We characterized 29 blaCTX-M-27-harboring plasmids of Escherichia coli sequence type 131 (ST131) sublineage C1/H30R isolates from healthy individuals and long-term-care facility (LTCF) residents. Most (27/29) plasmids were of the FIA, FIB, and FII multireplicon type with the same plasmid multilocus sequence typing (pMLST). Several plasmids (7/23) from LTCF residents harbored only blaCTX-M-27 as the resistance gene; however, their fundamental structures were very similar to those of previously isolated blaCTX-M-27/F1:A2:B20 plasmids, suggesting their prevalence as a newly arising public health concern.
KEYWORDS: blaCTX-M-27, F1:A2:B20 plasmid, elderly people, next-generation sequencing analysis
ABSTRACT
We characterized 29 blaCTX-M-27-harboring plasmids of Escherichia coli sequence type 131 (ST131) sublineage C1/H30R isolates from healthy individuals and long-term-care facility (LTCF) residents. Most (27/29) plasmids were of the FIA, FIB, and FII multireplicon type with the same plasmid multilocus sequence typing (pMLST). Several plasmids (7/23) from LTCF residents harbored only blaCTX-M-27 as the resistance gene; however, their fundamental structures were very similar to those of previously isolated blaCTX-M-27/F1:A2:B20 plasmids, suggesting their prevalence as a newly arising public health concern.
TEXT
Escherichia coli isolates harboring CTX-M-type extended-spectrum β-lactamase (ESBL) genes have become a global health concern in clinical and community settings (1). Particularly, E. coli sequence type 131 (ST131) is among the most important globally disseminated bacterial lineages because ST131 strains cause severe hospital-acquired and community-onset multidrug-resistant infections (2). Moreover, the ST131 lineage has been identified in healthy individuals, livestock, companion animals, food, and the environment, and some studies have suggested that food, wastewater, and migrating birds constitute potential transmission routes for ST131 distribution and circulation (3–5).
Within ST131, different sublineages have been identified and organized according to their phylogenetic clade (A to C), fimH allele (type 1 fimbrial adhesion-encoding gene), and antimicrobial resistance profile (6). Clade A is associated with fimH41, clade B with fimH22, and clade C with fimH30; H30 is the most prevalent variant of the fimH allele implicated in recent worldwide ST131 expansion. Recent studies using whole-genome sequencing (WGS) analysis revealed two main subsets, H30R1 and H30Rx (also known as clades C1 and C2, respectively), which commonly show resistance to fluoroquinolones (7, 8). The globally distributed H30Rx subset is closely associated with blaCTX-M-15, and some H30R1 isolates harbor blaCTX-M-27 and blaCTX-M-14.
Globally, the ST131 sublineage C2/H30Rx harboring blaCTX-M-15 is the pandemic clone (9); however, this is not the case in Japan. Since 2010, the prevalence of ST131 C1/H30R with blaCTX-M-27 has increased in Japanese clinical settings (10). In addition, a high fecal carriage rate of this sublineage (23/59 [39%]) has recently been demonstrated in long-term-care facility (LTCF) residents (11), and the sublineage has been also identified in healthy individuals in some European countries (12–14). Although the ST131 sublineage C1/H30R harboring blaCTX-M-27 has rapidly disseminated worldwide (15, 16), its plasmid distribution is unclear. This study was conducted to characterize plasmids harboring blaCTX-M-27 obtained from healthy individuals and LTCF residents living in nonacute-care settings using next-generation sequencing (NGS) and to determine the complete nucleotide sequence of a representative plasmid.
In this study, we used only one isolate from a single resident and characterized 29 plasmids from nonrepetitive CTX-M-27-producing E. coli ST131 C1/H30R isolates (six isolates from healthy individuals [average age, 43.8 years] and 23 isolates from LTCF residents [average age, 83.6 years]). Of the 29 isolates, 25 and 29 isolates were identified as the extraintestinal pathogenic E. coli (ExPEC) pathotype and uropathogenic E. coli (UPEC) pathotype, respectively, by multiplex PCR, as previously described (see Fig. S1 in the supplemental material) (17, 18). The genetic diversity among the 29 isolates was analyzed by pulsed-field gel electrophoresis (PFGE) using the Chef-DR II system (Bio-Rad, Hercules, CA, USA) after digestion with XbaI (19). Strain H9812 of Salmonella enterica serotype Braenderup was used as the control. A dendrogram showing genetic relatedness among the isolates was constructed using the Fingerprinting II software (Bio-Rad), and isolates from each sample were considered to have the same background if their pulsotypes showed ≥85% similarity. The 29 ST131 C1/H30R isolates harboring blaCTX-M-27 were assigned to six distinct groups based on their pulsotype. Group I contained four isolates from healthy people and three isolates from LTCF residents (B, H, and I). In contrast, group V contained six isolates from six residents of facility A, suggesting that the same clone had disseminated among residents of facility A (Fig. S1). Next, a conjugation experiment was performed using the broth mating method with the azide-resistant E. coli J53 strain as a recipient. Transconjugants were selected on LB agar (BD Biosciences, Franklin Lakes, NJ, USA) containing 1 mg/liter cefotaxime (CTX; Wako Pure Chemical Industries, Osaka, Japan) and 150 mg/liter sodium azide (Kanto Chemical Co., Inc., Tokyo, Japan). As a result, no transconjugants carrying blaCTX-M-27 were obtained from among the 29 isolates, and the ability of conjugal transmission of the plasmids mediating CTX resistance was not