Abstract
Background.
The purpose of this study was to determine the effect of algorithmic physiologic management on patients undergoing head and neck free tissue transfer and reconstruction.
Methods.
Ninety-four adult patients were randomized to treatment and control groups. The blood pressure of the control group was managed consistent with contemporary standards. The treatment group was managed using an algorithm based on blood pressure and calculated physiologic values derived from arterial waveform analysis. Primary outcome was intensive care unit (ICU) length of stay.
Results.
ICU length of stay was decreased in the treatment group (33.7 vs 58.3 hours; p = .026). The complication rate was not increased in the treatment group.
Conclusion.
The goal-directed hemodynamic management algorithm decreased the ICU length of stay. Judicious use of vasoactive drugs and goal-directed fluid administration has a role in improving perioperative outcomes for patients undergoing head and neck free tissue transfer. © 2016 Wiley Periodicals, Inc. Head Neck 38: E1974–E1980, 2016
Keywords: free tissue transfer, hemodynamics, length of stay, goal-directed therapy, critical care
INTRODUCTION
Free tissue transfer (FTT) reconstruction is a standard procedure for reconstruction of significant defects after head and neck cancer ablation.1 Since the 1970s, these procedures have been refined extensively with sequential improvements of technology and technique, yet this surgical population is known to have frequent postoperative complications and often endures a significant length of stay in the intensive care unit (ICU) and hospital.2–4
Perioperative use of vasopressors in this patient population has been discouraged for many years because of theoretical concerns about reduction in graft perfusion.5 Concerns about vasoconstriction negatively impacting perfusion to the transferred tissue caused many institutions to empirically avoid administration of vasoactive medications during or after surgery.6 Anesthesia providers have been faced with the difficult task of maintaining tissue perfusion in patients with multiple comorbidities while using a limited set of pharmacologic tools for blood pressure management. The most common method of maintaining blood pressure without the use of vasoactive substances has been aggressive intravenous (i.v.) fluid administration combined with judicious doses of anesthetics. However, recent work has repeatedly shown an association between fluid overload and increased perioperative morbidity, leaving the anesthesia team with few evidence-based options for blood pressure management in these operations.7–10 In addition, retrospective studies have shown that vasopressor use occurs at a high frequency (>50%) in FTT reconstruction cases, despite surgical policies discouraging their use.6,11
In an effort to manage blood pressure in a more rational way during these long, complex procedures, goal-directed hemodynamic therapy has been proposed.12 Goal-directed hemodynamic therapy utilizes technology and physiologic modeling to provide accurate estimates of multiple hemodynamic variables and predictions of responsiveness to potential therapeutic interventions. In particular relevance to FTT surgery, this information could facilitate better decision-making by the anesthesia provider with regard to whether a hypotensive patient would best respond to i.v. fluid, vasopressors, or inotropes. The effectiveness of goal-directed hemodynamic therapy algorithms and technology has been previously validated for intermediate to high-risk surgery.13–16 However, this method of blood pressure management has not been prospectively studied in cases requiring FTT with microvascular reconstruction. Accordingly, we tested the hypothesis that maintaining normotension during surgery via a goal-directed hemodynamic therapy algorithm, including i.v. fluids, inotropes, or vasoactive drugs will lead to shortened ICU length of stay without increasing flap-related morbidity.
MATERIALS AND METHODS
Approval was obtained from the institutional review board at the Medical University of South Carolina (MUSC IRB-II). The trial was registered with clinical-trials.gov (NCT02186938). All adult patients scheduled for primary FTT reconstruction with the head and neck oncologic surgeons at the Medical University of South Carolina, Charleston, SC, were candidates for enrollment and provided the opportunity to enroll and give informed consent. Exclusion criteria included: cognitive limitation, New York Heart Association congestive heart failure class >III or ejection fraction (EF) <30%, pulmonary disease expected to prevent 8 cc/kg tidal volumes, patient weight <55 kg or >160 kg, or patients with an active cardiac dysrhythmia (eg, rate-controlled atrial fibrillation) at the time of preoperative visit or the day of surgery; some of these features preclude valid physiologic assessments using arterial pulse contour analysis.17 The study enrolled patients beginning April 1, 2012, through September 2014 performed by 3 ablative and 3 reconstructive surgeons. Ninety-four patients were enrolled over this time and assigned to the control group or treatment group (goal-directed hemodynamic therapy) on the day of surgery by random numerical identifier. The study was not blinded. The cases were all overseen by attending anesthesiologists (3) and staffed by nurse anesthetists (7) who were recruited and educated before enrollment to ensure adherence to the control and treatment algorithms and to assist with data collection.
