Scabies is a frequent cutaneous infection caused by the mite Sarcoptes scabiei in a large number of mammals, including humans. As the resistance of S. scabiei against several chemical acaricides has been previously documented, the establishment of alternative and effective control molecules is required. In this study, the potential acaricidal activity of beauvericin was assessed against different life stages of S. scabiei var. suis and in comparison with dimpylate and ivermectin, two commercially available molecules used for the treatment of S. scabiei infection in animals and/or humans.
KEYWORDS: Sarcoptes scabiei, acaricide, beauvericin, in vitro test, mycotoxin, scabies
ABSTRACT
Scabies is a frequent cutaneous infection caused by the mite Sarcoptes scabiei in a large number of mammals, including humans. As the resistance of S. scabiei against several chemical acaricides has been previously documented, the establishment of alternative and effective control molecules is required. In this study, the potential acaricidal activity of beauvericin was assessed against different life stages of S. scabiei var. suis and in comparison with dimpylate and ivermectin, two commercially available molecules used for the treatment of S. scabiei infection in animals and/or humans. The toxicity of beauvericin against cultured human fibroblast skin cells was evaluated using an MTT proliferation assay. In our in vitro model, developmental stages of S. scabiei were placed in petri dishes filled with Columbia agar supplemented with pig serum and different concentrations of the drugs. Cell sensitivity assays demonstrated low toxicity of beauvericin against primary human fibroblast skin cells. At 0.5 and 5 mM, beauvericin showed higher activity against adults and eggs of S. scabiei compared to dimpylate and ivermectin. These results revealed that the use of beauvericin is promising and might be considered for the treatment of S. scabiei infection.
INTRODUCTION
Scabies is a frequent cutaneous infection caused by the mite Sarcoptes scabiei in a large number of mammals, including humans (1). Human scabies was recently recognized as a neglected tropical disease by the WHO, due to its high global prevalence, estimated to be around 100 million to 200 million cases a year (2), and high morbidity (3). Although primary infection with S. scabiei is limited to severe itching and allergic rash, secondary infections with bacteria such as group A streptococcus or Staphylococcus aureus could lead to severe acute infectious complications and even death (4)—hence, the importance of early and efficient eradication of the mites (5). There is a limited number of drugs that can be used for the treatment of scabies. Furthermore, the resistance of S. scabiei to some of the drugs is emerging, caused by reinfection or incorrect use of acaricides, and may lead to an excessive and random use of treatments posing a threat to patient health (6). Less susceptible populations of S. scabiei may emerge from the repeated exposure to a single type of acaricide. The resistance development may involve a mutation to the target site of the acaricide molecule or an upregulation for genes encoding detoxification enzymes. Therefore, the development of new acaricides with a new mode of action against S. scabiei is required.
Many secondary metabolites produced by fungi have been used in medicine and agriculture (7). The entomopathogenic fungus Beauveria bassiana is known to produce beauvericin, a secondary metabolite belonging to the enniatin family (8). This cyclic hexadepsipeptide was proven to have many biological effects, including insecticidal, antitumor, antibacterial, and antifungal activity (9). Its mechanism of action is thought to be ionophore-induced apoptosis and DNA fragmentation (7). Accordingly, beauvericin could be considered a potential new acaricide for the treatment of human and animal scabies. The objective of this study was to evaluate the in vitro efficacy of beauvericin against the different life stages of S. scabiei. In addition, this study evaluated the toxicity of beauvericin against cultured human fibroblast skin cells.
RESULTS
The cytotoxic effect of beauvericin was assessed at 48 h postexposure. The mycotoxin caused a dose-dependent reduction in cell viability, and a 50% lethal concentration (LC50) was calculated. The human fibroblast cells were moderately sensitive to beauvericin toxicity, and the LC50 was 4.8 μM. Based on this result, two concentrations were selected (0.5 and 5 μM) for in vitro efficacy tests.
