Dalbavancin offers a possible treatment option for infectious peritonitis associated with peritoneal dialysis (PD) due to its coverage of Gram-positive bacteria and pharmacokinetic properties. We aimed to evaluate the clinical pharmacokinetics (PK) and pharmacodynamics of dalbavancin in a prospective, randomized, open-label, crossover PK study of adult patients with end-stage renal disease ESRD who were receiving PD. Sampling occurred prior to a single 30-min infusion of dalbavancin at 1,500 mg and at 1, 2, 3, 4, and 6 h and 7 and 14 days postadministration.
KEYWORDS: dalbavancin, peritoneal dialysis, pharmacodynamics, pharmacokinetics
ABSTRACT
Dalbavancin offers a possible treatment option for infectious peritonitis associated with peritoneal dialysis (PD) due to its coverage of Gram-positive bacteria and pharmacokinetic properties. We aimed to evaluate the clinical pharmacokinetics (PK) and pharmacodynamics of dalbavancin in a prospective, randomized, open-label, crossover PK study of adult patients with end-stage renal disease ESRD who were receiving PD. Sampling occurred prior to a single 30-min infusion of dalbavancin at 1,500 mg and at 1, 2, 3, 4, and 6 h and 7 and 14 days postadministration. Concentration-time data were analyzed via noncompartmental analysis. Pharmacodynamic parameters against common infectious peritonitis-causing pathogens were evaluated. Ten patients were enrolled. Patients were a median of 55 years old and had a median weight of 78.2 kg, 50% were female, and 70% were Caucasian. The terminal plasma half-life of dalbavancin was 181.4 ± 35.5 h. The day 0 to day 14 dalbavancin mean area under the curve (AUC) was 40,573.2 ± 9,800.3 mg·h/liter. The terminal-phase half-life of dalbavancin within the peritoneal fluid was 4.309 × 108 ± 1.140 × 109 h. The day 0 to day 14 dalbavancin mean peritoneal fluid AUC was 2,125.0 ± 1,794.3 mg·h/liter. The target plasma AUC/MIC was attained with the intravenous dose in all 10 patients for all Staphylococcus and Streptococcus species at the recommended MIC breakpoints. The intraperitoneal arm of the study was stopped early, because the first 3 patients experienced moderate to severe pain and bloating within 1 h following the administration of dalbavancin. Dalbavancin at 1,500 mg administered intravenously can be utilized without dose adjustment in peritoneal dialysis patients and will likely achieve the necessary peritoneal fluid concentrations to treat peritonitis caused by typical Gram-positive pathogens.
TEXT
Every year in the United States approximately 110,000 to 115,000 patients are newly diagnosed with end-stage renal disease (ESRD) and begin treatment with renal replacement therapy. Nearly 10,000 of these patients are started on peritoneal dialysis (PD) (1). Home dialysis has increased by 35% in the last decade, with 95% of home dialysis patients undergoing PD (1). Infectious peritonitis is a leading cause of morbidity associated with PD, and it accounts for approximately 18% of cases of infection-related mortality in PD patients. Severe or prolonged peritonitis can lead to peritoneal membrane failure, ultimately causing the technical failure of PD and requiring a transition to traditional hemodialysis methodologies. The rapid and effective treatment of peritonitis is required to reduce inflammation and preserve peritoneal membrane function (2, 3). Gram-positive bacteria are responsible for approximately 65% of cases of infectious peritonitis (4). The current standard of care involves empirical antibiotics covering Gram-positive organisms, as Staphylococcus aureus, coagulase-negative Staphylococcus spp., and Enterococcus spp. are common infecting pathogens (2, 3). Vancomycin can be used as an empirical regimen, particularly if there is a risk of methicillin-resistant S. aureus (MRSA) infections in this patient population. Addition of a second drug for the coverage of Gram-negative bacteria is recommended until culture and sensitivity results are available. Intravenous or intraperitoneal administration of antibiotics can be utilized; however, guidelines recommend the intraperitoneal administration of antibiotics with a dwell time of at least 6 h because it provides instant, high, and sustained concentrations of the antibiotic in the peritoneal fluid (2, 3).
Although vancomycin covers the majority of Gram-positive organisms that cause peritonitis, its use can be cumbersome in PD patients due to the need for therapeutic drug monitoring and transitions of care. Plasma vancomycin concentration monitoring has not been associated with clinical cure or relapse rates but may be utilized to ensure appropriate therapeutic troughs or to evaluate for vancomycin accumulation (5). Additionally, vancomycin must be readministered every 3 to 5 days in order to maintain steady-state trough concentrations of about 15 μg/ml (2, 3). Similarly to vancomycin, dalbavancin interferes with cell wall synthesis, through the binding of the d-alanyl-d-alanine terminus of the cell wall peptidoglycan (6). Dalbavancin has been shown to rapidly reach therapeutic systemic concentrations and to have an extended half-life of ∼8.5 days (7–11). Dalbavancin has been shown to be safe and effective with a treatment dose of 1,500 mg once or 1,000 mg followed by a 500-mg dose on day 7 (12–14). Dalbavancin offers a unique option for PD patients with peritonitis, due to its excellent coverage of Gram-positive bacteria, it is administered in a single-dose option, it does not require dose adjustment for patients in renal dysfunction on dialysis, and there is no need for routine plasma concentration monitoring.