Preoperative management of both groups was identical in terms of testing and perioperative medical optimization. Serial laboratory tests were obtained from both groups every 3 hours, or more frequently, if clinically necessary. Patients could receive preoperative anxiolysis with midazolam. Induction of anesthesia was executed with similar drugs in both groups at the anesthesiologist’s discretion, depending on comorbidities and potential airway concerns. In all patients, an arterial line and adequate i.v. access was obtained; for patients with difficult peripheral access and all patients in the treatment group, a central line was placed (often femoral). Maintenance of anesthesia utilized a multimodal technique with all patients being administered isoflurane (range, 0.4% to 1.1%), ketamine infusion (5 mcg/kg/min), and sufentanil infusion titrated to analgesic effect (range, 0.1–0.5 mcg/kg/hr). During the dissection phase of surgery, the use of non-depolarizing neuromuscular blocking drugs was usually avoided to allow for nerve monitoring. In both groups, maintenance i.v. fluid (Plasmalyte-A; Baxter Healthcare, Deerfield, IL) was administered based on the patient’s body weight and estimated evaporative losses based on incision. Albumin (25%) was given if the patient’s preoperative albumin is <2.5 g/dL based on the following formula: (2.5 g/dL – actual albumin g/dL) × weight (kg) × 0.8. Fixed transfusion criteria were enforced, allowing red blood cell transfusion for Hgb <6.5 gm/dL or heart rate >100% or >33% from baseline and lactate >2 mmol/L. Ventilation in both groups was set to 8 cc/kg tidal volume with a rate to keep CO2 between 33 and 40 cm H2O and FiO2 to keep SpO2 >97% and PaO2 >80 mm Hg.
The control group followed “standard” management of hypotension as historically practiced at our institution, utilizing only i.v. fluid (crystalloid and colloid). For this group, goal blood pressure was set as mean arterial pressure >70 or within 20% of baseline, also institutional standards for this patient population. To maintain these blood pressures, anesthesia practitioners titrated the volatile anesthetic to cause as little a drop in systemic vascular resistance and cardiac output as possible.
The treatment group (goal-directed hemodynamic therapy) used the Vigileo and EV-1000 (Edwards Lifesciences LLC, Irvine, CA) devices to calculate the physiologic parameters used to direct treatment of hypotension. The treatment algorithm seen in Figure 1 depicts the treatment algorithm based on blood pressure, stroke volume variation, cardiac index, and systemic vascular resistance. Hypotension in the treatment group was defined as the greater of mean arterial pressure <75 mm Hg or >10% below baseline.18 The baseline mean arterial pressure was defined as the lowest pressure recorded at the surgical history and physical, preoperative clinic visit, or morning of surgery. If a patient became hypotensive, the in-room provider, in communication with the attending anesthesiologist, would follow the treatment algorithm in Figure 1. The management protocol used well established and validated arterial pulse contour analysis algorithms to calculate stroke volume and predict fluid responsiveness; our group modified the existing algorithms to use phenylephrine (alpha agonist treating low systemic vascular resistance) as the final step because of the ongoing debate related to its safety in FTT.13,19 The exact values for each parameter were chosen with the intent to optimize perfusion and minimize potential risk to the patient or tissue being transferred.
