Fluoroquinolones are reported to possess immunomodulatory activity; hence, a novel benzoquinolizine fluoroquinolone, levonadifloxacin, was evaluated in lipopolysaccharide-stimulated human whole-blood (HWB) and mouse acute lung injury (ALI) models. Levonadifloxacin significantly mitigated the inflammatory responses in an HWB assay through inhibition of proinflammatory cytokines and in the ALI model by lowering lung total white blood cell count, myeloperoxidase, and cytokine levels. The immunomodulatory effect of levonadifloxacin, along with promising antibacterial activity, is expected to provide clinical benefits in the treatment of infections.
KEYWORDS: fluoroquinolone, lipopolysaccharide, acute lung injury, immunomodulatory, cytokine, myeloperoxidase
ABSTRACT
Fluoroquinolones are reported to possess immunomodulatory activity; hence, a novel benzoquinolizine fluoroquinolone, levonadifloxacin, was evaluated in lipopolysaccharide-stimulated human whole-blood (HWB) and mouse acute lung injury (ALI) models. Levonadifloxacin significantly mitigated the inflammatory responses in an HWB assay through inhibition of proinflammatory cytokines and in the ALI model by lowering lung total white blood cell count, myeloperoxidase, and cytokine levels. The immunomodulatory effect of levonadifloxacin, along with promising antibacterial activity, is expected to provide clinical benefits in the treatment of infections.
TEXT
Levonadifloxacin is a novel benzoquinolizine fluoroquinolone active against respiratory Gram-positive and Gram-negative pathogens, including methicillin- and quinolone-resistant Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, and Moraxella catarrhalis and atypical pathogens (1–7). Additionally, it exhibits clinically relevant activity against quinolone-susceptible Gram-negative species, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas, and Acinetobacter (7). Enabling preclinical and clinical features of levonadifloxacin, such as potent bactericidal action, enhancement of activity in an acidic environment, intracellular activity, and excellent lung pharmacokinetics, demonstrates promising potential for treating respiratory infections such as community-acquired bacterial pneumonia (CABP) and hospital-acquired bacterial pneumonia (HABP) (4, 8). Because of growing antibiotic resistance, the rate of treatment failure in CABP and HABP is on the rise and persistently calls for new effective antibiotics (9–11). In hospitalized patients with pneumonia or sepsis, the overproduction of proinflammatory cytokines by host immune cells in response to toxins secreted by bacteria is responsible for acute lung injury (ALI). In the past, several antibacterial drugs, including fluoroquinolones, exhibited good clinical outcome in infected patients due to immunomodulatory activity (12–14). Nonclinical in vitro and in vivo studies have also revealed immunomodulatory activity of fluoroquinolones (15). Considering these findings, the anti-inflammatory potential of levonadifloxacin was evaluated using clinically relevant ex vivo (lipopolysaccharide [LPS]-stimulated human whole-blood [HWB] assay) and in vivo (LPS-induced ALI in mice) models (16, 17).
For ex vivo study, heparinized HWB was collected from healthy adults after receipt of written informed consent. For in vivo study, healthy male Swiss albino mice kept under standard laboratory conditions were used. All of the experimental protocols were approved by the Institutional Review Board of Wockhardt Ltd., India. HWB was isolated from five individual healthy donors for the cytokine assessment. The freshly collected HWB from each donor was diluted 1:3 with sterile RPMI 1640 containing l-glutamine and 25 mM HEPES. Each diluted HWB sample was stimulated with an LPS concentration of 10 ng/ml for the cytokine (interleukin 6 [IL-6], tumor necrosis factor α [TNF-α], and IL-1β) assay and a concentration of 50 ng/ml for the granulocyte-macrophage colony-stimulating factor [GM-CSF] assay. The test drugs were subsequently added to the samples, which were incubated for 6 and 48 h to assess cytokine and GM-CSF levels, respectively. The collected samples were then analyzed using enzyme-linked immunosorbent assay (ELISA) kits (R & D systems, USA). Levonadifloxacin and levofloxacin concentrations for the study were selected from the initial dose-response study (data not shown). Based on these results, levonadifloxacin effective concentrations of 15 and 30 μg/ml, which correspond with the plasma therapeutic maximum concentration (Cmax) range, were selected for subsequent comparative study with levofloxacin (8). Levofloxacin concentrations of 25 and 100 μg/ml were selected because their cytokine inhibitory effects were comparable with those of levonadifloxacin concentrations of 15 and 30 μg/ml, respectively.