confirmed. Therefore, transformation by electroporation was performed to analyze the genetic backbones of the 29 plasmids. Plasmid DNA was extracted from the 29 CTX-M-27-producing E. coli isolates using the PureYield plasmid midiprep system (Promega, Madison, WI, USA), and purified plasmids were introduced into E. coli DH10B by electroporation. Transformants were screened on LB agar plates containing 1 mg/liter CTX, and the presence of blaCTX-M was checked by PCR. Additionally, the MICs of CTX, ceftazidime, erythromycin, tetracycline, and sulfamethoxazole-trimethoprim for the resultant transformants were determined (Table S1). To perform WGS, plasmid DNA was extracted from each transformant using the PureYield plasmid midiprep system and subjected to PFGE. A piece of agarose gel containing the plasmid DNA was cut out, and the plasmid DNA was reextracted and purified by using both the Wizard SV gel and PCR clean-up system kits (Promega). The quantity of DNA was estimated using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Nucleotide sequencing sample libraries were prepared using a Nextera XT DNA sample preparation kit (Illumina). The genetic backbones of the 29 plasmids were examined by NGS analysis using the MiSeq desktop sequencer (Illumina, San Diego, CA, USA) and MiSeq reagent Nano kit v2 (500 cycles; Illumina). Sequence data were assembled using the A5-miseq pipeline (20). Characterization of antimicrobial resistance genes, plasmid replicon type, and plasmid multilocus sequence typing (pMLST) was performed using the Center for Genomic Epidemiology server for ResFinder 3.0 (https://cge.cbs.dtu.dk/services/ResFinder/) (21), PlasmidFinder 1.3 (https://cge.cbs.dtu.dk/services/PlasmidFinder/) (22), and pMLST 1.4 (https://cge.cbs.dtu.dk/services/pMLST/) (22), respectively. Additionally, the plasmid size was estimated by S1-nuclease treatment PFGE (23). NGS analysis revealed that 6/6 (100%) plasmids from healthy individuals and 20/23 (87.0%) plasmids from LTCF residents were multireplicon, including FII, FIA, and FIB, and that their pMLSTs were all F1:A2:B20 (Tables 1 and 2). Of the 26 plasmids with F1:A2:B20 replicons, the sizes of 14 plasmids ranged from 120 to 130 kbp, which are similar to the sizes of the complete sequences of blaCTX-M-27-carrying plasmids pH105 (134,499 bp) (24) and pEC-81009 (135,720 bp) (25) from E. coli ST131 sublineage C1/H30R. Several previous studies showed that F1:A2:B20 replicons are associated with blaCTX-M-27 and the H30R1 clone (also known as H30R) (26–28). We also confirmed a close association between blaCTX-M-27 and F1:A2:B20 replicons in CTX-M-27-producing E. coli ST131 H30R isolates recovered from both healthy individuals and LTCF residents in Japan.
TABLE 1.
Characteristics of 23 plasmids harboring blaCTX-M-27 from ST131 sublineage C1/H30R E. coli isolates from elderly people living in LTCFsa
| Sample ID | Plasmid replicon type(s) | Plasmid MLST | Size (kbp) by: |
Resistance gene(s) | Facility ID | |
|---|---|---|---|---|---|---|
| MinION | S1-PFGE | |||||
| C0044 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 83.426 | 76.16 | blaCTX-M-27 | C |
| C0122 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 83.408 | 77.20 | blaCTX-M-27 | C |
| G0138 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 95.228 | 84.23 | aadA5, blaCTX-M-27, sul1, dfrA17 | G |
| F0090 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 97.569 | 89.33 | aadA5, blaCTX-M-27, sul1, dfrA17 | F |
| H0005 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 103.959 | 93.78 | strA, strB, blaCTX-M-27, mph(A), sul1, sul2, tet(A) | H |
| B0018 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 105.446 | 98.62 | blaCTX-M-27 | B |
| C0079 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 108.489 | 104.82 | blaCTX-M-27 | C |
| H0106 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 118.769 | 111.84 | blaCTX-M-27 | H |
| A0140 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 120.219 | 114.90 | blaCTX-M-27 | A |
| A0022 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | — | 120.52 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0146 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | — | 120.6 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0024 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | — | 120.83 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0060 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | — | 121.05 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0150 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 126.885 | 121.48 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0084 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 126.860 | 121.52 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0145 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 126.885 | 122.34 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| A0095 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | — | 122.70 | strA, strB, blaCTX-M-27, sul2, tet(A) | A |
| H0130 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 135.733 | 127.42 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 | H |
| A0086 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 135.670 | 127.65 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 | A |
| I0128 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 130.479 | 127.74 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 | I |
| H0063 | IncFII, IncFIB | F1:A-:B20 | — | 109.83 | blaCTX-M-27 | H |
| I0082 | IncFIA, IncFIB | F-:A2:B20 | — | 82.96 | strA, strB, blaCTX-M-27, mph(A), sul2, tet(A) | I |
| C0031 | IncFIA | F-:A2:B- | — | 40.47 | strB, blaCTX-M-27, tet(A) | C |
ID, identifier.
