Balamuthia mandrillaris is an under-reported, pathogenic free-living amoeba that causes Balamuthia amoebic encephalitis (BAE) and cutaneous skin infections. Although cutaneous infections are not typically lethal, BAE with or without cutaneous involvement is usually fatal. This is due to the lack of drugs that are both efficacious and can cross the blood-brain barrier. We aimed to discover new leads for drug discovery by screening the open-source Medicines for Malaria Venture (MMV) Malaria Box and MMV Pathogen Box, with 800 compounds total.
KEYWORDS: phenotypic screening, drug discovery, Balamuthia mandrillaris, granulomatous amoebic encephalitis, Balamuthia amoebic encephalitis, antiparasitic agents, Balamuthia, MMV Malaria Box, MMV Pathogen Box
ABSTRACT
Balamuthia mandrillaris is an under-reported, pathogenic free-living amoeba that causes Balamuthia amoebic encephalitis (BAE) and cutaneous skin infections. Although cutaneous infections are not typically lethal, BAE with or without cutaneous involvement is usually fatal. This is due to the lack of drugs that are both efficacious and can cross the blood-brain barrier. We aimed to discover new leads for drug discovery by screening the open-source Medicines for Malaria Venture (MMV) Malaria Box and MMV Pathogen Box, with 800 compounds total. From an initial single point screen at 1 and 10 μM, we identified 54 hits that significantly inhibited the growth of B. mandrillaris in vitro. Hits were reconfirmed in quantitative dose-response assays and 23 compounds (42.6%) were confirmed with activity greater than miltefosine, the current standard of care.
INTRODUCTION
Balamuthia mandrillaris has been identified as one of the etiological agents that cause granulomatous amoebic encephalitis (GAE), also referred to as Balamuthia amoebic encephalitis (BAE) (1). The first report of Balamuthia causing disease in animals or humans was of a pregnant mandrill baboon at the San Diego Zoo in 1986, which died from encephalitis (2). Later, retrospective analysis of previous human cases diagnosed as encephalitis that were caused by Hartmanella, Acanthamoeba, or an unknown amoeba (1974 to 1989) led to the discovery of B. mandrillaris as the cause of BAE in humans (3). The difficulty with diagnosis, isolation, and axenic cultivation of Balamuthia from environmental sampling or infected patients was due to a lack of research and unknown requirements to support the growth of this opportunistic parasite in vitro. Unlike Naegleria fowleri or Acanthamoeba spp., B. mandrillaris does not grow on heat-killed bacteria on agar plates and requires a host cell monolayer on which to feed (4). Recently, a novel culture medium was developed that supported the growth and development of 10 B. mandrillaris environmental isolates. Balamuthia mandrillaris ITSON (BMI) medium is easily and cheaply prepared and can be used for axenization of environmental isolates, as well as supporting robust growth of B. mandrillaris trophozoites for drug discovery studies (5).
Since the discovery of Balamuthia as an etiological agent of disease in 1986, there has been a significant lack of drug discovery efforts to identify clinically effective drugs for the treatment of cutaneous skin lesions or BAE. In the United States, 109 Balamuthia cases in both immunocompetent and immunocompromised individuals have been reported, with at least twice that many occurring worldwide and inflicting disease (3, 6, 7). For the BAE infections in the US, only 9 cases have been successfully treated; all other documented cases were unsuccessful, thus giving a 92% mortality rate. There are several contributing factors that likely lead to the observed high mortality rates; these include the need for better diagnostics, increased awareness of disease, and discovery and development of new active therapeutics.
Herein we describe the results of screening 800 open-source compounds from the Medicines for Malaria (MMV) Malaria and Pathogen Boxes for activity against trophozoites of Balamuthia mandrillaris. From this effort, we discovered 23 compounds that serve as starting points for drug discovery against this neglected pathogen.
RESULTS
Optimal seeding densities.