In the ALI model, mice (n = 6 per time point) were subcutaneously administered vehicle (for saline and LPS control), levonadifloxacin 200 mg/kg, and dexamethasone 10 mg/kg and, after 1 h, were anaesthetized and treated intranasally with LPS solution (100 μg/mouse) or normal saline. After 12 and 24 h of LPS or saline administration, mice were euthanized; the trachea was cannulated with a catheter and instilled with normal ice cold saline (0.6 ml) to collect bronchoalveolar lavage (BAL) fluid. The BAL fluid samples were centrifuged, and the supernatant was transferred in separate tubes leaving behind the cell pellets. The supernatant was analyzed for cytokines using ELISA kits and myeloperoxidase (MPO) activity using the o-dianisidine method (18). The cell pellet was suspended in saline, stained with trypan blue dye, and counted with a hemocytometer using a microscope. For histopathological assessment, a separate set of animals were sacrificed after 24 h of LPS challenge, and then their lungs were perfused, excised, and stored in 10% formalin. The lungs were embedded in paraffin, sliced into 4-μm sections, and stained with hematoxylin and eosin. The pathological changes were observed via microscope and scored by assigning different grades, as described previously (19).
Statistical analyses were performed using the GraphPad prism (version 5). The paired t test was used for the ex vivo study and one-way analysis of variance followed by Dunnett’s test for the in vivo study to compare drug treatment groups with the LPS control group.
In the HWB assay, LPS evoked a significant increase in cytokine levels at 6 h and GM-CSF at 48 h, indicating that this experimental setting is feasible for studying the early phase of endotoxemia. Levonadifloxacin inhibited TNF-α, IL-6, IL-1β, and GM-CSF production in a concentration-dependent manner. The absolute levels of IL-6, TNF-α, IL-1β, and GM-CSF after incubation of HWB with LPS or LPS plus levonadifloxacin 15 and 30 μg/ml and levofloxacin 25 and 100 μg/ml are depicted in Table 1. Levonadifloxacin 15 and 30 μg/ml exhibited significant inhibition of TNF-α, IL-6, and IL-1β that was comparable to that with levofloxacin concentrations of 25 and 100 μg/ml, respectively. The GM-CSF was significantly inhibited by levonadifloxacin 30 μg/ml, which was comparable to levofloxacin 100 μg/ml.
TABLE 1.
Effect of levonadifloxacin and levofloxacin on proinflammatory cytokine release in LPS-stimulated HWB samples
| Treatment | Release (pg/ml) of cytokinesa
: |
|||
|---|---|---|---|---|
| IL-6b | TNF-αb | IL-1βb | GM-CSFc | |
| Vehicle control, LPS + dextrose | 11,170.5 ± 1,773.1 | 4,371.6 ± 743.0 | 4,414.8 ± 892.6 | 15.4 ± 4.0 |
| Levofloxacin 25 μg/ml | 9,093.7 ± 1,684.6** | 3,393.7 ± 659.7*** | 1,964.5 ± 506.1** | 11.7 ± 3.7 |
| Levofloxacin 100 μg/ml | 5,209.5 ± 1,236.7*** | 1,986.9 ± 456.0** | 214.3 ± 90.9** | 4.5 ± 1.3* |
| Vehicle control, LPS + 1% arginine | 10,446.9 ± 1,657.8 | 4,402.6 ± 743.7 | 4,182.0 ± 815.9 | 14.4 ± 3.8 |
| Levonadifloxacin 15 μg/ml | 8,430.3 ± 1,746.7*** | 3,146.9 ± 686.2** | 2,615.2 ± 567.3** | 10.8 ± 2.5 |
| Levonadifloxacin 30 μg/ml | 5,714.7 ± 1,415.4*** | 2,183.8 ± 465.4** | 1,438.9 ± 392.7** | 9.2 ± 2.3* |
Values are means ± SEM; n = 5. *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus respective control calculated by paired t test.
