We investigated dose-fractionated polymyxin B (PB) on acute kidney injury (AKI). PB at 12 mg of drug/kg of body weight per day (once, twice, and thrice daily) was administered in rats over 72 h. The thrice-daily group demonstrated the highest KIM-1 increase (P = 0.018) versus that of the controls (P = 0.99) and histopathological damage (P = 0.013). A three-compartment model best described the data (bias, 0.
KEYWORDS: polymyxins, polymyxin B, colistin, pharmacokinetic, pharmacodynamic, toxicodynamic, animal model, acute kidney injury, urinary biomarker
ABSTRACT
We investigated dose-fractionated polymyxin B (PB) on acute kidney injury (AKI). PB at 12 mg of drug/kg of body weight per day (once, twice, and thrice daily) was administered in rats over 72 h. The thrice-daily group demonstrated the highest KIM-1 increase (P = 0.018) versus that of the controls (P = 0.99) and histopathological damage (P = 0.013). A three-compartment model best described the data (bias, 0.129 mg/liter; imprecision, 0.729 mg2/liter2; R2, 0.652,). Area under the concentration-time curve at 24 h (AUC24) values were similar (P = 0.87). The thrice-daily dosing scheme resulted in the most PB-associated AKI in a rat model.
TEXT
The widespread use of broad-spectrum antimicrobial agents has led to an increasing rate of resistant infections and associated morbidity and mortality (1–4). Although agents in the pipeline are promising, there is a prudent need to maximize the clinical efficacy and safety of currently available antibiotics (5). As a result of the paucity of active antibiotics, the polymyxins remain last-line options despite their associated nephrotoxicity (6–16). While the various mechanisms of polymyxin toxicity are being elucidated, dosing strategies that mitigate toxicity remain poorly defined. Toxicity thresholds for the plasma 24-h area under the curve (AUC24) of polymyxin B and colistin have recently been highlighted, yet approaches to minimize nephrotoxicity risk resulted in mixed outcomes (16–21). More specifically, it remains unclear whether dividing the total daily dose of polymyxins into fractions (e.g., giving twice or thrice daily) can circumvent kidney injury. This study aimed to examine the impact of dose-fractionated polymyxin B (PB) on acute kidney injury (AKI) in a preclinical model and describe the polymyxin B pharmacokinetic (PK) profile (22, 23).
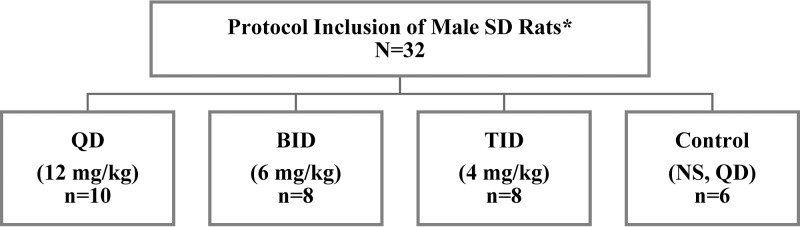
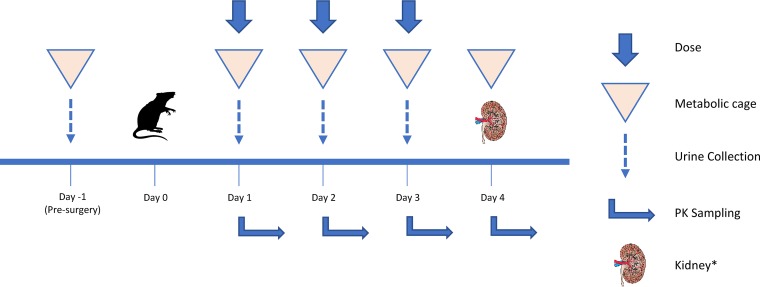
Male Sprague-Dawley rats were divided into three experimental groups (Fig. 1) as follows: once daily (QD), twice daily (BID), and thrice daily (TID) and received subcutaneous clinical grade PB. PB was fixed at 12 mg of drug/kg of body weight per day (allometrically scaled) for experimental groups for 72 h (24, 25). Control groups received equal volumes of normal saline (NS) as per QD protocol. Blood sampling strategies were similar in frequency and volume across groups (Fig. 2) (26, 27). Plasma creatinine content and PB1 were quantified (see the supplemental material) (28). Urine samples were analyzed for biomarkers (KIM-1, IP-10, TIMP-1, CLN, and OPN) (27). Histopathological examination of kidney tissues (n = 32) was performed (23, 29, 30). Midwestern University Institutional Animal Care and Use Committee approved this study (protocol 2677). PK models were fitted using the nonparametric adaptive grid (NPAG) algorithm within the Pmetrics (version 1.5.2) package (3) for R (3, 31). The best-fit PK model is described in the supplemental material. Best fit models were selected according to −2 log-likelihood (−2LL), Akaike’s information criterion (AIC), and rule of parsimony. Goodness of fit of the competing models was evaluated by observed versus predicted plots, bias and imprecision, and visual examination of individual concentration-time profiles. Bayesian posterior predicted concentrations identified maximum concentration of drug in serum (Cmax) and minimum concentration of drug in serum (Cmin); AUC24 was calculated across 24-h intervals using noncompartmental analysis (3). Analysis of variance (with Geisser and Greenhouse epsilon hat correction method accounting for subjects, treatments, and repeated measures over time) or mixed-effects models were utilized to compare urinary biomarkers, plasma creatinine, and PK indices between study groups (GraphPad Prism 8.2.1, La Jolla, CA). Ordinal logistical regressions were performed on histopathological scores (Stata 13, College Station, TX) (32). All tests were two-tailed with an α level of 0.05.