Despite the potential clinical and social advantages, no pharmacokinetic (PK) or pharmacodynamic data are available to determine if utilizing dalbavancin in patients receiving PD is safe and effective. To address this gap in knowledge, we conducted an open-label pharmacokinetic study to evaluate the clinical PK of dalbavancin administered either intravenously or intraperitoneally in healthy patients receiving PD.
RESULTS
A total of 10 patients were enrolled in the study. Patients were a median of 55 years old and had a median weight of 78.2 kg, 50% were female, and 70% were Caucasian. The baseline demographics of the patients are presented in Table 1. All patients completed the intravenous administration arm of the study, and 3 patients were able to complete the intraperitoneal administration arm of the study. The intraperitoneal administration arm of the study was stopped early due to adverse drug reactions. These patients represent a broad heterogeneous range of the patients receiving renal replacement therapy via PD.
TABLE 1.
Baseline characteristics
| Characteristic | All patients (n = 10) |
|---|---|
| No. (%) of female patients | 5 (50) |
| Median (IQRa ) age (yr) | 55.5 (36, 65.5) |
| Median (IQR) ht (cm) | 159 (154, 177) |
| Median (IQR) wt (kg) | 78.2 (58.9, 91) |
| No. (%) of patients by race | |
| White | 7 (70) |
| Hispanic | 3 (30) |
| Dalbavancin dose (mg) | 1,500 |
IQR, interquartile range.
Plasma pharmacokinetics.
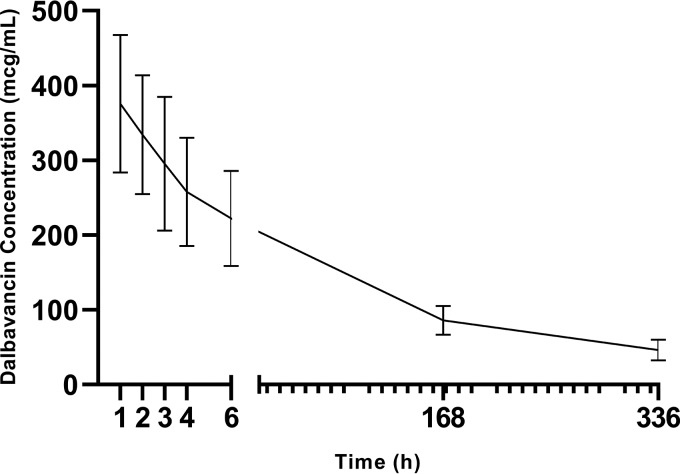
Complete pharmacokinetic parameters for dalbavancin plasma evaluations are reported in Table 2. The mean R2 value for the calculated elimination rate constant (kel) was 0.94 ± 0.07. The terminal plasma half-life of dalbavancin was 181.4 ± 35.5 h in the evaluated patients undergoing PD. The mean day 0 to day 14 area under the curve (AUC0–day 14) for dalbavancin was 40,573.2 ± 9,800.3 mg·h/liter.
TABLE 2.
Dalbavancin plasma noncompartmental analysis
| Compartment and pharmacokinetic parametera | Mean ± SD value |
|---|---|
| Plasma | |
| Cmax (mg/liter) | 357.64 ± 91.78 |
| Cmin (mg/liter) | 46.29 ± 13.97 |
| Terminal half-life (h) | 181.4 ± 35.5 |
| CL (liter/h) | 0.0298 ± 0.00765 |
| V (liter) | 7.71 ± 2.07 |
| AUC0–24 (mg·h/liter) | 5,473.0 ± 1,494.1 |
| AUC0–day 14 (mg·h/liter) | 40,573.2 ± 9,800.3 |
| AUC0–inf (mg·h/liter) | 52,897.3 ± 11,564.7 |
| Dialysate | |
| Cmax (mg/liter) | 9.16 ± 6.66 |
| Cmin (mg/liter) | 6.12 ± 7.2 |
| Terminal half-life (h) | 4.309 × 108 ± 1.140 × 109 |
| CL (liter/h) | 0.586 ± 0.192 |
| V (liter) | 2,142,484.1 ± 5,667,676.3 |
| AUC0–24 (mg·h/liter) | 134.3 ± 84.1 |
| AUC0–day 14 (mg·h/liter) | 2,125.0 ± 1,794.3 |
| AUC0–inf (mg·h/liter) | 66,998.2 ± 162,584.6 |
Calculated dialysate pharmacokinetic parameters are based upon a 1,500 mg dalbavancin dose administered intravenously. The exact dalbavancin dose reaching the peritoneal space is unknown and varied by patient. The median plasma-to-dialysate AUC0-day 14 ratio was 25 (interquartile range, 9 to 65 mg·h/liter). Therefore, interpretation of calculated dialysate pharmacokinetic parameters (e.g., V and CL) should be interpreted within this context. Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; CL, clearance; V, volume of distribution; AUC0–24, area under the curve from 0 to 24 h; AUC0–day 14, area under the curve from day 0 to day 24; AUC0–inf, area under the curve from 0 h to infinity.