LPS 10 ng/ml was used to estimate cytokine level.
LPS 50 ng/ml was used to estimate cytokine level.
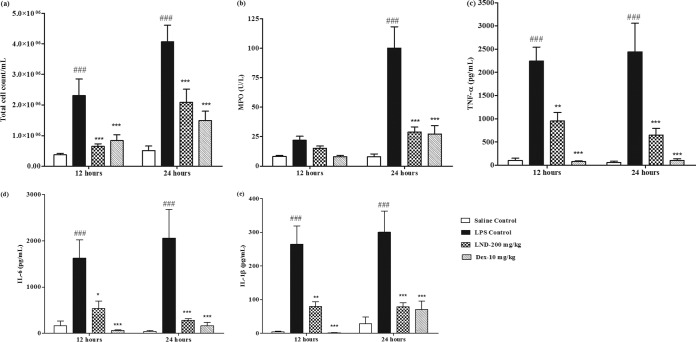
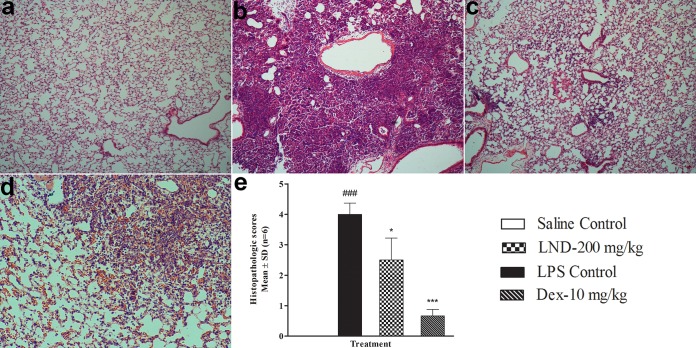
These findings were further confirmed in LPS-induced ALI in mice, where levonadifloxacin at a clinically relevant dose significantly countered the inflammatory responses. Intranasal treatment of LPS in mice produced progressive elevation of total white blood cell (WBC) count, cytokines, and MPO in the BAL fluid, with peak effect observed mostly at 24 h (Fig. 1). An increase in total WBC count in the BAL fluid of LPS-treated mice indicated inflammation because most of the cells were neutrophils. Neutrophil accumulation at the site of infection plays a vital role in the progression of ALIs because neutrophils are responsible for releasing free radicals, inflammatory mediators, and proteases (20). Levonadifloxacin significantly lowered the total WBC count compared with that in the LPS control group. To further confirm these findings, MPO, an indirect marker of neutrophil accumulation, was measured in BAL fluid. MPO is a peroxidase enzyme expressed in neutrophil granulocytes and is released on stimulation by proinflammatory factors and oxidative stress. Excessive release of MPO is reported to cause tissue damage (21, 22). In this study, levonadifloxacin significantly reduced MPO activity. It is widely recognized that excessive secretion of proinflammatory cytokines are implicated in the pathogenesis of ALI. TNF-α, IL-1β, and IL-6 are proinflammatory cytokines released during the early phase of ALI by macrophages and monocytes. They are responsible for triggering an inflammatory cascade and instigating neutrophil recruitment at the site of infection (23, 24). Hence, effective therapies in controlling the release of these cytokines may significantly impede the progression of lung damage. LPS-induced lung injury in mice is also associated with higher levels of cytokines. The levonadifloxacin groups in this study exhibited significant reductions in TNF-α, IL-1β, and IL-6 levels in BAL fluid compared with those in the LPS control group. Furthermore, this immunomodulatory finding was corroborated using histopathological evidence that suggested minimal infiltration of neutrophils into the lung tissue by levonadifloxacin after an LPS challenge (Fig. 2). As expected, dexamethasone demonstrated significant anti-inflammatory activity in this model (Fig. 1 and 2).
FIG 1.
Effect of levonadifloxacin (LND) and dexamethasone (Dex) on total cell count (a) and MPO (b), TNF-α (c), IL-6 (d), and IL-1β (e) levels in mouse BAL fluid (n = 6). Statistical significance for LPS versus saline control, P < 0.001 (###); statistical significance for LPS versus levonadifloxacin and dexamethasone, P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
FIG 2.