FIG 1.
Allocation and design of experimental groups. QD, once daily; BID, twice daily; TID, thrice daily; a total of 3, 6, and 9 doses for QD/control, BID, and TID groups, respectively, over 72 hours. Route of administration was subcutaneous. *, Envigo, Indianapolis, IN.
FIG 2.
Schematic of study design for each study group. Day 0 included surgical cannulation of catheter for blood sampling and recovery period. A maximum of 16 PK samples during the 3-day period with no more than 8 samples in a single day. Each sample was replaced with an equal volume of NS to maintain euvolemia. Day 4 included terminal sampling and terminal anesthesia. The left kidney was fixed in 10% formalin for histopathology examination, and the right kidney was stored at −80°C. Animals were maintained in plastic cages on a 12-h light and 12-h dark cycle; food and water were freely accessible at all times. *, Kidney image from Open Michigan (https://commons.wikimedia.org/wiki/File:Kidney_Cross_Section.png).
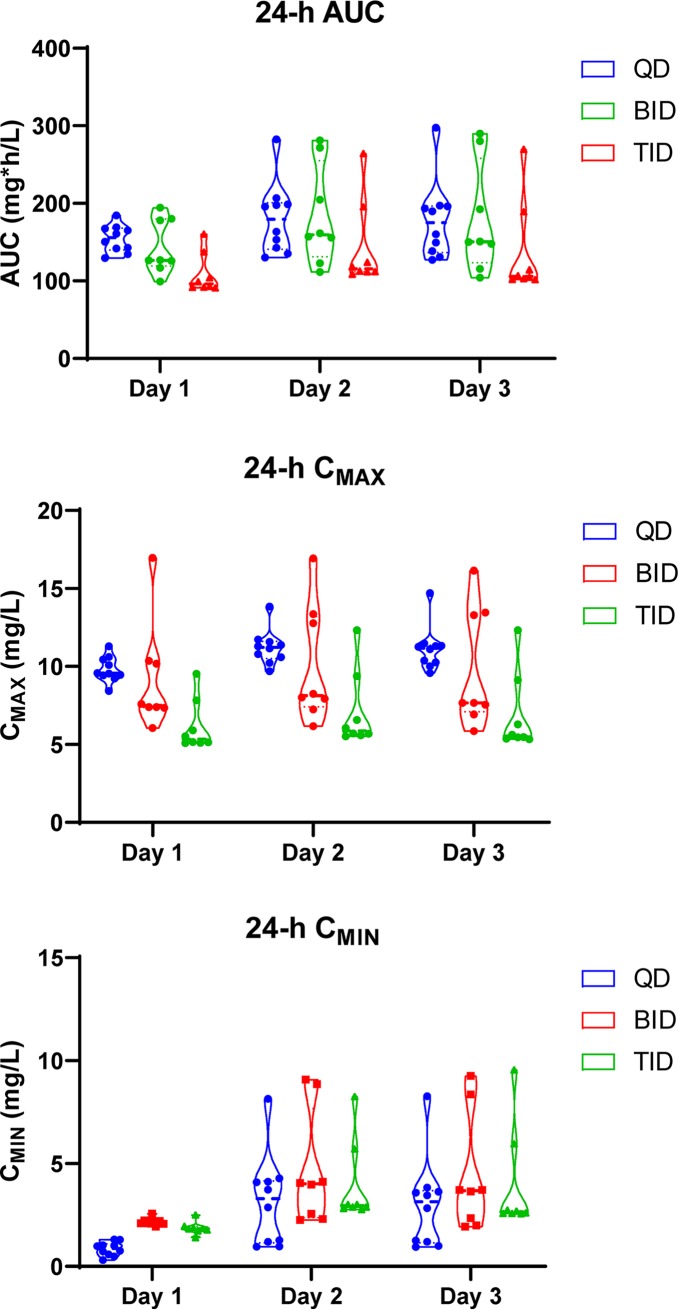
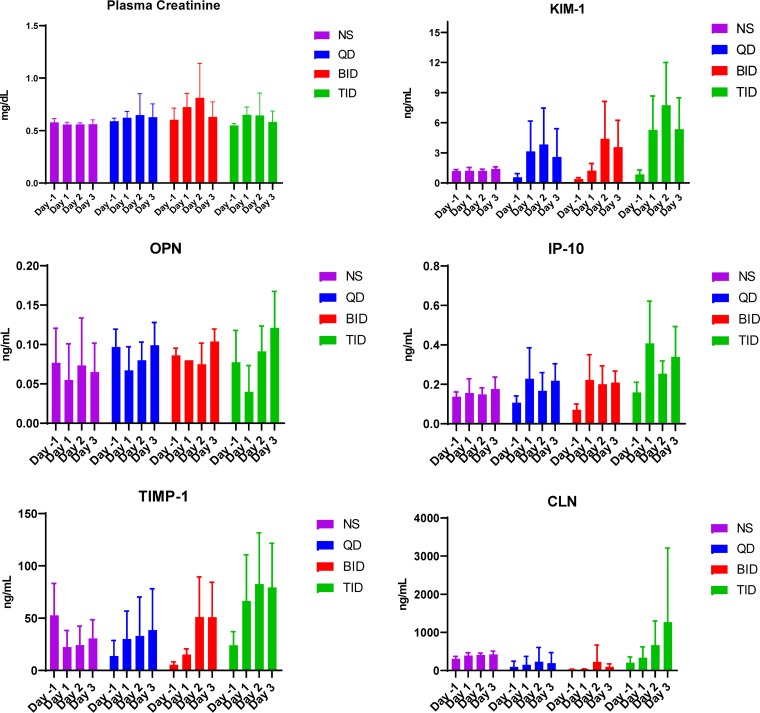
All 32 animals weighed between 291.1 and 321.1 g. The QD group had significantly lower urine volume compared to the BID group on day −1 (P = 0.01). All experimental groups produced significantly more urine on study days versus the control, except the BID group on day 1 (P = 0.23) (Fig. 3). Tables 1 and 2 and Fig. 4 describe PK models and population parameters. Means for AUC24 (P = 0.15) and Cmin (P = 0.19) for QD, BID, and TID groups were similar over the study period while mean Cmax differed (P = 0.0063) (Fig. 5). Although the QD group exhibited higher AUC24 than the TID group (P = 0.003) on