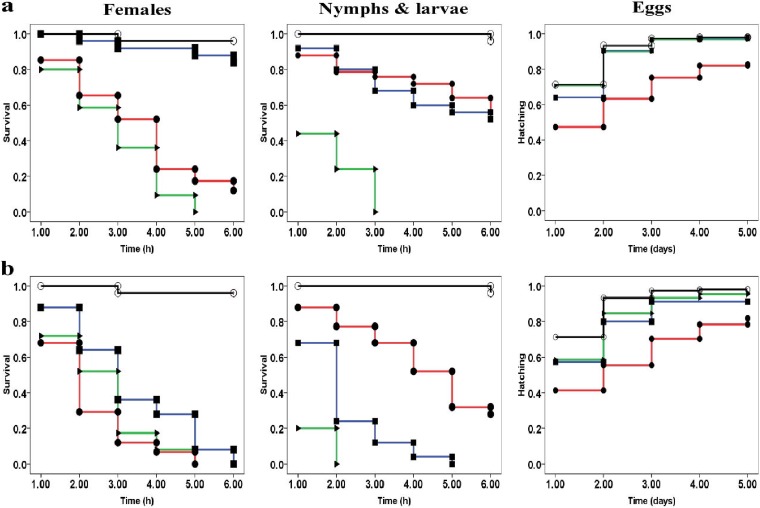
The three molecules, beauvericin, dimpylate, and ivermectin, were highly efficient against motile stages of S. scabiei mites (Table 1 and Fig. 1). The mortality rates in the control group were below 5% during the first 8 h postexposure. The survival and hatching curves of mites and eggs exposed to different drugs are presented in Fig. 1. In all tests, significant differences were found between each molecule and the control, except for the tests against S. scabiei eggs with 0.5 μM dimpylate and ivermectin. Overall, a differential effect between the molecules and the concentration being used was notable 1 h postexposure (Table 2).
TABLE 1.
Lethal time (LT50) to kill Sarcoptes scabiei females, nymphs, and larvae or eggs and statistical differences between data obtained with each drug (beauvericin, dimpylate, and ivermectin)a
| Drugs and concentrations | Females |
Nymphs and larvae |
Eggs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| X2 | P | LT50 ± SE (h) | X2 | P | LT50 ± SE (h) | X2 | P | HT50 ± SE (h) | |
| Beauvericin | |||||||||
| 0.5 μM | 112 | <0.05 | 3.4 ± 0.1 | 34.4 | <0.05 | 4.7 ± 0.2 | 39 | <0.05 | 2.3 ± 0.1 |
| 5 μM | 159.7 | <0.05 | 1.9 ± 0 | 79 | <0.05 | 4.1 ± 0.2 | 54.2 | <0.05 | 2.5 ± 0.1 |
| Dimpylate | |||||||||
| 0.5 μM | 5.9 | <0.05 | 5.6 ± 0.17 | 39.7 | <0.05 | 4.5 ± 0.2 | 1.3 | >0.05 | 1.5 ± 0 |
| 5 μM | 154.3 | <0.05 | 3.2 ± 0.1 | 172 | <0.05 | 2 ± 0.1 | 12.1 | <0.05 | 1.8 ± 0 |
| Ivermectin | |||||||||
| 0.5 μM | 151.4 | <0.05 | 2.8 ± 0.1 | 166.5 | <0.05 | 1.6 ± 0 | 0.3 | >0.05 | 1.4 ± 0 |
| 5 μM | 156.8 | <0.05 | 2.4 ± 0.1 | 166.9 | <0.05 | 1.2 ± 0 | 6.5 | <0.05 | 1.6 ± 0 |
X2, chi-square value; LT50, lethal time to kill 50% of mites; HT50, median time of 50% hatching of the eggs.
FIG 1.
Curves representing survival (of motile stages) and hatching (of eggs) of S. scabiei exposed to acaricide molecules at two concentrations, (a) 0.5 μM and (b) 5 μM. For each treatment, observed survival and hatching are presented using curves. Red lines with filled circles, beauvericin; blue lines with filled squares, dimpylate; green lines with filled triangles, ivermectin; black lines with open circles, control.