TABLE 2.
Characteristics of 6 plasmids harboring blaCTX-M-27 from ST131 sublineage C1/H30R E. coli isolates from healthy people
| Sample ID | Plasmid replicon types | Plasmid MLST | Size (kbp) by: |
Resistance genes | |
|---|---|---|---|---|---|
| MinION | S1-PFGE | ||||
| HP003 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 124.596 | 120.53 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
| HP030 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 134.908 | 130.26 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
| HP050 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 117.603 | 112.63 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
| HP129 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 135.628 | 129.93 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
| HP223 | Col156, IncFII, IncFIA, IncFIB | F1:A2:B20 | 102.959 | 97.04 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
| HP243 | IncFII, IncFIA, IncFIB | F1:A2:B20 | 98.916 | 90.76 | strA, strB, aadA5, blaCTX-M-27, mph(A), sul1, sul2, tet(A), dfrA17 |
All six plasmids (HP003, HP030, HP050, HP129, HP223, and HP243 in Table 2) from healthy individuals carried the same sets of resistance genes. In silico analysis of the six plasmids using ResFinder revealed the presence of several antimicrobial resistance genes, such as aminoglycoside resistance genes (strA, strB, and aadA5), sulfonamide resistance genes (sul1 and sul2), a trimethoprim resistance gene (dfrA17), a macrolide resistance gene [mph(A)], and a tetracycline resistance gene [tet(A)], on plasmids harboring blaCTX-M-27 (Table 2). This is consistent with recent genome sequencing data demonstrating that plasmids mediating blaCTX-M carry numerous resistance genes, such as aadA, mph(A), tet(A), and dfrA (29–31). Similar results were observed in plasmids derived from various food sources (vegetables) and environments (wastewater) (29–31), suggesting that the isolates harboring this type of plasmid successfully spread via different pathways into various sources, as the type of plasmid is advantageous under the conditions of selection pressure exerted by various antimicrobials.
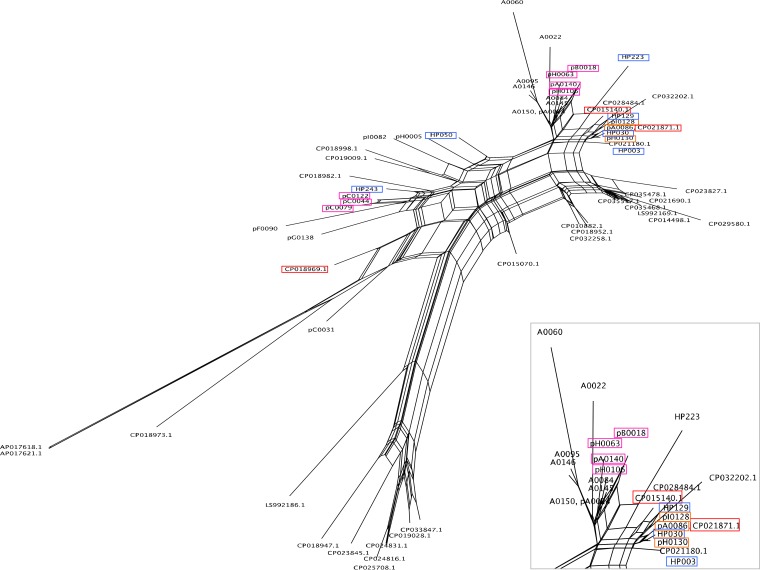
The plasmids from LTCF residents were more diverse than those from healthy people. In general, LTCF residents tend to be treated empirically with various antimicrobials; the residents (6/23 [26.1%]) in this study also had a history of antimicrobial administration within 3 months before testing (Fig. S1). Therefore, the plasmids from LTCF residents may mediate multiple resistance genes, enabling their survival under the pressure from antimicrobial agents. However, seven plasmids (7/23 [30.4%]) harbored blaCTX-M-27 as the only resistance gene. These seven plasmids were obtained from bacterial strains recovered from four different facilities (Fig. S2), and the plasmid sizes and genetic structures of the region surrounding blaCTX-M-27 were different (Tables 1 and S2). We performed open reading frame-based binarized structure network analysis of plasmids (OSNAp) to compare the molecular sequence similarities of the plasmids, and the OSNAp network was drawn using SplitsTree4 (https://uni-tuebingen.de/fakultaeten/mathematisch-naturwissenschaftliche-fakultaet/fachbereiche/informatik/lehrstuehle/algorithms-in-bioinformatics/software/splitstree/) (32) (Table S3). OSNAp is a holistic phylogenetic network analysis method that utilizes ORF-based binary sequence data. ORFs with ≥80% nucleotide sequence identity and coverage were determined to be identical. As shown in Fig. 1, three plasmids, pC0044, pC0079, and pC0122, were quite similar, with dice indices of 0.926 to 0.951, suggesting that this plasmid spread within the facility. The phylogenetic relationships between pB0018, pH0063, pH0106, and pA0140 were relatively similar to the dice indices of 0.872 to 0.904, although they were isolated from different facilities, suggesting that the sources and proliferation pathways of plasmids carrying blaCTX-M were different; however, this type of plasmid may have disseminated among Japanese LTCFs.