Peritoneal dialysate pharmacokinetics.
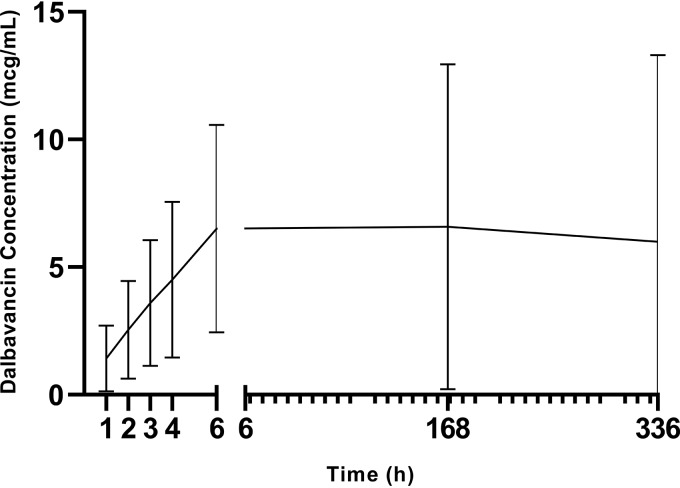
Complete pharmacokinetic parameters for dalbavancin peritoneal dialysate evaluations are reported in Table 2. The mean R2 value for the calculated kel was 0.67 ± 0.44. The terminal-phase half-life of dalbavancin within the peritoneal fluid was 4.309 × 108 ± 1.140 × 109 h. The mean day 0 to day 14 AUC for dalbavancin was 2,125.0 ± 1,794.3 mg·h/liter.
The median concentrations measured at each time point for both plasma and dialysate concentrations are reported in Table 3 and Fig. 1 to 3. Additionally, the plasma concentration-to-dialysate concentration ratio is also reported in Table 3, with the ratios ranging from 247 at 1 h postinfusion to 9 on day 14. The plasma-to-dialysate AUC0–day 14 ratio was 25 (interquartile range, 9 to 65 mg·h/liter).
TABLE 3.
Hourly evaluation of plasma and dialysate concentrations following intravenous administrationa
| Time of evaluation | Concn (mg/liter) in: |
Dialysate concn/plasma concn ratio | |
|---|---|---|---|
| Plasma | Dialysate | ||
| 1 h | 340.5 (331.1, 413.3) | 1.15 (0.46, 2.05) | 0.0032 (0.00089, 0.0057) |
| 2 h | 298.6 (288.8, 365.5) | 2.3 (1.01, 4.03) | 0.007 (0.0023, 0.0117) |
| 3 h | 287.3 (238.9, 323.3) | 3.2 (1.4, 5.7) | 0.0105 (0.0044, 0.0187) |
| 4 h | 235.1 (204.2, 287.1) | 4.4 (1.7, 6.1) | 0.0156 (0.0073, 0.0254) |
| 6 h | 202.8 (174.8, 271.6) | 6.8 (2.6, 8.5) | 0.0266 (0.0128, 0.0483) |
| Day 7 | 89.8 (78.6, 101.0) | 4.6 (1.8, 10.7) | 0.0527 (0.0261, 0.155) |
| Day 14 | 49.7 (35.5, 58.0) | 3.7 (0.9, 9.3) | 0.0815 (0.0152, 0.1732) |
The data are presented as the median (interquartile range).
FIG 1.
Mean plasma dalbavancin concentration following intravenous dalbavancin administration.
FIG 2.
Mean dialysate dalbavancin concentration following intravenous dalbavancin administration.
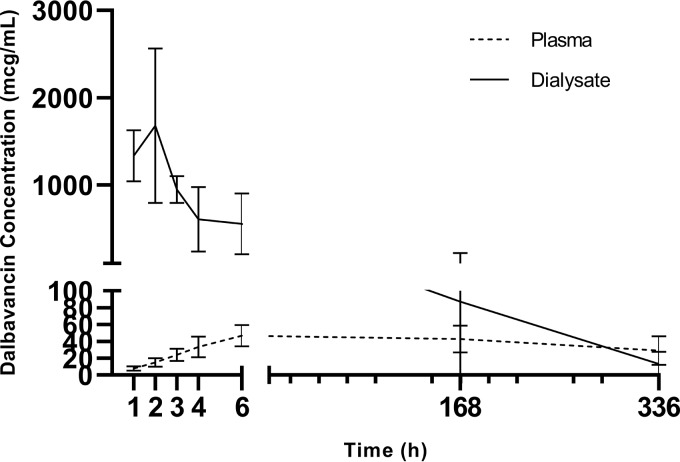
FIG 3.
Mean plasma and dialysate dalbavancin concentrations following intraperitoneal dalbavancin administration.
Pharmacodynamic target attainment.
