The aim of this work was to evaluate the pharmacokinetics of amikacin in Mexican patients with different renal functions receiving once-daily dosing regimens and the influence of clinical and demographical covariates that may influence the optimization of this antibiotic. A prospective study was performed in a total of 63 patients with at least one determination of amikacin plasma concentration. Population pharmacokinetic (PK) parameters were estimated by nonlinear mixed-effects modeling; validations were performed for dosing recommendation purposes based on PK/pharmacodynamic simulations.
KEYWORDS: amikacin, population pharmacokinetics, PK/PD modeling, augmented renal clearance
ABSTRACT
The aim of this work was to evaluate the pharmacokinetics of amikacin in Mexican patients with different renal functions receiving once-daily dosing regimens and the influence of clinical and demographical covariates that may influence the optimization of this antibiotic. A prospective study was performed in a total of 63 patients with at least one determination of amikacin plasma concentration. Population pharmacokinetic (PK) parameters were estimated by nonlinear mixed-effects modeling; validations were performed for dosing recommendation purposes based on PK/pharmacodynamic simulations. The concentration-versus-time data were best described by a one-compartment open model with proportional interindividual variability associated with amikacin clearance (CL) and volume of distribution (V); residual error followed a homoscedastic trend. Creatinine clearance (CLCR) and ideal body weight (IBW) demonstrated significant influence on amikacin CL and V, respectively. The final model [CL (liters/h) = 7.1 × (CLCR/130)0.84 and V (liters) = 20.3 × (IBW/68)2.9] showed a mean prediction error of 0.11 mg/liter (95% confidence interval, −3.34, 3.55) in the validation performed in a different group of patients with similar characteristics. There is a wide variability in amikacin PK parameters in Mexican patients. This leads to inadequate dosing regimens, especially in patients with augmented renal clearance (CLCR of >130 ml/min). Optimization based on the final population PK model in Mexican patients may be useful, since reliability and clinical applicability have been demonstrated in this study.
INTRODUCTION
Amikacin is an aminoglycoside antibiotic of wide clinical use to treat serious infectious caused mainly by Gram-negative pathogens. Aminoglycoside bactericidal activity has been strongly associated with the ratio of maximum concentration (Cmax) to MIC as the pharmacodynamic (PD) parameter related to favorable clinical outcome. Specifically, for amikacin, a Cmax/MIC ratio of ≥8 has been established to predict treatment efficacy considering postantibiotic effect, which is also concentration dependent and extends from approximately 4 to 6 h (1–3).
The pharmacokinetics (PK) of this antimicrobial is altered by the pathophysiology associated with patient condition, which leads to fluctuations in the plasma concentrations, requiring the use of different strategies to ensure effective pharmacotherapy (4, 5).
Augmented renal clearance (ARC) is a physiological condition defined by creatinine clearance (CLCR) greater than normal values (130 ml/min/1.73 m2), which recently has been identified and may lead to therapeutic failure in antibiotics mainly eliminated by the renal pathway (6–8). One of the main factors associated with failure in antibacterial therapy in patients with ARC is the inadequate dosage of the drug, which generates subtherapeutic concentrations of the antibiotic, with the consequent lack of efficacy of treatment and the possible emergence of resistant strains. On the other hand, it should be noted that the administration of excessive doses can generate toxic effects, which can increase the length of hospital stay and generate a greater expense in patient care (9).
The aim of this work was to characterize the PK parameters of amikacin in Mexican patients with different levels renal function, especially ARC, and to evaluate the influence of other anthropometric and clinical covariates to propose a dosage regimen achieving therapeutic targets.
RESULTS
Demographics.
A total of 80 plasma concentrations (Cp) from 50 patients were included for the development of a population PK model of amikacin. Clinical and demographic characteristics of the patients are shown in Table 1. Based on CLCR, 50% of patients from population and validation study groups showed ARC (>130 ml/min/1.73 m2). Amikacin quantification ranged from 2 to 48.7 mg/liter; 25% of the blood samples were drawn from 0.6 to 2 h (mean Cp of 30.86 mg/liter), 40% from 2 to 8 h (Cp mean of 16.4 mg/liter), and 35% up to 16.5 h (mean Cp of 2.9 mg/liter) after the last dose.
TABLE 1.