TABLE 2.
Concentrations of different molecules required to kill 50% of S. scabiei mites (females, nymphs/larvae and eggs)a,b
| Molecule |
LC50 (μM) ± SE for time points: |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h |
2 h |
3 h |
4 h |
5 h |
6 h |
7 h |
8 h |
5 days |
|||||||||
| Acaricide |
Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Females | Nymphs/larvae | Eggs |
| Beauvericin | 34.7 ± 3.9c | 48.0 ± 1.7b | 7.8 ± 5.1a | 39.5 ± 3b | 2 ± 0.9a | 28.4 ± 2.6b | 0.7 ± 0.2b | 18.9 ± 1.9b | 0.3 ± 0b | 13.7 ± 1.3b | 0.3 ± 0b | 10.4 ± 1.2b | 0.3 ± 0a | 1.7 ± 0.6b | 0.3 ± 0a | 0.9 ± 0a | 77.5 ± 1.8a |
| Dimpylate | 7.09 ± 0a | 6.9 ± 1a | 5.9 ± 0.2a | 3.3 ± 0.2a | 4.1 ± 0.1a | 2.3 ± 0.7a | 3.9 ± 0.1a | 1.7 ± 0a | 2.5 ± 0.2a | 0.5 ± 0a | 1.5 ± 0.1a | 0.5 ± 0a | 1.1 ± 0.4a | 0.4 ± 0a | 0.8 ± 0.1b | 0.4 ± 0a | 77.5 ± 1.8a |
| Ivermectin | 45.1 ± 0.9b | 5.7 ± 0.8a | 26 ± 1.5b | 0.4 ± 0a | 4.7 ± 3.9a | 0.3 ± 0a | 0.3 ± 0b | 0.3 ± 0a | 0.3 ± 0b | 0.3 ± 0a | 0.3 ± 0b | 0.3 ± 0a | 0.3 ± 0a | 0.3 ± 0a | 0.3 ± 0a | 0.3 ± 0a | 202.6 ± 16.5b |
Note: LC50: lethal concentration to kill 50% of mites.
Values followed by the same superscript letter in the same column are not significantly different at the 5% threshold.
The molecules can be put into the following order based on the chi-square value of their efficacy: beauvericin > ivermectin > dimpylate and ivermectin > dimpylate > beauvericin against females and immature forms (Table 1). The survival capacity seemed to be different according to the developmental stage of the mites. The efficacy of dimpylate and ivermectin seemed to be higher on S. scabiei immature motile stages, whereas that of beauvericin seemed to be higher on females at both concentrations (Table 1).
The activity of all three molecules against S. scabiei eggs was evaluated for 5 days. Results obtained with beauvericin (at 0.5 and 5 μM), dimpylate (at 5 μM), or ivermectin (at 5 μM) were significantly different from those in the control group (Table 1). Among all the molecules tested against the eggs of S. scabiei, beauvericin demonstrated the best inhibition of hatching when testing a concentration of 5 μM. The lowest activity was recorded at a concentration of 0.5 μM of dimpylate or ivermectin, with no significant statistical difference compared to the negative control (Table 1).
Time necessary to kill half of the mite’s population (LT50) values were different between treatment groups against the motile stages of S. scabiei (Table 1). The highest LT50 values (5.6 and 4.7 h) were observed with a concentration of 0.5 μM of beauvericin and dimpylate against females and nymphs/larvae, respectively. The lowest LT50 values (1.1 and 1 h) were observed with a concentration of 50 μM of dimpylate and ivermectin against females and nymphs/larvae, respectively. The median time for hatching of 50% of the eggs was also recorded in this study (Table 1).