FIG 1.
Comparison of structural similarities between 29 blaCTX-M-27/F1:A2:B20 plasmids used in this study and reference plasmids for which a complete sequence is already available from the NCBI databases was performed. As the reference plasmids, F1:A2:B20 plasmids and the plasmids belonging to a 1-locus different MLST from F1:A2:B20 were used (Table S3). Light-blue rectangles indicate plasmids from healthy individuals used in this study. Orange rectangles indicate plasmids harboring nine resistance genes the same as those in plasmids from healthy individuals. Pink rectangles indicate plasmids harboring only blaCTX-M-27 as a resistance gene. Red rectangles indicate reference plasmids (pH105, pEC732_2, and pEC542_1) or their accession numbers.
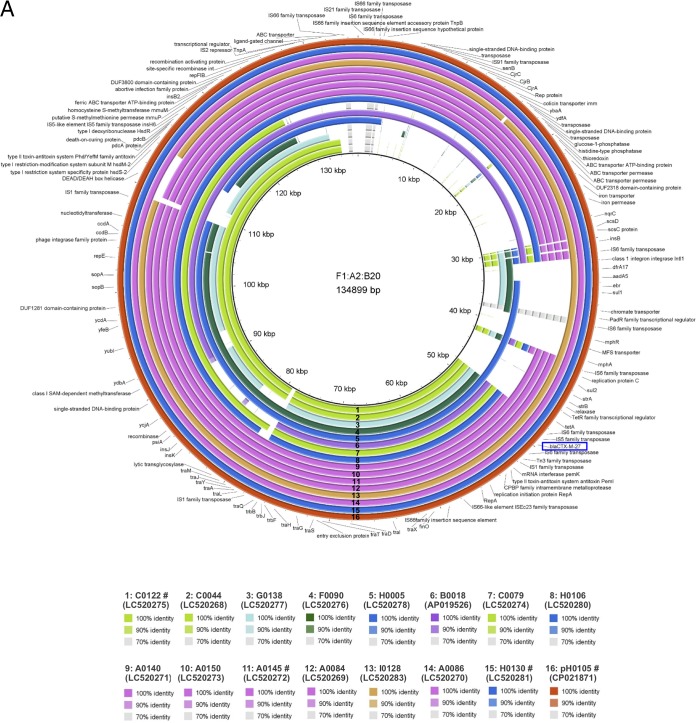
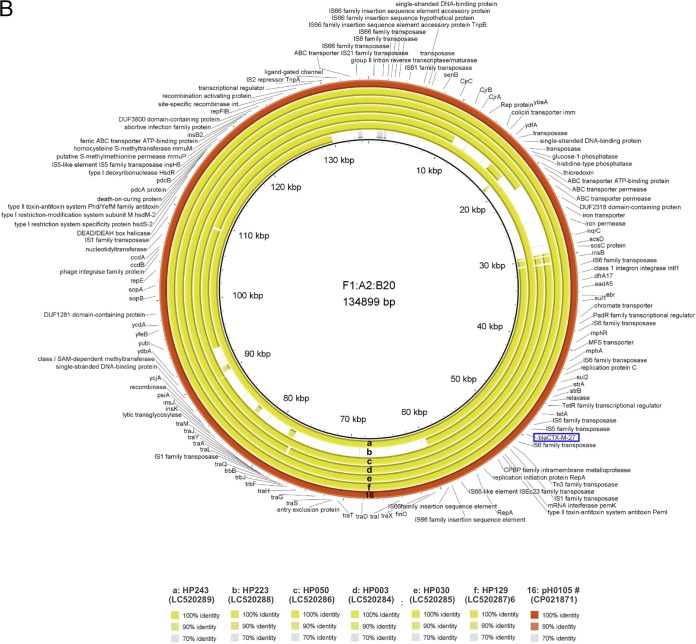
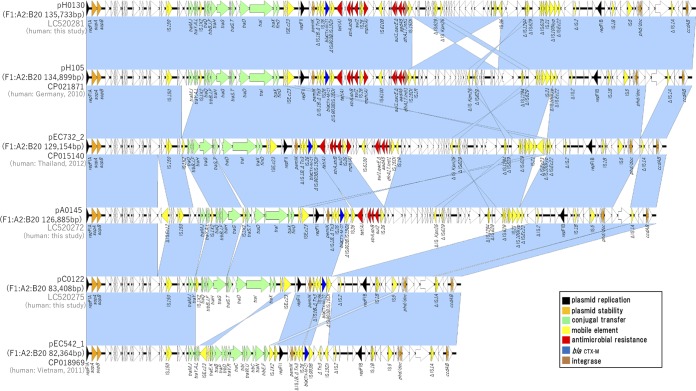
To further evaluate the genetic backbone of blaCTX-M-27-harboring plasmids, the complete nucleotide sequences of representative blaCTX-M-27/F1:A2:B20 plasmids were determined by Nanopore long-read sequencing. Six isolates (A0022, A0024, A0060, A0095, A0145, and A0146) belonging to group V and 2 isolates (A0140 and A0150) belonging to group VI were isolated from same facility and showed very similar PFGE profiles (Fig. S1). The OSNAp network analysis (32) indicated that the phylogenetic relationships between A0024, A0060, A0095, A0146, and A0150 were similar. Additionally, network analysis indicated a phylogenetic relationship between A0022 and A0145. Therefore, we selected pA0145 and A0150 as representative plasmids based on these results. Finally, 21 representative blaCTX-M-27/F1:A2:B20 plasmids (15 from LTCF residents and 6 from healthy people) were evaluated to determine their complete nucleotide sequences. Genomic DNA was extracted using the Qiagen Gentra Puregene Yeast/Bact kit and Qiagen Genomic-tip 100/G (Qiagen, Hilden, Germany). The MinION sequencing libraries were prepared with 1D native barcoding genomic DNA (with EXP-NBD104, EXP-NBD114, and SQK-LSK109; Oxford Nanopore Technologies, Oxford, UK) for 24 samples, according to the manufacturer’s instructions. Briefly, genomic DNA was repaired using NEBNext formalin-fixed paraffin-embedded (FFPE) DNA repair mix (catalog no. M6630; New England BioLabs, Ipswich, MA, USA) and Ultra II end prep enzyme mix, followed by barcode ligation and adaptor ligation. After priming, the library was loaded into the R9.4.1 flow cell (FLO-MIN106D), and a sequencing script was executed on MinKNOW version 19.06.7. Raw reads were base called into fastq files using the Guppy basecalling software 3.0.3. Demultiplexing/debarcoding was performed using guppy_barcoder 3.0.3. (Oxford Nanopore Technologies). Fastq sequences of 4,000 bp or more in length were filtered using an in-house python script. The blaCTX-M-27/F1:A2:B20 plasmids were compared based on pH105 (GenBank accession number CP021871, from Germany). As shown in Fig. 2A, 15 plasmids from 15 LTCF residents (facilities A to C and F to I) contained the stability and maintenance genes sopAB, but some plasmids lacked several genes and were divided into three groups, as follows: group 1 plasmids harbored only blaCTX-M-27 as a resistance gene and lacked the large sequence regions of an approximately 30-kbp region, including the colicin Colla immunity-encoding gene (cjrABC), enterotoxin-encoding senB gene, and several genes encoding the ABC transporter permease and iron permease. Of these, the cjrABC and senB genes are known to be involved in the virulence of UPEC (33). Group 2 plasmids harbored several resistance genes, such as tet(A), strAB, and sul2, together with blaCTX-M-27 based on group 1. Group 3 plasmids were similar to pH105 and harbored additional resistance genes, such as mph(A), aasA5, and dfrA. In contrast, the plasmids from healthy people lacked several genes encoding