Demographic and clinical characteristics of critically ill patients included for the development and validation of amikacin population pharmacokinetic model
| Characteristica | Value(s)b
for: |
|
|---|---|---|
| Population group (n = 50) | Validation group (n = 13) | |
| Sex (male/female) (n) | 45/5 | 11/2 |
| Amikacin plasma concn (n) | 80 | 21 |
| Age (yr) | 33.5 (18.0–64.0) | 33.0 (18.0–67.0) |
| Wt (kg) | 70.0 (44.0–138.0) | 70.0 (45.0–127.0) |
| Height (cm) | 170.1 ± 7.9 | 168.0 ± 5.8 |
| BMI (kg/m2) | 24.0 (16.0–38.2) | 23.7 (18.5–41.5) |
| Adjusted wt (kg) | 68.6 (46.0–107.3) | 70.2 (48.6–94.6) |
| Ideal wt (kg) | 68.1 ± 8.1 | 65.9 ± 6.7 |
| Serum creatinine (mg/dl) | 0.8 (0.4–1.8) | 0.8 (0.2–1.4) |
| CLCR (ml/min) | 130.0 ± 40.6 | 140.4 ± 70.3 |
| Urea (mg/dl) | 27.8 (8.5–129.2) | 28.9 (11.9–54.8) |
| Ureic nitrogen (mg/dl) | 13.0 (4.0–60.4) | 14.0 (5.6–25.6) |
| Total proteins (g/dl) | 6.5 ± 1.4 | 4.9 ± 0.9 |
| Albumin (g/dl) | 3.7 ± 0.8 | 2.5 ± 0.6 |
| Total bilirubin (mg/dl) | 0.7 ± 0.3 | 0.4 ± 0.3 |
| Sodium (mmol/liter) | 137.4 ± 4.8 | 140.4 ± 2.9 |
| Potassium (mmol/liter) | 3.8 (3.3–5.0) | 3.9 (3.0–4.5) |
| Chloride (mmol/liter) | 106.6 ± 5.8 | 110.0 ± 4.1 |
| Calcium (mg/dl) | 8.5 ± 0.9 | 8.2 ± 1.1 |
| Phosphorus (mg/dl) | 3.1 ± 1.0 | 3.7 ± 0.8 |
| Magnesium (mEq/liter) | 1.7 ± 0.2 | 1.8 ± 0.2 |
| APACHE II score | 7.8 ± 4.1 | 16.5 ± 2.1 |
| Amikacin dose (mg/day) | 1,000 (500–1,000) | 1,000 (750–1,000) |
| Mechanical ventilation (%) | 14 | 15.4 |
| Pharmacotherapy (%) | ||
| NSAIDs | 88 | 85 |
| Opioid analgesics | 80 | 70 |
| Cephalosporines | 88 | 62 |
| Diuretics | 2 | 15 |
| Antimycotics | 56 | 54 |
| Inotropics | 8 | 8 |
| Glucocorticoids | 20 | 15 |
BMI, body mass index; CLCR, creatinine clearance.
Data are shown as means ± standard deviations or as medians (ranges).
Development and validation of population PK model.
Amikacin plasma concentrations after intravenous infusion are best described by a one-compartment open model with proportional interindividual variability (IIV) associated with CL and V; residual variability (RV) was fitted to a homoscedastic model (additive) for the full range of concentrations. This base structural model estimated initial values of CL (6.9 liters/h) and V (20.9 liters), with an IIV of 34.6% and 41%, respectively, and an RV of 1.27 mg/liter.
The PK behavior of amikacin in Mexican patients showed an adequate adjustment to a one-compartment open model with first-order elimination, which corresponds to that previously described by other authors (10–13). It should be noted that it was not feasible to characterize the adjustment of amikacin kinetics to a two-compartment open model due to the small number of plasma samples per patient obtained for the purpose of routine monitoring of plasma concentrations of this antibiotic.
Continuous covariates that showed significant influence and, therefore, were included in this base model were CLCR and ideal body weight (IBW), affecting amikacin CL and V, respectively.
