Helicobacter pylori is an important risk factor for gastric ulcers. However, antibacterial therapies increase the resistance rate and decrease the eradication rate of H. pylori. Inspired by the microaerophilic characteristics of H. pylori, we aimed at effectively establishing an oxygen-enriched environment to eradicate and prevent the recurrence of H. pylori. The effect and the mechanism of an oxygen-enriched environment in eradicating H. pylori and preventing the recurrence were explored in vitro and in vivo.
KEYWORDS: Helicobacter pylori, oxygen, eradication, resistance, disruption
ABSTRACT
Helicobacter pylori is an important risk factor for gastric ulcers. However, antibacterial therapies increase the resistance rate and decrease the eradication rate of H. pylori. Inspired by the microaerophilic characteristics of H. pylori, we aimed at effectively establishing an oxygen-enriched environment to eradicate and prevent the recurrence of H. pylori. The effect and the mechanism of an oxygen-enriched environment in eradicating H. pylori and preventing the recurrence were explored in vitro and in vivo. During oral administration and after drug withdrawal, H. pylori counts were evaluated by Giemsa staining in animal cohorts. An oxygen-enriched environment in which H. pylori could not survive was successfully established by adding hydrogen peroxide into several solutions and rabbit gastric juice. Hydrogen peroxide effectively killed H. pylori in Columbia blood agar and special peptone broth. Minimum inhibition concentrations and minimum bactericidal concentrations of hydrogen peroxide were both relatively stable after promotion of resistance for 30 generations, indicating that hydrogen peroxide did not easily promote resistance in H. pylori. In models of Mongolian gerbils and Kunming mice, hydrogen peroxide has been shown to significantly eradicate and effectively prevent the recurrence of H. pylori without toxicity and damage to the gastric mucosa. The mechanism of hydrogen peroxide causing H. pylori death was related to the disruption of bacterial cell membranes. The oxygen-enriched environment achieved by hydrogen peroxide eradicates and prevents the recurrence of H. pylori by damaging bacterial cell membranes. Hydrogen peroxide thus provides an attractive candidate for anti-H. pylori treatment.
INTRODUCTION
Helicobacter pylori is a Gram-negative, spiral-shaped, and microaerophilic bacterium that causes gastritis, peptic ulcer, and gastric adenocarcinoma (1). H. pylori is wide spread and has afflicted nearly 50% of the world’s population, approximately 4.4 billion people (2, 3). Antibacterials are the most important and primary treatment for H. pylori-related diseases. Treatment options are mainly focused on the standard triple, bismuth/nonbismuth quadruple, concomitant, and sequential therapies (4–7). However, these antibacterials easily promote resistance in H. pylori (6), which results in a significant reduction of the eradication rate. Worldwide, the overall eradication rate of H. pylori fluctuates between 55% and 75% (8). The rates are 75% in South America (9), 80% in Asia (10, 11), 80% to 83% in Africa (9, 12), 57% to 80% in Europe (13–15), and 84% in North America (16). As H. pylori is much harder to kill after recurrence (17–20), more effective therapies are desired. To address this challenge, scholars have proposed some novel strategies, such as probiotic supplementation (21, 22) and vaccines (23, 24), which are under way. Inspired by the discovery that a microaerophilic environment is a prerequisite for the survival of H. pylori (25), we proposed that H. pylori could not survive in normal oxygen or oxygen-enriched environments. Here, we used hydrogen peroxide to create an environment with increased oxygen concentration in gastric juice to achieve the goals of killing and eradicating H. pylori.
RESULTS
The effect of hydrogen peroxide on oxygen concentration in solutions.
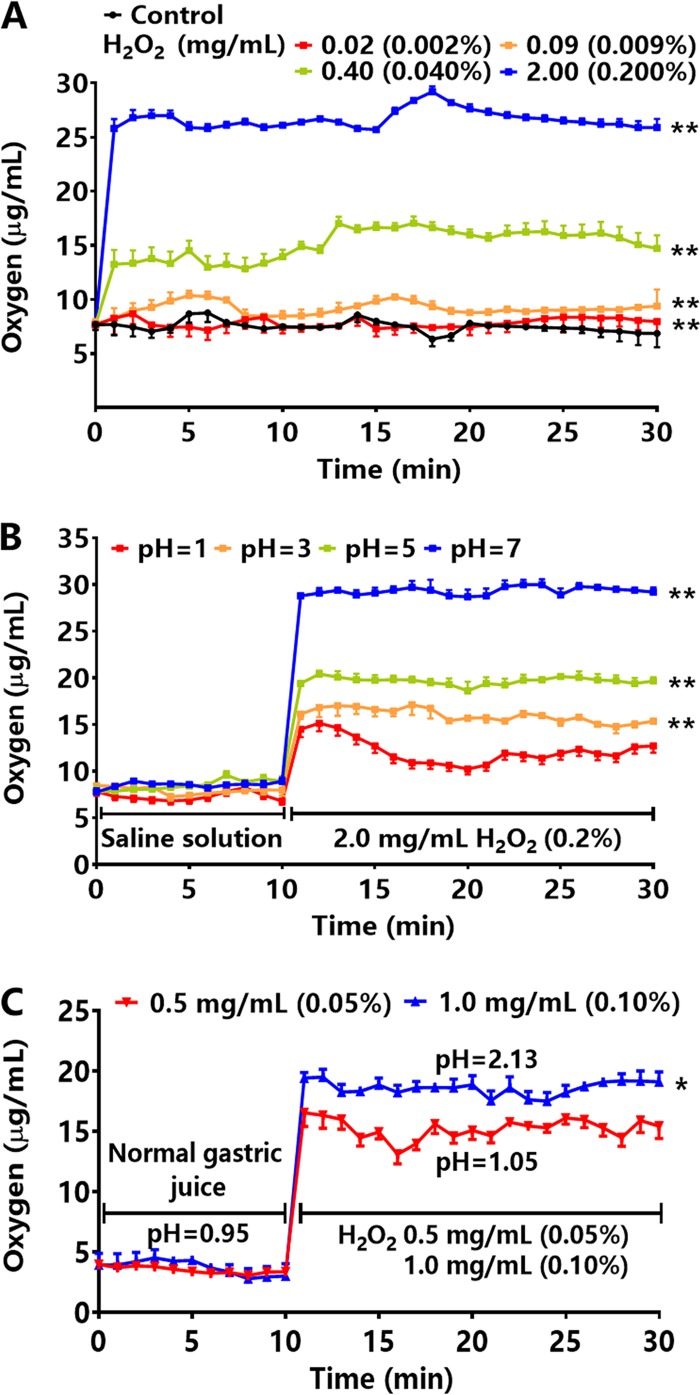
After a series of concentrations of hydrogen peroxide were prepared in saline, the oxygen concentrations were detected in the saline solutions and in rabbit gastric juice. The average oxygen concentration in the saline solutions was 6.7 μg/ml at 37°C, which was consistent with the results of research by El-Sherif and El-Feky (26). When hydrogen peroxide was diluted with different volumes of saline to yield solutions of 0.02, 0.09, 0.40, and 2.00 mg/ml (0.002%, 0.009%, 0.040%, and 0.200%), the average oxygen concentrations in the solutions at 37°C were 7.8, 9.1, 14.9, and 26.0 μg/ml, respectively, which were 1.2- to 3.9-fold higher than the control group (Fig. 1A). Altering pH affected the oxygen concentrations of solutions containing hydrogen peroxide. As the pH increased from 1 to 7, the oxygen concentrations increased to 12.9, 16.0, 19.7, and 29.3 μg/ml, suggesting that a less acidic environment allowed increased dissolved-oxygen concentrations in solutions by hydrogen peroxide (Fig. 1B). After a 24-h fast, the average oxygen concentration of rabbit gastric juice was 3.6 μg/ml. After hydrogen peroxide at 0.5 and 1.0 mg/ml (0.05% and 0.10%) was added to the rabbit stomach, the average oxygen concentrations in the gastric juice rose to 15.2 and 18.7 μg/ml, respectively, which were 4.2- and 5.2-fold higher than in normal gastric juice, respectively (Fig. 1C).
