KBP-7072 is a novel third-generation tetracycline (aminomethylcycline) antibacterial that overcomes common efflux and ribosomal protection resistance mechanisms that cause resistance in older-generation tetracyclines. KBP-7072 completed phase 1 clinical development studies for safety, tolerability, and pharmacokinetics (ClinicalTrials.gov identifier NCT02454361) and multiple ascending doses in healthy subjects (ClinicalTrials.gov identifier NCT02654626) in December 2015. Both oral and intravenous formulations of KBP-7072 are being developed.
KEYWORDS: Acinetobacter, KBP-7072, aminomethylcycline, susceptibility, tetracycline
ABSTRACT
KBP-7072 is a novel third-generation tetracycline (aminomethylcycline) antibacterial that overcomes common efflux and ribosomal protection resistance mechanisms that cause resistance in older-generation tetracyclines. KBP-7072 completed phase 1 clinical development studies for safety, tolerability, and pharmacokinetics (ClinicalTrials.gov identifier NCT02454361) and multiple ascending doses in healthy subjects (ClinicalTrials.gov identifier NCT02654626) in December 2015. Both oral and intravenous formulations of KBP-7072 are being developed. In this study, we evaluated the in vitro activities of KBP-7072 and comparator agents by CLSI document M07 (2018) broth microdilution against 531 recent geographically diverse and/or molecularly characterized Acinetobacter baumannii-A. calcoaceticus species complex (A. baumannii) isolates from the United States, Europe, Asia-Pacific (excluding China), and Latin America. A. baumannii isolates included carbapenem-resistant, colistin-resistant, tetracycline-resistant, and extended-spectrum-β-lactamase (ESBL)- and metallo-β-lactamase (MBL)-producing isolates. Overall, KBP-7072 (MIC50/90, 0.25/1 mg/liter) was comparable in activity to colistin (92.8%/92.8% susceptible [S] [CLSI/EUCAST]) against A. baumannii isolates, inhibiting 99.2% of isolates at ≤2 mg/liter and 97.6% of isolates at ≤1 mg/liter. KBP-7072 was equally active against A. baumannii isolates, including carbapenem-resistant, colistin-resistant, and tetracycline-resistant isolates, regardless of geographic location, and maintained activity against ESBL- and MBL-producing isolates. KBP-7072 outperformed comparator agents, including ceftazidime (40.3% S [CLSI]), gentamicin (48.2%/48.2% S [CLSI/EUCAST]), levofloxacin (39.5%/37.9% S [CLSI/EUCAST]), meropenem (42.0%/42.0% S [CLSI/EUCAST]), piperacillin-tazobactam (33.3% S [CLSI]), and all tetracycline-class comparator agents, which include doxycycline (67.3% S [CLSI]), minocycline (73.8% S [CLSI]), tetracycline (37.2% S [CLSI]), and tigecycline (79.5% inhibited by ≤2 mg/liter). The potent in vitro activity of KBP-7072 against recent geographically diverse, molecularly characterized, and drug-resistant A. baumannii isolates supports continued clinical development for the treatment of serious infections, including those caused by A. baumannii.
INTRODUCTION
Tetracycline antibacterials with broad-spectrum activity became available beginning in the late 1940s (1, 2). Tetracyclines have also been used to treat atypical infections, including those caused by Chlamydia spp., Mycoplasma spp., Rickettsia spp., and some protozoans (1). Over decades of use, resistance mechanisms, including efflux and ribosomal protection, have developed and substantially reduced how effective tetracyclines are against many important bacterial pathogens (2–4).
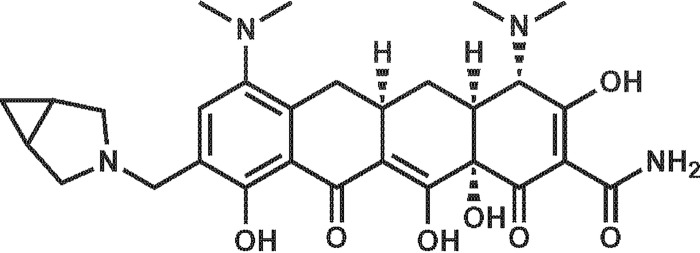
KBP-7072 (Fig. 1) is a potent, broad-spectrum, third-generation tetracycline (aminomethylcycline) antibacterial being developed (oral and intravenous formulations) for the treatment of community-acquired pneumonia (CAP) that overcomes many of these common tetracycline resistance mechanisms (5, 6). KBP-7072 has demonstrated potent in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci and has received qualified infectious disease product (QIDP) and fast-track status for CAP (7). In addition, KBP-7072 has completed phase 1 clinical development (December 2015) for safety, tolerability, pharmacokinetics, and multiple ascending doses in healthy subjects (8, 9).
FIG 1.
KBP-7072 compound structure.