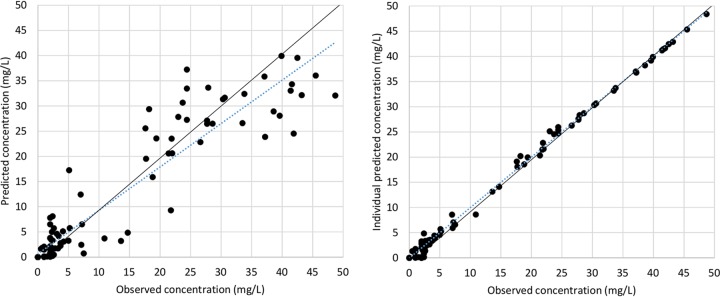
Sequentially, univariate analysis, including each categorical covariate, was performed, but none of them remained in the full model. The parameters estimated for this final model are shown in Table 2, as are the results from the bootstrapping of the original data set (n = 1,000), which confirm the stability and precision of the PK parameters, since all of the final estimates are close to the median and within the nonparametric 95% confidence interval (CI). The IIV associated with CL and V were reduced to 28.7% and 33.2%, respectively, from the base model variability. RV was finally estimated as 12.21% for a mean amikacin plasma concentration of 15.3 mg/liter. Representative mean plots of goodness of fit are shown in Fig. 1. Figure 2 represents a visual predictive check performed with 1,000 simulations of the original database.
TABLE 2.
Final population pharmacokinetic model and internal validation for amikacin administered once daily
| Modela | Parameter | Mean (% RSEb) | Bootstrap (n = 1000) |
||
|---|---|---|---|---|---|
| Median | Percentile |
||||
| 2.5th | 97.5th | ||||
| CL (liters/h) = θ1 × (CLCR/130)θ3 | θ1 | 7.1 (7) | 7.2 | 6.2 | 8.4 |
| θ3 | 0.84 (29) | 0.86 | 0.34 | 1.26 | |
| V (liters) = θ2 × (IBW/68)θ4 | θ2 | 20.3 (8) | 20 | 15.6 | 23.2 |
| θ4 | 2.94 (34) | 3.13 | 1.27 | 5.40 | |
| Interindividual variability associated with CL (CV%) | ω2CL | 27.2 (18) | 27.2 | 12.2 | 38.2 |
| Interindividual variability associated with V (CV%) | ω2V | 33.6 (18) | 30.2 | 9.7 | 41.4 |
| Residual variability (mg/liter) | σ | 1.78 (32) | 1.75 | 1.07 | 3.09 |
CV, coefficient of variation. CLi = CL × (1+ηCLi). Vdi = V × (1+ηVdi).
RSE, relative standard error.
FIG 1.
Scatterplots of goodness of fit of population and individual predicted versus observed amikacin concentrations (including the identity line) for the final one-compartment open model for critically ill patients receiving amikacin administered once daily by intermittent intravenous infusion (n = 50).
FIG 2.
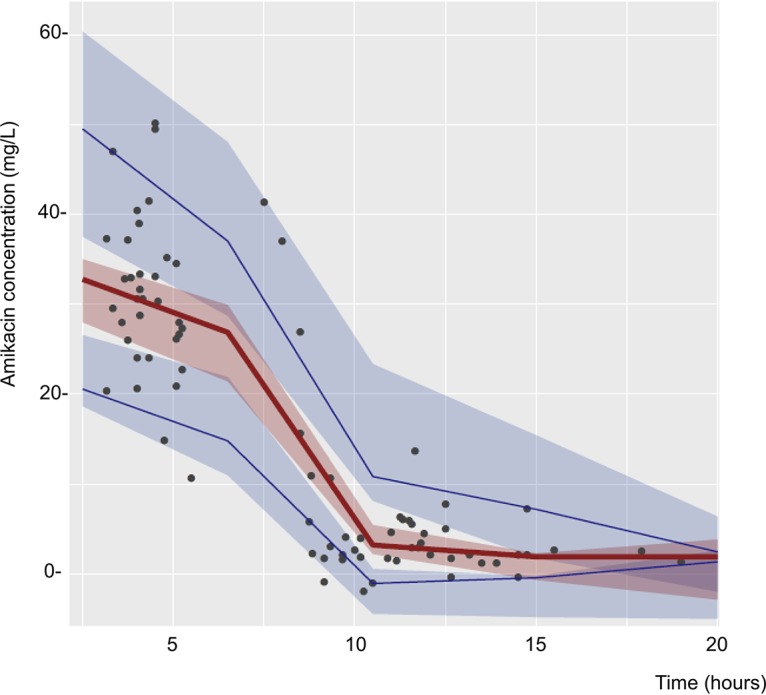
Visual predictive check for amikacin plasma concentration-time profiles. Solid lines represent the median (red) and the 95th and 5th percentiles (blue) of the observations, which are overlapped by the 90% confidence intervals for the median (red area) and the 95th and 5th percentiles (blue areas) of the simulated profiles (n = 1,000).