the ABC transporter permease and tra regions (Fig. 2B) but never lacked regions including resistance genes (Fig. 2B and S3). Thus, although these plasmids lacked some sequences, their fundamental structures were similar to those of blaCTX-M-27/F1:A2:B20 plasmids isolated outside Japan. Moreover, the sequences of three plasmids representing each group (pC0122, pH0130, and pA0145) were submitted to DFAST (https://dfast.nig.ac.jp/) for annotation, and a comparison with the complete sequences of pH105 (GenBank accession number CP021871, from Germany), pEC732_2 (GenBank accession number CP015140, from Thailand), and pEC542_1 (GenBank accession number CP018969, from Vietnam) was performed using Easyfig 2.2.2 and BLASTn (conditions of minimum length, 1,000 bp; maximum E value, 0.001; minimum identity value, 90%) (Fig. 3). pH0130, which is the largest among 21 F1:A2:B20 plasmids, is 135,733 bp, and it has an average G+C content of 51.9% and harbors 152 protein-coding genes. The nucleotide sequence of pH0130 showed 100% query coverage and >98% nucleotide identity compared to pH105 and pEC732_2, although reversal of the insertion sequence (IS) region was observed, and the similarity to pEC542_1 was high (>98% identity, 80% query coverage; Fig. 3). pB0145 is 126,885 bp, with an average G+C content of 51.3%. Although pB0145 lacked 15 open reading frames including several resistance genes, such as aadA5, dfrA, and sul1, the nucleotide sequence of pB0145 was very similar to those of pH105 and pEC732_2 (91% query coverage, >98% nucleotide identity). Furthermore, pB0122 is composed of 83,408 bp, with an average G+C content of 50.8%. pB0122 completely lacked resistance genes other than blaCTX-M-27, and the nucleotide sequence showed 79% query coverage and >98% nucleotide identity compared to pEC542_1. Although the tra regions were quite different from those of pEC542_1, pB0122 was more similar to pEC542_1 rather than to pH105 or pEC732_2 (62 to 69% query coverage, >98.0% nucleotide identity). Antimicrobial resistance, either through mutations or plasmid-mediated transfer, can impair the biological fitness of bacteria by negatively affecting their growth rate, survival ability, and virulence capacity (34). Thus, it may be advantageous for microorganisms to have few resistance determinants on the plasmids. Our plasmids harbored only blaCTX-M-27, which may well adapt to LTCF residents, who are sometimes treated with broad-spectrum antimicrobial agents, such as third-generation cephalosporins and fluoroquinolones. The F1:A2:B20 plasmids contained large numbers of ISs, which play major roles in plasmid evolution through replicative transposition and cointegrate formation (26, 35). The plasmids are commonly subjected to microevolutionary changes via homologous recombination, leading to gene loss and rearrangements of larger DNA segments under various environments and more diverse characteristics. blaCTX-M-27/F1:A2:B20 plasmids have also been reported from some countries, such as the Czech Republic and France (36, 37). Our results demonstrate that some plasmids have fundamental structures similar to these plasmids isolated outside Japan but have diverse characteristics and are already disseminated among elderly Japanese living in nonacute-care settings. In this study, we did not observe conjugate transmission of the plasmids mediating CTX resistance. Johnson et al. reported that the transfer frequency of ST131-source F1:A2:B20 plasmids in vitro was comparatively low (26). However, some ST131 strains can retain F plasmids over time by undergoing compensatory mutations to reduce this fitness cost, allowing resistant isolates to adapt and spread, even in the absence of antimicrobial selection pressure (34). Therefore, the ST131 isolates harboring blaCTX-M-27/F1:A2:B20 may be disseminated among elderly Japanese living in nonacute-care settings.