The target plasma AUC/MIC was attained with the 1,500-mg intravenous dose in all 10 patients for Staphylococcus spp. and Streptococcus spp. up to the currently recommended MIC breakpoints. The population Monte Carlo simulations revealed greater than 99% target attainment up to an MIC breakpoint of 2 μg/ml in plasma when dalbavancin at 1,500 mg was administered intravenously or directly into the peritoneum. Population Monte Carlo simulations demonstrated >80% pharmacodynamic target attainment for Staphylococcus spp. in peritoneal fluid for a 14-day treatment course when dalbavancin was administered intravenously at 1,500 mg. The peritoneal fluid concentrations remained >0.25 μg/ml in all study patients. Complete population simulation data are reported in Table 4 and Table 5.
TABLE 4.
Population simulation of dalbavancin at 1,500 mg administered intravenously on pharmacodynamic targets for clinically relevant bacteria
| Compartment | % of Staphylococcus isolates |
% of Streptococcus isolates |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
EUCAST breakpoint |
MIC (mg/liter) |
EUCAST breakpointc | ||||||||||
| 0.25 | 0.5 | 1 | 1.5 | 2 | MRSAa | Staphylococcus spp.b | 0.25 | 0.5 | 1 | 1.5 | 2 | ||
| Plasma | 100 | 100 | 100 | 100 | 99.99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Peritoneal dialysate | 96.67 | 92.74 | 83.85 | 72.07 | 60.06 | 92.96 | 95.64 | 99.59 | 99.44 | 98.65 | 98.09 | 97.11 | 99.98 |
The MIC distribution for MRSA strains was as follows: 0.125 mg/liter, 4%; 0.25 mg/liter, 29%; 0.5 mg/liter, 54%; 1 mg/liter, 13%.
The MIC distribution for Staphylococcus spp. was as follows: 0.064 mg/liter, 2%; 0.125 mg/liter, 22%; 0.25 mg/liter, 51%; 0.5 mg/liter, 23%; 1 mg/liter, 2%.
The MIC distribution for Streptococcus spp. according to EUCAST breakpoints was as follows: 0.002 mg/liter, 1%; 0.004 mg/liter, 1%; 0.008 mg/liter, 17%; 0.016 mg/liter, 53%; 0.032 mg/liter, 14%; 0.064 mg/liter, 10%; 0.125 mg/liter, 3%; 0.25 mg/liter, 1%.
TABLE 5.
Population simulation of dalbavancin at 1,500 mg administered intraperitoneally on pharmacodynamic targets for clinically relevant bacteria
| Compartment | % of Staphylococcus isolates |
% of Streptococcus isolates |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
EUCAST breakpoint |
MIC (mg/liter) |
EUCAST breakpointc | ||||||||||
| 0.25 | 0.5 | 1 | 1.5 | 2 | MRSAa | Staphylococcus spp.b | 0.25 | 0.5 | 1 | 1.5 | 2 | ||
| Plasma | 99.97 | 99.92 | 99.81 | 99.62 | 99.38 | 99.88 | 99.9 | 99.99 | 100 | 99.99 | 99.99 | 99.96 | 100 |
| Peritoneal dialysate | 99.91 | 99.8 | 99.6 | 99.44 | 99.33 | 99.86 | 99.93 | 100 | 99.96 | 99.94 | 99.93 | 99.94 | 100 |
The MIC distribution for MRSA strains was as follows: 0.125 mg/liter, 4%; 0.25 mg/liter, 29%; 0.5 mg/liter, 54%; 1 mg/liter, 13%.
The MIC distribution for Staphylococcus spp. was as follows: 0.064 mg/liter, 2%; 0.125 mg/liter, 22%; 0.25 mg/liter, 51%; 0.5 mg/liter, 23%; 1 mg/liter, 2%.
The MIC distribution for Streptococcus spp. according to EUCAST breakpoints was as follows: 0.002 mg/liter, 1%; 0.004 mg/liter, 1%; 0.008 mg/liter, 17%; 0.016 mg/liter, 53%; 0.032 mg/liter, 14%; 0.064 mg/liter, 10%; 0.125 mg/liter, 3%; 0.25 mg/liter, 1%.
Adverse events.
Three patients received dalbavancin at 1,500 mg by intraperitoneal administration. All 3 patients noted abdominal discomfort, which was self-described as moderate to severe bloating with diffuse pain at a level ranging from 4 to 8 out of 10 within 1 h following the administration of dalbavancin. Abdominal exams of all three patients, performed by the study medical doctor, were benign, and the abdomens were described as soft and mildly distended, consistent with fluid in the peritoneal space. Two of the three patients received acetaminophen at 650 mg for 1 dose. All patients noted decreasing pain and bloating following acetaminophen treatment. Dialysate samples drawn following intraperitoneal administration appeared hazy on visual inspection. The discomfort was notably reduced per patient report following draining of the dialysate solution containing dalbavancin. All three patients reported no continued discomfort beyond 48 h. Due to the consistency and reproducibility of the discomfort among the three patients, the research team, in conjunction with the safety review committee, determined to halt the intraperitoneal administration of dalbavancin arm of the study. Following further analysis, it was determined that dalbavancin and dextrose had undergone an adduct reaction, forming an insoluble precipitant. Complete plasma and peritoneal dialysate PK are reported in Table 6 and Table 7.
TABLE 6.