FIGURE 1.
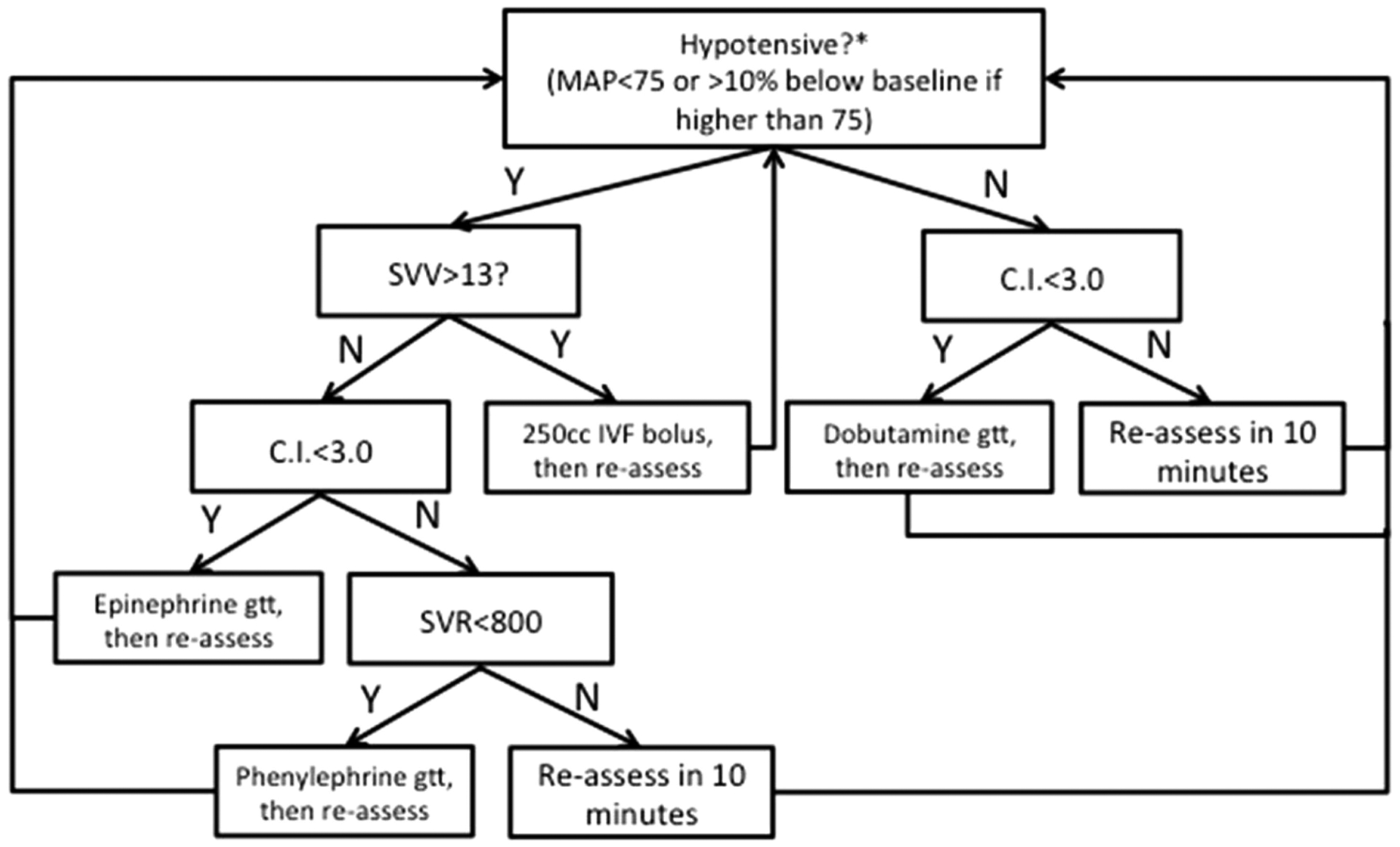
Goal-directed therapy algorithm directed by utilizing calculated physiologic parameters based on the arterial waveform. MAP, mean arterial pressure; SVV, stroke volume variation; CI, cardiac index; IVF, intravenous fluid; SVR, systemic vascular resistance.
Patients were withdrawn from the research study if the decision was made intraoperatively not to perform FTT reconstruction. For patients in the treatment group, records were noted as “withdrawn” for transient failure to meet requirements for goal-directed hemodynamic therapy (reliable stroke volume variation calculation requires tidal volume of 8 cc/kg and maintenance of sinus rhythm).16 The goal-directed hemodynamic therapy algorithm was applied as soon as all requirements were met.
Postoperative care was not controlled by this research, however, the same team of critical care physicians managed patients from both the treatment and control groups, and the care patterns were presumably similar within groups (eg, ventilator weaning protocols, insulin management, and hemodynamic management). Patients in both groups had routine monitoring of flap perfusion and serial examinations for edema, discoloration, and bleeding. Standard postoperative complications (eg, myocardial infarction, wound infection, or the need for reintubation) were documented as well as flap-related complications unique to this patient population (flap dehiscence, need for reoperation, donor site complications, and flap failure). Data was queried from electronic medical records, dictations, and billing records. Chart review data was collected in duplicate and discrepancies were verified (by author W.H.) whenever necessary.
Statistical analysis
Based on an average ICU length of stay of 4.3 days (non-normal distribution; SD = 5 5.21 days) a power analysis was performed with the goal of a 40% decreased ICU length of stay (range, 4.3–2.5 days) that yielded a sample size of 94 patients. This power analysis did not account for attrition.
Neither ICU length of stay nor hospital length of stay was normally distributed a priori, therefore, the test for association between ICU length of stay and treatment type was conducted using the Wilcoxon rank sum test. Patient characteristics in the treatment and control groups were compared to ensure that the groups were similar. Univariate tests of association between treatment and categorical variables in the dataset were conducted using chi-square tests or the Fisher’s exact test where appropriate. Associations between treatment group and all continuous variables were examined using Student’s t test or the Wilcoxon rank sum test where appropriate. The analysis was conducted as an intention-to-treat analysis and, thus, included the patients who were “withdrawn” from the study. The primary outcome of interest was ICU length of stay (in hours). ICU length of stay was analyzed using an intention-to-treat analysis and on a complete case basis.