FIG 2.
Genetic comparison of blaCTX-M-27/F1:A2:B20 plasmids with pH105 (GenBank accession no. CP021871). (A) Comparison of 16 plasmids (numbered) from LTCF residents by using the BRIG software. The comparison was performed based on pH105 (134,899 bp), whose nucleotide sequences were completely determined by Ghosh et al. (24). Red coding is pH105, and the other colors are based on facility, as follows: pink, facility A; purple, facility B; yellowish-green, facility C; dark green, facility F; light blue, facility G; blue, facility H; dark yellow, facility I. MFS, major facilitator superfamily; CAAX proteases and bacteriocin-processing; SAM, S-adenosyl-l-methionine. (B) Comparison of 6 plasmids (a to f) from healthy people and with the plasmids from panel A (labeled 16) by using the BRIG software. The comparison was performed based on pH105 and plasmids from LTCF residents. Plasmids marked with the # symbol are also shown in Fig. 3. blaCTX-M-27 is boxed in blue.
FIG 3.
Genetic comparison of representative plasmids from LTCF residents. The complete sequences of pH00130, pA0145, pC0122, pH105, pEC732_2, and pEC542_1 were compared using Easyfig 2.2.2 and BLASTn. The open reading frames are represented by arrows and color coded according to their functions. Nucleotide sequence identities between the plasmid sequences are indicated by color shading, and gene functions are indicated on the right. blaCTX-M-27 is blue, insertion sequences are yellow, and antimicrobial resistance genes are red.
We characterized the blaCTX-M-27/F1:A2:B20 plasmids harbored by E. coli ST131 sublineage C1/H30R spreading among nonacute-care settings. These plasmids harbor several genes conferring resistance to aminoglycosides, macrolides, tetracycline, and trimethoprim. However, we also identified F1:A2:B20 plasmids harboring blaCTX-M-27 as the only resistance gene. Although the plasmids lacked genes encoding some transposases, as well as antimicrobial resistance genes, the fundamental structures of the plasmids were similar to those of blaCTX-M-27/F1:A2:B20 plasmids isolated worldwide. Our findings demonstrate that blaCTX-M-27/F1:A2:B20 plasmids previously identified outside Japan have already disseminated among elderly Japanese staying in nonacute-care settings. These results suggest that public health problems can arise from the worldwide dissemination of this plasmid type; however, further studies are necessary to clarify the process of blaCTX-M-27/F1:A2:B20 plasmid dissemination.
Data availability.
The complete nucleotide sequences of 21 plasmids were deposited in the DDBJ database under accession numbers LC520268, LC520269, LC520270, LC520271, LC520272, LC520273, LC520274, LC520275, LC5202676, LC520277, LC520278, LC520280, LC520281, LC520283, LC520284, LC520285, LC520286, LC520287, LC520288, LC520289, and AP019526.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Research Program of the Health Labor Sciences Research grant H27-Iryo-Ippan-012. The plasmid sequencing was partially supported by AMED under grant 18jk0210004h0001.