Pharmacokinetic parameters for plasma and dialysate following intraperitoneal administration of dalbavancina
| Pharmacokinetic parameter | Value for: |
|
|---|---|---|
| Plasma (n = 3) | Dialysate (n = 3) | |
| Cmax (mg/liter) | 46.6 ± 12.6 | 1,697.0 ± 865.2 |
| Cmin (mg/liter) | 29.0 ± 16.9 | 13.3 ± 14.4 |
| Terminal half-life (h) | 522.4 ± 400.3 | 122.2 ± 55.2 |
| CL (liter/h) | 0.059 ± 0.039 | 0.034 ± 0.027 |
| V (liters) | 30.7 ± 7.5 | 7.17 ± 7.15 |
| AUC0–24 (mg·h/liter) | 973.6 ± 277.0 | 14,969.4 ± 5,786.1 |
| AUC0–day 14 (mg·h/liter) | 13,532.3 ± 4,913.4 | 69,654.3 ± 55,486.5 |
| AUC0–inf (mg·h/liter) | 41,843.2 ± 39,549.3 | 71,238.4 ± 56,492.2 |
Data are presented as mean ± standard deviation. Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; CL, clearance; V, volume of distribution; AUC0–24, area under the curve from 0 to 24 h; AUC0–day 14, area under the curve from day 0 to day 24; AUC0–inf, area under the curve from 0 h to infinity.
TABLE 7.
Hourly evaluation of plasma and dialysate concentrations following intraperitoneal administrationa
| Time of evaluation | Concn (mg/liter) in: |
Dialysate concn/plasma concn ratio | |
|---|---|---|---|
| Plasma | Dialysate | ||
| 1 h | 7.77 (5.17, 10.2) | 1,414.7 (1,008.1, 1,580.1) | 194.92 (138.97, 203.35) |
| 2 h | 14.76 (9.94, 19.9) | 1,404.0 (960.6, 2,667.9) | 96.65 (70.40, 180.82) |
| 3 h | 22.07 (17.48, 31.94) | 919.74 (809.89, 1,114.4) | 36.69 (34.88, 52.61) |
| 4 h | 30.3 (23.1, 47.1) | 759.9 (182.5, 873.2) | 16.15 (6.023, 37.84) |
| 6 h | 44.7 (35.0, 60.1) | 559.8 (206.1, 899.9) | 9.317 (4.613, 25.71) |
| Day 7 | 42.0 (26.7, 59.1) | 21.1 (2.14, 238.6) | 0.357 (0.0511, 8.922) |
| Day 14 | 22.3 (16.5, 48.3) | 9.62 (1.02, 29.2) | 0.1992 (0.0459, 1.768) |
The data are presented as the median (interquartile range).
DISCUSSION
This study is the first to evaluate the PK of dalbavancin in patients with end-stage renal disease receiving PD as renal replacement therapy. Dalbavancin pharmacokinetics were evaluated in patients who had hepatic or renal impairment. These previously published data demonstrated that, in comparison with the dalbavancin exposure in individuals with no renal impairment, dalbavancin exposure was not increased in patients with mild renal impairment and that exposure was ∼50% higher in patients with moderate renal impairment, 100% higher in patients with severe renal impairment, and not significantly different in end-stage renal disease patients undergoing dialysis (15). Based on these data, only patients with severe renal dysfunction (creatinine clearance, <30 ml/min) and not on hemodialysis should have their dalbavancin dose reduced (6). The maximum plasma concentration (Cmax) found within our patient population was 357.64 ± 91.78 mg/liter, and the plasma area under the curve from 0 h to 24 h (AUC0–24) was 5,473.0 ± 1,494.1 mg·h/liter, which is approximately 15% less than the reported Cmax and approximately 13% greater than the reported AUC0–24 in healthy subjects administered 1,500 mg of dalbavancin (6). Given these relatively small pharmacokinetic differences in Cmax and AUC0–24 and given that no adverse events were reported in any of the patients in the intravenous administration arm, the dosing strategy of 1,500 mg once may be utilized in patients undergoing PD as renal replacement therapy. The pharmacodynamic targets, for a 14-day treatment course, of an AUC/MIC ratio of ≥1,000 for Staphylococcus aureus and an AUC/MIC ratio of ≥100 for Streptococcus species have been proposed based on previous in vitro studies (16, 17). A previously reported pharmacodynamic model, based on phase II and phase III study pharmacokinetic parameters and bacterial isolates from these studies, proposed MIC breakpoints of 0.5 μg/ml for Staphylococcus aureus and 2 μg/ml for Streptococcus spp.; however, EUCAST has designated breakpoints of 0.125 μg/ml and 0.25 μg/ml, depending on the bacterial species (9). Additionally, the MIC50 and MIC90 are 0.03 μg/ml and 0.03 μg/ml, respectively, for Staphylococcus aureus and 0.03 μg/ml and 0.06 μg/ml, respectively, for Streptococcus spp. Recent pharmacodynamic evaluations have suggested a free drug AUC/MIC ratio of ≥27.1, using a population protein binding estimate of 93% bound and 7% free dalbavancin plasma concentrations (18, 19). Using pharmacodynamic attainment estimates combined with the AUC0–day 14 data generated from this study, the pharmacodynamic targets are far exceeded for both Staphylococcus aureus and Streptococcus spp., regardless of our current use of more conservative pharmacodynamic targets or the more recent pharmacodynamic target recommendation determined using population-based protein binding estimates.