Newer aminomethylcycline tetracyclines, such as KBP-7072 and omadacycline, have shown potent in vitro activity against Enterobacteriaceae isolates that produce extended-spectrum β-lactamases (ESBLs) and carbapenemases as well as multidrug-resistant (resistant to ≥3 classes of agents) and carbapenem-resistant Acinetobacter baumannii (CRAB) isolates (10). Carbapenem-resistant A. baumannii was recognized by the World Health Organization in 2017 as a critical (priority 1) pathogen to help guide the research, discovery, and development of new antibacterials (11, 12). In this study, the in vitro activities of KBP-7072 and comparator agents were evaluated against 531 recent geographically diverse and/or molecularly characterized A. baumannii isolates, which included carbapenem-resistant, colistin-resistant, tetracycline-resistant, and ESBL- and metallo-β-lactamase (MBL)-producing A. baumannii isolates.
RESULTS
Overall activity of KBP-7072.
The in vitro activities of KBP-7072 and comparator agents against 531 recent geographically diverse and/or molecularly characterized A. baumannii isolates are detailed in Table 1 as MIC range, MIC50/90, percent susceptible (S), percent intermediate (I), and percent resistant (R) according to CLSI/EUCAST breakpoint interpretive criteria and in Table 2 according to frequency and cumulative percent inhibition. KBP-7072 (MIC50/90, 0.25/1 mg/liter; 97.6% and 99.2% inhibited at ≤1 mg/liter and ≤2 mg/liter, respectively) and colistin (MIC50/90, ≤0.5/1 mg/liter; 92.8%/92.8% S [CLSI/EUCAST]) were the most active agents tested against A. baumannii (Table 1). A. baumannii susceptibilities to tetracycline-class comparators were 67.3% S, 73.8% S, and 37.2% S for doxycycline, minocycline, and tetracycline, respectively (Table 1). In addition, only 79.5% of A. baumannii isolates were inhibited by ≤2 mg/liter of tigecycline (FDA susceptibility breakpoint for Enterobacteriaceae) (Tables 1 and 2). Based on MIC90 values, KBP-7072 (MIC90, 1 mg/liter) was 4-fold more potent than tigecycline (MIC90, 4 mg/liter) and more than 8-fold more potent than doxycycline, minocycline, and tetracycline (MIC90 values of >8 mg/liter). Comparator agents were only marginally active against A. baumannii isolates. The comparator agents included ceftazidime (40.3% S [CLSI]), gentamicin (48.2%/48.2% S [CLSI/EUCAST]), levofloxacin (39.5%/37.9% S [CLSI/EUCAST]), meropenem (42.0%/42.0% S [CLSI/EUCAST]), and piperacillin-tazobactam (33.3% S [CLSI]) (Table 1).
TABLE 1.
Activities of KBP-7072 and comparators against 531 recent geographically diverse Acinetobacter baumannii isolates
| Antimicrobial agent (no. of isolates tested) | MIC range (mg/liter) | MIC50/90 (mg/liter) | CLSIa
|
EUCASTa
|
||||
|---|---|---|---|---|---|---|---|---|
| % S | % I | % R | % S | % I | % R | |||
| KBP-7072 (531) | ≤0.015 to 4 | 0.25/1 | 99.2b | |||||
| Tigecycline (531) | 0.06 to 16 | 1/4 | 79.5c | 18.2 | 2.3 | |||
| Doxycycline (453) | ≤0.06 to >8 | 1/>8 | 67.3 | 2.0 | 30.7 | |||
| Minocycline (454) | ≤0.03 to >8 | 1/>8 | 73.8 | 6.8 | 19.4 | |||
| Tetracycline (457) | 0.5 to >8 | >8/>8 | 37.2 | 7.2 | 55.6 | |||
| Ceftazidime (531) | 0.5 to >16 | >16/>16 | 40.3 | 6.0 | 53.7 | |||
| Colistin (528) | ≤0.5 to >8 | ≤0.5/1 | 92.8 | 7.2 | 92.8 | 7.2 | ||
| Gentamicin (531) | ≤2 to >8 | 8/>8 | 48.2 | 4.9 | 46.9 | 48.2 | 51.8 | |
| Levofloxacin (531) | 0.03 to >4 | >4/>4 | 39.5 | 2.8 | 57.6 | 37.9 | 1.3 | 60.8 |
| Meropenem (531) | 0.12 to >8 | >8/>8 | 42.0 | 0.8 | 57.3 | 42.0 | 2.3 | 55.7 |
| Piperacillin-tazobactam (520) | ≤0.06 to >128 | >64/>64 | 33.3 | 5.2 | 61.5 | |||
TABLE 2.
Antimicrobial activities of KBP-7072 and tetracycline comparators against Acinetobacter baumannii isolates
| Antimicrobial (no. of isolates) | No. (cumulative %) of isolates inhibited at MIC (mg/liter) of: |
MIC50 (mg/liter) | MIC90 (mg/liter) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >a | |||
| KBP-7072 (531) | 5 (0.9) | 62 (12.6) | 121 (35.4) | 46 (44.1) | 49 (53.3) | 165 (84.4) | 70 (97.6) | 9 (99.2) | 4 (100.0) | 0.25 | 1 | ||||||
| Tigecycline (531) | 0 (0.0) | 5 (0.9) | 53 (10.9) | 97 (29.2) | 62 (40.9) | 58 (51.8) | 147 (79.5) | 97 (97.7) | 9 (99.4) | 3 (100.0) | 1 | 4 | |||||
| Doxycycline (453) | 0 (0.0) | 30 (6.6) | 80 (24.3) | 66 (38.9) | 28 (45.0) | 35 (52.8) | 30 (59.4) | 36 (67.3) | 9 (69.3) | 139 (100.0) | 1 | >8 | |||||
| Minocycline (454) | 0 (0.0) | 8 (1.8) | 34 (9.3) | 64 (23.3) | 74 (39.6) | 42 (48.9) | 52 (60.4) | 40 (69.2) | 21 (73.8) | 31 (80.6) | 88 (100.0) | 1 | >8 | ||||
| Tetracycline (457) | 0 (0.0) | 5 (1.1) | 37 (9.2) | 55 (21.2) | 73 (37.2) | 33 (44.4) | 254 (100.0) | >8 | >8 | ||||||||
>, greater than the highest concentration tested.