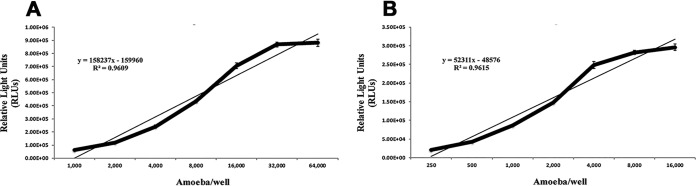
Balamuthia mandrillaris growth and development was standardized by seeding known concentrations of amoebae in triplicate in 96- and 384-well opaque white solid bottom (Thermo Fisher Scientific 136102 and Corning 3570) and clear plastic plates (Corning 3628 and Nunc 242757). Optimal seeding concentrations were defined as the maximum relative light units (RLUs) produced while B. mandrillaris cells were still in the logarithmic phase. The seeding densities were found to be 16,000 cells per well and 4,000 cells per well for 96- and 384-well plates, respectively (Fig. 1).
FIG 1.
Optimal seeding densities of B. mandrillaris trophozoites for drug susceptibility testing in vitro in 96- and 384-well plates. RLU was determined in white plates, microscopy was performed in clear plates. Using RLU production, timing of the logarithmic phase, and cell health as determined by microscopy, for 96-well plates (left) we determined an optimum of 16,000 B. mandrillaris per well and for 384-well plates (right) we determined an optimum of 4,000 B. mandrillaris per well for 72 h experimentation within all future drug experiments.
Screening results for initial activity.
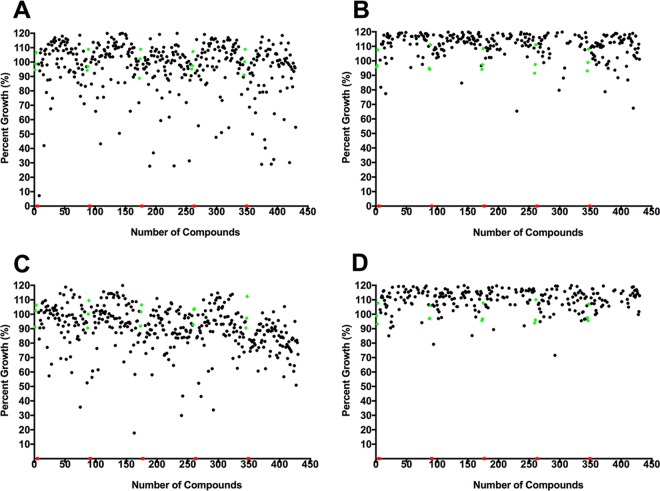
Two libraries comprised of 400 compounds each were assembled at 10 mM in dimethyl sulfoxide (DMSO) by Medicines for Malaria Venture (MMV, Switzerland) for open-access pathogen screening. Initially, we screened each sample at 10 and 1 μM against trophozoites of pathogenic B. mandrillaris (V039). We identified one inhibitor, MMV007875, that inhibited amoeba growth between 33 and 67% at 1 μM and a further 53 inhibitors that produced similar inhibition or better at 10 μM. Of these 54 hits, 27 compounds were from the MMV Malaria Box and 27 were from the MMV Pathogen Box (Fig. 2).
FIG 2.
Results from primary single-point screen of Malaria and Pathogen Box compounds at 10 and 1 μM. Single-point assays of MMV Malaria Box (A and B) and MMV Pathogen Box (C and D) compounds from the Medicines for Malaria Venture (MMV) at 10 μM and 1 μM, respectively. All screening was carried out using the pathogenic B. mandrillaris V039 isolate. Each black point represents individual compounds that were screened, the red points are positive controls (chlorhexidine at 12.5 μM), and the green points are negative controls (0.1% DMSO [A and C] or 0.01% DMSO [B and D]) included in each plate. We identified 54 hits from the MMV total library, with 27 hits from the MMV Malaria Box and 27 hits from the MMV Pathogen Box.
Quantitative dose-response assays to confirm hits.
We used viability, as determined by the CellTiter-Glo assay, as the endpoint for quantitative dose-response assays to validate potency of the identified compounds against B. mandrillaris. All 54 hits from the primary screen were assessed in duplicate, 2-fold serial dilutions to determine the 50% inhibitory concentration (IC50). Of the 54 samples tested, 23 (42.6%) compounds were reconfirmed and validated with IC50s of <10 μM. Of these, 17 (63%) were from the Malaria Box and 6 (22%) from the Pathogen Box. Pentamidine was included in the boxes and the activity we observed for this standard drug served as a control for the studies. The lists of confirmed hits are described in Table 1 and the initial 54 hits are documented in Table S1 in the supplemental material.