A multivariable model of ICU length of stay was also developed. All variables with a univariate p value of < .2 were considered in the multivariable model. Model assumptions of normality and homoscedasticity were checked graphically and were not met, therefore, ICU length of stay was log transformed before fitting the regression model to improve normality. The log transformation was selected using the Box–Cox approach considering lambda values from −3 to 3 in increments of 0.25. The final model was selected using backward selection retaining only those variables significant at the 0.2 level.
RESULTS
There were 47 patients recruited to both treatment and control groups of this study, see Figure 2, and pertinent demographic information and surgical information is shown in Table 1. There were no significant differences between the groups in terms of age, sex, body mass index, or smoking status (active, prior, or never). Similarly, there was no difference in the distribution of pathologic tumor staging by group using the TNM system (only 1 patient with M classification 1 disease, not shown in Table 1).20 There was a significant difference in racial distribution between the treatment groups. Specifically, there were significantly more white subjects in the treatment group compared with other races (p = .009). Preoperative hemoglobin levels were similar between the 2 groups. Table 2 illustrates anesthesia-related factors associated with each group. There was no significant difference in total i.v. fluid administered based on grouping, however, patients in the treatment group received vasoactive drugs more frequently (20.5% vs 78.1%; p < .001). Blood product transfusion was indifferentiable between groups and no patient received any blood component other than packed red blood cells. Phenylephrine was the most common vasoactive drug used in the treatment group (61.5% of subjects in the treatment group). No patient in the control group had continuous infusion of vasoactive substances. Finally, the groups were similar in terms of FTT characteristics, such as donor site and number of bone-containing flaps.
FIGURE 2.
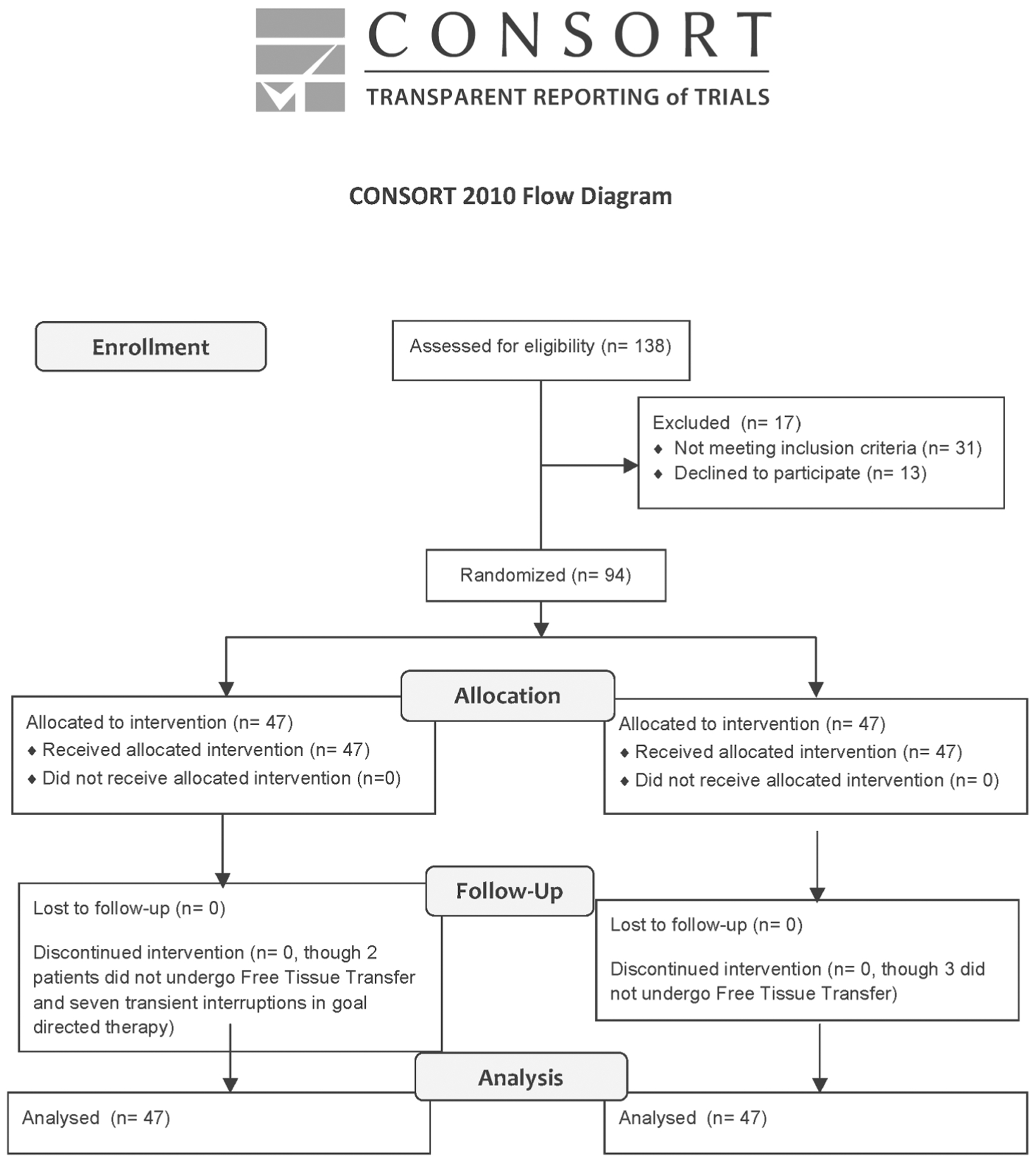
Consolidated Standards of Reporting Trails diagram showing enrollment and disposition.