FIG 1.
The effect of hydrogen peroxide (H2O2) on oxygen concentration in solutions and gastric juice. (A) The oxygen concentrations in saline solution after adding a series of concentrations of hydrogen peroxide at 37°C (n = 3). (B) The oxygen concentrations in saline solution after adding hydrogen peroxide at 2.0 mg/ml (0.2%) in solutions of pH 1, 3, 5, and 7 at 37°C (n = 3). (C) The oxygen concentrations in rabbit gastric juice after adding hydrogen peroxide at 0.5 and 1.0 mg/ml (0.05% and 0.10%, n = 4). Values given are mean ± SE; *, P < 0.05; and **, P < 0.01 versus control (saline solution, normal gastric juice), as determined by one-way ANOVA and Student’s t test.
The effect of hydrogen peroxide on H. pylori.
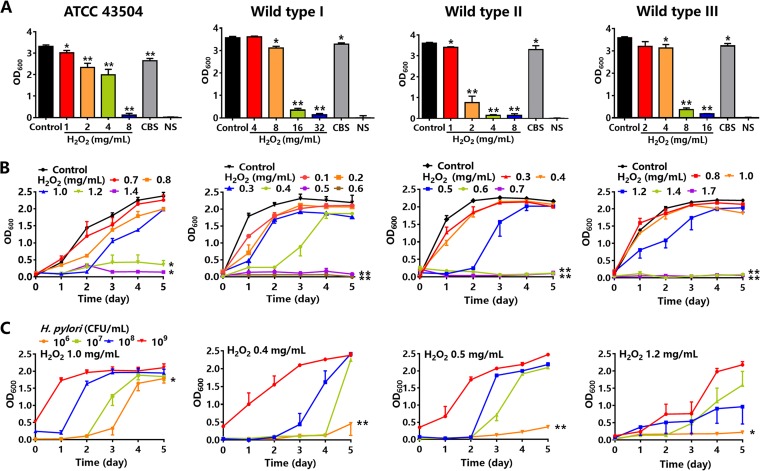
To confirm the effect of hydrogen peroxide on cultures on agars and in peptone broth, four H. pylori strains (ATCC 43504, wild type I, II, and III) were cultivated on Columbia blood agar, chocolate agar, H. pylori special agar, and H. pylori special peptone broth. All H. pylori strains were susceptible to hydrogen peroxide and were verified to be positive in assays of urease, catalase, and oxidase. The turbidity of H. pylori suspensions by optical density at 600 nm (OD600) was detected, which represented bacterial proliferation. The numbers of colonies and turbidity of colony suspensions were compared in Columbia blood agar, chocolate agar, and H. pylori special agar. The results showed that both the number of colonies and OD600 were highest in Columbia blood agar. H. pylori suspensions at 1 × 106, 107, 108, and 109 CFU/ml were also incubated into special peptone broth to detect bacterial growth. The optimal incubation concentration of H. pylori was 1 × 108 CFU/ml (Fig. S1-2 in the supplemental material). Colloidal bismuth subcitrate (CBS), an anti-H. pylori drug (27), was used as the positive control. Normal saline (NS) solution was the negative control. The results showed that the OD600 of an H. pylori ATCC 43504 colony suspension in Columbia blood agar was 3.3 in the control group (i.e., normal growth control). In the agars containing hydrogen peroxide at 1, 2, 4, and 8 mg/ml, OD600 decreased to 3.0, 2.3, 2.0, and 0.1, respectively, while the OD600 with CBS was 2.6. The downward trends of OD600 of H. pylori wild type I, II, and III in hydrogen peroxide and CBS were similar to ATCC 43504 (Fig. 2A). For H. pylori ATCC 43504 in special peptone broth, OD600 on the 5th day in the control group (normal growth control) was 2.4. In the broth containing hydrogen peroxide at 0.7, 0.8, 1.0, 1.2, and 1.4 mg/ml, OD600 decreased to 2.3, 2.0, 2.0, 0.4, and 0.1, respectively. The times for reaching the 50% maximal effect for hydrogen peroxide at 1.2 and 1.4 mg/ml were 1.7 and 0.9 days, respectively, which were shorter than the 1.9 days seen for the control. OD600 of other wild-type strains was similar to ATCC 43504. The MICs were 1.4, 0.5, 0.6, and 1.4 mg/ml for four H. pylori strains (ATCC 43504, wild type I, II, and III), respectively (Fig. 2B). The times for hydrogen peroxide at 1.0 mg/ml to reach the 50% maximal effect on ATCC 43504 at incubation concentrations of 1 × 106, 107, 108, and 109 CFU/ml were 3.5, 2.8, 1.6, and 0.4 days, respectively. Similar data were also found in three wild-type strains (Fig. 2C).
FIG 2.
The effect of hydrogen peroxide (H2O2) on H. pylori. (A) OD600 of H. pylori colony suspensions in Columbia blood agars containing a series of concentrations of hydrogen peroxide. Colloidal bismuth subcitrate (CBS) 7.3 mg/ml was the positive control, and normal saline (NS) solution was the negative control (n = 5). (B) OD600 of H. pylori suspensions in special peptone broth containing a series of concentrations of hydrogen peroxide (n = 7). (C) OD600 of H. pylori suspensions in special peptone broth containing hydrogen peroxide as follows: 1.0 mg/ml for ATCC 43504; 0.3 mg/ml for wild type I; 0.5 mg/ml for wild type II; and 1.2 mg/ml for wild type III, with H. pylori inoculum concentrations of 1 × 106, 107, 108, and 109 CFU/ml, respectively (n = 7). Values given are mean ± SE; *, P < 0.05; and **, P < 0.01 versus control, as determined by one-way ANOVA and Student’s t test.
Resistance to hydrogen peroxide by H. pylori.
Hydrogen peroxide at 0.8, 0.3, 0.4, and 1.0 mg/ml was used to promote resistance to four H. pylori strains (ATCC 43504, wild type I, II, and III), respectively. Clarithromycin, an important component of standard triple therapy (28), was used as positive control. A 24-h culture was recorded as a generation, and H. pylori strains were continuously cultured for 30 generations. The MICs of H. pylori to clarithromycin significantly increased on the 20th and 30th generations, and were approximately 4- and 10-fold higher than that on the 0th generation. The minimum bactericidal concentrations (MBCs) of H. pylori to clarithromycin increased from the 0th to the 10th, 20th, and 30th generations by 2.6-, 5.4-, and 17.0-fold, respectively. The resistance of H. pylori strains to hydrogen peroxide was mainly detected. The MICs revealed there was no increase on the 20th generation for all H. pylori strains. When comparing the 30th generation with the 0th generation, the MIC of wild type I did not change, while the MICs of ATCC 43504, wild type II, and III were no more than 3-fold than that on the 0th generation. Meanwhile, the MBCs of hydrogen peroxide in the four H. pylori strains did not show an increase on the 20th generation. On the 30th generation, MBCs of wild type I and III did not increase, and the MBCs of ATCC 43504 and wild type II were approximately 2-fold than that on the 0th generation (Table 1).
TABLE 1.