TABLE 1.
Confirmed hits with activity against trophozoites of Balamuthia mandrillaris in vitroa
| MMV ID | IC50 (μM) ± SEM | Disease set/companyb | Synonym(s) | Mol wt (Da) | ACD/LogPc |
|---|---|---|---|---|---|
| MMV665941 | 2.93 ± 0.03 | St. Jude | SJ000140980; methylrosaniline; gentian violet | 389.5 | 4.91 |
| MMV007875 | 3.14 ± 0.04 | GNF; GSK | GNF-Pf-376; TCMDC-124236 | 298.8 | 4.12 |
| MMV006169 | 3.42 ± 0.53 | GNF | GNF-Pf-4180 | 326.4 | 3.63 |
| MMV676401 | 3.44 ± 0.21 | Tuberculosis | Several; CHEMBL1622353 | 308.4 | 2.68 |
| MMV676588 | 3.5 ± 0.7 | Tuberculosis | Several; CHEMBL1504142 | 258.4 | 5.93 |
| MMV000304 | 3.71 ± 1.06 | St. Jude | SJ000026232 | 314.4 | 4.39 |
| MMV019110 | 3.75 ± 0.69 | GSK | TCMDC-123639 | 250.3 | 1.89 |
| MMV011522 | 4.12 ± 1.27 | GNF; St. Jude | GNF-Pf-1374; SJ000201287 | 284.7 | 2.92 |
| MMV009085 | 4.38 ± 1.33 | GNF | GNF-Pf-3184 | 410.4 | −1.30 |
| MMV006522 | 4.44 ± 1.25 | GNF | GNF-Pf-2807 | 357.2 | 6.06 |
| MMV676512 | 4.65 ± 1.14 | Tuberculosis | GSK1691553A; TCMDC-142919 | 347.4 | 2.73 |
| MMV676602 | 4.85 ± 0.79 | Kinetoplastids | Milciclib; PHA-848125 | 460.6 | 1.75 |
| MMV080034 | 4.89 ± 0.68 | Commercial library | Several; CHEMBL1623028 | 254.3 | 1.92 |
| MMV687749 | 4.95 ± 0.34 | Tuberculosis | CHEMBL3637866 | 412.5 | 5.28 |
| MMV000326 | 4.97 ± 0.77 | St. Jude | SJ000029230 | 352.8 | 5.95 |
| MMV019199 | 5.23 ± 0.56 | GSK | TCMDC-123746 | 266.3 | 1.69 |
| MMV006825 | 5.41 ± 0.38 | GNF | GNF-Pf-2776 | 320.4 | 3.15 |
| MMV019918 | 5.57 ± 0.31 | GSK | TCMDC-124617 | 383.7 | 4.28 |
| MMV019690 | 5.84 ± 0.84 | GSK | TCMDC-124340 | 470.6 | 3.69 |
| MMV019017 | 5.89 ± 0.03 | GSK | TCMDC-123535 | 367.3 | 4.82 |
| MMV001041 | 5.99 ± 0.46 | GNF; St. Jude | GNF-Pf-3259; SJ000201788 | 349.2 | 3.21 |
| MMV665810 | 6.15 ± 0.33 | GSK; St. Jude | Imidocarb dipropionate; TCMDC-124304; SJ000113875 | 348.4 | −0.45 |
| MMV675993 | 6.35 ± 0.67 | Cryptosporidiosis | CHEMBL1242290 | 313.4 | 1.79 |
| MMV000062 | 20.65 ± 2.39 | Sigma-Aldrich | Pentamidine; GNF-Pf-3680; CHEMBL55 | 340.4 | 2.47 |
| MMV000062 | 20.91 ± 2.32 | Sigma-Aldrich | Pentamidine; GNF-Pf-3680; CHEMBL55 | 340.4 | 2.47 |
Sample size n = 2.