TABLE 1.
Patient characteristics and surgical information.
| Variables | Control group (n = 47) | Treatment group (n = 47) | p value |
|---|---|---|---|
| Age, y | 57.6 (14.7) | 59.1 (12.0) | .599 |
| Sex, male | 35 (74.5) | 35 (74.5) | 1.00 |
| BMI | 25.4 (5.99) | 25.6 (5.28) | .907 |
| Race | |||
| White | 33 (70.2) | 43 (91.5) | .002 |
| African American | 12 (25.5) | 1 (2.13) | |
| Other | 2 (4.26) | 3 (6.38) | |
| Smoking status | |||
| Former | 19 (40.4) | 23 (48.9) | .342 |
| Current | 18 (38.3) | 10 (21.3) | |
| Never | 8 (17.0) | 11 (23.4) | |
| ASA | |||
| 2 | 7 (15.2) | 9 (20.0) | .793 |
| 3 | 38 (82.6) | 35 (77.8) | |
| 4 | 1 (2.17) | 1 (2.22) | |
| Surgery duration, h | 10.6 (3.01) | 11.3 (3.40) | .321 |
| Flap ischemia time, h | 2.44 (1.18) | 2.72 (1.58) | .561 |
| Baseline mean arterial pressure | 83.4 (14.1) | 83.7 (12.3) | .922 |
| Flap type | |||
| Fasciocutaneous | 23 (51.1) | 17 (43.6) | .791 |
| Musculocutaneous | 9 (20.0) | 10 (25.6) | |
| Osteocutaneous | 12 (26.7) | 12 (30.8) | |
| Other | 1 (1.19) | 0 (0) | |
| Flap stagingT classification | .137 | ||
| 0 | 7 (14.9) | 3 (6.38) | |
| 1 | 6 (17.0) | 9 (19.2) | |
| 2 | 1 (10.8) | 8 (17.0) | |
| 3 | 1 (2.13) | 7 (14.9) | |
| 4 | 25 (55.6) | 20 (42.6) | |
| N classification | .130 | ||
| 0 | 26 (55.3) | 24 (51.1) | |
| 1 | 2 (4.26) | 9 (19.2) | |
| 2 | 17 (36.0) | 13 (27.7) | |
| 3 | 2 (4.06) | 1 (2.13) | |
| Preoperative Hgb, SD | 11.5 (2.1) | 12.2 (2.11) | .11 |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists (physical status); Hbg, Hemoglobin g/dL.
Note continuous variables are reported as mean (SD) and categorical variables are reported as number (%).
TABLE 2.
Intraoperative totals.
| Variable | Control group (n = 47) | Treatment group (n = 47) | p value |
|---|---|---|---|
| i.v. fluid, mL | 6318.6 (2519.5) | 5886.9 (2290.7) | .462 |
| Colloid, mL | 108.0 (219.4) | 31.5 (111.8) | .283 |
| EBL, mL | 433.3 (316.3) | 406.3 (326.3) | .232 |
| RBC, % yes | 10 (23.3) | 7 (18.0) | .554 |
| UOP, mL | 1436.3 (1163.6) | 1342.1 (742.4) | .915 |
| Vasopressor, % yes | 9 (20.5) | 32 (78.1) | < .001 |
Abbreviations: EBL, estimated blood loss; RBC, red blood cell transfusion; UOP, urine output.