A significant difference was recorded between LC50 values of the drugs at 1 h (F [variance ratio] = 68.779, df = 2, P < 0.05), 2 h (F = 12.809, df = 2, P < 0.05), 4 h (F = 145.902, df = 2, P < 0.05), 5 h (F = 59.758, df = 2, P < 0.05), and 6 h postexposure against females (F = 12.809, df = 2, P < 0.05). The effect of the drugs was not notable 3 h postexposure against females (F = 0.39, df = 2, P > 0.05). A significant difference was also notable between the LC50 values of molecules at any given time during the test against larvae 1 h (F = 367.273, df = 2, P < 0.05), (F = 1423.942, df = 2, P < 0.05), 3 h (F = 108.356, df = 2, P < 0.05), 4 h (F = 86.263, df = 2, P < 0.05), 5 h (F = 94.776, df = 2, P < 0.05), and 6 h postexposure (F = 68.387, df = 2, P < 0.05). A significant difference was recorded between the LC50 values of the molecules against eggs at 5 days postexposure (F = 42.709, df = 2, P < 0.05).
DISCUSSION
Data about beauvericin cytotoxicity, especially against skin cell lines, are lacking. This lack of data could explain why there are no maximum guidance levels. The present study assessed for the first time the cytotoxic activity of beauvericin against primary fibroblast human skin cells. Heilos et al. (10) investigated the cytotoxic activity of beauvericin against the principal constituent of the epidermis, HEK2 keratinocyte (IC50 = 5.4 μM). The impact of beauvericin was also assessed against several nucleated human cells (IC50s included human intestinal cell line Caco 2 [3.9 μM], human liver cell line HEPG2 [3.4 μM], and human normal vascular endothelial cells [HUVEC; 2.4 μM]) (10). The present study demonstrated that the therapeutic index of beauvericin for scabies infection could be high. The susceptible dose against motile stages of S. scabiei are extremely low compared that of all human cell lines. Furthermore, a study conducted by Taevernier et al. (11) demonstrated that the beauvericin concentration was 21 times higher in the epidermis than in the dermis after topical application of the mycotoxin. Transdermal kinetics is mediated by the outermost layer of the skin, providing a protective reservoir for cyclic depsipeptides (11).
The mortality rate in the control groups was low (<5%) during the first 8 h of the test, indicating suitable conditions of the bio-assays. The survival of S. scabiei outside its host is considered to be the first limitation of in vivo studies. After 24 h, the “natural” mortality rate at room temperature reached 19%, which indicates that mortalities recorded at 24 h may not be caused only by the acaricide effect of the tested molecules; therefore, results recorded at 24 h postexposure were discarded.
The efficacies of macrocyclic lactones (including ivermectin) or organophosphates (such as dimpylate) against S. scabiei have been previously evaluated (12–15). However, most studies evaluated the efficacy of such molecules against motile stages of S. scabiei with no differentiation of the life stage. Studies evaluating the efficacy of acaricides against specific life stages of S. scabiei are few. The present study demonstrated that the application of dimpylate and ivermectin caused lower mortality rates among females than among immature forms. These results are in accordance with those from Mounsey et al. (16), who observed that S. scabiei nymphs and larvae were more vulnerable than females to ivermectin and moxidectin. Generally, larvae are more sensitive due to their greater surface area/volume ratio, meaning more drug is absorbed through the cuticle during in vitro exposure. On the contrary, in the present study, S. scabiei females seemed to be more susceptible to beauvericin than immature forms.
The resistance of S. scabiei to commercial products exists and might increase in the future, whereas treatment failures of scabies infections in animals and humans have been previously reported (15, 17–19). Beauvericin is known to be an ionophoric cyclodepsipeptide which forms complexes with cations and increases the permeability of biological membranes (20–22). Given the nonsimilarity of its mode of action to that of the commonly used neuro-inhibitors, a cross-resistance of S. scabiei mites against beauvericin is unlikely to happen.
The three drugs selected for the bioassays had a low LT50 value, indicating a rapid effect on the mites. For beauvericin and dimpylate, LT50 values were related to the concentration of the molecules (death occurred more rapidly among mites treated with the higher concentration).