GNF, Genomics Institute of the Novartis Research Foundation; GSK, GlaxoSmithKline; St. Jude, St. Jude Children's Research Hospital.
ACD/LogP, partition coefficient.
DISCUSSION
Cutaneous as well as severe central nervous system infections with B. mandrillaris have been diagnosed from immunocompetent as well as immunocompromised patients, and treatment of BAE has been with antiviral, antibacterial, and antifungal drugs. Unfortunately, these drugs are not very effective and the mortality rate still remains high at ∼92%. Miltefosine, the newest drug added to the cocktail of drugs used to treat various pathogenic free-living amoebae infections has not improved the treatment of BAE (3). Clearly new drugs are needed and the dire situation is compounded by the lack of known starting points for new drug discovery or new repurposed drugs with good potency. In this study, we have used the Cell-Titer Glo assay previously developed for N. fowleri drug evaluation to screen 800 open-source compounds from the Medicines for Malaria Venture (8). These compounds included known bioactive compounds with drug-like or probe-like properties. The goal of screening these libraries was to provide starting points for drug discovery against neglected tropical diseases, as this method has been highly successful for other diseases (9).
A significant problem hampering drug discovery for pathogenic free-living amoebae is the lack of good, bioactive chemical starting points to fuel the drug discovery pipeline. As shown in Table 1, we validated 23 compounds that produce IC50s of <6.35 μM. All of these compounds are more potent than the standard drugs pentamidine and miltefosine against trophozoites of B. mandrillaris. Included in the validated hits are compounds with known activity against mycobacteria, kinetoplastids, and Plasmodium spp. Interestingly, the hits from a previous screen of N. fowleri with the MMV Malaria box set do not overlap the B. mandrillaris hits described herein (10). These data suggest that unbiased screening of compound libraries to discover a new drug for BAE is a preferable strategy over prescreening for activity against another amoeba. In fact, the data thus far suggest that finding a “magic bullet” that targets multiple pathogenic free-living amoebae may not be possible.
An important consideration for prioritizing the compounds we identified is relative potency against the amoeba. Given that the drugs currently used to treat BAE are not very potent (e.g., IC50s of >10 μM), we propose that good starting points for hit-to-lead projects should be activity in the low μM range (e.g., IC50s of <10 μM), molecular weights of < 500 Da, reasonable physiochemical properties, and absence of having known pan-assay interference compounds (PAINS) with nonspecific activity against multiple cell types. From our list of validated hits in Table 1, there are several interesting compounds that meet these criteria and could be used as starting points for hit-to-lead studies. Many of the hits with anti-Balamuthia activity were previously shown to be active against P. falciparum asexual blood stages (9). For example, MMV007875 is a methylquinoline-4-amine that is active against P. falciparum (IC50 = 0.95 μM) and had an IC50 of 3.14 μM against the amoebae. Importantly, only one hit (gentian violet) from this study has any potential for repurposing, given that a topical formulation is used as an antifungal and could be used for treating cutaneous infections with B. mandrillaris. The rest of the validated hits are more likely to serve as inspiration for hit-to-lead studies or to identify potential mechanisms of action for prioritizing target-based drug discovery.
Despite the high mortality rate of BAE and the lack of effective therapeutics, there are few studies that focus on discovery of new drugs for this disease. In a recent screen of 2,177 FDA-approved compounds, nitroxoline was identified as a possible drug for repurposing (11). Nitroxoline is an aminoquinoline derivative with antimicrobial activity, perhaps via metal chelation. Twenty structural analogs of nitroxoline were tested, yet the parent compound was the most potent with an IC50 of 4.77 μM (11). Interestingly, we identified numerous drugs with better or similar potency as nitroxoline and all were more potent than pentamidine. Interestingly, MMV007875 is a methylquinoline-4-amine derivative that we found to be more potent (IC50 = 3.14 μM) than nitroxoline.
In summary, we validated 23 compounds with activities of ≤ 6.35 μM potency with potential to accelerate drug discovery for BAE. In addition to using these scaffolds to initiate structure-activity optimization studies, these compounds could also be used as chemical probes for discovering potential targets and could provide opportunities for structure-based drug design of new drugs with specific activity against these neglected pathogenic amoebic infections.
