAT 1/3 substrates (4). However, the contributions of renal uptake and efflux transporters in the active renal tubular secretion of morinidazole and its conjugated metabolites remain unclear. The competition or noncompetition inhibition of OAT transport function reduces renal clearance and causes bad clinical outcomes (8).
Tenofovir disoproxil fumarate (TDF) is an oral prodrug of tenofovir and a potent substrate of OATs, which are responsible for its tubular secretion into urine (9). TDF and morinidazole are likely to be used concomitantly for the treatment of mixed infections. Therefore, it was important to exclude the drug-drug interaction (DDI) potential when morinidazole was administered with TDF.
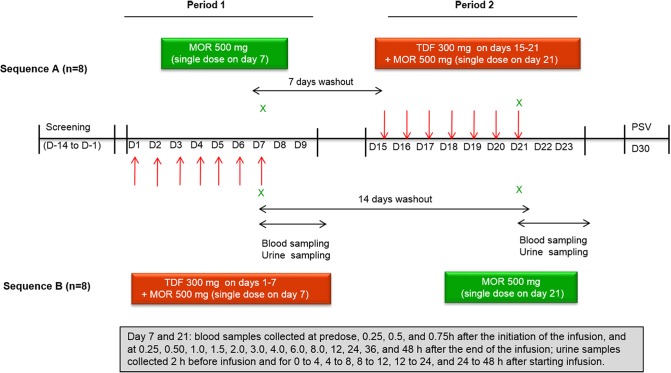
A prospective single-center, open-label crossover study was performed to assess the effect of tenofovir on the pharmacokinetics of morinidazole in healthy adults. The current study conformed to regulatory guidelines on the conduction of DDI studies (https://www.fda.gov/media/82734/download). The study protocol and informed consent documents were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University (approval no. 2017-EC-70). The Chinese Clinical Trial Registry number was ChiCTR-IIR-17012161.
The subjects were randomized into two treatment sequences, and each subject received two treatments, which included morinidazole alone and morinidazole in combination with TDF (Fig. 1). Sixteen healthy subjects (8 men and 8 women) were enrolled, but 1 woman withdrew from the study before initiation of period 1 because of herpesvirus infection. Fifteen subjects received 300 mg of TDF once daily for 6 days, 1 h before breakfast. They received the seventh TDF dose 1 h before the single 45-minute intravenous infusion of morinidazole. No breakfast was supplied, and no food was allowed for 4 h after administration of the drug on days 7 and 21. Blood and urine samples were collected at the specified time points (Fig. 1). Morinidazole, M2, M7, M8-1, and M8-2 were determined by liquid chromatography-tandem mass spectrometry. The main pharmacokinetic parameters were calculated by noncompartmental methods using WinNonlin 6.3 software.
FIG 1.
Study design.
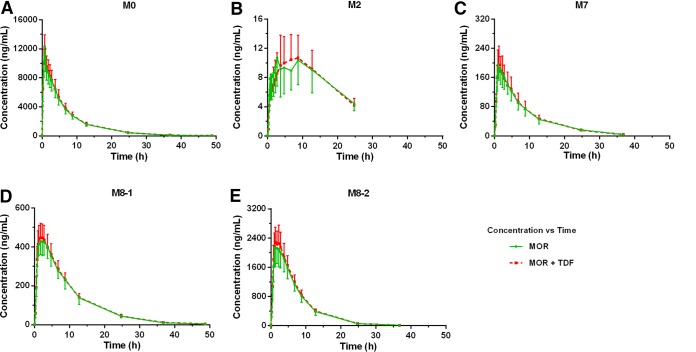
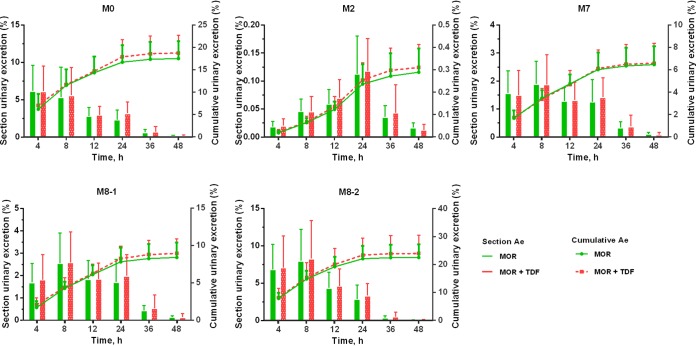