day 1, no significant effects were observed for overall exposure versus time (P = 0.87). Only the QD group had a lower mean Cmin than that of the BID group (P < 0.0001) and TID group (P < 0.0001) during the first 24 h. Compared to that of the TID group, QD and BID groups showed significantly higher Cmax during the first 24 h (P = 0.0083 and P = 0.049, respectively). The QD group exhibited the highest Cmax on days 2 and 3 (P = 0.0025 and P = 0.0017, respectively). KIM-1 (P < 0.0001), OPN (P = 0.029), IP-10 (P = 0.046), and TIMP-1 (P < 0.0001) exhibited significant treatment effect over the study period while plasma creatinine did not (P = 0.18) (Fig. 6). As a representative biomarker, KIM-1 rose rapidly on days 1 and 2 for experimental groups. The TID group experienced the largest KIM-1 increase (mean increase [95% confidence interval {CI}] day 1 from day −1, 4.44 [0.89, 8.00] ng/ml; P = 0.018) compared to that of the control (0.03 [−0.42, 0.49] ng/ml; P = 0.99). Nonsignificant increases were observed with QD (P = 0.06) and BID (P = 0.06) groups. Further, the TID group exhibited a significant KIM-1 increase on day 2 (mean increase [95% CI], 2.44 [1.22, 3.67] ng/ml; P = 0.0013), and a decrease was observed on day 3 (2.39 [−0.053, 4.83] ng/ml; P = 0.055). Mean KIM-1 changes did not differ on days 2 and 3 between QD and BID groups. Similar trends and significant treatment and time effects were also observed for OPN (P = 0.029), IP-10 (P = 0.046), and TIMP-1 (P < 0.0001) but not CLN (P = 0.093). Significant histopathological damage was observed in the TID group (median score, 2; P = 0.013) (Table 3; see also Fig. S1 to S5 in the supplemental material).