Resistance of H. pylori to hydrogen peroxidea
| Generation | MIC (mean ± SE [mg/ml]) |
MBC (mean ± SE [mg/ml]) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2O2 |
Clarithromycin |
H2O2 |
Clarithromycin |
|||||||
| ATCC 43504 | Wild type I | Wild type II | Wild type III | ATCC 43504 | ATCC 43504 | Wild type I | Wild type II | Wild type III | ATCC 43504 | |
| 0 | 1.750 ± 0.350 | 0.650 ± 0.150 | 1.100 ± 0.500 | 0.950 ± 0.450 | 0.003 ± 0.001 | 2.100 ± 0.700 | 1.550 ± 0.850 | 2.050 ± 1.150 | 2.300 ± 0.900 | 0.005 ± 0.002 |
| 10 | 2.100 ± 0.400 | 0.800 ± 0.000 | 1.550 ± 0.250 | 1.400 ± 0.000 | 0.006 ± 0.003 | 2.950 ± 0.450 | 2.250 ± 1.250 | 2.700 ± 1.100 | 2.600 ± 1.200 | 0.013 ± 0.005* |
| 20 | 2.300 ± 0.600 | 0.800 ± 0.000 | 2.250 ± 0.950 | 2.100 ± 0.700 | 0.013 ± 0.008* | 3.500 ± 0.600 | 3.200 ± 1.800 | 3.200 ± 1.400 | 3.000 ± 1.600 | 0.027 ± 0.009** |
| 30 | 3.600 ± 0.100** | 1.000 ± 0.200 | 3.150 ± 0.850* | 2.350 ± 0.650* | 0.032 ± 0.023* | 4.700 ± 1.300* | 3.700 ± 2.300 | 4.600 ± 0.900* | 3.750 ± 1.750 | 0.085 ± 0.040** |
MBC, minimum bactericidal concentration; H202; hydrogen peroxide; sample number n = 3; generation was defined as a 24-h culture; clarithromycin was a positive control (n = 4). Significance: *, P < 0.05; **, P < 0.01 versus the 0th generation as determined by one-way ANOVA and Student’s t test.
The effect of hydrogen peroxide on H. pylori-infected Mongolian gerbils.
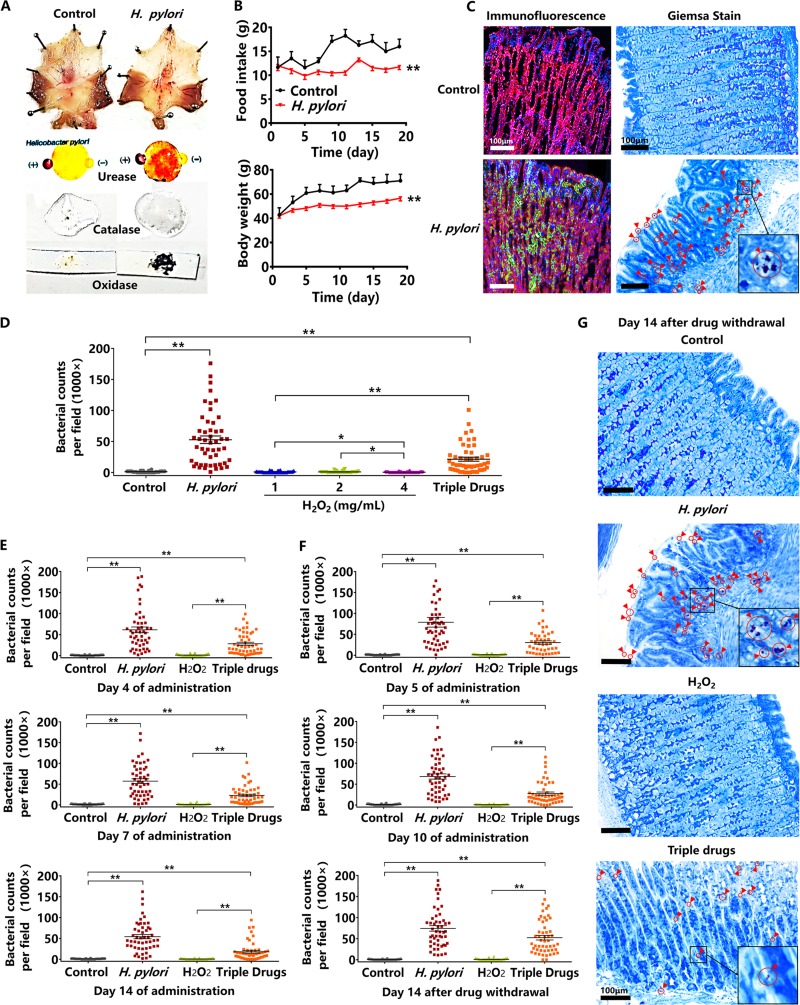
For establishing an H. pylori-infected model of Mongolian gerbils, the suspension (1 × 108 CFU/ml) of H. pylori ATCC 43504 was orally administered to Mongolian gerbils for 20 days. In order to verify the success of the gerbil model, the following test indicators were assessed. The morphology of gastric mucosa in the control group was normal and smooth. However, the gastric mucosa of H. pylori-infected gerbils showed obvious hyperemia and edema, and H. pylori from the infected mucosa was positive for urease, catalase, and oxidase assays, which showed an obvious red spot, rich foams, and a dark blue smear, respectively (Fig. 3A). During 20 days of modeling, the daily food intake and weight of Mongolian gerbils was recorded every 2 days. The daily food intake and body weight of H. pylori-infected gerbils both significantly decreased, compared with the control gerbils which were orally administered the same volume of saline solution (Fig. 3B). The stomach tissues of Mongolian gerbils were fixed in paraffin and in frozen sections. For localization by Giemsa staining, the areas colonized by H. pylori will stain violet, while gastric mucosal cells will stain blue (29). The results showed that H. pylori scattered on the surface of the gastric mucosa and in the gastric pits. For immunofluorescence, H. pylori was stained by polyclonal IgG/FITC-IgG, the cytoskeleton was stained by ActinRed, and the nucleus was stained by 4’, 6-diamidino-2-phenylindole (DAPI). H. pylori colonizing the gastric mucosa showed green fluorescence, the cytoskeleton appeared red, and the nucleus was blue. Similarly, H. pylori was observed distributed in the glandular cavity or scattered on the surface of the gastric mucosa (Fig. 3C). After successful establishment of the model, H. pylori counts of the infected mucosa after 14 days of oral administration by hydrogen peroxide at 1, 2, and 4 mg/ml were tested. The counts of H. pylori significantly decreased upon administration of hydrogen peroxide. There was no significant difference in the counts of H. pylori between the control and the hydrogen peroxide groups, indicating that H. pylori had been eradicated. However, the counts of H. pylori in the triple-drug group were more than those of the hydrogen peroxide group and less than that of the H. pylori-infected control group (Fig. 3D). On the 4th, 7th and 14th days of oral administration of hydrogen peroxide at 2 mg/ml, the counts of H. pylori rapidly dropped, and were significantly smaller than those of the triple-drug group (Fig. 3E). We further shortened the administration period from 14 to 10 days, and obtained a similar result, which showed the counts of H. pylori significantly decreased on the 5th and 10th day of oral administration of hydrogen peroxide at 2 mg/ml. After drug withdrawal (hydrogen peroxide and the triple drugs), Mongolian gerbils were observed for another 14 days to determine if there was a recurrence of H. pylori. The counts of H. pylori in the hydrogen peroxide group did not increase compared with the control group, which did not show H. pylori recurrence. However, compared with the control, the bacterial counts significantly increased in the triple-drug group, demonstrating the recurrence of H. pylori occurred due to the failing eradication during the initial drug administration (Fig. 3F and G).
FIG 3.
The effect of hydrogen peroxide (H2O2) on H. pylori-infected Mongolian gerbils. (A) The morphology of H. pylori-infected mucosa and assays for urease, catalase, and oxidase (H. pylori ATCC 43504). (B) The alterations of daily food intake and body weight of H. pylori-infected Mongolian gerbils (H. pylori ATCC 43504). (C) Giemsa staining and immunofluorescence of H. pylori-infected gastric mucosa. Giemsa staining shows H. pylori as purple and gastric mucosa as blue. Immunofluorescence shows H. pylori ATCC 43504 as green, cytoskeleton as red, and the nucleus as blue. (D) The bacterial counts of H. pylori-infected mucosa after 14 days of oral administration by hydrogen peroxide at 1, 2, and 4 mg/ml. The triple-drug group (colloidal bismuth subcitrate, clarithromycin, and tinidazole) was a positive control. (E) The bacterial counts of H. pylori-infected mucosa on the 4th, 7th, and 14th days of oral administrations of hydrogen peroxide at 2 mg/ml. The triple-drug group was a positive control. (F) The bacterial counts of H. pylori-infected mucosa on the 5th and 10th days of oral administrations of hydrogen peroxide at 2 mg/ml, and on the 14th day after hydrogen peroxide withdrawal. (G) Giemsa staining of gastric mucosa on the 14th day after hydrogen peroxide withdrawal. Red circles and arrows indicate H. pylori ATCC 43504, as magnified within the black frame (n = 10 animals; 5 dots per animal; 50 dots per group). The bar length measurement was 100 μm. Values given are mean ± SE; *, P < 0.05; and **, P < 0.01 versus control, as determined by one-way ANOVA and Student’s t test.