Validation was performed with plasma concentrations retrieved from 13 patients with characteristics like those of the population study group (Table 1).
The prediction ability of the final model was compared with that of the base structural model. The mean values and 95% CIs of bias and imprecision are shown in Table 3. Lower values were obtained for the final model, and the mean prediction error (MPE) was well distributed around zero. Based on a mean amikacin plasma concentration of 21.7 mg/liter measured for this group, the average error diminished from 36% for concentrations predicted with the base model to 30% for concentrations predicted with the final population PK model.
TABLE 3.
Mean prediction errors and 95% confidence intervals estimated for the validation group with the base model and the final one-compartment open model (n = 13)
| Parameter | Modela
|
|
|---|---|---|
| Base | Final | |
| Mean prediction error | −0.98 (−6.68, 4.71) | 0.11 (−3.34, 3.55) |
| Absolute mean prediction error | 7.83 (5.83, 9.82) | 6.56 (4.75, 8.36) |
| Median absolute error (%) | 46 | 44 |
| Root mean square prediction error | 13.04 | 7.86 |
Data are shown as means (95% confidence intervals). Errors are measured in milligrams per liter.
Dosing recommendations.
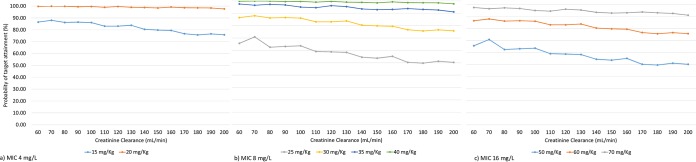
PK/PD simulations were performed to evaluate standard amikacin doses administered to patients with different renal function and considering the final population pharmacokinetic model developed. Figure 3 shows initial dosing recommendations based on Cmax/MIC ratio of >8 for different MICs and patients with different grades of renal clearance (based on CLCR).
FIG 3.
Amikacin dose suggested for critically ill patients based on final population pharmacokinetic model and bacterial MIC (n = 1,000 PK/PD simulations).
Based on the simulations performed, a priori dosing guidelines for amikacin were obtained. It should be noted that the proposed doses are recommended for subjects with an ideal weight of approximately 68 kg, because it was the average ideal weight of the patients who were included in the study for the development of the population PK model.
DISCUSSION
The final one-compartment population PK model for amikacin administered once daily obtained in this study shows that CLCR has a statistically significant influence on amikacin CL, and ideal weight is a covariate with significant influence on the V of the drug.
Water-soluble antibiotics, such as amikacin, are distributed mainly in the extracellular fluid and are almost completely eliminated through the kidneys by glomerular filtration; therefore, modifications in renal function directly affect the clearance of this drug (14).
Several methods have been described for estimating renal function. Among these, inulin clearance is the most widely accepted method for estimating glomerular filtration rate (GFR); however, due to its complexity, it cannot be applied routinely in clinical settings (15). The CLCR can be determined based on serum and urinary creatinine concentrations; however, it has been reported that through this method, the GFR is overestimated by 5% to 15% (16). In the present study, the Cockcroft-Gault equation which includes age, total body weight, sex, and serum creatinine concentration was used for the calculation of CLCR. While the MDRD (modification of diet in renal disease) and CKD-EPI (chronic kidney disease epidemiology collaboration) equations for the calculation of CLCR have been widely used in recent years, CLCR calculated based on the Cockcroft-Gaul equation showed better association with amikacin CL, as demonstrated by the current study and the previous one report by Boidin et al. (17), which also included patients with and without ARC.
It is important to highlight that patients with ARC show a dynamic renal function that is difficult to characterize, so the formulas applied for the calculation of GFR give rise to estimates that must be interpreted based on the clinical context of each patient (18).
Several authors have reported the influence of CLCR on the PK of amikacin, so this covariate has been incorporated into different population models for this aminoglycoside (19–21). The knowledge of the degree of renal function of the patient is fundamental for the establishment of amikacin dosing regimens, since patients with high CLCR will require high doses of the antibiotic to reach optimal plasma concentrations.