The dose-response test recorded low LC50 values, indicating high efficacy of all drugs at killing S. scabiei mites. Moreover, LC50 values obtained in the present study were low compared to those obtained by Mounsey et al. (16). The latter study reported that 50.5 μM of ivermectin is required to kill 50% of S. scabiei mites 1 h postexposure (versus 45.1 μM in our study). This result could be explained by the fact that the strain of S. scabiei and/or the assay conditions used in the present study were different.
The next generation of scabicide molecules needs to target S. scabiei eggs and ensuing developmental stages. In accordance with the results of the present study, previous investigations (23, 24) demonstrated that currently used acaricides have a very limited inhibition of hatching activity. In contrast, beauvericin seemed to be active against the eggs of S. scabiei.
In conclusion, this study presented the first evidence of in vitro efficacy of the mycotoxin beauvericin against different developmental stages of S. scabiei mites. Beauvericin showed higher efficacy against females and eggs of S. scabiei compared to dimpylate and ivermectin. These are in vitro results, and further investigations are required to better understand the bioavailability, toxicokinetic properties, distribution, absorption, metabolization, and excretion of beauvericin in the skin of mammals. Studies measuring and confirming that the maximum concentrations of beauvericin in the skin are within the in vitro susceptibility range are crucial.
MATERIALS AND METHODS
Ethics.
All animals were maintained in strict accordance with good animal practices as defined by the French and European code of practice for the care and use of animals for scientific purposes (approval no. 02515.01). The biopsy specimens were obtained after a written consent form was secured from each individual according to an approved protocol by the Institution Review Board (IRB) at the American University of Beirut (protocol no. DER.MK.01). The experiments were conducted in accordance with good clinical practice and the ethical principles of the Helsinki Declaration.
Primary fibroblast culture.
Human skin biopsy specimens from healthy volunteer patients were delivered to the laboratory in culture medium (RPMI 1640, 450 ml; fetal bovine serum [FBS], 50 ml; and penicillin-streptomycin solution ×100, 1 ml). Each biopsy specimen was transferred into a sterile petri dish and rinsed with phosphate-buffered saline (PBS) to eliminate blood and debris. Then, 2 ml of collagenase (Worthington) was added to the medium before the tissue was minced with a scalpel. After incubation at 37°C for 1 h, the digested tissue was transferred to a 15-ml conical tube, the petri dish was rinsed twice with 2 ml of the medium, and the liquid was collected in the same tube and spun at 200 × g for 5 min at room temperature. The pellet was washed twice with 3 ml of the medium to remove the collagenase, resuspended with 5 ml of the same medium, and transferred to a T25 flask. Finally, the cells were cultured in a 37°C humidified air incubator with 5% CO2. When there were sufficient cells, the latter were detached with trypsin and plated in another dish for further proliferation.
Molecules to be evaluated.
Beauvericin 97% was purchased from Sigma-Aldrich. Dimpylate (diazinon) was purchased from Huvepharma (Segre en Anjou, France) (Dimpygal solution; 100 mg/ml). Ivermectin was purchased from Boehringer-Ingelheim (Lyon, France) (Ivomec injectable solution; 10 mg/ml).
Beauvericin cytotoxicity assessment.
The cell death rates of treated fibroblast cells were used as an indicator to assess the cytotoxicity of beauvericin. Cultured cells were transferred to a 96-well plate and treated with 12 different concentrations of beauvericin in triplicate (0 to 50 μM) when they reached 50% to 60% confluence. Cells were continuously exposed to the drugs for 48 h and subsequently assessed for cell death. The viability of the cells was assessed based on their metabolic activity using the MTT proliferation assay (25) as follows: 24 h pretreatment, fibroblast cells were starved in 100 μl FBS-free medium. After starvation, fibroblast cells were exposed to the drugs as described above. Four hours prior to the end of the treatment, 10 μl MTT dye (100 mg thiazolyl blue tetrazolium bromide [Sigma-Aldrich] and 20 ml PBS) was added to the wells. In addition, 100 μl MTT stop solution (12 mM HCl, 0.05% isobutanol, and 10% SDS) was added to cells before incubation at 37°C overnight. After 24 h, the absorbance was measured at 550 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader. All tests were carried out in triplicates of three biological replicates.