Activity of KBP-7072 against 38 colistin-resistant isolates.
The in vitro activities of KBP-7072 and comparator agents against a subset of 38 colistin-resistant A. baumannii isolates are detailed in Table 3. KBP-7072 (MIC50/90, 0.5/1 mg/liter) was the most active agent tested, inhibiting 92.1% and 100.0% of these isolates at ≤1 mg/liter and ≤2 mg/liter, respectively (Table 3). Tetracycline-class comparators demonstrated limited activity against colistin-resistant A. baumannii isolates, and these comparators included doxycycline (MIC50/90, >8/>8 mg/liter; 39.4% S [CLSI]), minocycline (MIC50/90, 8/32 mg/liter; 45.5% S [CLSI]), tetracycline (MIC50/90, >16/>16 mg/liter; 21.2% S [CLSI]), and tigecycline (MIC50/90, 2/8 mg/liter; 52.6% inhibited at ≤2 mg/liter) (Table 3). Other comparator agents that were largely inactive against colistin-resistant A. baumannii isolates included ceftazidime (28.9% S [CLSI]), gentamicin (28.9%/28.9% S [CLSI/EUCAST]), levofloxacin (23.7%/21.1% S [CLSI/EUCAST]), meropenem (26.3%/26.3% S [CLSI/EUCAST]), and piperacillin-tazobactam (13.9% S [CLSI]) (Table 3).
TABLE 3.
Activities of KBP-7072 and comparators against 38 colistin-resistant Acinetobacter baumannii isolates
| Antimicrobial agent (no. of isolates tested) | MIC range (mg/liter) | MIC50/90 (mg/liter) | CLSIa
|
EUCASTa
|
||||
|---|---|---|---|---|---|---|---|---|
| % S | % I | % R | % S | % I | % R | |||
| KBP-7072 (38) | 0.03 to 2 | 0.5/1 | 100.0b | |||||
| Tigecycline (38) | 0.12 to 8 | 2/8 | 52.6c | 36.9 | 10.5 | |||
| Doxycycline (33) | ≤0.06 to >8 | >8/>8 | 39.4 | 3.0 | 57.6 | |||
| Minocycline (33) | 0.06 to 32 | 8/32 | 45.5 | 15.2 | 39.4 | |||
| Tetracycline (33) | 1 to >16 | >16/>16 | 21.2 | 6.1 | 72.7 | |||
| Ceftazidime (38) | 2 to >32 | >32/>32 | 28.9 | 5.3 | 65.8 | |||
| Colistin (38) | 4 to >8 | >8/>8 | 0.0 | 100.0 | 0.0 | 100.0 | ||
| Gentamicin (38) | 0.25 to >16 | >16/>16 | 28.9 | 2.6 | 68.4 | 28.9 | 71.1 | |
| Levofloxacin (38) | 0.06 to >32 | 32/>32 | 23.7 | 2.6 | 73.7 | 21.1 | 2.6 | 76.3 |
| Meropenem (38) | 0.25 to >32 | >32/>32 | 26.3 | 0.0 | 73.7 | 26.3 | 0.0 | 73.7 |
| Piperacillin-tazobactam (36) | ≤0.06 to >128 | >128/>128 | 13.9 | 8.3 | 77.8 | |||
Activity of KBP-7072 against 304 carbapenem (meropenem)-resistant isolates.
The in vitro activities of KBP-7072 and comparator agents against a subset of 304 carbapenem (meropenem)-resistant A. baumannii isolates are detailed in Table 4. KBP-7072 (MIC50/90, 0.5/1 mg/liter; 95.7% and 98.7% inhibited at ≤1 mg/liter and ≤2 mg/liter, respectively) was the most active agent tested against meropenem-resistant A. baumannii isolates (Table 4). Tetracycline-class comparators, which included doxycycline (MIC50/90, 8/>8 mg/liter; 49.3% S [CLSI]), minocycline (MIC50/90, 2/>8 mg/liter; 57.8% S [CLSI]), tetracycline (MIC50/90, >8/>8 mg/liter; 8.6% S [CLSI]), and tigecycline (MIC50/90, 2/4 mg/liter; 65.5% inhibited at ≤2 mg/liter), demonstrated limited activity against the meropenem-resistant A. baumannii isolates (Table 4). With the exception of colistin (90.7%/90.7% S [CLSI/EUCAST]), comparator agents, including ceftazidime (5.3% S [CLSI]), gentamicin (16.8%/16.8% S [CLSI/EUCAST]), levofloxacin (1.6%/1.6% S [CLSI/EUCAST]), and piperacillin-tazobactam (0.0% S [CLSI]), were inactive against meropenem-resistant A. baumannii isolates (Table 4).