The interindividual variability of the CL of amikacin obtained in the final model was 28.7%, similar to that reported by Romano et al. (13), in a study conducted in Spanish critically ill patients, and inferior to that described by Jang et al. (11), Lugo and Castañeda-Hernández (12), and Debord et al. (10), in Korean, Mexican, and French critically ill patients, respectively. According to the final model, the CLCR explains 17% of the variability in the amikacin CL established by the basic model.
It can be observed that the mean CL of amikacin (7.5 liters/h) in Mexican patients included in the development of the population PK model of the present work is higher than that reported in other populations, except for that shown by Garraffo et al. (33), who obtained a similar CL value (7.6 liters/h) in a study conducted in healthy French volunteers. The high CL value obtained in the present study could be attributed to the fact that a high percentage of the included patients with ARC is the young adult population, male, and with a renal function apparently not compromised.
It has been described that obese patients show modifications in the PK parameters of various aminoglycosides compared to those of patients with normal weight (Bauer et al. [34]). Amikacin is distributed mainly in the extracellular fluid, and as this volume increases, the V of the drug increases. It is important to point out that the critically ill patient usually presents cardiovascular dysfunction, which could generate a third compartment corresponding to the interstitial fluid, whose volume is significantly increased due to the administration of large volumes of resuscitation fluids in response to hypotension in the critically ill patient (5, 22). Therefore, the V of amikacin in critically ill patients with normal weight is usually increased.
It should be emphasized that the critically ill patient presents multiple physiopathological changes that lead to modifications in the PK behavior of the drugs. In addition to the renal system, the liver, lung, and cardiovascular system can be affected by critical illness. Some authors have shown that the use of mechanical ventilation reduces cardiac output, hepatic and renal flow, and the GFR and urine flow (22, 23). In this study, the influence of assisted mechanical ventilation was evaluated; however, this covariate was not included in the final model.
Romano et al. (13) reported that critically ill patients with a diagnosis of trauma presented amikacin CL that was significantly higher than that of patients with other diagnoses. The influence of concomitant pharmacotherapy on the PK of amikacin has been reported in a very limited way. Lugo and Castañeda-Hernández (12), in a study conducted in Mexican critically ill patients, reported the impact of the administration of catecholamines in the CL of amikacin. The use of high doses of these drugs compromises renal flow due to its adrenergic effect. In the present study, the influence of drugs coadministered on the PK of this aminoglycoside was not identified.
The process in the construction of the final model followed from the basic model initially established, adjusting the data to a one-compartment open model, has led to a reduction in the interindividual variability of CL and V by 5.9 and 7.8%, respectively. This result suggests that the final model includes factors that have an important influence on the interindividual variability of the PK parameters of amikacin in patients with or without ARC.
However, no decrease in residual variability was observed in the final model. In this context, it is necessary to consider that this error quantifies all the possible sources of variability of the concentrations observed with respect to those predicted with the proposed model and includes not only the intraindividual biological variability but also the analytical errors in the measurement of the blood concentrations of the drug and in the blood sampling times.
Nevertheless, validations performed guarantee the validity of the model for clinical prediction purposes, which will allow us to realize, by means of Bayesian estimations, the readjustment of the doses according to therapeutic amikacin blood concentrations.
Given that the proposed final model allows us to estimate the CL and V of amikacin based on the CLCR and ideal weight, we can predict the concentrations that the drug will reach in each patient or establish the most appropriate dosage regimen.
The amikacin dosing regimens suggested in this study were established based on once-daily administration; previous studies have shown that this regimen is more effective than the multiple daily doses. This is based on the importance of achieving high bactericidal concentrations of this aminoglycoside during the initial stage of treatment to improve clinical effectiveness in intensive care unit patients (24). Figure 3 shows the initial amikacin dose proposed for different bacterial MICs; results suggest that dosage recommendations based on IBW reach a minimum concentration of drug in serum (Cmin) below 1 mg/liter even in patients with lower CLCR (60 to 80 ml/min) who show a high probability of a Cmin of <4 ml/min, which is still safe. However, during treatment it is important to monitor the Cmax and Cmin of the antibiotic to minimize the risk of subtherapeutic concentrations or the presence of toxic side effects.
One of the main limitations of the current study is the limited number of samples obtained for each patient; furthermore, the proposed dosing regimens have not been prospectively validated, and extrapolation to different populations should be done with caution.