FIG 3.
Mean 24-h urine volume between experimental and control groups. Presurgery mean (standard deviation [SD]) urine volumes were 5.2 (1.7), 8.6 (2.2). 6.4 (2.3), and 5.8 (2.2) ml for QD, BID, TID, and control groups, respectively.
TABLE 1.
Model selection summarya
| Compartmental model | −2LL | OFV change | AIC | Bias (mg/liter) | Imprecision (mg2/liter2) | Bayesian R2 |
|---|---|---|---|---|---|---|
| One | 1,709 | Ref | 1,715 | −0.48 | 0.95 | 0.10 |
| Two | 1,709 | 0 | 1,720 | −0.05 | 0.90 | 0.20 |
| Three | 1,450 | 259 | 1,463 | 0.13 | 0.73 | 0.65 |
One-compartment model served as the base model to derive two- and three-compartment models; the three-compartment model is the final model. −2LL, −2 log-likelihood; OFV, objective function value; AIC, Akaike’s information criterion.
TABLE 2.
Mean population parameters from the final model
| Population parametera | Mean | SD |
|---|---|---|
| Ka (h−1) | 0.290 | 0.460 |
| Ke (h−1) | 0.411 | 0.076 |
| Vc (liter) | 0.056 | 0.079 |
| K23 (h−1) | 6.214 | 11.430 |
| K32 (h−1) | 3.163 | 43.00 |
Ka, absorption rate constant; Ke, elimination rate constant; VC, central volume of distribution; K23, intercompartmental transfer rate (central to peripheral); K32, intercompartmental transfer rate (peripheral to central).
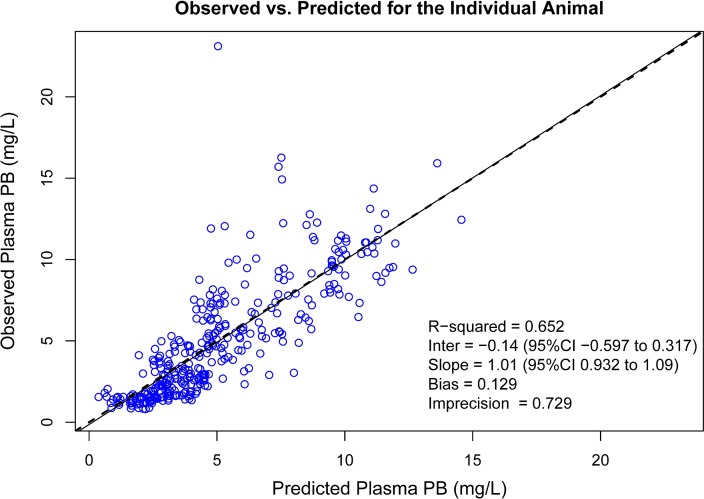
FIG 4.
Goodness-of-fit plot for Bayesian observed versus predicted plasma PB concentrations utilizing the final three-compartment model. Bias was defined as mean weighted prediction error; imprecision was defined as bias-adjusted mean weighted squared prediction error.
FIG 5.
Violin plots of PK indices from the final best-fit model by days for AUC24, Cmax, and Cmin.
FIG 6.
Plasma creatinine and urinary biomarkers.
TABLE 3.
Ordinal logistic regression of histopathology scorings by groupa
| Group | Median score (range) | P value | 95% CI |
|---|---|---|---|
| Control | 1.5 (1–2) | Referent | Referent |
| QD | 2 (1–3) | 0.156 | −0.61–3.77 |
| BID | 2 (1–3) | 0.092 | −0.34–4.50 |
| TID | 2 (2–3.5) | 0.013 | 0.70–5.88 |
Histopathology examination was conducted by IDEXX BioAnalytics (Columbia, MO, USA); the final histopathology score for an individual animal was calculated based on the highest score from the anatomical structural segment.