The mean concentration-time profiles of morinidazole, M2, M7, M8-1, and M8-2 were not affected by TDF coadministration (Fig. 2, Table 1). Drug excretion rates and renal clearance (CLR) values were also unaffected by TDF coadministration (Fig. 3). The potential DDI risk of tenofovir on morinidazole major metabolites was also evaluated based on FDA guidelines (10). Consistent with previous studies, morinidazole was found to be the major circulating drug-related component, whereas the systemic exposures of M8-2, M7, and M8-1 were approximately 14.4%, 1.8%, and 4.0% of the parent drug, respectively (4, 11). The metabolic ratio of M2 (C9H16N4O4, the loss of C2H2 from morinidazole) in plasma and urine was similar in each group (Fig. 2 and 3). Exposure results for the primary endpoint area under the curve from 0 h to infinity (AUC0–∞) and other pharmacokinetic measures indicated that concomitant administration with TDF had no clinically relevant effect on the exposure of morinidazole and its major metabolites.
FIG 2.
Mean plasma concentration-time profiles of morinidazole (MOR) (A), M2 (B), M7 (C), M8-1 (D), and M8-2 (E) after intravenous infusion of morinidazole 500 mg in healthy Chinese subjects with or without pretreatment with TDF. Data expressed as means ± standard deviation (n = 15). M0, morinidazole.
TABLE 1.
Pharmacokinetic parameters of morinidazole and its major metabolites after intravenous infusion of morinidazole 500 mg in healthy subjects with or without treatment with TDF
| Parametera | Result (mean ± SD) of treatment with: |
MOR+TDF:MOR (GLSM ratio [90% CI]) | |
|---|---|---|---|
| MOR (n = 15) | MOR+TDF (n = 15) | ||
| Morinidazole | |||
| Cmax (ng · ml−1) | 12,514.09 ± 1,913.83 | 12,462.65 ± 1,459.58 | 1.00 (0.92–1.07) |
| AUC0–t (ng · h · ml−1) | 76,590.73 ± 11,262.1 | 77,787.11 ± 11,918.50 | 1.01 (0.97–1.06) |
| AUC0–∞ (ng · h · ml−1) | 76,830.62 ± 11,263.1 | 78,074.47 ± 11,928.26 | 1.01 (0.97–1.06) |
| Tmax (h)b | 0.75 (0.50–1.25) | 0.75 (0.50–1.00) | |
| t1/2 (h) | 5.95 ± 0.36 | 6.20 ± 0.35 | |
| V (liters) | 57.34 ± 11.23 | 58.87 ± 11.90 | |
| CL (liters · h−1) | 6.65 ± 1.05 | 6.56 ± 1.09 | |
| CLR (liters · h−1) | 1.16 ± 0.26 | 1.22 ± 0.25 | |
| M2 | |||
| Cmax (ng · ml−1) | 12.47 ± 3.69 | 11.45 ± 3.72 | 0.91 (0.82–0.99) |
| AUC0–t (ng · h · ml−1) | 180.43 ± 75.40 | 183.96 ± 70.79 | 1.03 (0.88–1.18) |
| AUC0–∞ (ng · h · ml−1) | 382.13 ± 202.48 | 294.32 ± 73.50 | 0.86 (0.56–1.18) |
| Tmax (h)b | 3.75 (0.75–12.75) | 8.75 (3.75–12.75) | |
| t1/2 (h) | 26.36 ± 28.42 | 10.99 ± 1.75 | |
| V (liters) | 43,181.69 ± 27,863.49 | 28,567.50 ± 9,595.22 | |