The application of the model in clinical practice could have a better predictive capacity in the Mexican population than the parameters described in the literature, suggesting its reliable application for the prediction of a priori blood concentrations of this antibiotic, as well as in the establishment of dosage regimens and in the readjustment of dosage regimens of this drug using strategies based on Bayesian algorithms. This may lead to the optimization of antimicrobial therapy with amikacin by minimizing the development of bacterial resistance by underdosing or the appearance of adverse events caused by high concentrations of amikacin in blood.
MATERIALS AND METHODS
Study design.
An observational prospective study was developed in Hospital Central “Dr. Ignacio Morones Prieto” (HCIMP) in San Luis Potosí, México. Patients with suspected or proven infection and under treatment with amikacin provided written informed consent according to the protocol approved by the Research and Ethics Committee of HCIMP (Register 37-16) and the Ethics Committee of the Chemical Sciences Faculty at the Autonomous University in San Luis Potosi, Mexico (Register CEID2016083-S).
Patients (>18 years old) receiving once-daily intravenous amikacin treatment were included consecutively in the current study. Amikacin dosing was prescribed by treating physicians, and two blood samples were drawn in EDTA tubes at least 30 min and up to 12 h after the infusion was finished. Amikacin concentrations were quantified by the clinical laboratory of HCIMP through particle-enhanced turbidimetric inhibition immunoassay (PETINIA; Architect ci8200 system; Abbot, Abbott Park, IL, USA). The calibration range was 2 to 50 mg/liter. Inter- and intra-assay precision showed a coefficient of variation of <5% and an average recovery of 100.1% ± 2.6%. Clinical information was retrieved from medical records.
Development of the population PK model.
The population PK model was built using nonlinear mixed-effects modeling via NONMEM software v7.3 (Icon Development Solutions, Dublin, Ireland) in conjunction with Perl-speaks-NONMEM (PsN) 3.5.3 (25). Compartmental PK models were coded using ADVAN1 TRANS2 and ADVAN3 TRANS4 subroutines in NONMEM. Data exploration, manipulation, and graphics were handled using Xpose 4.3.5 embedded in R 3.1.0 (http://cran.r-project.org/; R is an open-source, S-based statistical software) (26, 27). The first-order conditional estimation with interaction method (FOCE-I) was used to estimate PK parameters. The variability of the parameter was estimated using the covariance step. Visual inspections of the amikacin blood concentration-versus-time profiles and the objective function value (OFV), calculated using likelihood ratio tests, were used to determine the base model. A heteroscedastic model (proportional) was selected to describe the interindividual variability in amikacin PK parameters, and the residual variability was modeled as a homoscedastic error model (additive).
Following model development, covariates were evaluated in a stepwise forward selection, and significant covariates were combined in a full model to characterize amikacin PK. This was followed by backward elimination, and significant covariates were retained in the final model. The continuous covariates tested were age, total body weight, adjusted and ideal body weight, body mass index (BMI), creatinine, creatinine clearance (estimated using the Cockcroft-Gault equation with total body weight [28], as well as CKD-EPI [29] and MDRD [30] estimations), urea, blood urea nitrogen, total proteins, albumin, total bilirubin, serum electrolytes (sodium, potassium, chloride, calcium, phosphorus, and magnesium), and acute physiology and chronic health evaluation II (APACHE II) scores. The categorical covariates evaluated were sex, mechanical ventilation, diabetes mellitus, arterial hypertension diagnosis, and/or concomitant administration of nonsteroidal anti-inflammatory drugs (NSAIDs), opioid analgesics, cephalosporins, diuretics, antimycotics, inotropics agents, and corticosteroids. The preliminary selection of covariates was performed by stepwise generalized additive model (GAM) analysis. Covariate selection was guided using likelihood ratio tests at a significance level (P value) of <0.05 for forward addition of covariates (ΔOFV > 3.84) and P value of <0.01 for backward elimination of covariates (ΔOFV > 6). Diagnostic plots and comparisons of changes in the minimum OFV between the nested models were used to evaluate the covariates and physiologically reasonable results.
Continuous covariate effects were introduced into the population model using linear, power, or exponential functions; parameters were also centered on the median value of the continuous variables in the database (allometric function). Categorical covariates were usually set to 1 for the most frequent classification and introduced to the model as described by Mould and Upton (31).
Validation of the PK model.
Internal validation was performed by bootstrapping, which was based on 1,000 resamples generated from the original database and by following the same structure of the final model. The model was considered stable if the mean parameters estimated with the original database are found into the 2.5th and 97.5th percentiles built, with the databases generated by a resampling technique.