TABLE 4.
Activities of KBP-7072 and comparators against 304 meropenem-resistant Acinetobacter baumannii isolates
| Antimicrobial agent (no. of isolates tested) | MIC range (mg/liter) | MIC50/90 (mg/liter) | CLSIa
|
EUCASTa
|
||||
|---|---|---|---|---|---|---|---|---|
| % S | % I | % R | % S | % I | % R | |||
| KBP-7072 (304) | 0.03 to 4 | 0.5/1 | 98.7b | |||||
| Tigecycline (304) | 0.12 to 16 | 2/4 | 65.5c | 30.9 | 3.6 | |||
| Doxycycline (274) | ≤0.06 to >8 | 8/>8 | 49.3 | 2.9 | 47.8 | |||
| Minocycline (275) | ≤0.03 to >8 | 2/>8 | 57.8 | 10.9 | 31.3 | |||
| Tetracycline (278) | 1 to >8 | >8/>8 | 8.6 | 7.2 | 84.2 | |||
| Ceftazidime (304) | 1 to >16 | >16/>16 | 5.3 | 6.2 | 88.5 | |||
| Colistin (301) | ≤0.5 to >8 | ≤0.5/2 | 90.7 | 9.3 | 90.7 | 9.3 | ||
| Gentamicin (304) | ≤2 to >8 | >8/>8 | 16.8 | 7.2 | 76.0 | 16.8 | 83.2 | |
| Levofloxacin (304) | 0.12 to >4 | >4/>4 | 1.6 | 3.3 | 95.1 | 1.6 | 0.0 | 98.4 |
| Meropenem (304) | 8 to >8 | >8/>8 | 0.0 | 0.0 | 100.0 | 0.0 | 2.6 | 97.4 |
| Piperacillin-tazobactam (304) | 64 to >64 | >64/>64 | 0.0 | 1.3 | 98.7 | |||
Activity of KBP-7072 against 254 tetracycline-resistant isolates.
The in vitro activities of KBP-7072 and comparator agents were evaluated against a subset of 254 tetracycline-resistant A. baumannii isolates (Table 5). KBP-7072 (MIC50/90, 0.5/1 mg/liter; 95.3% and 98.4% inhibited at ≤1 mg/liter and ≤2 mg/liter, respectively) was the most active agent tested against tetracycline-resistant A. baumannii isolates (Table 5). Tetracycline-class comparators, including doxycycline (MIC50/90, >8/>8 mg/liter; 41.3% S [CLSI]), minocycline (MIC50/90, 4/>8 mg/liter; 52.8% S [CLSI]), and tigecycline (MIC50/90, 2/4 mg/liter; 63.0% inhibited at ≤2 mg/liter), demonstrated limited activity against tetracycline-resistant A. baumannii isolates (Table 5). Except for colistin (90.5%/90.5% S [CLSI/EUCAST]), other comparator agents, including ceftazidime (9.1% S [CLSI]), gentamicin (16.9%/16.9% S [CLSI/EUCAST]), levofloxacin (6.3%/4.7% S [CLSI/EUCAST]), meropenem (7.4%/7.4% S [CLSI/EUCAST]), and piperacillin-tazobactam (3.9% S [CLSI]), were inactive against these isolates (Table 5).
TABLE 5.
Activities of KBP-7072 and comparators against 254 tetracycline-resistant Acinetobacter baumannii isolates
| Antimicrobial agent (no. of isolates tested) | MIC range (mg/liter) | MIC50/90 (mg/liter) | CLSIa
|
EUCASTa
|
||||
|---|---|---|---|---|---|---|---|---|
| % S | % I | % R | % S | % I | % R | |||
| KBP-7072 (254) | 0.03 to 4 | 0.5/1 | 98.4b | |||||
| Tigecycline (254) | 0.25 to 16 | 2/4 | 63.0c | 32.3 | 4.7 | |||
| Doxycycline (252) | 0.25 to >8 | >8/>8 | 41.3 | 3.6 | 55.2 | |||
| Minocycline (252) | 0.25 to >8 | 4/>8 | 52.8 | 12.3 | 34.9 | |||
| Tetracycline (254) | >8 to >8 | >8/>8 | 0.0 | 0.0 | 100.0 | |||
| Ceftazidime (254) | 4 to >16 | >16/>16 | 9.1 | 5.1 | 85.8 | |||
| Colistin (253) | ≤0.5 to >8 | ≤0.5/2 | 90.5 | 9.5 | 90.5 | 9.5 | ||
| Gentamicin (254) | 0.25 to >8 | >8/>8 | 16.9 | 5.9 | 77.2 | 16.9 | 83.1 | |
| Levofloxacin (254) | 0.12 to >4 | >4/>4 | 6.3 | 3.1 | 90.6 | 4.7 | 1.2 | 94.1 |
| Meropenem (254) | 0.12 to >8 | >8/>8 | 7.4 | 0.4 | 92.1 | 7.5 | 1.2 | 91.3 |
| Piperacillin-tazobactam (254) | ≤0.06 to >64 | >64/>64 | 3.9 | 3.5 | 92.5 | |||
Activities of KBP-7072 and tetracycline comparators stratified by geographic region.