Effects of levonadifloxacin (LND) and dexamethasone (DEX) on the histological changes in lung tissue in mice with LPS-induced ALI. Illustrative histological changes in lungs observed in saline control (a), LPS control (b), dexamethasone 10 mg/kg + LPS (c), and levonadifloxacin 200 mg/kg + LPS (d). (e) The comparative lung histopathological scores are presented as means ± standard deviation (n = 6). Statistical significance for LPS versus saline control, P < 0.001 (###); statistical significance for LPS versus levonadifloxacin and dexamethasone, P < 0.05 (*) and P < 0.001 (***).
Structurally, levonadifloxacin is different from other marketed fluoroquinolones. It is a benzoquinolizine, which is a novel subclass of the quinolone class of antibiotics with spectral coverage against Gram-positive bacteria, especially quinolone-resistant and methicillin-resistant Staphylococcus aureus (MRSA). Being a novel molecule with structural modifications, it may have enhanced potential to alter anti-inflammatory activity compared with levofloxacin. The production and release of a number of pro- and anti-inflammatory cytokines were reported in the systemic circulation of all patients with severe pneumococcal pneumonia. Treatment with levofloxacin exhibited clinical stability in a short time, in parallel with lower levels of cytokines, such as TNF-α, IL-6, and IL-1β (25). Based on these observations, our ex vivo findings with levonadifloxacin in HWB predict an enhanced inhibitory effect on proinflammatory cytokines in patients. Levonadifloxacin’s immunomodulatory effect, along with its antibacterial activity, is expected to provide clinical benefits in the treatment of severe respiratory infections. These findings need to be confirmed in future clinical studies.
ACKNOWLEDGMENTS
We are all employees of Wockhardt Research Centre.
We have no conflicts of interest to declare.
REFERENCES
- 1.Jacobs MR, Bajaksouzian S, Windau A, Appelbaum PC, Patel MV, Gupte SV, Bhagwat SS, De Souza NJ, Khorakiwala HF. 2004. In vitro activity of the new quinolone WCK 771 against Staphylococci. Antimicrob Agents Chemother 48:3338–3342. doi: 10.1128/AAC.48.9.3338-3342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum PC, Pankuch GA, Bozdogan B, Lin G, Jacobs MR, Patel MV, Gupte SV, Jafri MA, De Souza NJ, Khorakiwala HF. 2005. Activity of the new quinolone WCK 771 against pneumococci. Clin Microbiol Infect 11:9–14. doi: 10.1111/j.1469-0691.2004.01017.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel MV, De Souza NJ, Gupte SV, Jafri MA, Bhagwat SS, Chugh Y, Khorakiwala HF, Jacobs MR, Appelbaum PC. 2004. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob Agents Chemother 48:4754–4761. doi: 10.1128/AAC.48.12.4754-4761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue G, Crabb DM, Xiao L, Liu Y, Waites KB. 2018. In vitro activities of the benzoquinolizine fluoroquinolone levonadifloxacin (WCK 771) and other antimicrobial agents against mycoplasmas and ureaplasmas in humans, including isolates with defined resistance mechanisms. Antimicrob Agents Chemother 62:e01348-18. doi: 10.1128/AAC.01348-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhagwat SS, McGhee P, Kosowska-Shick K, Patel MV, Appelbaum PC. 2009. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob Agents Chemother 53:811–813. doi: 10.1128/AAC.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peric M, Jacobs MR, Appelbaum PC. 2004. Antianaerobic activity of a novel fluoroquinolone, WCK 771, compared to those of nine other agents. Antimicrob Agents Chemother 48:3188–3192. doi: 10.1128/AAC.48.8.3188-3192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flamm RK, Farrell DJ, Sader HS, Rhomberg PR, Jones RN. 2016. In vitro activity of WCK 771, a benzoquinolizine fluoroquinolone (levonadifloxacin) when tested against contemporary Gram-positive and -negative bacteria from a global surveillance program, poster Sunday-456. ASM Microbe 2016, Boston, MA. [Google Scholar]