Peritoneal dialysate dalbavancin concentrations were also evaluated to assess the penetration of dalbavancin from the plasma into the peritoneal space. These concentrations have not been previously evaluated and will provide foundational data for the possible use of dalbavancin as a treatment modality for PD-associated peritonitis. The Cmax following intravenous infusion was seen at 6 h following the infusion of 6.8 mg/liter and represented approximately 3% of the plasma concentration at 6 h. The median concentrations at day 7 and day 14 were 4.6 mg/liter and 3.7 mg/liter, respectively, and were 5.1% and 7.4% of the plasma concentrations, respectively. While the absolute concentration decreases over time, as expected, there was a proportional increase in the plasma concentration to peritoneal dialysate concentration. This suggests that there is a proportion of dialysate accumulation within the peritoneal space over the course of treatment. The total estimated exposure within the peritoneal dialysate over 14 days was 2,125.0 ± 1,794.3 mg·h/liter, representing approximately 5% of the plasma exposure over the same period of time. The previously described MIC breakpoints for Staphylococcus aureus and Streptococcus spp. were generated based on plasma AUC/MIC target attainment goals. However, if the same proposed targets are utilized, the AUC/MIC ratio for Staphylococcus aureus was approximately 4,250 and the AUC/MIC ratio for Streptococcus spp. was 1,062. Peritoneal dialysate concentrations achieved the proposed pharmacodynamic targets for both common pathogens of peritonitis. Given the pharmacokinetics generated and previous reports of probable pharmacodynamic targets, dalbavancin is a likely treatment option for patients receiving consistent PD for renal replacement therapy.
Full pharmacokinetic and pharmacodynamic evaluations of the intraperitoneal administration of dalbavancin were not performed due to the termination of this arm of the study. Prior to study initiation, stability testing was performed with both dextrose and icodextrin solutions, and both physical stability and chemical stability were determined for up to 48 h. Given the reproducibility of the adverse reaction in the first two study subjects, an icodextrin dialysate solution was utilized for one patient receiving dalbavancin by intraperitoneal administration. This alteration to the study protocol subjectively reduced the appearance of precipitant. However, precipitant was still present, likely due to a small volume of dextrose dialysate solution remaining from the patient’s overnight prescription, and the patient experienced discomfort. Therefore, the authors recommend against utilizing the intraperitoneal administration of dalbavancin in patients who receive dextrose-containing peritoneal dialysate as any portion of their dialysis regimen. It is unknown whether the use of smaller intraperitoneal dalbavancin dosages would allow this event to be avoided; however, testing of intraperitoneal administration strategies may be unnecessary, given the ability of a single intravenous dose to achieve reliable intraperitoneal concentrations over a 14-day period.
Several limitations need to be considered when evaluating our study results. While the data from this study indicate that dalbavancin concentrations may be appropriately high to utilize dalbavancin in the treatment of peritonitis, this was a PK study and did not evaluate patients with clinical peritonitis. Protein binding and free concentrations in plasma and peritoneal fluid were not measured. Alterations in protein content in patients with peritonitis could affect PK/pharmacodynamic estimates. Given the sample size, the data presented may not be representative of all PD patients or patients with clinical infective peritonitis. The terminal half-life observed in our study would project adequate dalbavancin plasma and peritoneal fluid concentrations above the MIC90s for Streptococcus and Staphylococcus species for up to a 28-day treatment duration in most patients. However, given the 14-day sampling strategy utilized by this study, there are not sufficient data to evaluate if a single-dose dosing strategy would be an appropriate alternative for treatments recommended for greater than 14 days. In the absence of therapeutic drug monitoring, careful patient observation with consideration of dosing alterations may be warranted in these patients. Further studies are needed to evaluate the clinical effectiveness of dalbavancin in comparison to that of known effective treatments in PD patients with infective peritonitis.
Conclusions.
Dalbavancin pharmacokinetic parameters in peritoneal dialysis-treated patients were similar to those previously reported in healthy subjects, with only a slight increase in drug exposure being seen over a 14-day treatment period. Dalbavancin penetration into the peritoneal space after intravenous administration was approximately 5% of the overall plasma exposure; however, the concentrations achieved remained above the designated MIC breakpoints throughout the treatment period. The administration of dalbavancin directly into the peritoneal space is not recommended, due to the moderate to severe pain and bloating experienced by the patients in this study. Dalbavancin administered intravenously at a dose of 1,500 mg can be utilized without dose adjustment in peritoneal dialysis patients and will likely achieve the peritoneal fluid concentrations necessary to treat peritonitis caused by typical Gram-positive pathogens.
MATERIALS AND METHODS
Patients.