There were a total of 12 patients who were classified as “withdrawn.” In the control group, 2 patients underwent pedicled tissue transfer and 1 patient had an unresectable cancer, and no FTT was performed. In the treatment group, 1 patient had a pedicled tissue transfer and 1 patient had an unresectable cancer that was not reconstructed with FTT. Seven patients in the treatment group had intraoperative periods during which the goal-directed hemodynamic therapy algorithm could not be applied (5 patients had intraoperative atrial fibrillation and 2 had inadequate pulmonary compliance). Of note, atrial fibrillation was noted in 4 patients in the control group (although hemodynamic management did not change). All patients who went into atrial fibrillation during surgery converted within 1 hour (spontaneously or pharmacologically).
Postoperative data is shown in Table 3. Under intention-to-treat analysis, ICU length of stay (primary endpoint) was significantly reduced in the goal-directed hemodynamic therapy group. Patients in the goal-directed hemodynamic therapy treatment group had an average 24.6 hour shorter ICU length of stay compared to the control group (33.7 vs 58.3 hours or 1.4 vs 2.4 days; p = .026). The median ICU length of stay was also shorter in the treatment group (21.1 vs 39.0 hours). Fewer patients in the treatment group required positive pressure ventilator support and there was a statistically significant difference in duration of mechanical ventilator requirement (1.72 vs 0.81 days; p = .006). Figure 3 is a boxplot of ICU length of stay. Variables considered in the multivariable model included treatment group, whether or not the patient had a reoperation, flap failure, flap death, smoking status, and baseline mean arterial pressure. We also controlled for patient age and sex in the model. ICU length of stay was log transformed before fitting the model. The final model included treatment status, sex, and age. Patients receiving treatment showed a trend toward shorter ICU length of stay after controlling for age and sex, but the association was not statistically significant (p = .066). Specifically, subjects in the control group had a 105% increase in their ICU length of stay relative to patients who receive treatment (p = 0.066; 95% CI −3.7% to 337%). Neither patient sex nor patient age was significantly associated with ICU length of stay controlling for treatment. The mean hospital length of stay was not significantly different between the 2 groups (276.0 vs 216.9 hours or 11.5 vs 9.0 days; p = .357). The frequency of flap-related complications was not significantly different between the treatment and control groups; however, each of these rare events occurred less frequently in the treatment group. There were no complications related to placement or use of central venous or arterial catheters.
TABLE 3.
Postoperative patient characteristics and outcomes.
| Variables | Control group (n = 47) | Treatment group (n = 47) | p value |
|---|---|---|---|
| ICU length of stay, h, intent-to-treat | 58.3 (63.6) | 33.7 (36.7) | .026 |
| Hospital length of stay, h | 276.0 (198.9) | 216.9 (87.7) | .357 |
| ICU length of stay, d | 2.64 (2.49) | 1.88 (2.01) | .104 |
| Hospital length of stay, d | 10.8 (7.65) | 9.11 (5.76) | .221 |
| Reoperation | 8 (17.4) | 4 (10.0) | .324 |
| Flap failure | 4 (9.30) | 2 (5.88) | .681 |
| Flap death | 3 (6.82) | 2 (5.00) | 1.00 |
| ICU ventilation | 18 (52.9) | 9 (31.0) | .125 |
| Ventilator days | 1.72 (1.82) | 0.81 (1.30) | .006 |
| Direct cost, $ | 30,047 (14,216) | 26,509 (10,577) | .174 |
Abbreviation: ICU, intensive care unit.
FIGURE 3.