KBP-7072 inhibited 96.5% to 100.0% and 98.8% to 100.0% of all A. baumannii isolates at ≤1 mg/liter and ≤2 mg/liter, respectively, regardless of geographic region, whereas tigecycline inhibition at ≤2 mg/liter varied from 69.1% for A. baumannii isolates from Latin America to 85.8% for isolates from North America (Table 6). In general, KBP-7072 and tigecycline MIC90 values were 1 mg/liter and 4 mg/liter, respectively, regardless of geographic region; however, KBP-7072 and tigecycline MIC50 values were lowest in North America (0.06 mg/liter and 0.5 mg/liter, respectively) and highest in Latin America (0.5 mg/liter and 2 mg/liter, respectively) (Table 6). Similarly, susceptibilities of tetracycline-class comparator agents, including doxycycline, minocycline, and tetracycline, were lowest for A. baumannii isolates from Latin America (51.3% S, 61.5% S, and 11.1% S, respectively) and highest against A. baumannii isolates from North America (85.4% S, 90.6% S, and 53.1% S, respectively) (Table 6). Comparator agents, including ceftazidime, gentamicin, levofloxacin, meropenem, and piperacillin-tazobactam, also demonstrated the lowest susceptibilities against A. baumannii isolates from Latin America (10.0% S to 21.0% S) and the highest susceptibilities against A. baumannii isolates from North America (48.8% S to 72.2% S) (Table 6).
TABLE 6.
Activities of KBP-7072 and comparators against Acinetobacter baumannii isolates stratified by geographic region
| Compound | North America (n = 169) |
Europe (n = 171) |
Latin America (n = 81) |
Asia-Pacific (n = 110) |
||||
|---|---|---|---|---|---|---|---|---|
| MIC50/90 (mg/liter) | % S | MIC50/90 (mg/liter) | % S | MIC50/90 (mg/liter) | % S | MIC50/90 (mg/liter) | % S | |
| KBP-7072a | 0.06/1 | 98.8 | 0.25/1 | 98.8 | 0.5/1 | 100.0 | 0.25/1 | 100.0 |
| Tigecyclineb | 0.5/4 | 85.8 | 1/4 | 78.4 | 2/4 | 69.1 | 1/4 | 79.1 |
| Doxycyclinec | 0.25/>8 | 85.4 | 1/>8 | 58.2 | 4/>8 | 51.3 | 1/>8 | 77.1 |
| Minocyclinec | 0.25/4 | 90.6 | 0.5/>8 | 66.1 | 2/32 | 61.5 | 1/8 | 79.8 |
| Tetracyclinec | 4/>16 | 53.1 | >8/>8 | 37.6 | >8/>8 | 11.1 | >8/>8 | 41.8 |
| Ceftazidime | 8/>32 | 64.5 | >32/>32 | 28.1 | >16/>16 | 17.3 | >32/>32 | 39.1 |
| Colistin | 0.25/2 | 91.7 | ≤0.5/4 | 89.5 | 0.25/0.5 | 98.7 | ≤0.5/1 | 95.5 |
| Gentamicin | 1/>16 | 72.2 | >8/>8 | 40.4 | >8/>8 | 21.0 | >8/>8 | 43.6 |
| Levofloxacin | 0.25/>16 | 60.4 | >4/>4 | 30.4 | >4/>4 | 12.4 | >4/>4 | 41.8 |
| Meropenem | 1/>32 | 62.1 | >8/>8 | 35.1 | >8/>8 | 14.8 | >8/>8 | 41.8 |
| Piperacillin-tazobactam | 32/>128 | 48.8 | >64/>64 | 26.9 | >64/>64 | 10.0 | >64/>64 | 37.4 |
Activity of KBP-7072 against molecularly characterized isolates.
MIC values for KBP-7072 and comparator agents against individual isolates of ESBL-, carbapenemase-, and/or MBL-producing A. baumannii isolates are presented in Table 7. KBP-7072 was the most active agent tested against individual A. baumannii isolates containing an ESBL (GES or CTX-M-), carbapenemase (KPC-3 or OXA), and/or MBL (IMP or NDM), with MIC values of 0.06 mg/liter to 0.5 mg/liter, 0.5 mg/liter, and 0.12 to 1 mg/liter, respectively (Table 7).
TABLE 7.