The effect of hydrogen peroxide on H. pylori-infected Kunming mice.
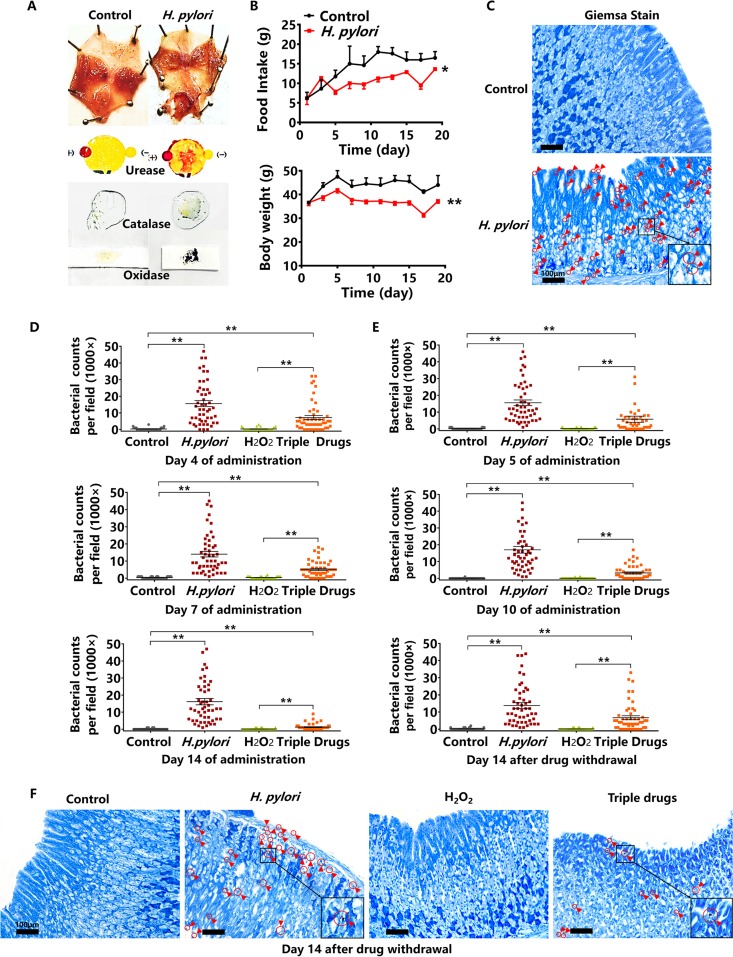
We further established the model of H. pylori-infected Kunming mice. Similar to what was seen in Mongolian gerbils, the gastric mucosa of infected mice presented hyperemia and edema, and was urease-, catalase-, and oxidase-positive (Fig. 4A). Both the daily food intake and body weight of H. pylori-infected mice significantly dropped compared with the control (Fig. 4B). Gastric mucosal cells and H. pylori-infected cells stained by Giemsa solution were blue and purple, respectively, but H. pylori counts in Kunming mice were less than those in Mongolian gerbils (Fig. 4C). After successfully establishing the H. pylori-infected model of Kunming mice, we detected the counts of H. pylori after treatment with hydrogen peroxide at 2 mg/ml. The counts of H. pylori significantly dropped in the hydrogen peroxide group on the 4th, 7th, and 14th days of administration. The counts of H. pylori in the triple-drug group were more than those of the hydrogen peroxide group and less than the H. pylori-infected group on the 4th, 7th, and 14th days of administrations (Fig. 4D). We shortened the administration period and observed that the counts of H. pylori in the hydrogen peroxide group significantly decreased on the 5th and 10th days of administration, which was similar to what was seen in Mongolian gerbils. After 10 days of oral administration, hydrogen peroxide and the triple drugs were both withdrawn. Similar to Mongolian gerbils, the mice were observed for H. pylori recurrence over another 14 days. The gastric tissues of Kunming mice were fixed in paraffin sections and stained by Giemsa solution. The counts of H. pylori in the gastric mucosa after hydrogen peroxide withdrawal did not rise, while there was a significant increase after the triple drug withdrawal. These results show the absence in recurrence of H. pylori in the hydrogen peroxide group, whereas H. pylori recurred in the triple-drug group (Fig. 4E and 4F).
FIG 4.
The effect of hydrogen peroxide (H2O2) on H. pylori-infected Kunming mice. (A) The morphology of H. pylori-infected mucosa and assays for urease, catalase, and oxidase (H. pylori ATCC 43504). (B) The alterations of daily food intake and body weight of H. pylori-infected Kunming mice (H. pylori ATCC 43504). (C) Giemsa staining of H. pylori-infected gastric mucosa. Giemsa staining shows H. pylori as purple and gastric mucosa as blue. (D) The bacterial counts of H. pylori-infected mucosa on the 4th, 7th, and 14th days of oral administrations of hydrogen peroxide at 2 mg/ml. The triple-drug group was the positive control. (E) The bacterial counts of H. pylori-infected mucosa on the 5th and 10th days of oral administrations of hydrogen peroxide at 2 mg/ml, and on the 14th day after hydrogen peroxide withdrawal. (F) Giemsa staining of gastric mucosa on the 14th day after hydrogen peroxide withdrawal. Red circles and arrows indicate H. pylori ATCC 43504, as magnified within the black frame (n = 10 animals; 5 dots per animal; 50 dots per group). Values given are mean ± SE; *, P < 0.05; and **, P < 0.01 versus control, as determined by one-way ANOVA and Student’s t test.
The effect of hydrogen peroxide on bacterial cell membranes and enzymes of H. pylori.