| CL (liters · h−1) | 1,541.17 ± 529.15 | 1,787.14 ± 433.47 | |
| CLR (liters · h−1) | 7.73 ± 2.96 | 8.60 ± 3.79 | |
| M7 | |||
| Cmax (ng · ml−1) | 208.42 ± 47.29 | 205.02 ± 49.55 | 1.00 (0.92–1.04) |
| AUC0–t (ng · h · ml−1) | 1,798.51 ± 409.09 | 1,807.14 ± 401.58 | 1.00 (0.94–1.08) |
| AUC0–∞ (ng · h · ml−1) | 1,842.16 ± 407.22 | 1,847.11 ± 399.83 | 1.00 (0.93–1.08) |
| Tmax (h)b | 1.00 (1.00–1.75) | 1.25 (1.00–2.75) | |
| t1/2 (h) | 6.44 ± 0.48 | 6.57 ± 0.43 | |
| V (liters) | 2,627.90 ± 583.84 | 2,668.14 ± 531.55 | |
| CL (liters · h−1) | 281.98 ± 52.99 | 280.73 ± 50.69 | |
| CLR (liters · h−1) | 23.50 ± 4.34 | 24.04 ± 6.26 | |
| M8-1 | |||
| Cmax (ng · ml−1) | 445.04 ± 61.59 | 465.54 ± 70.31 | 1.00 (0.99–110.6) |
| AUC0–t (ng · h · ml−1) | 5,065.55 ± 807.37 | 5,176.49 ± 694.49 | 1.00 (0.98–1.08) |
| AUC0–∞ (ng · h · ml−1) | 5,120.78 ± 800.43 | 5,235.34 ± 695.45 | 1.00 (0.98–1.08) |
| Tmax (h)b | 2.25 (1.00–3.75) | 1.75 (1.00–2.75) | |
| t1/2 (h) | 6.47 ± 0.55 | 6.55 ± 0.46 | |
| V (liters) | 932.14 ± 169.12 | 916.50 ± 138.01 | |
| CL (liters · h−1) | 99.80 ± 15.06 | 97.01 ± 12.33 | |
| CLR (liters · h−1) | 13.86 ± 3.12 | 14.44 ± 3.42 | |
| M8-2 | |||
| Cmax (ng · ml−1) | 2,248.67 ± 481.29 | 2,376.93 ± 473.02 | 1.00 (1.00–1.12) |
| AUC0–t (ng · h · ml−1) | 18,203.89 ± 3,139.73 | 18,953.68 ± 2,511.84 | 1.00 (1.00–1.11) |
| AUC0–∞ (ng · h · ml−1) | 18,466.67 ± 3,103.75 | 19,231.04 ± 2,532.43 | 1.00 (1.00–1.10) |
| Tmax (h)b | 1.30 (1.00–2.75) | 1.75 (1.00–2.75) | |
| t1/2 (h) | 4.19 ± 0.43 | 4.10 ± 0.29 | |
| V (liters) | 168.60 ± 34.40 | 156.64 ± 26.25 | |
| CL (liters · h−1) | 27.73 ± 4.23 | 26.24 ± 3.43 | |
| CLR (liters · h−1) | 10.40 ± 2.41 | 10.48 ± 2.54 | |
AUC0–t, area under the concentration-time curve from 0 h to the last sampling time; AUC0–∞, area under the concentration-time curve from 0 h to infinity; CI, confidence interval; CL, apparent clearance; CLR, renal clearance; Cmax, maximum observed concentration; GLSM, geometric least-squares mean; MOR, morinidazole; V, volume of distribution; TDF, tenofovir disoproxil fumarate; Tmax, time to Cmax; t1/2, terminal elimination-phase half-life.
Data expressed as median (range).
FIG 3.
Urinary excretion of morinidazole (MOR) and its major metabolites after intravenous infusion of morinidazole 500 mg, with or without pretreatment with TDF, in healthy Chinese subjects. Data expressed as means ± standard deviation (n = 15). Ae, amount of drug excreted; M0, morinidazole.