Activities of KBP-7072 and comparator agents against ESBL- and MBL-producing Acinetobacter baumannii isolatesa
| Collection no. | MIC (mg/liter) |
β-Lactamase profile | |||||
|---|---|---|---|---|---|---|---|
| KBP-7072 | TGC | CAZ | GM | LEV | MEM | ||
| 674334 | 0.12 | 1 | >32 | ≤1 | >4 | >8 | GES-22, OXA-23, OXA-51 |
| 689088 | 0.06 | 0.5 | >32 | 8 | >4 | >8 | GES-11, OXA-23, OXA-51 |
| 764370 | 0.5 | 2 | >32 | >8 | >4 | >8 | CTX-M-15, KPC-3, OXA-23, OXA-66, OXA-9, SHV-11, TEM-1 |
| 919444 | 0.06 | 0.25 | >32 | >8 | >4 | >32 | CTX-M-115, CARB-16, OXA-72, OXA-90 TEM-1 |
| 1003787 | 0.06 | 0.5 | >32 | >16 | >16 | >32 | CTX-M-115, CARB-16, OXA-72, OXA-90 |
| 143038 | 0.5 | 2 | >16 | >8 | >4 | >8 | IMP-1, OXA-51 |
| 143675 | 0.5 | 2 | >16 | >8 | >4 | >8 | IMP-1, OXA-51 |
| 177190 | 0.12 | 0.5 | >16 | ≤2 | >4 | >8 | IMP-1, OXA-51 |
| 602690 | 0.5 | 2 | >32 | >8 | >4 | >8 | NDM-1, OXA-51, TEM-1 |
| 602847 | 1 | 2 | >32 | >8 | >4 | >8 | NDM-1, OXA-23, OXA-51, TEM-1 |
TGC, tigecycline; CAZ, ceftazidime; GM gentamicin; LEV, levofloxacin; MEM, meropenem; ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase.
All ESBL- and MBL-producing A. baumannii isolates were resistant to ceftazidime, levofloxacin, and meropenem, with MIC values of >16 mg/liter, >4 mg/liter, and >8 mg/liter, respectively (Table 7).
DISCUSSION
Approximately one-half of the estimated 1,000,000 global A. baumannii infections that occur annually are carbapenem resistant; of those 500,000 infections, approximately 22,950 cases occur annually in the United States (13). Few therapeutic options currently exist for treating carbapenem-resistant A. baumannii infections, which tend to be extensively drug resistant or pandrug resistant, mostly due to the worldwide expansion of international clones well adapted to the nosocomial environment (14–16).
Previous studies by Lepak et al. (5) in a neutropenic murine pneumonia model against Staphylococcus aureus and Streptococcus pneumoniae have shown that the area under the concentration-time curve (AUC)/MIC ratio is the pharmacokinetic/pharmacodynamic (PK/PD) parameter that correlates best with KBP-7072 in vivo efficacy. In that study, KBP-7072 epithelial lining fluid (ELF) concentrations ranged from 82% to 238% compared to free plasma concentrations. KBP-7072 phase 2 dose selection discussions for CAP are ongoing with the U.S. Food and Drug Administration (FDA). A similar aminomethylcycline (omadacycline) received FDA approval in October 2018, with a susceptibility breakpoint of 4 mg/liter for CAP against Klebsiella pneumoniae. Based on this information and available Gram-positive PK/PD data for KBP-7072, conservative estimates of 1 mg/liter and 2 mg/liter were applied for the in vitro analysis of KBP-7072 MIC results against A. baumannii.
As observed with other newer tetracycline antibacterials, including eravacycline, omadacycline, and tigecycline, KPB-7072 is able to overcome many of the common efflux and ribosomal protection resistance mechanisms that cause resistance in older-generation tetracyclines (6). Combination treatments consisting of carbapenems, colistin, rifampin, and tigecycline have been studied; however, each of these drugs has limitations (14). New antibacterial agents in clinical development with in vitro activity against A. baumannii include cefiderocol (17), sulbactam-durlobactam (18), and KBP-7072.
Overall, KBP-7072 (MIC50/90, 0.25/1 mg/liter) was comparable in activity to colistin (92.8% S) against A. baumannii isolates, inhibiting 99.2% of A. baumannii isolates at ≤2 mg/liter and 97.6% of isolates at ≤1 mg/liter. Compared to other recent tetracyclines, KBP-7072 (MIC50/90, 0.25/1 mg/liter) was comparable in activity to eravacycline (MIC50/90, 0.25/1 mg/liter) (19, 20), 4-fold more potent than tigecycline (MIC50/90, 1/4 mg/liter), and 8- to 16-fold more potent than omadacycline (MIC50/90, 4/8 mg/liter) (10, 21). KBP-7072 was equally active against carbapenem-resistant, colistin-resistant, and tetracycline-resistant isolates of A. baumannii; maintained activity against ESBL-phenotype and MBL-producing isolates; and remained active against A. baumannii isolates regardless of geographic location. Interestingly, KBP-7072 outperformed, based on MIC90 values, all comparator agents, including tigecycline, that are often clinically used against carbapenem-resistant and multidrug-resistant A baumannii isolates. In general, KBP-7072 MIC50 and MIC90 results were similar to those observed for colistin.
In summary, these data document the potent in vitro activity of KBP-7072 against a challenge set of recent geographically diverse and molecularly characterized A. baumannii isolates and support the agent’s continued clinical development for the treatment of serious infections, including those caused by drug-susceptible and -resistant A. baumannii isolates.
MATERIALS AND METHODS
Organisms.
A collection of 531 recent (98.1% from 2018) geographically diverse A. baumannii isolates were recovered from patients with documented infections in 34 countries, including the United States (61 medical centers; 169 isolates [31.8%]) and countries in Europe (29 medical centers; 171 isolates [32.2%]), Latin America (9 medical centers; 81 isolates [15.3%]), and the Asia-Pacific region (17 medical centers; 110 isolates [20.7%]), as part of the SENTRY Antimicrobial Surveillance Program. A. baumannii isolates were collected from patients with bloodstream infections (115 isolates [21.7%]), patients with skin and skin structure infections (113 isolates [21.3%]), hospitalized patients with pneumonia (301 isolates [56.7%]), and patients with urinary tract infections (2 isolates [0.4%]) and included only 1 isolate/patient/infection episode. Bacterial identifications were confirmed by JMI Laboratories using matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany).