- 8.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Yeole R, Patel A, Bhatia A. 2018. Intrapulmonary pharmacokinetics of levonadifloxacin following oral administration of alalevonadifloxacin to healthy adult subjects. Antimicrob Agents Chemother 62:e02297-17. doi: 10.1128/AAC.02297-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassoun A, Linden PK, Friedman B. 2017. Incidence, prevalence, and management of MRSA bacteremia across patient populations—a review of recent developments in MRSA management and treatment. Crit Care 21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claeys KC, Lagnf AM, Hallesy JA, Compton MT, Gravelin AL, Davis SL, Rybak MJ. 2016. Pneumonia caused by methicillin-resistant Staphylococcus aureus: does vancomycin heteroresistance matter? Antimicrob Agents Chemother 60:1708–1716. doi: 10.1128/AAC.02388-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SH, Liao WH, Lai CC, Liao CH, Tan CK, Wang CY, Huang YT, Hsueh PR. 2010. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J Antimicrob Chemother 65:1792–1798. doi: 10.1093/jac/dkq188. [DOI] [PubMed] [Google Scholar]
- 12.Nie W, Li B, Xiu Q. 2014. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother 69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 13.Zapater P, González-Navajas JM, Such J, Francés R. 2015. Immunomodulatory effects of antibiotics used in the prophylaxis of bacterial infections in advance cirrhosis. World J Gastroenterol 21:11493–11501. doi: 10.3748/wjg.v21.i41.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badari MS, Abd El-Fatah SG, Kamel SI, Mohamed AS. 2014. Immunomodulatory action of levofloxacin on cytokine production in adults with community-acquired pneumonia. Med J Cairo Univ 82:127–132. [Google Scholar]
- 15.Dalhoff A. 2005. Immunomodulatory activities of fluoroquinolones. Infection 33:55–70. doi: 10.1007/s15010-005-8209-8. [DOI] [PubMed] [Google Scholar]
- 16.Zeitlinger M, Marsik C, Steiner I, Sauermann R, Seir K, Jilma B, Wagner O, Joukhadar C. 2007. Immunomodulatory effects of fosfomycin in an endotoxin model in human blood. J Antimicrob Chemother 59:219–223. doi: 10.1093/jac/dkl464. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Dong L, Zhao D, Gao F, Yan J. 2019. Classical dendritic cells regulate acute lung inflammation and injury in mice with lipopolysaccharide-induced acute respiratory distress syndrome. Int J Mol Med 44:617–629. doi: 10.3892/ijmm.2019.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciragil P, Kurutas EB, Miraloglu M. 2014. New markers: urine xanthine oxidase and myeloperoxidase in the early detection of urinary tract infection. Dis Markers 2014:269362. doi: 10.1155/2014/269362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiaona A, Xiaotong S, Yonghao H, Xiaomei Y, Hongli C, Peng Z, Jianbo W. 2019. Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci Rep 9:2836. doi: 10.1038/s41598-019-39349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, He H, Zhang B, Chen Q, Yao S, Gui P. 2018. Amelioration of lipopolysaccharide-induced lung injury in rats by Na-H exchanger-1 inhibitor amiloride is associated with reversal of ERK mitogen-activated protein kinase. Biomed Res Int 2018:3560234. doi: 10.1155/2018/3560234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reber LL, Gillis CM, Starkl P, Jönsson F, Sibilano R, Marichal T, Gaudenzio N, Bérard M, Rogalla S, Contag CH, Bruhns P, Galli SJ. 2017. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J Exp Med 214:1249–1258. doi: 10.1084/jem.20161238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan A, Alsahli M, Rahmani A. 2018. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel) 6:E33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yelena L, Minsoo K. 2016. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets 15:19–28. doi: 10.2174/1871529x15666150108113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte W, Bernhagen J, Bucala R. 2013. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm 2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calbo E, Alsina M, Rodríguez-Carballeira M, Lite J, Garau J. 2008. Systemic expression of cytokine production in patients with severe pneumococcal pneumonia: effects of treatment with a β-lactam versus a fluoroquinolone. Antimicrob Agents Chemother 52:2395–2402. doi: 10.1128/AAC.00658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]