There are several potential explanations for the finding that tenofovir did not cause a clinical DDI. First, the systemic concentration of tenofovir may not have been high enough to inhibit the transport of morinidazole-conjugated metabolites. Second, it may take ≥2 weeks of daily TDF administration to achieve the maximum level of inhibition. Third, other uptakes or efflux transporters, such as MRP4, might have led to competing inhibition and be involved in morinidazole and its metabolite renal excretion. Finally, merely the total drug plasma concentration was determined. Thus, plasma protein binding may have covered up a possible clinical DDI.
The pharmacokinetic properties of morinidazole were similar to those in previous studies (3, 11). The renal clearance of morinidazole and its conjugated metabolites in healthy volunteers was much higher than the values previously reported for patients with severe renal impairment (Table 1). Furthermore, the study conducted by Kong F et al. (12) clarified that accumulated uremic toxins may inhibit transporters, particularly OAT 3, leading to plasma exposure changes in relevant substrates. Thus, future investigations are needed to evaluate the effect of TDF administration on morinidazole pharmacokinetics in patients.
Adverse events (AEs), vital signs, pregnancy tests, clinical laboratory tests, and electrocardiograms (ECGs) were monitored to assess safety. Morinidazole alone and coadministered with TDF were well tolerated by the volunteers. As shown in Table 2, all AEs were transient and were grade 1 (mild) in severity. No serious AEs occurred during the study, and 15 patients were in good compliance with the protocol. No clinically significant changes in physical exams or ECGs were observed.
TABLE 2.
Summary of adverse events during study period
| Adverse event parametera | Treatment |
Total | |
|---|---|---|---|
| MOR | MOR+TDF | ||
| No. (%) of subjects with ≥1 AE | 5 (33.3%) | 4 (26.7%) | 9 (60.0%) |
| No. of AEs | 10 | 6 | 16 |
| No. (%) of subjects with ≥1 TEAE | 3 (20.0%) | 4 (33.3%) | 7 (46.7%) |
| TEAE, total (n) | 4 | 6 | 10 |
| Leucopenia | 1 | 0 | |
| Neutropenia | 1 | 0 | |
| Urine protein positive | 0 | 2 | |
| Urinary occult blood test positive | 0 | 1 | |
| Fasting venous glucose increased | 0 | 1 | |
| Serum triglycerides increased | 1 | 0 | |
| Constipation | 0 | 1 | |
| Abdominal distension | 0 | 1 | |
| Fever | 1 | 0 | |
AE, adverse event; TEAE, treatment-emergent adverse event.
However, the current study has some limitations. First, the sample size was small, and the sample consisted of young and healthy subjects exposed to TDF for a short period in contrast to elderly patients with hepatic impairment. Second, tenofovir is not recommended by the FDA as an OAT 1/3 inhibitor. Therefore, further clinical studies are needed to evaluate the clinical DDI risk that involves OAT 1/3 between morinidazole and the recommended inhibitors. Although previous studies have shown no significant accumulation after multiple doses and no need for dose adjustment of TDF in patients with hepatic impairment (13–15), caution should be exercised when extrapolating these data for patients with abnormal liver function under long-term TDF treatment.
In conclusion, this study demonstrated that coadministration of the approved clinical dose of 300 mg of TDF has little effect on systemic exposure of morinidazole or its main metabolites at a single intravenous dose of 500 mg. Morinidazole and TDF, alone and combined, were well tolerated.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant 81703615) and the National Science and Technology Major Projects for Major New Drug Innovation and Development (no. 2017ZX09303002).
We thank Huidi Jiang (College of Pharmaceutical Sciences, Zhejiang University) for assistance in study design. We also thank the clinical site staff for their contributions and Calibra-Teddy Alliance Laboratory (Shanghai) for bioanalytical analysis. We are grateful for the contributions of the study sponsor, Shanghai Hansoh BioMedical Co., Ltd.
We have no conflicts of interest to declare.