MATERIALS AND METHODS
Maintenance of Balamuthia mandrillaris.
The pathogenic Balamuthia mandrillaris (CDC V039; ATCC 50209), a granulomatous amoebic encephalitis (GAE) strain, was isolated from a pregnant baboon at the San Diego Zoo, San Diego, USA, 1986. Trophozoites were routinely grown axenically at 37°C in BMI (5). Balamuthia mandrillaris ITSON (BMI) medium is composed of bacto casitone (20 g/liter, pancreatic digest of casein from animal origin) and 68 ml/liter of Hanks’ balanced salts (12.61 mM CaCl2 [1.4 g/liter]; 4.93 mM MgCl2·6H2O [1 g/liter]; 4.06 mM MgSO4·7H2O [1 g/liter]; 53.33 mM KCl [4 g/liter]; 4.41 mM KH2PO4 [600 mg/liter]; 1.38 M NaCl [8 g/liter]; 3.36 mM Na2HPO4·7H2O [900 mg/liter]; 55.55 mM d-glucose [dextrose] [10 g/liter]) in 912 ml of deionized water. After autoclaving, complete BMI medium was produced by the addition of 10% fetal bovine serum and 125 μg of penicillin/streptomycin antibiotics. All experiments were performed using logarithmic phase trophozoites.
Optimal seeding densities.
Optimal seeding densities were performed in BMI complete medium using logarithmic phase trophozoites. B. mandrillaris was routinely cultured as described above and, once the trophozoites were 90% confluent, the seeding density of Balamuthia mandrillaris was standardized using the CellTiter-Glo 2.0 luminescent viability assay (Promega, Madison, WI) described by Rice et al. for Naegleria fowleri (8). In brief, Balamuthia trophozoites cultured in BMI complete medium were seeded in triplicate at cell concentrations varying from 1 × 104 to 6.4 × 105 cells/ml for 96-well white plates and 5 × 103 to 3.2 × 105 cells/ml for 384-well white plates to determine the concentration of cells that produce the most luminescence while still in the logarithmic stage of growth. Total well volumes for 96-well plates were 100 μl and for 384-well plates were 40 μl. Optimization was standardized over a period of 72 h at 37°C. At the 72-h time point, 25 μl or 15 μl of CellTiter-Glo 2.0 reagent (Promega, Madison, WI) was added to all wells of the 96-well or 384-well plates, respectively. Plates were protected from light and contents were mixed using an orbital shaker at 300 rpm at room temperature for 2 min to induce cell lysis. After shaking, the plates were equilibrated at room temperature for 10 min to stabilize the luminescent signal. The ATP luminescent signal (relative light units; RLUs) were measured at 490 nm by using a SpectraMax i3X (Molecular Devices, Sunnyvale, CA). The same cell concentrations were plated into clear well plates for microscopic analysis and imaging by microscopy (data not shown).
Compound libraries.
The Malaria Box (https://www.mmv.org/mmv-open/malaria-box/about-malaria-box) was distributed as an open-source drug library comprised of 400 drug-like and probe-like compounds. The criteria for drug placement into this box included a broad diversity of compounds with commercial availability and confirmed nanomolar to low micromolar antimalarial activity. Over 250 copies of this drug box have been distributed globally to 30 countries. The MMV Pathogen Box (https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box) is also an open-source drug library comprising of 400 diverse compounds specifically comprised of molecules that are biologically active against several neglected tropical diseases. To date 315 copies of the Pathogen Box have been distributed globally to 44 countries. All stock compounds were supplied at 10 mM in dimethyl sulfoxide (DMSO).
In vitro CellTiter-Glo trophocidal assay.