Colistin-resistant A. baumannii isolates were defined as having colistin MIC values at ≥4 mg/liter (22). MBL-producing A. baumannii isolates were defined as containing an IMP, VIM, or NDM β-lactamase-encoding gene and displaying a meropenem MIC value of ≥8 mg/liter. ESBL-producing A. baumannii isolates were defined as containing an ESBL gene, e.g., blaCTX-M or blaGES, and displaying a ceftazidime MIC value of ≥32 mg/liter, and carbapenemase-producing A. baumannii isolates were defined as containing a carbapenemase (KPC-3 or OXA) and having a meropenem MIC value of ≥8 mg/liter. In this study, test organisms consisted of 490 colistin-susceptible A. baumannii isolates, 38 colistin-resistant A. baumannii isolates, 5 MBL-producing (IMP and NDM) A. baumannii isolates, and 5 ESBL (CTX-M and GES)- and/or carbapenemase (KPC and OXA)-producing A. baumannii isolates. Colistin susceptibility results were not available for three of the MBL-producing A. baumannii isolates. Colistin-resistant and MBL- and ESBL-producing A. baumannii isolates were molecularly characterized using next-generation sequencing and high-resolution in silico analysis (16).
Antimicrobial susceptibility testing.
Reference broth microdilution susceptibility testing was performed at JMI Laboratories according to CLSI document M07 guidelines (23) and interpreted using CLSI document M100 (22), EUCAST (24), and FDA (25) breakpoint interpretive criteria. Freshly prepared cation-adjusted Mueller-Hinton broth (CLSI document M100) (22) was used to inoculate test panels containing KBP-7072 (KBP BioSciences, Princeton, NJ) and tigecycline (lot number R09410; U.S. Pharmacopeia, Rockville, MD). KBP-7072 and tigecycline test ranges were 32 mg/liter to 0.015 mg/liter and 16 mg/liter to 0.015 mg/liter, respectively. Historical susceptibility data from the SENTRY Antimicrobial Surveillance Program were included for ceftazidime, colistin, doxycycline, gentamicin, levofloxacin, meropenem, minocycline, piperacillin-tazobactam, and tetracycline. Tigecycline was included in this study as well as the SENTRY Antimicrobial Surveillance Program and was used as a bridge compound for the comparator agent susceptibility data. Broth microdilution MIC values were validated by concurrently testing CLSI quality control reference strains (22). The bacterial inoculum density was monitored by colony counts.
ACKNOWLEDGMENTS
This study was performed by JMI Laboratories and supported by KBP Biosciences, which included funding for services related to preparing the manuscript. JMI Laboratories contracted to perform services in 2018 for Achaogen, Inc.; the Albany College of Pharmacy and Health Sciences; Allecra Therapeutics; Allergan; AmpliPhi Biosciences Corp.; Amplyx; Antabio; the American Proficiency Institute; Arietis Corp.; Arixa Pharmaceuticals, Inc.; Astellas Pharma, Inc.; Athelas; Basilea Pharmaceutica, Ltd.; Bayer AG; Becton, Dickinson and Company; bioMérieux SA; Boston Pharmaceuticals; Bugworks Research, Inc.; CEM-102 Pharmaceuticals; Cepheid; Cidara Therapeutics, Inc.; CorMedix, Inc.; DePuy Synthes; Destiny Pharma; Discuva Ltd.; Dr. Falk Pharma GmbH; Emery Pharma; Entasis Therapeutics; Eurofarma Laboratorios SA; the U.S. Food and Drug Administration; the Fox Chase Chemical Diversity Center, Inc.; Gateway Pharmaceutical, LLC; GenePOC, Inc.; Geom Therapeutics, Inc.; GlaxoSmithKline plc; Harvard University; Helperby; HiMedia Laboratories; F. Hoffmann-La Roche, Ltd.; Icon plc; Idorsia Pharmaceuticals, Ltd.; Iterum Therapeutics plc; Laboratory Specialists, Inc.; Melinta Therapeutics, Inc.; Merck & Co., Inc.; Microchem Laboratory; Micromyx; MicuRx Pharmaceuticals, Inc.; Mutabilis Co.; Nabriva Therapeutics plc; NAEJA-RGM; Novartis AG; Oxoid, Ltd.; Paratek Pharmaceuticals, Inc.; Pfizer, Inc.; Polyphor, Ltd.; Pharmaceutical Product Development, LLC; Prokaryotics, Inc.; Qpex Biopharma, Inc.; Ra Pharmaceuticals, Inc.; Roivant Sciences, Ltd.; Safeguard Biosystems; Scynexis, Inc.; SeLux Diagnostics, Inc.; Shionogi and Co., Ltd.; SinSa Labs; Spero Therapeutics; Summit Pharmaceuticals International Corp.; Synlogic; T2 Biosystems, Inc.; Taisho Pharmaceutical Co., Ltd.; TenNor Therapeutics, Ltd.; Tetraphase Pharmaceuticals; The Medicines Company; Theravance Biopharma; the University of Colorado; the University of Southern California—San Diego; the University of North Texas Health Science Center; VenatoRx Pharmaceuticals, Inc.; Vyome Therapeutics, Inc.; Wockhardt; Yukon Pharmaceuticals, Inc.; the Zai Lab; and Zavante Therapeutics, Inc. There are no speakers’ bureaus or stock options to declare. V.J.B., J.Z., L.L., M.Z., X.T., and Q.L. are employees of and may hold stock options in KBP Biosciences.