Additionally, the final PK model was validated with a new group of patients with characteristics similar to those included in the population study group. The predictive performance of the population PK model was evaluated using an a priori method. Amikacin concentrations of the validation group were compared with predicted values to estimate the precision of the final population model. The bias was evaluated via the mean prediction error. Accuracy was estimated by the absolute relative error and the root mean squared prediction error (32).
Dosing recommendations.
PK/PD simulations (n = 1,000) were performed with the final population model for dose selection, considering clinical characteristics influencing amikacin PK parameters and different MICs. Based on the clinical breakpoints recently reported by EUCAST (http://www.eucast.org/clinical_breakpoints/), MICs for susceptible microorganisms are distributed below 8 mg/liter, but 16 mg/liter has also been reported. Cmax/MIC of >8 and area under the concentration-time curve (AUC)/MIC of >75 were evaluated as PK/PD targets for different scenarios. Typical patients were simulated to predict Cmax, AUC, and Cmin with different initial doses of amikacin administered once daily. Dosage regimens were chosen at the level of the higher percentage of patients who achieved therapeutic index in terms of efficacy and safety for each simulation performed.
ACKNOWLEDGMENTS
We thank the pharmacists, analytical technicians, nurses, and medical staff from the Hospital Central Dr. Ignacio Morones Prieto (San Luis Potosí, Mexico) for their contributions to the present study.
We acknowledge the support given by the Technological Research Council of Science and Technology through the Institutional Funding “National Problems” (project CONACYT-248716-2015), as well as the fellowship given to Norma Aréchiga-Alvarado (register 589805). Susanna E. Medellín-Garibay was promoted for Retention at Universidad Autónoma de San Luis Potosí through the Support Program for the Incorporation of Scientists, linked to the Institutional Consolidation of Research Groups and/or National Postgraduate Programs of CONACYT (grant C-891/2018).
We have no conflicts of interest to declare.
REFERENCES
- 1.Isaksson B, Hanberger H, Maller R, Nilsson LE, Nilsson M. 1990. The postantibiotic effect of amikacin alone and in combination with piperacillin on gram-negative bacteria. Scand J Infect Dis Suppl 74:129–132. [PubMed] [Google Scholar]
- 2.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother 43:623–629. doi: 10.1128/AAC.43.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, Massias L, Papy E, Tubach F, Wolff M, Sonneville R. 2014. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 40:998–1005. doi: 10.1007/s00134-014-3276-x. [DOI] [PubMed] [Google Scholar]
- 5.Marsot A, Guilhaumou R, Riff C, Blin O. 2017. Amikacin in critically ill patients: a review of population pharmacokinetic studies. Clin Pharmacokinet 56:127–138. doi: 10.1007/s40262-016-0428-x. [DOI] [PubMed] [Google Scholar]
- 6.Baptista JP, Roberts JA, Udy AA. 2019. Augmented renal clearance: a real phenomenon with an uncertain cause. Anaesth Crit Care Pain Med 38:335–336. doi: 10.1016/j.accpm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. 2010. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet 49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Cook AM, Hatton-Kolpek J. 2019. Augmented renal clearance. Pharmacotherapy 39:346–354. doi: 10.1002/phar.2231. [DOI] [PubMed] [Google Scholar]
- 9.Kovacevic T, Avram S, Milakovic D, Spiric N, Kovacevic P. 2016. Therapeutic monitoring of amikacin and gentamicin in critically and noncritically ill patients. J Basic Clin Pharm 7:65–69. doi: 10.4103/0976-0105.183260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debord J, Pessis C, Voultoury JC, Marquet P, Lotfi H, Merle L, Lachatre G. 1995. Population pharmacokinetics of amikacin in intensive care unit patients studied by NPEM algorithm. Fundam Clin Pharmacol 9:57–61. doi: 10.1111/j.1472-8206.1995.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 11.Jang SB, Lee YJ, Park MS, Song YG, Kim JH, Kim HK, Ahn BS, Park K. 2011. Population pharmacokinetics of amikacin in a Korean clinical population. Int J Clin Pharmacol Ther 49:371–381. doi: 10.5414/cp201520. [DOI] [PubMed] [Google Scholar]