REFERENCES
- 1.Cao C, Luo A, Wu P, Weng D, Zheng H, Wang S. 2017. Efficacy and safety of morinidazole in pelvic inflammatory disease: results of a multicenter, double-blind, randomized trial. Eur J Clin Microbiol Infect Dis 36:1225–1230. doi: 10.1007/s10096-017-2913-z. [DOI] [PubMed] [Google Scholar]
- 2.Lv A. October 2008 Usage of alpha-(morpholin-1-base) methyl-2-methyl-5-azathio-1-alcohol in preparation of anti-trichomoniasis and anti-ameba medicines. China patent ZL 200510134254.3.
- 3.Gao R, Li L, Xie C, Diao X, Zhong D, Chen X. 2012. Metabolism and pharmacokinetics of morinidazole in humans: identification of diastereoisomeric morpholine N+-glucuronides catalyzed by UDP glucuronosyltransferase 1A9. Drug Metab Dispos 40:556–567. doi: 10.1124/dmd.111.042689. [DOI] [PubMed] [Google Scholar]
- 4.Zhong K, Li X, Xie C, Zhang Y, Zhong D, Chen X. 2014. Effects of renal impairment on the pharmacokinetics of morinidazole: uptake transporter-mediated renal clearance of the conjugated metabolites. Antimicrob Agents Chemother 58:4153–4161. doi: 10.1128/AAC.02414-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng B, El-Kattan AF, Radi ZA. 2012. Renal transporters in drug disposition, drug-drug interactions, and nephrotoxicity. Curr Protoc Toxicol Chapter 23:unit 23.3.1-15. doi: 10.1002/0471140856.tx2303s53. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich KJ. 1999. Affinity of drugs to the different renal transporters for organic anions and organic cations. Pharm Biotechnol 12:159–179. doi: 10.1007/0-306-46812-3_5. [DOI] [PubMed] [Google Scholar]
- 7.International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. 2010. Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo X, Liu K. 2018. Renal organic anion transporters in drug-drug interactions and diseases. Eur J Pharm Sci 112:8–19. doi: 10.1016/j.ejps.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 10.Grime K, Pehrson R, Nordell P, Gillen M, Kuhn W, Mant T, Brannstrom M, Svanberg P, Jones B, Brealey C. 2017. An S-warfarin and AZD1981 interaction: in vitro and clinical pilot data suggest the N-deacetylated amino acid metabolite as the primary perpetrator. Br J Clin Pharmacol 83:381–392. doi: 10.1111/bcp.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang X, Zhang Y, Gao R, Zhong K, Zhong D, Chen X. 2014. Effects of rifampin and ketoconazole on pharmacokinetics of morinidazole in healthy Chinese subjects. Antimicrob Agents Chemother 58:5987–5993. doi: 10.1128/AAC.03382-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong F, Pang X, Zhong K, Guo Z, Li X, Zhong D, Chen X. 2017. Increased plasma exposures of conjugated metabolites of morinidazole in renal failure patients: a critical role of uremic toxins. Drug Metab Dispos 45:593–603. doi: 10.1124/dmd.116.074492. [DOI] [PubMed] [Google Scholar]
- 13.Hu CY, Liu YM, Liu Y, Chen Q, Wang W, Wu K, Dong J, Li J, Jia JY, Lu C, Sun SX, Yu C, Li X. 2013. Pharmacokinetics and tolerability of tenofovir disoproxil fumarate 300 mg once daily: an open-label, single- and multiple-dose study in healthy Chinese subjects. Clin Ther 35:1884–1889. doi: 10.1016/j.clinthera.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Wenning LA, Friedman EJ, Kost JT, Breidinger SA, Stek JE, Lasseter KC, Gottesdiener KM, Chen J, Teppler H, Wagner JA, Stone JA, Iwamoto M. 2008. Lack of a significant drug interaction between raltegravir and tenofovir. Antimicrob Agents Chemother 52:3253–3258. doi: 10.1128/AAC.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song I, Min SS, Borland J, Lou Y, Chen S, Ishibashi T, Wajima T, Piscitelli SC. 2010. Lack of interaction between the HIV integrase inhibitor S/GSK1349572 and tenofovir in healthy subjects. J Acquir Immune Defic Syndr 55:365–367. doi: 10.1097/QAI.0b013e3181e67909. [DOI] [PubMed] [Google Scholar]