We demonstrated that fractionating the PB dose into three daily aliquots resulted in the most AKI as measured by urinary biomarkers (KIM-1, OPN, IP-10, and TIMP-1) with significant differences within 24 h. These markers have demonstrated high sensitivity for kidney injury (33–35). Further, KIM-1 and OPN are directly linked to proximal tubular toxicity and are concordant with previous reports (17, 33, 36). Histopathological findings also indicated that TID dosing led to the most severe AKI. PK analyses demonstrated that the PB exposures were similar across experimental groups.
Contemporary clinical dosing recommendations for PB suggest two divided doses based on a weight-based total daily dose (18, 37). It has been suggested that PB-induced AKI could be minimized by optimizing dosing intervals, similar to that observed with aminoglycosides (5, 38). Wallace et al. utilized a rat model to explore this possibility for colistin methanesulfonate and observed that every 12-h dosing led to a greater number and severity of renal lesions compared to those of every 8-h dosing. They concluded that higher and less fractionated doses of colistin methanesulfonate resulted in more extensive renal damage (19). Abdelraouf et al. administered PB to rats at 20 mg/kg/day or 5 mg/kg every 6 h (17). In contrast to Wallace et al. (but similar to our data), they observed a lower rate of nephrotoxicity in the once-daily group while the split-dosage group experienced a quicker onset of nephrotoxicity (serum creatinine elevation). The authors suggested that a nonpassive, saturable uptake may be responsible for the higher rate of renal injury when PB was given repeatedly (versus daily). Most recently, Okoduwa et al. conducted a retrospective, propensity score-matched study on patients treated with once-daily or twice-daily PB (20). In contrast to our findings, a higher proportion of nephrotoxicity (using clinical criteria) was observed in the once-daily group than in the twice-daily group (47% versus 17%, respectively; P = 0.0005). Our preclinical data agree with those from Abdelraouf et al. that dose fractionation consistently led to more AKI. Our study has limitations, including the use of a preclinical model and a 72-h study window; however, we employed highly sensitive biomarkers and histopathology to define AKI.
To date, this is the first preclinical, dose fractionation study of PB with a rich PK sampling design that allowed PK estimates at an individual level, demonstrating that thrice daily dosing worsened AKI more than once-daily dosing. Further studies are warranted to explore PB exposure linked to toxicity while maximizing efficacy.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by a grant (LEE17GO) awarded from the Cystic Fibrosis Foundation (CFF).
The funding agency had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank the Midwestern University CORE for use and access to the liquid chromatography-tandem mass spectrometer (LC-MS/MS).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Waterer GW, Wunderink RG. 2001. Increasing threat of Gram-negative bacteria. Crit Care Med 29:N75–N81. doi: 10.1097/00003246-200104001-00004. [DOI] [PubMed] [Google Scholar]
- 2.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. 2018. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Interagency Coordination Group on Antimicrobial Resistance. 2019. No time to wait: securing the future from drug-resistant infections. Report to the secretary-general of the United Nations. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.The Pew Charitable Trusts. 2019. Antibiotics currently in global clinical development. The Pew Charitable Trusts, Philadelphia, PA. [Google Scholar]
- 6.Evans ME, Feola DJ, Rapp RP. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant Gram-negative bacteria. Ann Pharmacother 33:960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 7.Zavascki AP, Nation RL. 2017. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 61:e02319-16. doi: 10.1128/AAC.02319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2011. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother 55:4044–4049. doi: 10.1128/AAC.00328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2012. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67:452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. 2013. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 57:6319–6324. doi: 10.1128/AAC.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justo JA, Bosso JA. 2015. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 35:28–33. doi: 10.1002/phar.1493. [DOI] [PubMed] [Google Scholar]