This was a prospective, randomized, open-label, crossover PK study of adult patients who were receiving management for PD at the University of Colorado Hospital Renal Clinic. The Colorado Multiple Institutional Review Board (COMIRB) approved the study prior to enrolling patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Patients were eligible for enrollment if they were receiving active PD, were receiving PD treatments ≥3 times per week for ≥3 months, and were managed at the University of Colorado Hospital’s Renal Clinic. No restrictions were placed on home PD prescription for enrollment in the trial. All patients were 18 to 89 years old. Patients were excluded from the study if they were currently receiving antimicrobial therapy; had received antibiotic therapy within 14 days prior to the study; had known hypersensitivity reactions to dalbavancin or other glycopeptides, active peritonitis, a body temperature of >38.3°C or <36°C at the time of the study, significant abdominal tenderness at the time of the study, or Child-Pugh class B or C hepatic dysfunction; or were a member of any vulnerable population.
Antibiotic administration and sample collection.
Patients were randomized to receive dalbavancin either intravenously or intraperitoneally as the initial route of administration followed by the alternative route of administration following the washout period. Randomization was based on the patient’s enrollment number and was generated prior to the enrollment of the first patients.
Prior to study initiation, patients had peritoneal dialysate fluid drained from their peritoneal space and 2.5 liters of 2.5% dextrose peritoneal dialysis solution was manually infused into the peritoneal space. Following the initial dalbavancin administration day, patients returned to their normally prescribed PD prescription, which was not directed by the study team. Dalbavancin at 1,500 mg was reconstituted with 75 ml of sterile water and was either diluted in 500 ml of 5% dextrose solution for intravenous infusion or split between two 60-ml slip-tip syringes for intraperitoneal administration. When dalbavancin was assigned to be administered intravenously, the diluted dalbavancin was infused over 30 min (6). When dalbavancin was assigned to be administered directly into the peritoneal space, each syringe was administered over 5 min and then the PD catheter was flushed with 50 ml of 2.5% dextrose peritoneal dialysis solution.
Eight venous blood samples and dialysate fluid samples were collected for each arm of the study. Blood samples were drawn from a peripherally inserted intravenous catheter, with 10 ml blood being removed prior to each sample being drawn for analysis. Peritoneal dialysate samples were collected from the patient’s permanent peritoneal dialysate catheter, with 30 ml of dialysate fluid being removed prior to 10 ml of fluid being drawn for analysis. Sampling occurred just prior to the start of the intravenous infusion or the peritoneal administration of dalbavancin and at hours 1, 2, 3, 4, and 6 on the date of administration, followed by the collection of one sample each at 7 days and 14 days following administration. Following the day 14 sampling, there was a washout period of 45 days or greater. The patients then received dalbavancin by the alternative administration method, followed by the same sampling strategy. Plasma and dialysate samples were processed and stored at −80°C until the completion of enrollment and sample collection. Dalbavancin concentrations in plasma and dialysate samples were determined via a previously published liquid chromatography-tandem mass spectrometry assay (20) at the University of Colorado Medicinal Chemistry Core Facility.
Pharmacokinetic analysis.
The plasma and dialysate concentration-time data for dalbavancin were analyzed via noncompartmental analysis utilizing linear trapezoidal linear interpolation calculation methods (Phoenix WinNonlin software, version 7.0; Certara, Princeton, NJ). Minimum plasma concentrations (Cmin) were determined by direct measurement. Ratios of the total area under the curve (AUC) and the concentration at each measured time point were analyzed by use of the mean concentrations to compare plasma and peritoneal dialysate concentrations.
Pharmacodynamic analysis.
Pharmacodynamic parameters were evaluated in order to determine whether dalbavancin dosing results in initial and steady-state plasma and peritoneal concentrations that are adequate for the treatment of infections due to common pathogens (Staphylococcus aureus, coagulase-negative Staphylococcus spp., and Enterococcus spp.). The goals for the AUC-to-MIC ratios to be achieved over the 14-day treatment period were ≥1,000 for Staphylococcus aureus and ≥100 for Streptococcus spp. Attainment of pharmacodynamic targets was evaluated for Staphylococcus aureus and Streptococcus spp. at the 0.25-μg/ml MIC breakpoint. Due to a lack of pharmacodynamic targets within the peritoneal fluid, attainment was considered achieved if the peritoneal fluid dalbavancin concentration remained above the MIC at day 14. Monte Carlo simulation (Crystal Ball, version 7; Decisioneering, Inc., Denver, CO) was used to calculate the probability of target attainment (PTA) for the pharmacodynamic goals. The model randomly applies values for Cmax and AUC0–24 derived from data obtained from the study patients. We utilized EUCAST MIC data for the common Gram-positive pathogens. We placed these MIC distributions into our Monte Carlo simulation for comparison to dalbavancin plasma concentrations and PK variability. From these studies, MIC frequency distributions were constructed and used in the Monte Carlo simulations. Ten thousand simulations were performed at each MIC value and for each of the selected pathogens.
Adverse event evaluation.
Treatment-emergent adverse events were evaluated by the research nurse by questioning at the beginning of the study visit and at each pharmacokinetic sampling time point. In addition to known common adverse events, special questioning regarding abdominal discomfort or peritoneal signs were conducted. The study team visually inspected the peritoneal fluid content at each sampling period. Adverse events were graded on a scale of from 1 to 5 (e.g., mild, moderate, severe, life-threatening, death). Reactions graded as severe or greater were reviewed by the members of a data safety monitoring board, consisting of an independent nephrologist, a nurse, and a pharmacist.