Sarcoptes mites.
Sarcoptes scabiei mites were collected from pigs maintained at the Centre de Recherche Bio Médicale (CRBM) (Maisons-Alfort, France). Pigs were experimentally infected as described by Mounsey et al. (26). Inoculation was done by directly introducing mite-infected skin crusts deep into the ear canals of 5-week-old female piglets. Glucocorticoid treatment was initiated in naive piglets 1 week prior to inoculation and continued. For the present study, mites were collected from the pigs in weeks 15 and 16. Crusts in the external ear canal were gently removed and collected in a sterile petri dish in the morning of the in vitro experiments. Mites crawled out of the crusts in about half an hour. Then, they were picked one by one with a needle under a dissecting stereomicroscope (Nikon SMZ645; Lisses, France).
In vitro efficacy tests on mites and eggs.
To assess the efficacy of drugs (including beauvericin) against S. scabiei motile stages (larvae/nymphs and females), petri dishes filled with Columbia agar supplemented with pig serum were used for bioassays. To prepare the medium, 42 g of Columbia agar (Bio-Rad, Marne-la-Coquette, France) was dissolved in 1 liter of distilled water. The solution was autoclaved for 15 min at 121°C and then cooled in a water bath at 53°C. Blood samples were obtained from pigs maintained at CRBM. Tubes of blood were centrifuged at 4,500 rpm for 10 min at 4°C. The resulting supernatant was designated serum. For the preparation of each petri dish, 1 ml of serum was added to 18 ml of Columbia agar medium at 53°C. Drugs to be tested were incorporated into the medium following the method described by Brimer (12, 27) with slight modifications. The mycotoxin beauvericin and two acaricide drugs (dimpylate and ivermectin) were tested with different concentrations. The absence of drug concentration was considered a negative control. The required volumes of serum-supplemented Columbia agar and molecules were pipetted in a tube and quickly transferred to a 9-cm sterile plastic petri dish under a flow cabinet. Petri dishes were kept under the flow cabinet until the agar was solidified and were stored upside down at 4°C until use. Based on the results of beauvericin cytotoxicity assessment, the efficacy of two different concentrations, 0.5 and 5 μM, was evaluated. Five females and five nymphs or larvae were inoculated in the middle of the plates and examined at 1, 2, 3, 4, 5, 6, 7, 8, and 24 h after exposure for survival assessment of motile stages at room temperature. Mites were considered dead when no movement occurred under the microscope during 5 min even after a gentle stimulation with a dissecting needle. After each inspection, mites were moved again to the center of the plate using a dissecting needle to lower chances of runaways.
To assess the efficacy of chemical products against S. scabiei eggs, 10 eggs were manually isolated using a fine needle and placed in the middle of beauvericin-, dimpylate-, or ivermectin-supplemented agar plates under the dissecting stereomicroscope as described above. Petri dishes were maintained at 37°C in an incubator for 5 days to promote egg development. Newly hatched larvae were recorded and removed from the petri dishes.
Five replications were performed in three biological replicates, for a total of 150 motile stages and 150 eggs observed for each treatment.
Statistical analyses.
The efficacy data of all treatments against S. scabiei were analyzed with Kaplan Meier survival curves using the software Statistical Package for the Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY). The statistical differences between data obtained with each treatment and the control for each experiment were measured with a log-rank test expressed by chi-square results and P values (degree of freedom [df]) = 1). A P value of ≤0.05 was considered significant. The lethal concentration (LC50) and lethal time (LT50) necessary to kill half of the mite’s population in addition to the lethal concentration (LC50) necessary to kill half of fibroblast cells and their standard error were calculated using the probit regression analysis in (SPSS). The median time of 50% hatching (HT50) of the eggs was assessed.