- 12.Kelesidis T, Falagas ME. 2015. The safety of polymyxin antibiotics. Expert Opin Drug Saf 14:1687–1701. doi: 10.1517/14740338.2015.1088520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crass RL, Rutter WC, Burgess DR, Martin CA, Burgess DS. 2017. Nephrotoxicity in patients with or without cystic fibrosis treated with polymyxin B compared to colistin. Antimicrob Agents Chemother 61:e02329-16. doi: 10.1128/AAC.02329-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. 2014. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58:2740–2746. doi: 10.1128/AAC.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temocin F, Erdinc S, Tulek N, Demirelli M, Bulut C, Ertem G. 2015. Incidence and risk factors for colistin-associated nephrotoxicity. Jpn J Infect Dis 68:318–320. doi: 10.7883/yoken.JJID.2014.223. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelraouf K, Braggs KH, Yin T, Truong LD, Hu M, Tam VH. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 56:4625–4629. doi: 10.1128/AAC.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nation RL, Rigatto MHP, Falci DR, Zavascki AP. 2019. Polymyxin acute kidney injury: dosing and other strategies to reduce toxicity. Antibiotics (Basel) 8:24. doi: 10.3390/antibiotics8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace SJ, Li J, Nation RL, Rayner CR, Taylor D, Middleton D, Milne RW, Coulthard K, Turnidge JD. 2008. Subacute toxicity of colistin methanesulfonate in rats: comparison of various intravenous dosage regimens. Antimicrob Agents Chemother 52:1159–1161. doi: 10.1128/AAC.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okoduwa A, Ahmed N, Guo Y, Scipione MR, Papadopoulos J, Eiras DP, Dubrovskaya Y. 2018. Nephrotoxicity associated with intravenous polymyxin B once- versus twice-daily dosing regimen. Antimicrob Agents Chemother 62:e00025-18. doi: 10.1128/AAC.00025-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavascki AP. 2014. Polymyxins for the treatment of extensively-drug-resistant Gram-negative bacteria: from pharmacokinetics to bedside. Expert Rev Anti Infect Ther 12:531–533. doi: 10.1586/14787210.2014.902307. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, Venkatesan N, Pais G, Cluff C, Lamar PC, Briyal S, Day JZ, Gulati A, Scheetz MH. 2017. 24-Hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e00416-17. doi: 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma V, McNeill JH. 2009. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair AB, Jacob S. 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. 2007. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes NJ, Liu J, McLaughlin MM, Qi C, Scheetz MH. 2015. Evaluation of clinical outcomes in patients with Gram-negative bloodstream infections according to cefepime MIC. Diagn Microbiol Infect Dis 82:165–171. doi: 10.1016/j.diagmicrobio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH. 2008. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis 60:163–167. doi: 10.1016/j.diagmicrobio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Sistare FD, Dieterle F, Troth S, Holder DJ, Gerhold D, Andrews-Cleavenger D, Baer W, Betton G, Bounous D, Carl K, Collins N, Goering P, Goodsaid F, Gu YZ, Guilpin V, Harpur E, Hassan A, Jacobson-Kram D, Kasper P, Laurie D, Lima BS, Maciulaitis R, Mattes W, Maurer G, Obert LA, Ozer J, Papaluca-Amati M, Phillips JA, Pinches M, Schipper MJ, Thompson KL, Vamvakas S, Vidal JM, Vonderscher J, Walker E, Webb C, Yu Y. 2010. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat Biotechnol 28:446–454. doi: 10.1038/nbt.1634. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leary R, Roger J, Schumitzky A, Van Guilder M. 2001. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models In Proceedings of the IEEE Symposium on Computer-Based Medical Systems. IEEE, Bethesda, MD, USA, USA. doi: 10.1109/CBMS.2001.941750. [DOI] [Google Scholar]
- 32.StataCorp. 2013. Stata statistical software: release 13. StataCorp LP, College Station, TX. [Google Scholar]
- 33.Vaidya VS, Ferguson MA, Bonventre JV. 2008. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musial K, Zwolinska D. 2014. Pleiotropic functions of TIMP-1 in patients with chronic kidney disease. Cell Mol Life Sci 71:1547–1548. doi: 10.1007/s00018-014-1592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erez DL, Sullivan KE, Denburg M, Bunin NJ, Jodele S, Afolayan S, Wallace G, Davies SM, Laskin B. 2019. Urinary CXCL10 and CXCL9 are associated with acute kidney injury in children after hematopoietic stem cell transplantation: results of a discovery and validation cohort. Biol Blood Marrow Transplant 25:S185–S186. doi: 10.1016/j.bbmt.2018.12.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avedissian SN, Pais GM, O'Donnell JN, Lodise TP, Liu J, Prozialeck WC, Joshi MD, Lamar PC, Becher L, Gulati A, Hope W, Scheetz MH. 2019. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 74:2326–2334. doi: 10.1093/jac/dkz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SteriMax, Inc. 2016. Polymyxin B for injection USP (package insert). SteriMax, Inc., Oakville, ON. [Google Scholar]
- 38.Barza M, Ioannidis JP, Cappelleri JC, Lau J. 1996. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ 312:338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.