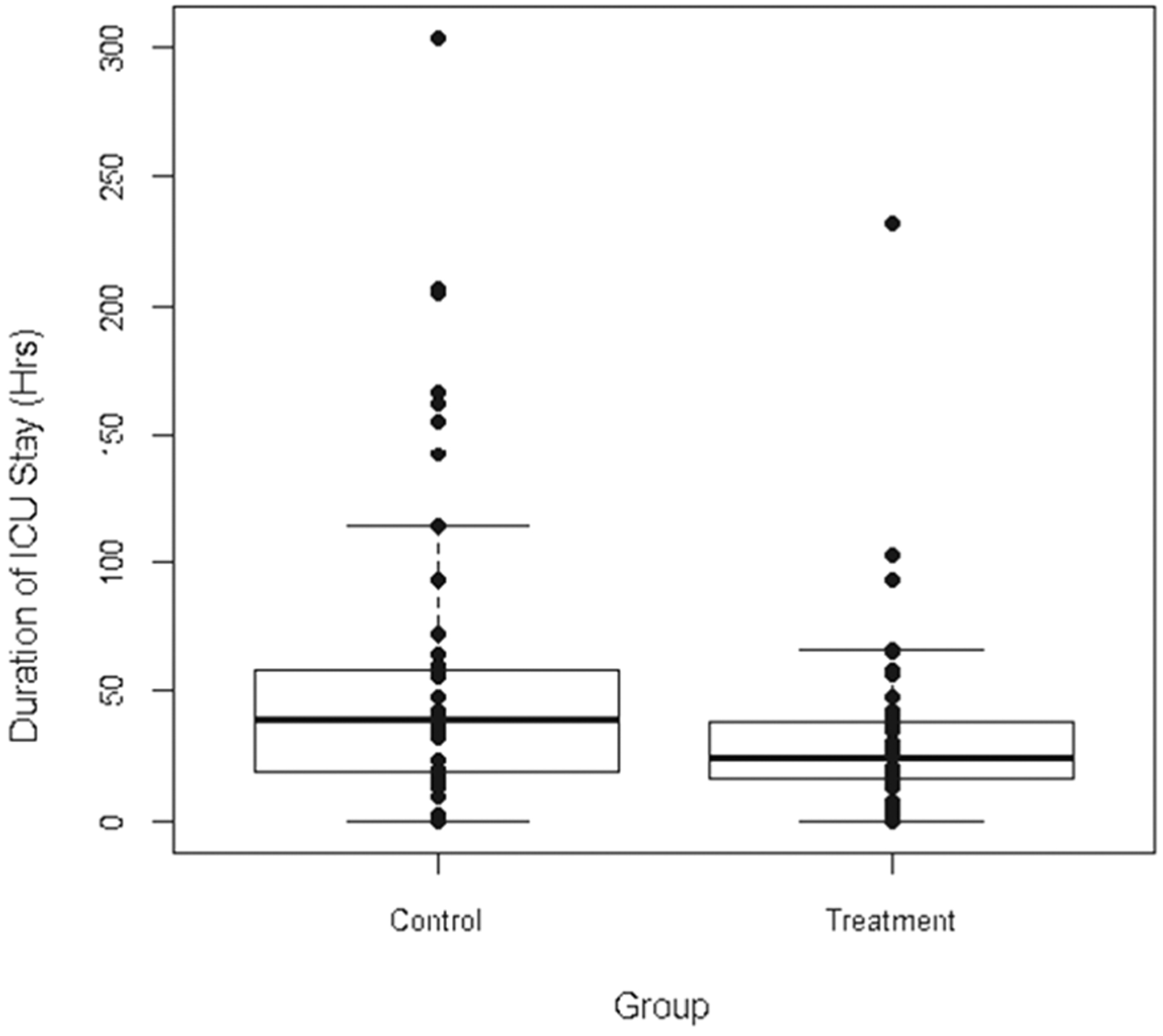
Boxplot showing intensive care unit (ICU) length of stay (in hours) in treatment and control groups based on intention-to-treat analysis.
Cost per treatment group was also evaluated. Information captured on each subject included total cost, operating room cost, pharmacy cost, radiation cost, laboratory cost, therapy cost, and supply cost. The mean total cost in the control group was $30,047 compared to $26,509 in the treatment group (p = .17). There were no statistically significant differences in cost of care between the treatment and control groups.
DISCUSSION
We believe this to be the first published prospective randomized trial evaluating the safety and efficacy of a goal-directed hemodynamic management algorithm, including the use of vasoactive drugs, in head and neck FTT reconstruction surgery. Our results confirm that the use of this treatment algorithm can decrease ICU length of stay, even without control of postoperative care (or continued intervention). Total and direct costs were not significantly decreased in the goal-directed hemodynamic therapy group, although the study was not powered to achieve significance for this outcome. This study was performed at a single institution, and it is our practice to recover all free tissue transfer patients in the surgical ICU. Our postoperative care is consistent with that reflected in the literature: ICU or FTT-specific high acuity unit care, hourly “flap checks,” implantable Doppler monitoring, and similar “standard” postoperative protocols (eg, deep venous thrombosis prophylaxis, insulin management, electrolyte replacement).21,22 We postulate the decreased ICU length of stay occurs because the treatment group patients arrive to the ICU with better physiologic function and therefore require less time before being stable for discharge to their regular floor.
Importantly, there was no measurable increased risk of flap-related complications with the use of the goal-directed hemodynamic therapy treatment algorithm. Although this study was not statistically powered to detect differences in flap outcomes, the fact that flap morbidity was not increased in absolute terms is a reassuring finding. Anecdotally, each category of flap-related complications occurred less commonly in the treatment group. In an effort to determine whether administration of vasoactive medication is safe in FTT requiring microvascular anastomosis, Banic et al23 demonstrated that systemic administration of phenylephrine caused an increase in blood pressure, but had no detrimental effect on blood flow to the flap; however, direct infusion into the flap pedicle decreased blood flow by 30%. All vasoactive drugs, in our study, were similarly administered systemically.