The LC50 of all treatments was analyzed by the statistical comparison test of means (analysis of variance [ANOVA]) using SPSS. The Tukey test was used at the 5% threshold for the separation of means.
ACKNOWLEDGMENTS
We are grateful to Radia Guechi and Francis Moreau for their laboratory assistance.
This work was funded by the Lebanese National Council for Scientific Research (CNRS) (grant CNRS-L/USEK) and “Coopération pour l’évaluation et le développement de la recherche” CEDRE (grant 37349SA).
We declare no conflicts of interest.
REFERENCES
- 1.Walton SF, Currie BJ. 2007. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev 20:268–279. doi: 10.1128/CMR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimkhani C, Colombara DV, Drucker AM, Norton SA, Hay R, Engelman D, Steer A, Whitfeld M, Naghavi M, Dellavalle RP. 2017. The global burden of scabies: a cross-sectional analysis from the global burden of disease study 2015. Lancet Infect Dis 17:1247–1254. doi: 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelman D, Cantey PT, Marks M, Solomon AW, Chang AY, Chosidow O, Enbiale W, Engels D, Hay RJ, Hendrickx D, Hotez PJ, Kaldor JM, Kama M, Mackenzie CD, McCarthy JS, Martin DL, Mengistu B, Maurer T, Negussu N, Romani L, Sokana O, Whitfeld MJ, Fuller LC, Steer AC. 2019. The public health control of scabies: priorities for research and action. Lancet 394:81–92. doi: 10.1016/S0140-6736(19)31136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynar S, Currie BJ, Baird R. 2017. Scabies and mortality. Lancet Infect Dis 17:1234. doi: 10.1016/S1473-3099(17)30636-9. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy JS, Kemp DJ, Walton SF, Currie BJ. 2004. Scabies: more than just an irritation. Postgrad Med J 80:382–387. doi: 10.1136/pgmj.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walton SF, Myerscough MR, Currie BJ. 2000. Studies in vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var. hominis. Trans R Soc Trop Med Hyg 94:92–96. doi: 10.1016/s0035-9203(00)90454-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Xu L. 2012. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules 17:2367–2377. doi: 10.3390/molecules17032367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Patocka J, Nepovimova E, Kuca K. 2018. A review on the synthesis and bioactivity aspects of beauvericin, a Fusarium mycotoxin. Front Pharmacol 9:1338. doi: 10.3389/fphar.2018.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallebrera B, Prosperini A, Font G, Ruiz MJ. 2018. In vitro mechanisms of beauvericin toxicity: a review. Food Chem Toxicol 111:537–545. doi: 10.1016/j.fct.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Heilos D, Rodríguez-Carrasco Y, Englinger B, Timelthaler G, van Schoonhoven S, Sulyok M, Boecker S, Süssmuth RD, Heffeter P, Lemmens-Gruber R, Dornetshuber-Fleiss R, Berger W. 2017. The natural fungal metabolite beauvericin exerts anticancer activity in vivo: a pre-clinical pilot study. Toxins 9:258. doi: 10.3390/toxins9090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taevernier L, Veryser L, Roche N, Peremans K, Burvenich C, Delesalle C, De Spiegeleer B. 2016. Human skin permeation of emerging mycotoxins (beauvericin and enniatins). J Expo Sci Environ Epidemiol 26:277–287. doi: 10.1038/jes.2015.10. [DOI] [PubMed] [Google Scholar]
- 12.Brimer L, Bønløkke L, Pontoppidan C, Henriksen SA, Gyrd-Hansen N, Rasmussen F. 1995. A method for in vitro determination of the acaricidal effect of ivermectin using Sarcoptes scabiei var. suis as test organism. Vet Parasitol 59:249–255. doi: 10.1016/0304-4017(94)00759-6. [DOI] [PubMed] [Google Scholar]