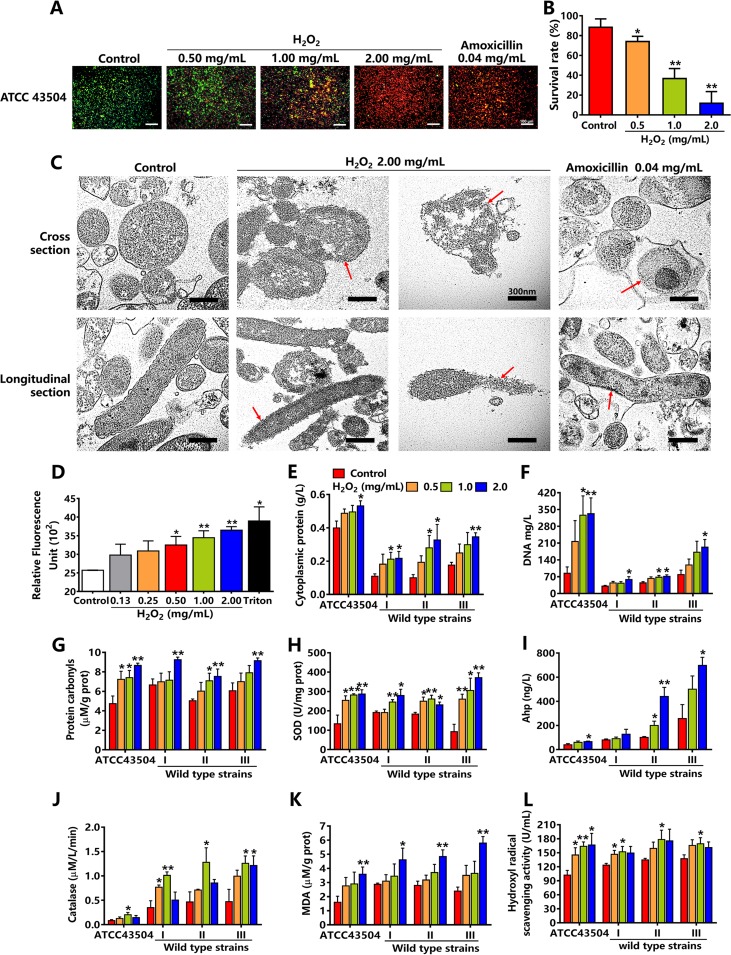
SYTO 9 and propidium iodide (PI) were used to stain the living and dead H. pylori, and emitted green and red fluorescence, respectively. The results showed that H. pylori in the control group (normal growth control) was living and showed green fluorescence. With the increasing concentrations of hydrogen peroxide, the red fluorescence gradually increased, and the green fluorescence decreased, demonstrating a trend of death in H. pylori. Red fluorescence appeared in both the group administered hydrogen peroxide at 2.0 mg/ml and the group administered amoxicillin, which was the positive control (Fig. 5A). Flow cytometry showed survival rates of H. pylori in hydrogen peroxide at 0.5 to 2.0 mg/ml decreased in a concentration-dependent manner (Fig. 5B). As H. pylori in hydrogen peroxide at 2.0 mg/ml were fully killed, the concentration of 2.0 mg/ml was used to determine morphological alterations of H. pylori cell membranes. Transmission electron microscopy showed that H. pylori cell membranes in the control were complete and clear, while the membranes in 2.0 mg/ml hydrogen peroxide were fragmentized, broken, and blurred. Amoxicillin damaged only bacterial cell walls, while bacterial cell membranes remained complete (Fig. 5C). Moreover, we used 1-N-phenyl-naphtylamine (NPN) (blue fluorescence) to detect the disruption of bacterial cell membranes. Triton X-100 was used as a positive control. With the increasing concentrations of hydrogen peroxide, blue fluorescence gradually increased, indicating that the cell membranes were damaged in a concentration-dependent manner (Fig. 5D). The disruption of bacterial cell membranes subsequently caused the leakage of cytoplasmic proteins and DNA. In four H. pylori strains, the leakage of cytoplasmic proteins and DNA in hydrogen peroxide at 2.0 mg/ml was 1.3, 2.0, 3.3, and 2.0 times higher (for cytoplasmic proteins) and 3.9, 1.9, 1.6, and 2.4 times higher (for DNA) than that of the control group (Fig. 5E and 5F). Additionally, cytoplasmic proteins were oxidized to be protein carbonyls by hydrogen peroxide. The results of the enzyme-linked immunosorbent assays (ELISAs) showed that carbonyl contents significantly increased in a concentration-dependent manner (Fig. 5G). Superoxide dismutase (SOD), alkyl hydroperoxide reductase (Ahp), and catalase are three key enzymes of H. pylori that are affected by oxidative stress (30–32). The SOD activities significantly increased in hydrogen peroxide at 0.5, 1.0, and 2.0 mg/ml (Fig. 5H). Similarly, Ahp activity in hydrogen peroxide at 2.0 mg/ml also rose significantly (Fig. 5I). Catalase activity initially increased and almost reached a peak in hydrogen peroxide at 1.0 mg/ml, while the activity decreased in hydrogen peroxide 2.0 mg/ml (Fig. 5J). Malondialdehyde (MDA) is a product of lipid peroxidation that reflects the extent of lipid oxidation of bacterial cell membranes. After incubation with hydrogen peroxide at 2.0 mg/ml, the level of MDA significantly increased in the bacterial membranes, proving that H. pylori cell membranes were severely damaged (Fig. 5K). As hydrogen peroxide produces large hydroxyl radicals that are strongly aggressive against bacterial cell membranes, H. pylori showed strong scavenging activities in hydrogen peroxide at 0.5 and 1.0 mg/ml but not at 2.0 mg/ml (Fig. 5L).
FIG 5.
The effect of hydrogen peroxide (H2O2) on bacterial cell membranes and enzymes of H. pylori. (A) SYTO 9/PI stain of H. pylori ATCC 43504 incubated with hydrogen peroxide at 0.5, 1, and 2 mg/ml. Amoxicillin was the positive control. Green fluorescence indicates living H. pylori ATCC 43504; red fluorescence indicates dead H. pylori ATCC 43504. (B) Survival rates of H. pylori ATCC 43504 incubated with hydrogen peroxide at 0.5, 1, and 2 mg/ml by flow cytometry (n = 6). (C) The morphology of H. pylori ATCC 43504 incubated with hydrogen peroxide at 2 mg/ml by transmission electron microscopy. Amoxicillin was the positive control, which damaged bacterial cell walls, not cell membranes (n = 3). Red arrows showed bacterial cell membranes. The bar length measurement was 300 nm. (D) The disruption of bacterial cell membranes in H. pylori ATCC 43504 incubated with hydrogen peroxide at 2 mg/ml by 1-N-phenyl-naphtylamine (blue fluorescence). Triton X-100 was the positive control (n = 4). (E and F) Leakage of cytoplasmic proteins and DNA in four H. pylori strains incubated with hydrogen peroxide at 0.5, 1, and 2 mg/ml (n = 6). (G to J) The protein carbonyls and activities of three enzymes: superoxide dismutase (SOD), alkyl hydroperoxide reductase (Ahp), and catalase of four H. pylori strains incubated with hydrogen peroxide at 0.5, 1, and 2 mg/ml (n = 6). (K and L) Malondialdehyde (MDA) and hydroxyl radical scavenging activities of four H. pylori strains incubated with hydrogen peroxide at 0.5, 1, and 2 mg/ml (n = 6). Values given are mean ± SE; *, P < 0.05; and **, P < 0.01 versus control, as determined by one-way ANOVA and Student’s t test.
The safety of hydrogen peroxide in animals.
Single-dose acute toxicity and gastric mucosal injury tests were used to determine the safety of hydrogen peroxide in Kunming mice and Mongolian gerbils. For single-dose acute toxicity tests in mice, hydrogen peroxide at 30 mg/ml with the maximum dose volume 40 ml/kg (1,200 mg/kg) was orally administered to the mice for once. After 14 days of observation, the mice in the hydrogen peroxide group all survived and their body weights did not decrease compared with the control mice. The gerbils were orally administered hydrogen peroxide at 30 mg/ml with the maximum dose volume of 30 ml/kg (900 mg/kg). After 14 days of observation, the gerbils all survived and their body weights did not alter. For a gastric mucosal injury test, after hydrogen peroxide at 2.5 mg/ml and 10.0 mg/ml with the dose volume of 20 ml/kg (50 mg/kg and 200 mg/kg) was orally administered to the mice for 7 days, the gastric mucosa was as complete as the controls, and there was no damage such as erosion or ulceration. However, the gastric mucosa of the aspirin group, the positive control, showed an obvious erosion (Fig. S3).