- 12.Lugo G, Castañeda-Hernández G. 1997. Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit Care Med 25:806–811. doi: 10.1097/00003246-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Romano S, Del Mar Fdez de Gatta M, Calvo V, Mendez E, Domínguez-Gil A, Lanao JM. 1998. Influence of clinical diagnosis in the population pharmacokinetics of amikacin in intensive care unit patients. Clin Drug Investig 15:435–444. doi: 10.2165/00044011-199815050-00008. [DOI] [PubMed] [Google Scholar]
- 14.Zaske DE, Strate RG, Kohls PR. 1991. Amikacin pharmacokinetics: wide interpatient variation in 98 patients. J Clin Pharmacol 31:158–163. doi: 10.1002/j.1552-4604.1991.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez Ocampo J, Torres RA, Rodriguez CF. 2010. Comparison of four methods for measuring glomerular filtration rate by inulin clearance in healthy individuals and patients with renal failure. Nefrologia 30:324–330. doi: 10.3265/Nefrologia.pre2010.Mar.10238. [DOI] [PubMed] [Google Scholar]
- 16.Park EJ, Pai MP, Dong T, Zhang J, Ko CW, Lawrence J, Crentsil V, Zhang L, Xu NN. 2012. The influence of body size descriptors on the estimation of kidney function in normal weight, overweight, obese, and morbidly obese adults. Ann Pharmacother 46:317–328. doi: 10.1345/aph.1Q374. [DOI] [PubMed] [Google Scholar]
- 17.Boidin C, Bourguignon L, Cohen S, Roger C, Lefrant JY, Roberts JA, Allaouchiche B, Lepape A, Friggeri A, Goutelle S. 2019. Amikacin initial dose in critically ill patients: a nonparametric approach to optimize a priori pharmacokinetic/pharmacodynamic target attainments in individual patients. Antimicrob Agents Chemother 63:e00993-19. doi: 10.1128/AAC.00993-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcazar R, Albalate M. 2010. New methods for estimating glomerular filtration rate. Achieving more precision in diagnosing chronic kidney disease. Nefrologia 30:143–146. doi: 10.3265/Nefrologia.pre2010.Mar.10263. [DOI] [PubMed] [Google Scholar]
- 19.Delattre IK, Musuamba FT, Nyberg J, Taccone FS, Laterre PF, Verbeeck RK, Jacobs F, Wallemacq PE. 2010. Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther Drug Monit 32:749–756. doi: 10.1097/FTD.0b013e3181f675c2. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, Hagihara M, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Yamagishi Y, Matsuura K, Mikamo H. 2017. Evaluation of amikacin pharmacokinetics and pharmacodynamics for optimal initial dosing regimen. Drugs R D 17:177–187. doi: 10.1007/s40268-016-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matar KM, Al-Lanqawi Y, Abdul-Malek K, Jelliffe R. 2013. Amikacin population pharmacokinetics in critically ill Kuwaiti patients. Biomed Res Int 2013:202818. doi: 10.1155/2013/202818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. 2012. Introduction to drug pharmacokinetics in the critically ill patient. Chest 141:1327–1336. doi: 10.1378/chest.11-1396. [DOI] [PubMed] [Google Scholar]
- 23.Perkins MW, Dasta JF, DeHaven B. 1989. Physiologic implications of mechanical ventilation on pharmacokinetics. DICP 23:316–323. doi: 10.1177/106002808902300408. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Lipman J, Kobilski S, Scribante J. 1991. A prospective randomized study comparing once- versus twice-daily amikacin dosing in critically ill adult and paediatric patients. J Antimicrob Chemother 28:753–764. doi: 10.1093/jac/28.5.753. [DOI] [PubMed] [Google Scholar]
- 25.Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. 2005. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Keizer RJ, Karlsson MO, Hooker A. 2013. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson EN, Karlsson MO. 1999. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 28.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA. 2010. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2:e38. doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheiner LB, Beal SL. 1981. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512. doi: 10.1007/bf01060893. [DOI] [PubMed] [Google Scholar]
- 33.Garraffo R, Drugeon HB, Dellamonica P, Bernard E, Lapalus P. 1990. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob Agents Chemother 34:614–621. doi: 10.1128/aac.34.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer LA, Edwards WD, Dellinger EP, Simonowitz DA. 1983. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur J Clin Pharmacol 24:643–647. doi: 10.1007/bf00542215. [DOI] [PubMed] [Google Scholar]