- 13.Huffam SE, Currie BJ. 1998. Ivermectin for Sarcoptes scabiei hyperinfestation. Int J Infect Dis 2:152–154. doi: 10.1016/s1201-9712(98)90118-7. [DOI] [PubMed] [Google Scholar]
- 14.Glaziou P, Cartel JL, Alzieu P, Briot C, Moulia-Pelat JP, Martin PM. 1993. Comparison of ivermectin and benzyl benzoate for treatment of scabies. Trop Med Parasitol 44:331–332. [PubMed] [Google Scholar]
- 15.Currie BJ, Harumal P, McKinnon M, Walton SF. 2004. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 39:e8–e12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- 16.Mounsey KE, Walton SF, Innes A, Cash-Deans S, McCarthy JS. 2017. In vitro efficacy of moxidectin versus ivermectin against Sarcoptes scabiei. Antimicrob Agents Chemother 61:e00381-17. doi: 10.1128/AAC.00381-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feyera T, Admasu P, Abdilahi Z, Mummed B. 2015. Epidemiological and therapeutic studies of camel mange in Fafan zone, eastern Ethiopia. Parasite Vector 8:612. doi: 10.1186/s13071-015-1228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aussy A, Houivet E, Hébert V, Colas-Cailleux H, Laaengh N, Richard C, Ouvry M, Boulard C, Léger S, Litrowski N, Benichou J, Joly P, investigators from the Normandy Association of Medical Education in Dermatology. 2019. Risk factors for treatment failure in scabies: a cohort study. Br J Dermatol 180:888–893. doi: 10.1111/bjd.17348. [DOI] [PubMed] [Google Scholar]
- 19.Mounsey KE, Holt DC, McCarthy JS, Currie BJ, Walton SF. 2009. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol 145:840–841. doi: 10.1001/archdermatol.2009.125. [DOI] [PubMed] [Google Scholar]
- 20.Toman P, Makrlík E, Vaňura P. 2011. On the complexation of the sodium cation with beauvericin: experimental and theoretical study. Monatsh Chem 142:779–782. doi: 10.1007/s00706-011-0499-1. [DOI] [Google Scholar]
- 21.Wätjen W, Debbab A, Hohlfeld A, Chovolou Y, Proksch P. 2014. The mycotoxin beauvericin induces apoptotic cell death in H4IIE hepatoma cells accompanied by an inhibition of NF-κB-activity and modulation of MAP-kinases. Toxicol Lett 231:9–16. doi: 10.1016/j.toxlet.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Lin H, Chen B, Jow GM. 2016. Beauvericin-induced cell apoptosis through the mitogen-activated protein kinase pathway in human nonsmall cell lung cancer A549 cells. J Toxicol Sci 41:429–437. doi: 10.2131/jts.41.429. [DOI] [PubMed] [Google Scholar]
- 23.Usha V, Nair TG. 2000. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol 42:236–240. doi: 10.1016/S0190-9622(00)90131-2. [DOI] [PubMed] [Google Scholar]
- 24.Bernigaud C, Fernando DD, Lu H, Taylor S, Hartel G, Guillot J, Chosidow O, Fischer K. 2019. In vitro ovicidal activity of current and under-development scabicides: which treatments kill scabies eggs? Br J Dermatol in press. doi: 10.1111/bjd.18517. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Mounsey K, Ho M, Kelly A, Willis C, Pasay C, Kemp DJ, McCarthy JS, Fischer K. 2010. A tractable experimental model for study of human and animal scabies. PLoS Negl Trop Dis 4:e756. doi: 10.1371/journal.pntd.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brimer L, Henriksen SA, Gyrd-Hansen N, Rasmussen F. 1993. Evaluation of an in vitro method for acaricidal effect: activity of parathion, phosmet and phoxim against Sarcoptes scabiei. Vet Parasitol 51:123–135. doi: 10.1016/0304-4017(93)90203-Y. [DOI] [PubMed] [Google Scholar]