DISCUSSION
H. pylori survives in a microaerophilic environment of 5% to 6% oxygen (vol/vol %) in air (25), and in aqueous solutions in which the oxygen concentrations are approximately 1.7 to 2.0 μg/ml at 37°C. H. pylori colonizes the surface of the gastric mucosa and is surrounded by the gastric juice, in which the oxygen concentration is approximately 2.0 μg/ml. H. pylori cannot survive at 15% oxygen in the air (vol/vol %), i.e., at oxygen of 5.0 μg/ml in aqueous solution at 37°C (33, 34). In this study, hydrogen peroxide at 0.02 to 2.00 mg/ml (0.002% to 0.200%) increased oxygen in aqueous solutions (or rabbit gastric juice) to 7.8 to 26.0 (15.2 to 18.7) μg/ml, which was a 1.2- to 3.9-fold (4.2- to 5.2-) increase to oxygen levels in which H. pylori cannot survive. In Columbia blood agar and H. pylori special peptone, hydrogen peroxide significantly inhibited the growth of H. pylori. The inhibition potency of hydrogen peroxide was significantly stronger than that of CBS. Furthermore, CBS, like other antibacterial drugs, easily promoted resistant strains of H. pylori, which sharply increased the MIC and MBC of antibacterial drugs (35, 36). However, our results showed the MIC and MBC of hydrogen peroxide to be relatively stable after attempting to promote resistance for 30 generations, revealing that hydrogen peroxide does not easily promote resistance in H. pylori. In addition to these experiments in vitro, we also verified the effect of hydrogen peroxide on H. pylori in vivo. Mongolian gerbils are the most commonly used animal for establishing an H. pylori-infection model (37, 38). The symptoms of H. pylori-infected gerbils, such as the loss of appetite and weight, were consistent with human patients. The in vivo results showed there were no colonizing H. pylori in the stomach of Mongolian gerbils in the hydrogen peroxide group, suggesting that hydrogen peroxide killed H. pylori, which was consistent with the in vitro results, and was better than that of the triple drugs. The recurrence of H. pylori often causes reinfection in humans and animals. In our study, the definition of recurrence was incomplete clearance. After establishing H. pylori models of Mongolian gerbils and Kunming mice, hydrogen peroxide and the triple drugs were orally administered to animals for 14 days. At this time, we did not know whether H. pylori had been cleared completely or not. Thus, all animals were stopped with drug administration and observed for another 14 days to determine if there existed residual H. pylori growth. In the hydrogen peroxide group, we did not observe H. pylori growth after hydrogen peroxide withdrawal and considered that H. pylori was completely eradicated, which showed that recurrence did not occur. However, in the triple-drug group, residual H. pylori significantly grew after the triple-drug withdrawal, demonstrating H. pylori was not completely cleared and recurrence occurred. Furthermore, we studied the mechanism of hydrogen peroxide in eradicating H. pylori and found it involved damage to bacterial cell membranes. Regarding H. pylori morphology, transmission electron microscopy directly confirmed the disruption of bacterial cell membranes after hydrogen peroxide treatment. As a positive control, amoxicillin only damaged bacterial cell walls of H. pylori, while cell membranes remained intact. Additionally, NPN bound to a phospholipid of the H. pylori cell membrane and produced blue fluorescence, which could be used to determine the degree of damage to bacterial cell membranes (39). With increasing concentrations of hydrogen peroxide, the intensity of blue fluorescence also increased, indicating that bacterial cell membranes of H. pylori were ruptured. After the disruption, the contents of H. pylori leaked out. As expected, our results showed that DNA and cytoplasmic proteins such as SOD, Ahp, and catalase increased in the hydrogen peroxide group. Leaked proteins could be oxidized to protein carbonyls, which showed increased levels in the hydrogen peroxide group. These results further supported that bacterial cell membranes had been ruptured. In addition to this disruption, hydrogen peroxide also altered the activities of key enzymes. Among the enzymes, SOD is a major antioxidant that scavenges superoxide radicals and inhibits lipid peroxidation of bacterial cell membranes caused by hydrogen peroxide. Ahp restores peroxide substrates to water and alcohols, thereby protection H. pylori against oxidative stress. Hydrogen peroxide-induced increases in the activities of SOD and Ahp might also be related to protecting H. pylori. Due to this self-protecting of H. pylori, the activity of catalase increased at lower concentrations of hydrogen peroxide because catalase could decompose hydrogen peroxide into oxygen and water, whereas this activity decreased due to oxidative inactivation in the presence of higher hydrogen peroxide levels. Hydrogen peroxide oxidized lipids of the H. pylori membrane to generate MDA. The increase in MDA reflected the oxidation of the H. pylori cell membrane. Simultaneously, hydroxyl radical scavenging activity reflected the ability of H. pylori to resist hydrogen peroxide, which increased in low concentrations of hydrogen peroxide to improve survival but decreased at higher concentration due to the death of H. pylori cells. In the single-dose acute toxicity test, we performed a single oral administration to Kunming mice of hydrogen peroxide at 30 mg/ml and did not observe the death of the mice after 14 days. Hydrogen peroxide at 30 mg/ml is used as a disinfectant for skin and oral mucosa in clinical practice (40), which is 15-fold higher than 2 mg/ml used in our study, and our results with hematoxylin and eosin (H&E) staining also showed that hydrogen peroxide at 10 mg/ml did not damage the gastric mucosa of Kunming mice. Additionally, all mice and gerbils survived without weight loss in the single-dose acute toxicity test. These data suggest that hydrogen peroxide is safe and feasible for oral administration to eradicate H. pylori. In conclusion, hydrogen peroxide can increase the oxygen concentration in the gastric juice and eventually eradicate H. pylori colonizing the gastric mucosa by damaging bacterial cell membranes.
MATERIALS AND METHODS
Drugs and reagents.
Hydrogen peroxide at 300 mg/ml was purchased from Jinhuada Chemical Reagent (China). H. pylori ATCC 43504 was purchased from ATCC (American Type Culture Collection; certificate of analysis 43504, USA). Wild-type strains (named H. pylori wild-type I, II, and III) were isolated from gastric tissues of peptic ulcer combined with C13 urea-positive patients. Columbia blood agar, chocolate agar, H. pylori special agar, and H. pylori special liquid broth were purchased from Hope Biotechnology (China). Microaerophilic bags and a culture tank were purchased from Mitsubishi Gas Chemical (Japan). Colloidal bismuth subcitrate, clarithromycin, and the triple drugs were purchased from Livzon Pharmaceutical Group (China). Urease test paper was purchased from Zhuhai Kedi Technology (China). Oxidase test paper was purchased from Hope Biotechnology (China). ELISA kits and ActinRed were purchased from Nanjing Jiancheng Bioengineering Institute (China). Live/Dead BacLight bacterial viability kits and horse serum were purchased from Thermo Fisher Scientific (USA). Polyclonal IgG (rabbit anti-H. pylori ATCC 43504) and FITC-IgG (goat anti-rabbit) were purchased from Bio-Rad Laboratories (USA). DAPI was purchased from Solarbio Life Sciences (China). Optimal cutting temperature compound was purchased from Sakura FineTek (Japan).
Animals.
Rabbits (2.0 to 3.0 kg, male and female) and Kunming mice (20 to 25 g, male and female) were provided by the Animal Medical Center of Xi’an Jiaotong University. Mongolian gerbils (60 to 70 g, male and female) were provided by the Animal Center of Zhejiang Academy of Medical Sciences. Animal experiments were done by permission of the Ethics Committee of Xi’an Jiaotong University (No. 2016-1033).
Oxygen concentration detection.
Oxygen concentrations in hydrogen peroxide solutions and rabbit gastric juice were detected by a portable dissolved oxygen meter (WTW, Germany). The probe was automatically calibrated by exposure to the air. The oxygen meter was placed in the solutions to be tested for 30 min at 37°C. After a 24-h fast, the rabbits were anesthetized by ketamine and medetomidine. A small incision was cut along the duodenum near the pylorus. The probe was quickly inserted into the pylorus and the incision was tightened. After injecting hydrogen peroxide solutions into the stomach, the oxygen concentrations were recorded on the oxygen meter.
Cultivation of H. pylori.
The suspensions (1 × 108 CFU/ml) of H. pylori strains were inoculated on Columbia blood agars, chocolate agars, H. pylori special agars, and the H. pylori special peptone broth. The agars and the broth were put into the sealed culture tank (containing microaerophilic bags and a vibrator of 150 rpm/min for the broth) at 37°C for 3 to 7 days.
Determination of the MICs and the MBCs.
The serial dilution method was used to determine the MICs and the MBCs of H. pylori strains in the H. pylori special peptone broth. H. pylori suspensions (1 × 108 CFU/ml) of each strain were prepared, equally inoculated in the peptone broth with a series of hydrogen peroxide concentrations, and cultivated (microaerophilic atmosphere, 150 rpm/min) at 37°C for 24 h. The minimum concentration of hydrogen peroxide in the peptone broth that did not allow H. pylori growth was recorded as the MIC. The peptone broths without H. pylori growth which contained hydrogen peroxide were separately inoculated on the blank agars and cultivated (microaerophilic atmosphere) at 37°C for 72 h. When there were no colonies on the agar, the minimum concentration of hydrogen peroxide was recorded as the MBC.