Statistical analysis.
To compare the two modalities of administration, a Fisher’s exact test or a chi-square test was used for all categorical data, and a Student t test was utilized for all continuous data comparisons (JMP, version 13; SAS Institute Inc., Cary, NC).
ACKNOWLEDGMENTS
This research was funded by an investigator-initiated research grant (to T.H.K.) from Allergan Pharmaceuticals. The Clinical Research Center (CRC) at the University of Colorado Hospital and the Medicinal Chemistry Core (MCC) are supported in part by the Colorado Clinical and Translational Sciences Institute (CCTSI) and Colorado CTSA grant UL1TR001082 from NCATS/NIH.
Patients were given payment to participate within the study, which was reviewed and approved by the Colorado Multiple Institutional Review Board. We all report that our institutions received grant support from Allergan Pharmaceuticals. The funding body played no role in any of the following: design and conduct of the study, data collection, management, analysis and interpretation of the data, the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
We thank the nurses and staff of the Clinical Research Center (CRC) at the University of Colorado Hospital for their assistance with this study. Additionally, we thank Michael F. Wempe from the Medicinal Chemistry Core (MCC) facility, housed within the Department of Pharmaceutical Sciences at the University of Colorado Anschutz Medical Campus, for analyzing the dalbavancin concentrations.
REFERENCES
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JLT, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert P, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, et al. 2017. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 69:A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. 2016. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szeto CC, Li PK, Johnson DW, Bernardini J, Dong J, Figueiredo AE, Ito Y, Kazancioglu R, Moraes T, Van Esch S, Brown EA. 2017. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int 37:141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 4.Whitty R, Bargman JM, Kiss A, Dresser L, Lui P. 2017. Residual kidney function and peritoneal dialysis-associated peritonitis treatment outcomes. Clin J Am Soc Nephrol 12:2016–2022. doi: 10.2215/CJN.00630117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson S, Tang W, Cho Y, Mudge DW, Hawley CM, Badve SV, Johnson DW. 2015. The role of monitoring vancomycin levels in patients with peritoneal dialysis-associated peritonitis. Perit Dial Int 35:222–228. doi: 10.3747/pdi.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durata Therapeutics U.S. Limited. 2016. Dalvance(R) package insert. Durata Therapeutics U.S. Limited, Parsippany, NJ. [Google Scholar]
- 7.Buckwalter M, Dowell JA. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol 45:1279–1287. doi: 10.1177/0091270005280378. [DOI] [PubMed] [Google Scholar]
- 8.Dorr MB, Jabes D, Cavaleri M, Dowell J, Mosconi G, Malabarba A, White RJ, Henkel TJ. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 55(Suppl 2):ii25–ii30. doi: 10.1093/jac/dki008. [DOI] [PubMed] [Google Scholar]
- 9.Dowell JA, Goldstein BP, Buckwalter M, Stogniew M, Damle B. 2008. Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. J Clin Pharmacol 48:1063–1068. doi: 10.1177/0091270008321273. [DOI] [PubMed] [Google Scholar]
- 10.Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C, Mroszczak EJ, Campbell KC, Kelly E. 2004. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 48:940–945. doi: 10.1128/aac.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA. 2007. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother 60:681–684. doi: 10.1093/jac/dkm263. [DOI] [PubMed] [Google Scholar]
- 12.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. 2014. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 13.Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. 2016. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis 62:545–551. doi: 10.1093/cid/civ982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jauregui LE, Babazadeh S, Seltzer E, Goldberg L, Krievins D, Frederick M, Krause D, Satilovs I, Endzinas Z, Breaux J, O'Riordan W. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 41:1407–1415. doi: 10.1086/497271. [DOI] [PubMed] [Google Scholar]
- 15.Marbury T, Dowell JA, Seltzer E, Buckwalter M. 2009. Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol 49:465–476. doi: 10.1177/0091270008330162. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2007. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 51:1150–1154. doi: 10.1128/AAC.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin G, Credito K, Ednie LM, Appelbaum PC. 2005. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob Agents Chemother 49:770–772. doi: 10.1128/AAC.49.2.770-772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrothers TJ, Chittenden JT, Critchley I. 2020. Dalbavancin population pharmacokinetic modeling and target attainment analysis. Clin Pharmacol Drug Dev 9:21–31. doi: 10.1002/cpdd.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepak A, Marchillo K, VanHecker J, Andes D. 2015. Impact of glycopeptide resistance in Staphylococcus aureus on the dalbavancin in vivo pharmacodynamic target. Antimicrob Agents Chemother 59:7833–7836. doi: 10.1128/AAC.01717-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alebic-Kolbah T, Demers R, Cojocaru L. 2011. Dalbavancin: quantification in human plasma and urine by a new improved high performance liquid chromatography-tandem mass spectrometry method. J Chromatogr B Analyt Technol Biomed Life Sci 879:2632–2641. doi: 10.1016/j.jchromb.2011.07.027. [DOI] [PubMed] [Google Scholar]