The trophocidal activity of compounds was assessed using the CellTiter-Glo 2.0 luminescent viability assay (Promega, Madison, WI). In brief, B. mandrillaris trophozoites cultured in BMI-complete media were seeded at 16,000 cells/well into 96-well plates (Costar 3370). Initially all compounds were assessed in a single-point drug screen at 10 and 1 μM. Using the Biomek NXP workstation, 1 μl of a 10 mM stock from the MMV drug plates was diluted into 99 μl of BMI incomplete media to produce an initial stock mother plate at a 100 μM concentration. Screening plates (Thermo Fisher Scientific 136102) were made by the addition of 10 and 1 μl of drugs from these initial mother stock plates. A further 9 μl of BMI-incomplete medium was added into the screening plates that received 1 μl of drug by using the Biomek workstation. Next, 90 μl of 1.77 × 105 B. mandrillaris cells/ml (16,000 cells/well) was seeded into these plates. Inhibitors were assessed for % inhibition using the criteria of: ≤33% growth inhibition (no inhibition); 33 to 67% growth inhibition (moderate inhibition); and ≥67% growth inhibition (strong inhibition). Control wells were supplemented with 0.1% (10 μl screening plate) or 0.01% (1 μl screening plate) DMSO or 12.5 μM chlorhexidine, as negative and positive controls, respectively. All inhibitors that met the predetermined criteria of inhibiting parasite growth by ≥33% were next confirmed in quantitative dose-response assays. Hit compounds were retrieved from original assay plates, dissolved in BMI-incomplete medium, and assessed in 2-fold serial dilutions from the highest concentration of 10 μM. Control wells were supplemented with 0.1% DMSO or 12.5 μM chlorhexidine, as negative and positive controls, respectively. All assays were incubated at 37°C for 72 h. At the 72-h time point, 25 μl of CellTiter-Glo 2.0 reagent was added to all wells of the 96-well plates using the Biomek workstation. The plates were protected from light and contents were mixed using an orbital shaker at 300 rpm at room temperature for 2 min to induce cell lysis. After shaking, the plates were equilibrated at room temperature for 10 min to stabilize the luminescent signal. The ATP luminescent signal (relative light units; RLUs) was measured at 490 nm by using a SpectraMax i3X (Molecular Devices, Sunnyvale, CA). Drug inhibitory concentration (IC50) curves were generated using total ATP RLUs, where controls were calculated as the average of replicates using the Levenberg-Marquardt algorithm, using DMSO as the normalization control, as defined in CDD Vault (Burlingame, CA, USA). Values reported are from a minimum of two biological replicates with standard error of the mean.
Statistical analysis.
We used the Z’-factor as a statistical measurement to assess the quality of our medium- to high-throughput screening assays (12). This factor uses the mean and standard deviation values of the positive and negative controls to assess data quality. The robustness of all of the plates screened had an excellent Z’-score value of 0.998 or above.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Belen Cassera, University of Georgia, for providing a copy of the MMV Malaria Box.
This study was supported by the Georgia Research Alliance.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Schuster FL, Visvesvara GS. 2004. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat 7:41–51. doi: 10.1016/j.drup.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M. 1990. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 28:2750–2756. doi: 10.1128/JCM.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, Moser M, Puttagunta R, Yoder JS, Ali IK, Roy SL. 2019. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. Clin Infect Dis 68:1815–1822. doi: 10.1093/cid/ciy813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lares-Jiménez LF, Booton GC, Lares-Villa F, Velázquez-Contreras CA, Fuerst PA. 2014. Genetic analysis among environmental strains of Balamuthia mandrillaris recovered from an artificial lagoon and from soil in Sonora, Mexico. Exp Parasitol 145 Suppl:S57–S61. doi: 10.1016/j.exppara.