Determination of resistance.
H. pylori strains were cultivated in peptone broth containing hydrogen peroxide concentrations that were slightly lower than the MICs. A 24-h culture was considered a generation. All H. pylori strains were continuously cultivated for 30 generations, and the MICs and MBCs of H. pylori strains were detected on the 10th, 20th, and 30th generations according to the serial dilution method.
Establishment of H. pylori-infected animal models and certification of colonized H. pylori.
H. pylori-infected animal models (Mongolian gerbils and Kunming mice) were established, and the numbers of H. pylori-infected animals were identified. After a fast for 24 h, Mongolian gerbils (60 to 70 g, male and female) and Kunming mice (20 to 25 g, male and female) were orally administered H. pylori suspensions (ATCC 43504) at a dose of 1.5 × 106 CFU/g and 2.0 × 106 CFU/g, respectively, once a day for 20 days. Giemsa staining and immunofluorescence were used to verify whether H. pylori infection had been successfully established. Then, Mongolian gerbils and Kunming mice were anesthetized by pentobarbital and ketamine, and the gastric tissues were removed. For the Giemsa staining, the gastric tissues of Mongolian gerbils and Kunming mice were fixed in paraffin sections and were stained by Giemsa solutions. For immunofluorescence, the gastric tissues were made into frozen sections and were stained by antibodies (polyclonal IgG rabbit anti-H. pylori ATCC 43504, and FITC-IgG goat anti-rabbit), ActinRed, and DAPI. Sections from the control group were also stained by the same Giemsa solutions or antibodies. The H. pylori numbers within each animal were counted via 5 random fields at 1000× magnification under the microscope (Olympus, Japan). The dots of the y axis showed H. pylori counts, and there were 50 dots per group (n = 10).
Determination of H. pylori viability.
Live/Dead BacLight bacterial viability kits (Thermo Fisher Scientific, USA) and flow cytometry (BD Biosciences, USA) were used to detect the viability of H. pylori ATCC 43504. H. pylori suspensions (1 × 108 CFU/ml) were incubated with a series of hydrogen peroxide for 20 min. After centrifuging and rinsing with saline solution, H. pylori suspensions were stained with fluorescent dyes (SYTO 9/PI), and were added to a clean glass slide with a coverslip. The fluorescence was observed under the microscope (Olympus, Japan). H. pylori suspensions stained by fluorescent dyes (SYTO 9/PI) were added to the flow cytometry. The proportion of live H. pylori was calculated based on the ratio of survival bacteria in the quadrants.
Determination of H. pylori cell membrane disruption.
H. pylori suspensions (ATCC 43504, 1 × 108 CFU/ml) were incubated with hydrogen peroxide at 2 mg/ml for 20 min. Then, H. pylori suspensions were centrifuged (15,000 rpm, 5 min) and rinsed 3 times with phosphate buffer, eventually becoming a precipitate with a volume of 1 mm3. After fixing with 2.5% glutaraldehyde for 2 h, the precipitate was washed 3 times with phosphate buffer. Again, after another fixation with 1% citric acid for 2 h, the precipitate was also washed by phosphate buffer. The was then dehydrated with 50%, 70%, 80%, and 90% ethanol for 15 min, and finally dehydrated 3 times with 100% ethanol. The precipitate was embedded with pure acetone and embedding solution. After slicing, the H. pylori samples were stained by 3% uranyl acetate and lead citrate. The morphology of the H. pylori was observed by the transmission electron microscope (Hitachi, Japan). The bar length measurement of H. pylori was 300 nm.
Determination of enzymes activities.
ELISA kits were used to determine the activities of enzymes in H. pylori, such as SOD, Ahp, and catalase. ELISAs were performed according to the manufacturer’s instructions.
The safety of hydrogen peroxide in animals.
Single-dose toxicity and gastric-mucosal injury tests were used to determine the safety of hydrogen peroxide. For single-dose toxicity, Kunming mice were orally administered with hydrogen peroxide (30 mg/ml, 40 ml/kg) at a dose of 1,200 mg/kg and were then observed for 14 days. Mongolian gerbils were orally administered with hydrogen peroxide at 30 mg/ml with the maximum dose volume of 30 ml/kg (900 mg/kg) and were also observed for 14 days. For the gastric-mucosal injury test, Kunming mice were orally administered with hydrogen peroxide (2.5 mg/ml and 10.0 mg/ml, 20 ml/kg) at a dose of 50 mg/kg and 200 mg/kg, once a day for 7 days. Then the mice were anesthetized by pentobarbital and diazepam. The gastric tissues were then removed and underwent fixation, grossing, tissue processing, embedding with paraffin, microtomy, and staining by hematoxylin and eosin (H&E) solution. The gastric tissues were observed under the microscope (Olympus, Japan).
Statistical analysis.
Figures were generated by GraphPad Prism 5.2. Statistical analyses were performed by Stata 12.0 and GraphPad Prism 5.2 of one-way ANOVA and the Student’s t test. Results are presented as the mean ± standard error (SE). As compared to control, P values were deemed significant at P < 0.05 (*) and P < 0.01 (**).
Supplementary Material
ACKNOWLEDGMENTS
We thank Pinghui Wang and Jing Tao for proofreading and editing the manuscript at different stages, as well as for valuable suggestions and comments.
The authors also thank the Zhejiang Academy of Medical Sciences for providing Mongolian gerbils, and the School of Energy and Power Engineering, Xi’an Jiaotong University, for providing the oxygen gas detector.
This work was supported by grants from the National Natural Science Foundation of China (grant number 81670001), the National Youth Foundation of China (grant numbers 81502840, 81903288, and 81900049), and the Natural Science Foundation of Shaanxi Province (grant number 2018JQ8051).