REFERENCES
- 1.Roberts MC. 2003. Tetracycline therapy: update. Clin Infect Dis 36:462–467. doi: 10.1086/367622. [DOI] [PubMed] [Google Scholar]
- 2.Chopra I. 2002. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist Updat 5:119–125. doi: 10.1016/s1368-7646(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 3.Grossman TH. 2016. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med 6:a025387. doi: 10.1101/cshperspect.a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra I. 2001. Glycylcyclines: third-generation tetracycline antibiotics. Curr Opin Pharmacol 1:464–469. doi: 10.1016/s1471-4892(01)00081-9. [DOI] [PubMed] [Google Scholar]
- 5.Lepak AJ, Zhao M, Liu Q, Wang P, Wang Y, Bader JC, Ambrose PG, Andes DR. 2019. Pharmacokinetic/pharmacodynamic evaluation of a novel aminomethylcycline antibiotic, KBP-7072, in the neutropenic murine pneumonia model against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 63:e02404-18. doi: 10.1128/AAC.02404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminishi T, Schedlbauer A, Ochoa-Lizarralde B, de Astigarraga E, Çapuni R, Yang F, Benn V, Liu Q, Tan X, Zhang M, Connell SR, Fucini P. 2018. The third-generation tetracycline KBP-7072 exploits and reveals a new potential of the primary tetracycline binding pocket. bioRxiv 508218 10.1101/508218. [DOI]
- 7.Wang Y, Liu Q, Zhang B. 2016. Antibacterial activity of KBP-7072 against clinical isolates of drug-resistant bacteria, abstr Monday-565. Abstr ASM Microbe, 2016, 16 to 20 June 2016, Boston, MA.
- 8.ClinicalTrials.gov. 2015. Safety, tolerability and pharmacokinetics of KBP-7072. National Library of Medicine, Bethesda, MD: https://clinicaltrials.gov/ct2/show/NCT02454361?cond=kBP-7072&draw=2&rank=1. [Google Scholar]
- 9.ClinicalTrials.gov. 2015. A multiple ascending dose study of KBP-7072 in healthy subjects. National Library of Medicine, Bethesda, MD: https://clinicaltrials.gov/ct2/show/NCT02654626?cond=kBP-7072&draw=2&rank=2. [Google Scholar]
- 10.Pfaller MA, Huband MD, Shortridge D, Flamm RK. 2018. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: report from the SENTRY Antimicrobial Surveillance Program, 2016. Antimicrob Agents Chemother 62:e02327-17. doi: 10.1128/AAC.02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. 2019. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect 25:951–957. doi: 10.1016/j.cmi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 12.WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. [Google Scholar]
- 13.Spellberg B, Rex JH. 2013. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viehman JA, Nguyen MH, Doi Y. 2014. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 74:1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 16.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997-2016). Open Forum Infect Dis 6:S34–S46. doi: 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazmierczak KM, Tsuji M, Wise MG, Hackel M, Yamano Y, Echols R, Sahm DF. 2019. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-beta-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 53:177–184. doi: 10.1016/j.ijantimicag.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Barnes MD, Kumar V, Bethel CR, Moussa SH, O’Donnell J, Rutter JD, Good CE, Hujer KM, Hujer AM, Marshall SH, Kreiswirth BN, Richter SS, Rather PN, Jacobs MR, Papp-Wallace KM, van den Akker F, Bonomo RA. 2019. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone beta-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 10:e00159-19. doi: 10.1128/mBio.00159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother 59:1802–1805. doi: 10.1128/AAC.04809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang S, Efimova E, Fyfe C, Hawser S, Morrissey I. 2019. Global in vitro surveillance of eravacycline against Gram-negative and Gram-positive clinical isolates, including multidrug-resistant pathogens, collected in 2017, abstr Friday-543. ASM Microbe, 20 to 24 June 2019, San Francisco, CA.
- 21.Huband MD, Pfaller MA, Shortridge D, Flamm RK. 2019. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme, 2017. J Glob Antimicrob Resist 19:56–63. doi: 10.1016/j.jgar.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 22.CLSI. 2019. Performance standards for antimicrobial susceptibility testing: 29th informational supplement. M100Ed29. CLSI, Wayne, PA. [Google Scholar]
- 23.CLSI. 2018. M07Ed11. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed CLSI, Wayne, PA. [Google Scholar]
- 24.EUCAST. 2019. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, January 2019 http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 25.FDA. 2019. US FDA-recognized antimicrobial susceptibility test interpretive criteria. FDA, Silver Spring, MD: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm410971.htm. [Google Scholar]