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Lares-Jiménez LF, Gámez-Gutiérrez RA, Lares-Villa F. 2015. Novel culture medium for the axenic growth of Balamuthia mandrillaris. Diagn Microbiol Infect Dis 82:286–288. doi: 10.1016/j.diagmicrobio.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Stidd DA, Root B, Weinand ME, Anton R. 2012. Granulomatous amoebic encephalitis caused by Balamuthia mandrillaris in an immunocompetent girl. World Neurosurg 78:715.e7-12. doi: 10.1016/j.wneu.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Jung S, Schelper RL, Visvesvara GS, Chang HT. 2004. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med 128:466–468. doi:. [DOI] [PubMed] [Google Scholar]
- 8.Rice CA, Colon BL, Alp M, Goker H, Boykin DW, Kyle DE. 2015. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob Agents Chemother 59:2037–2044. doi: 10.1128/AAC.05122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Voorhis WC, Adams JH, Adelfio R, Ahyong V, Akabas MH, Alano P, Alday A, Alemán Resto Y, Alsibaee A, Alzualde A, Andrews KT, Avery SV, Avery VM, Ayong L, Baker M, Baker S, Ben Mamoun C, Bhatia S, Bickle Q, Bounaadja L, Bowling T, Bosch J, Boucher LE, Boyom FF, Brea J, Brennan M, Burton A, Caffrey CR, Camarda G, Carrasquilla M, Carter D, Belen Cassera M, Chih-Chien Cheng K, Chindaudomsate W, Chubb A, Colon BL, Colón-López DD, Corbett Y, Crowther GJ, Cowan N, D'Alessandro S, Le Dang N, Delves M, DeRisi JL, Du AY, Duffy S, Abd El-Salam El-Sayed S, Ferdig MT, Fernández Robledo JA, Fidock DA, Florent I, Fokou PVT, Galstian A, Gamo FJ, Gokool S, Gold B, Golub T, Goldgof GM, Guha R, Guiguemde WA, Gural N, Guy RK, Hansen MAE, Hanson KK, Hemphill A, Hooft van Huijsduijnen R, Horii T, Horrocks P, Hughes TB, Huston C, Igarashi I, Ingram-Sieber K, Itoe MA, Jadhav A, Naranuntarat Jensen A, Jensen LT, Jiang RHY, Kaiser A, Keiser J, Ketas T, Kicka S, Kim S, Kirk K, Kumar VP, Kyle DE, Lafuente MJ, Landfear S, Lee N, Lee S, Lehane AM, Li F, Little D, Liu L, Llinás M, Loza MI, Lubar A, Lucantoni L, Lucet I, Maes L, Mancama D, Mansour NR, March S, McGowan S, Medina Vera I, Meister S, Mercer L, Mestres J, Mfopa AN, Misra RN, Moon S, Moore JP, Morais Rodrigues da Costa F, Müller J, Muriana A, Nakazawa Hewitt S, Nare B, Nathan C, Narraidoo N, Nawaratna S, Ojo KK, Ortiz D, Panic G, Papadatos G, Parapini S, Patra K, Pham N, Prats S, Plouffe DM, Poulsen S-A, Pradhan A, Quevedo C, Quinn RJ, Rice CA, Abdo Rizk M, Ruecker A, St Onge R, Salgado Ferreira R, Samra J, Robinett NG, Schlecht U, Schmitt M, Silva Villela F, Silvestrini F, Sinden R, Smith DA, Soldati T, Spitzmüller A, Stamm SM, Sullivan DJ, Sullivan W, Suresh S, Suzuki BM, Suzuki Y, Swamidass SJ, Taramelli D, Tchokouaha LRY, Theron A, Thomas D, Tonissen KF, Townson S, Tripathi AK, Trofimov V, Udenze KO, Ullah I, Vallieres C, Vigil E, Vinetz JM, Voong Vinh P, Vu H, Watanabe N-A, Weatherby K, White PM, Wilks AF, Winzeler EA, Wojcik E, Wree M, Wu W, Yokoyama N, Zollo PHA, Abla N, Blasco B, Burrows J, Laleu B, Leroy D, Spangenberg T, Wells T, Willis PA. 2016. Open source drug discovery with the Malaria Box compound collection for neglected diseases and beyond. PLoS Pathog 12:e1005763. doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colon BL, Rice CA, Guy RK, Kyle DE. 2019. Phenotypic screens reveal posaconazole as a rapidly acting amebicidal combination partner for treatment of primary amoebic meningoencephalitis. J Infect Dis 219:1095–1103. doi: 10.1093/infdis/jiy622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurie MT, White CV, Retallack H, Wu W, Moser MS, Sakanari JA, Ang K, Wilson C, Arkin MR, DeRisi JL. 2018. Functional assessment of 2,177 U.S. and international drugs identifies the quinoline nitroxoline as a potent amoebicidal agent against the pathogen Balamuthia mandrillaris. mBio 9:e02051-18. doi: 10.1128/mBio.02051-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.