Rina Nonogaki contributed to the additional experiments and analysis in response to the reviewer’s comments.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Spellberg B, Bartlett JG, Gilbert DN. 2013. The future of antibiotics and resistance. N Engl J Med 368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peirano G, Pitout JD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents 35:316–321. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Mora A, Herrera A, Mamani R, López C, Alonso MP, Blanco JE, Blanco M, Dahbi G, García-Garrote F, Pita JM, Coira A, Bernárdez MI, Blanco J. 2010. Recent emergence of clonal group O25b:K1:H4-B2-ST131 ibeA strains among Escherichia coli poultry isolates, including CTX-M-9-producing strains, and comparison with clinical human isolates. Appl Environ Microbiol 76:6991–6997. doi: 10.1128/AEM01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amos GCA, Hawkey PM, Gaze WH, Wellington EM. 2014. Waste water effluent contributes to the dissemination of CTX-M-15 in the natural environment. J Antimicrob Chemother 69:1785–1791. doi: 10.1093/jac/dku079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Báez J, Hernández-García M, Guamparito C, Díaz S, Olave A, Guerrero K, Cantón R, Baquero F, Gahona J, Valenzuela N, Del Campo R, Silva J. 2015. Molecular characterization and genetic diversity of ESBL-producing Escherichia coli colonizing the migratory Franklin’s gulls (Leucophaeus pipixcan) in Antofagasta, North of Chile. Microb Drug Resist 21:111–116. doi: 10.1089/mdr.2014.0158. [DOI] [PubMed] [Google Scholar]
- 6.Schembri SA, Zakour NLB, Phan M-D, Forde BM, Stanton-Cook M, Beatson SA. 2015. Molecular characterization of the multidrug resistant Escherichia coli ST131 clone. Pathogens 4:422–430. doi: 10.3390/pathogens4030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30Rx. mBio 4:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan M-D, Gomes Moriel D, Peters KM, Davies M, Rogers BA, Dougan G, Rodriguez-Baño J, Pascual A, Pitout JDD, Upton M, Paterson DL, Walsh TR, Schembri MA, Beatson SA. 2014. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas-Chanoine M-H, Bertrand X, Madec J-Y. 2014. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Johnson JR, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S, the Kyoto-Shiga Clinical Microbiology Study Group. 2015. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J Antimicrob Chemother 70:1639–1649. doi: 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura K, Hayashi K, Matsuo N, Kitaoka K, Kimura K, Wachino J, Kondo T, Iinuma Y, Murakami N, Fujimoto S, Arakawa Y. 2018. Prevalence of CTX-M-type extended-spectrum β-Lactamase-producing Escherichia coli B2-O25-ST131 H30R among residents in nonacute care facilities in Japan. Microb Drug Resist 24:1513–1520. doi: 10.1089/mdr.2018.0068. [DOI] [PubMed] [Google Scholar]
- 12.Merino I, Hernández-García M, Turrientes M-C, Pérez-Viso B, López-Fresneña N, Diaz-Agero C, Maechler F, Fankhauser-Rodriguez C, Kola A, Schrenzel J, Harbarth S, Bonten M, Gastmeier P, Canton R, Ruiz-Garbajosa P, R-GNOSIS Study Group. 2018. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother 73:2973–2980. doi: 10.1093/jac/dky296. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh H, Doijad S, Falgenhauer L, Fritzenwanker M, Imirzalioglu C, Chakraborty T. 2017. blaCTX-M-27-encoding Escherichia coli sequence type 131 lineage C1-M27 clone in clinical isolates, Germany. Emerg Infect Dis 23:1754–1756. doi: 10.3201/eid2310.170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birgy A, Bidet P, Levy C, Sobral E, Cohen R, Bonacorsi S. 2017. CTX-M-27-producing Escherichia coli of sequence type 131 and clade C1-M27, France. Emerg Infect Dis 23:885. doi: 10.3201/eid2305.161865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S, Kyoto-Shiga Clinical Microbiology Study Group. 2013. Association of fluoroquinolone resistance, virulence genes, and IncF plasmids with extended-spectrum-beta-lactamase-producing Escherichia coli sequence type 131 (ST131) and ST405 clonal groups. Antimicrob Agents Chemother 57:4736–4742. doi: 10.1128/AAC.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doumith M, Dhanji H, Ellington MJ, Hawkey P, Woodford N. 2012. Characterization of plasmid encoding extended-spectrum-beta-lactamases and their addiction systems circulating among Escherichia coli clinical isolates in the UK. J Antimicrob Chemother 67:878–885. doi: 10.1093/jac/dkr553. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JR, Gajewski A, Lesse AJ, Russo TA. 2003. Extraintestinal pathogenic Escherichia coli as a cause of invasive nonurinary infections. J Clin Microbiol 41:5798–5802. doi: 10.1128/jcm.41.12.5798-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JR, Porter S, Johnson B, Kuskowski MA, Spurbeck RR, Mobley HL, Williamson DA. 2015. Host characteristics and bacterial traits predict experimental virulence for Escherichia coli bloodstream isolates from patients with uroseptis. Open Forum Infect Dis 2:ofv083. doi: 10.1093/ofid/ofv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakane K, Kawamura K, Goto K, Arakawa Y. 2016. Long-term colonization by blaCTX-M-producing Escherichia coli in healthy Japanese people engaged in food handing. Appl Environ Microbiol 82:1818–1827. doi: 10.1128/AEM.02929-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coil D, Jospin G, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina Miseq data. Bioinformatics 31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 21.