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kamboj AK, Cotter TG, Oxentenko AS. 2017. Helicobacter pylori: the past, present, and future in management. Mayo Clin Proc 92:599–604. doi: 10.1016/j.mayocp.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Zabala Torrres B, Lucero Y, Lagomarcino AJ, Orellana-Manzano A, George S, Torres JP, O'Ryan M. 2017. Review: prevalence and dynamics of Helicobacter pylori infection during childhood. Helicobacter 22:e12399. O’Ryan M. doi: 10.1111/hel.12399. [DOI] [PubMed] [Google Scholar]
- 4.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. 2016. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Chey WD, Leontiadis GI, Howden CW, Moss SF. 2017. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European Helicobacter and Microbiota Study Group and Consensus panel. 2017. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 7.Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, Czinn S, Gold BD, Guarner J, Elitsur Y, Homan M, Kalach N, Kori M, Madrazo A, Megraud F, Papadopoulou A, Rowland M, ESPGHAN, NASPGHAN. 2017. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr 64:991–1003. doi: 10.1097/MPG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 8.Lau CS, Ward A, Chamberlain RS. 2016. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis. Infect Drug Resist 9:275–289. doi: 10.2147/IDR.S117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu RP, Romero R, Sarosiek J, Dodoo C, Dwivedi AK, Zuckerman MJ. 2018. Eradication rate of Helicobacter pylori on the US-Mexico border using the urea breath test. South Med J 111:51–55. doi: 10.14423/SMJ.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 10.Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AHR, Goh KL, Das R, Lu H, Lin JT, Tu YK, Yamaoka Y, Wu MS, Asian Pacific Alliance on Helicobacter and Microbiota. 2017. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 11.Kim SE, Park MI, Park SJ, Moon W, Choi YJ, Cheon JH, Kwon HJ, Ku KH, Yoo CH, Kim JH, Lee GW, Song SE. 2015. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med 30:801–807. doi: 10.3904/kjim.2015.30.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benajah DA, Lahbabi M, Alaoui S, El Rhazi K, El Abkari M, Nejjari C, Amarti A, Bennani B, Mahmoud M, Ibrahimi SA. 2013. Prevalence of Helicobacter pylori and its recurrence after successful eradication in a developing nation (Morocco). Clin Res Hepatol Gastroenterol 37:519–526. doi: 10.1016/j.clinre.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Di Ciaula A, Scaccianoce G, Venerito M, Zullo A, Bonfrate L, Rokkas T, Portincasa P. 2017. Eradication rates in Italian subjects heterogeneously managed for Helicobacter pylori infection. Time to abandon empiric treatments in Southern Europe. J Gastrointestin Liver Dis 26:129–137. doi: 10.15403/jgld.2014.1121.262.itl. [DOI] [PubMed] [Google Scholar]
- 14.Boal Carvalho P, Magalhaes J, Dias de Castro F, Rosa B, Cotter J. 2017. Randomized controlled trial for Helicobacter pylori eradication in a naive Portuguese population: is sequential treatment superior to triple therapy in real world clinical setting? Acta Med Port 30:185–189. doi: 10.20344/amp.8072. [DOI] [PubMed] [Google Scholar]
- 15.Haider RB, Brennan DE, Omorogbe J, Holleran G, Hall B, O'Morain C, Breslin N, O'Connor HJ, Smith SM, McNamara D. 2015. A randomized-controlled study to compare the efficacy of sequential therapy with standard triple therapy for Helicobacter pylori eradication in an Irish population. Eur J Gastroenterol Hepatol 27:1265–1269. doi: 10.1097/MEG.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 16.Nayar DS. 2018. Current eradication rate of Helicobacter pylori with clarithromycin-based triple therapy in a gastroenterology practice in the New York metropolitan area. Infect Drug Resist 11:205–211. doi: 10.2147/IDR.S153617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Megraud F. 2016. Failed eradication for Helicobacter pylori. What should be done? Dig Dis 34:505–509. doi: 10.1159/000445230. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. 2017. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 46:773–779. doi: 10.1111/apt.14319. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Zhu Y, Lu NH. 2017. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front Cell Infect Microbiol 7:168. doi: 10.3389/fcimb.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez Cuén JA, Irineo Cabrales AB, León Sicairos NM, Calderón Zamora L, Monroy Higuera L, Canizalez Román VA. 2017. Recurrence of infection and diversity of Helicobacter pylori strains in an adult population in Mexico treated with empirical standard triple therapy. Rev Esp Enferm Dig 109:749–756. doi: 10.17235/reed.2017.4994/2017. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Yu S, Deng J, Yan Q, Yang C, Xia G, Zhou X. 2016. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: a meta-analysis of randomized controlled trials. PLoS One 11:e0163743. doi: 10.1371/journal.pone.0163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng JR, Wang F, Qiu X, McFarland LV, Chen PF, Zhou R, Liu J, Zhao Q, Li J. 2017. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol 73:1199–1208. doi: 10.1007/s00228-017-2291-6. [DOI] [PubMed] [Google Scholar]
- 23.Robinson K, Kaneko K, Andersen LP. 2017. Helicobacter: inflammation, immunology and vaccines. Helicobacter 22 Suppl 1 doi: 10.1111/hel.12406. [DOI] [PubMed] [Google Scholar]
- 24.Velin D, Straubinger K, Gerhard M. 2016. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter 21 Suppl 1:26–29. doi: 10.1111/hel.12336. [DOI] [PubMed] [Google Scholar]
- 25.Wallace N, Zani A, Abrams E, Sun Y. 2016. The impact of oxygen on bacterial enteric pathogens. Adv Appl Microbiol 95:179–204. doi: 10.1016/bs.aambs.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 26.El-Sherif MS, Mohamed A, El-Feky AMI. 2009. Performance of Nile tilapia (Oreochromis niloticus) fingerlings. II. Influence of different water temperatures. Int J Agric Biol 11:301–305. [Google Scholar]
- 27.Wang R, Lai T-P, Gao P, Zhang H, Ho P-L, Woo PC-Y, Ma G, Kao RY-T, Li H, Sun H. 2018. Bismuth antimicrobial drugs serve as broad-spectrum metallo-beta-lactamase inhibitors. Nat Commun 9:439. doi: 10.1038/s41467-018-02828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramas M, Donday MG, McNicholl AG, Gisbert JP. 2017. Efficacy and safety of rifaximin associated with standard triple therapy (omeprazole, clarithromycin and amoxicillin) for H. pylori eradication: a phase IV pilot clinical trial. Gastroenterol Hepatol 40:658–662. doi: 10.1016/j.gastrohep.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Arachchi P-O, Weerasekera MM, Seneviratne B, Weerasekera D, Fernando N, Gunasekara C-O. 2018. Imprint cytology: a useful screening test for diagnosis of Helicobacter pylori in resource poor settings. BMC Res Notes 11:481. doi: 10.1186/s13104-018-3592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter BM, Gancz H, Gonzalez-Nieves RP, West AL, Whitmire JM, Michel SL, Merrell DS. 2009. A single nucleotide change affects fur-dependent regulation of sodB in H. pylori. PLoS One 4:e5369. doi: 10.1371/journal.pone.0005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YY, Cheng T, Yang X, Jin L, Sun H, Li H. 2017. Functional disruption of peroxiredoxin by bismuth antiulcer drugs attenuates Helicobacter pylori survival. J Biol Inorg Chem 22:673–683. doi: 10.1007/s00775-017-1452-5. [DOI] [PubMed] [Google Scholar]
- 32.Harris AG, Wilson JE, Danon SJ, Dixon MF, Donegan K, Hazell SL. 2003. Catalase (KatA) and KatA-associated protein (KapA) are essential to persistent colonization in the Helicobacter pylori SS1 mouse model. Microbiology 149:665–672. doi: 10.1099/mic.0.26012-0. [DOI] [PubMed] [Google Scholar]
- 33.Bury-Moné S, Kaakoush NO, Asencio C, Mégraud F, Thibonnier M, De Reuse H, Mendz GL. 2006. Is Helicobacter pylori a true microaerophile? Helicobacter 11:296–303. doi: 10.1111/j.1523-5378.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 34.Thorell K, Yahara K, Berthenet E, Lawson DJ, Mikhail J, Kato I, Mendez A, Rizzato C, Bravo MM, Suzuki R, Yamaoka Y, Torres J, Sheppard SK, Falush D. 2017. Rapid evolution of distinct Helicobacter pylori subpopulations in the Americas. PLoS Genet 13:e1006546. doi: 10.1371/journal.pgen.1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi I, Saika T, Muraoka H, Murakami K, Fujioka T. 2006. Helicobacter pylori isolated from patients who later failed H. pylori eradication triple therapy readily develop resistance to clarithromycin. J Med Microbiol 55:737–740. doi: 10.1099/jmm.0.46316-0. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, O'Morain C, McNamara D. 2014. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol 20:9912–9921. doi: 10.3748/wjg.v20.i29.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Hong D, Wang S, Zhang F, Tang F, Wu T, Chu Y, Liu H, He M, Yang H, Yin R, Liu K. 2019. Therapeutic protection against H. pylori infection in Mongolian gerbils by oral immunization with a tetravalent epitope-based vaccine with polysaccharide adjuvant. Front Immunol 10:1185. doi: 10.3389/fimmu.2019.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckett AC, Loh JT, Chopra A, Leary S, Lin AS, McDonnell WJ, Dixon B, Noto JM, Israel DA, Peek RM Jr, Mallal S, Algood HMS, Cover TL. 2018. Helicobacter pylori genetic diversification in the Mongolian gerbil model. Peer J 6:e4803. doi: 10.7717/peerj.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckholtz GA, Reger NA, Anderton WD, Schimoler PJ, Roudebush SL, Meng WS, Miller MC, Gawalt ES. 2016. Reducing Escherichia coli growth on a composite biomaterial by a surface immobilized antimicrobial peptide. Mater Sci Eng C Mater Biol Appl 65:126–134. doi: 10.1016/j.msec.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Ikai H, Odashima Y, Kanno T, Nakamura K, Shirato M, Sasaki K, Niwano Y. 2013. In vitro evaluation of the risk of inducing bacterial resistance to disinfection treatment with photolysis of hydrogen peroxide. PLoS One 8:e81316. doi: 10.1371/journal.pone.0081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.