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carattoli A, Zankari E, Garcia-Fernandez A, Larsen MV, Lund O, Villa L, Aarestrup FM, Hasman H. 2014. In silico detection and typing of plasmids using Plasmid-Finder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norizuki C, Wachino J, Suzuki M, Kawamura K, Nagano N, Kimura K, Arakawa Y. 2017. Specific blaCTX-M-8/IncI1 plasmid transfer among genetically diverse Escherichia coli isolates between humans and chickens. Antimicrob Agents Chemother 61:e00663-17. doi: 10.1128/AAC.00663-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh H, Bunk B, Doijad S, Schmiedel J, Falgenhauer L, Sproer C, Imirzalioglu C, Overmann J, Chakraborty T. 2017. Complete genome sequence of blaCTX-M-27 encoding Escherichia coli strain H105 of sequence type 131 lineage C1/H30R. Genome Announc 5:e00736-17. doi: 10.1128/genomeA.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutti M, Sonnevend Á, Pál T, Junttila S, Ekker H, Galik B, Gyenesei A, Nagy G, Nagy E, Szijártó V . 2018. Complete genome sequence of Escherichia coli 81009, a representative of the sequence type 131 C1-M27 clade with a multidrug-resistant phenotype. Genome Announc 6:e00056-18. doi: 10.1128/genomeA.00056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson TJ, Danzeisen JL, Youmans B, Case K, Llop K, Munoz-Aguayo J, Flores-Figueroa C, Aziz M, Stoesser N, Sokurenko E, Price LB, Johnson JR. 2016. Separate F-type plasmids have shaped the evolution of the H30 subclone of Escherichia coli sequence type 131. mSphere 1:e00121-16. doi: 10.1128/mSphere.00121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitout JD, DeVinney R. 2017. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000Res 6:195. doi: 10.12688/f1000research.10609.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PNA, Ben Zakour NL, Roberts LW, Wailan AM, Zowawi HM, Tambyah PA, Lye DC, Jureen R, Lee TH, Yin M, Izharuddin E, Looke D, Runnegar N, Rogers B, Bhally H, Crowe A, Schembri MA, Beatson SA, Paterson DL, Harris-Brown T, Lorenc P, McNamara J, Underwood N, Eisenmann J, Stewart J, Henderson A, Ali J, Chiang D, Hwa SS, Kang Y, Pei OS, Ying D, Holland U, Korman T, MERINO Trial Investigators. 2018. Whole genome analysis of cephalosporin-resistant Escherichia coli from bloodstream infections in Australia, New Zealand and Singapore: high prevalence of CMY-2 producers and ST131 carrying blaCTX-M-15 and blaCTX-M-27. J Antimicrob Chemother 73:634–642. doi: 10.1093/jac/dkx466. [DOI] [PubMed] [Google Scholar]
- 29.Zurfluh K, Stevens MJA, Stephan R, Nuesch-Inderbinen M. 2018. Complete and assembled genome sequence of an NDM-5-and CTX-M-15-producing Escherichia coli sequence type 617 isolated from wastewater in Switzerland. J Glob Antimicrob Resist 15:105–106. doi: 10.1016/j.jgar.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Tadesse DA, Tadesse DA, Li C, Mukherjee S, Hsu CH, Jones SB, Gaines SA, Kabera C, Loneragan GH, Torrence M, Harhay DM, McDermott PF, Zhao S. 2018. Whole-genome sequence analysis of CTX-M containing Escherichia coli isolates from retail meats and cattle in the United States. Microb Drug Resist 24:939–948. doi: 10.1089/mdr.2018.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Founou LL, Founou RC, Allam M, Ismail A, Essack SY. 2018. Draft genome sequence of an extended-spectrum β-lactamase (CTX-M-15)-producing Escherichia coli ST10 isolated from a nasal sample of an abattoir worker in Cameroon. J Glob Antimicrob Resist 14:68–69. doi: 10.1016/j.jgar.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Doi Y, Arakawa Y. 2020. ORF-based binarized structure network analysis of plasmids (OSNAp), a novel approach to core gene-independent plasmid phylogeny. Plasmid 108:102477. doi: 10.1016/j.plasmid.2019.102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cusumano CK, Hung CS, Chen SL, Hultgren SJ. 2010. Virulence plasmid harbored by uropathogenic Escherichia coli functions in acute stages of pathogenesis. Infect Immun 78:1457–1467. doi: 10.1128/IAI.01260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol 8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 35.He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F. 2015. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 6:e00762-15. doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamborova I, Johnston BD, Papousek I, Kachlikova K, Micenkova L, Clabots C, Skalova A, Chudejova K, Dolejska M, Literak I, Johnson JR. 2018. Extensive genetic commonality among wildlife, wastewater, community, and nosocomial isolates of Escherichia coli sequence type 131 (H30R1 and H30Rx subclones) that carry blaCTX-M-27 or blaCTX-M-15. Antimicrob Agents Chemother 62:e00519-18. doi: 10.1128/AAC.00519-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birgy A, Levy C, Nicolas-Chanoine M-H, Cointe A, Hobson CA, Magnan M, Bechet S, Bidet P, Cohen R, Bonacorsi S. 2019. Independent host factors and bacterial genetic determinants of the emergence and dominance of Escherichia coli sequence type 131 CTX-M-27 in a community pediatric cohort study. Antimicrob Agents Chemother 63:e00382-19. doi: 10.1128/AAC.00382-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete nucleotide sequences of 21 plasmids were deposited in the DDBJ database under accession numbers LC520268, LC520269, LC520270, LC520271, LC520272, LC520273, LC520274, LC520275, LC5202676, LC520277, LC520278, LC520280, LC520281, LC520283, LC520284, LC520285, LC520286, LC520287, LC520288, LC520289, and AP019526.