Careful review of the results reveals that ICU length of stay was reported in 2 ways. The primary endpoint was ICU length of stay in hours: time of admission until time until the discharge order placed, regardless of bed availability. When accumulating cost data, we were provided the number of “days” a patient was billed for an ICU bed. Our hospital policy is to count ICU days according to the patient in the bed at midnight. This low resolution of data may affect the lack of statistical significant in ICU length of stay measured in ICU days, but also may affect the lack of difference in cost. A patient may be admitted to the ICU at 11:45 PM and stay only 6 hours, but this patient would not be differentiated in the cost system from someone who required ICU care until 11:59 pm the following day. For this reason, we endorse ICU length of stay in hours to be the most precise descriptor of overall clinical improvement.
We report ICU length of stay in hours both via an intent-to-treat and cases completed. All but 2 patients underwent complex resection and reconstructions. The 7 patients in the treatment group who were designated as “withdrawn” from the protocol each spent less than an hour in a condition that prevented application of the goal-directed hemodynamic therapy algorithm. For example, 2 patients entered into atrial fibrillation (chronic diagnosis for both, but normal sinus rhythm in the preoperative clinic) after induction of anesthesia. Both patients spontaneously converted with normalization of blood pressure (ie, coronary perfusion pressure) almost instantly. Nonetheless, we had to deviate from the protocol and reported the outcomes separately. The absolute difference and average ICU length of stay for both the cases completed and intent-to-treat groups was similar, with statistical significance a matter of sample size rather than meaningful difference.
Goal-directed fluid administration is different than “fluid restriction.”12 As seen in prior work assessing intraoperative goal-directed hemodynamic therapy protocols, there was not a significant decrease in average volume of i.v. fluid administered.14,24 In goal-directed hemodynamic therapy, i.v. fluid is administered to patients only when it is indicated, as opposed to empiric administration based on generalized overestimates of maintenance needs.12,25,26 We believe that this patient-specific approach avoids the complications associated with excessive or restrictive fluid administration.8,27 As described above, the goal-directed hemodynamic therapy algorithm used in this study was specifically designed for patients undergoing FTT. The intended outcome of goal-directed hemodynamic therapy algorithms is to optimize perfusion by assessing and treating the component of blood pressure that is measurably deficient. Although volatile anesthetics are known vasodilators, patients undergoing FTT are potentially sensitive to vasoconstrictors, therefore, assessment of systemic vascular resistance was the last step in the goal-directed hemodynamic therapy algorithm and would not be treated unless the patient was hypotensive, euvolemic, and had an appropriate cardiac output. In this way, the treatment was both patient-specific and procedure-specific.
The impetus for a more cogent approach to hemodynamic management in microvascular FTT cases has been apparent in the literature.6,28 This study represents important progress toward improving the anesthetic and hemodynamic management and outcomes of patients undergoing head and neck FTT, akin to the iterations of improvements made by our surgical colleagues in this specialized field. The results of this study demonstrate that judicious use of vasoactive drugs on patients with euvolemic FTT does not produce untoward consequences in the microvascular perfusion of a free flap as measured by critical clinical outcome parameters, such as the rate of return to the operating room for flap-related complications or flap failure rate. Euvolemia, however, is admittedly a moving target, and it is the application of technology that calculates physiologic parameters in a goal-directed hemodynamic therapy context that has created this opportunity for clinical improvement.15,16
Our group recently published a retrospective review undertaken in an attempt to determine risk factors associated with suboptimal outcomes after head and neck FTT.11 We found little evidence that further optimization could be accomplished without challenging the existing treatment paradigm, namely i.v. fluid administration and strict avoidance of vasoactive medication, that has been implicated in the development of the most common perioperative complications in this population. The treatment algorithm seems to have improved the perioperative pulmonary function of patients, as fewer required ventilator support, and patients in the goal-directed hemodynamic therapy treatment group maintained stable spontaneous ventilation more rapidly. The return to unsupported spontaneous ventilation in patients with head and neck FTT is arduous. At our institution, tracheostomy rates are consistent with rates reflected in literature (>70%), and the decision to extubate patients who do not have a surgical airway is related to the difficulty of original airway management and the subjective assessment of airway and pharyngeal edema. For patients requiring positive pressure ventilator support, ventilator settings are guided by pulmonary mechanics and arterial blood gas values, consistent with current literature.29,30 At the conclusion of each surgery, anesthesia was weaned in an attempt to awaken and extubate; for those patients who required postoperative ventilator support, sedation was continued.
At the time of writing this manuscript, there are 2 additional clinical trials registered on ClinicalTrials.gov examining the safety and efficacy of similar protocols on microvascular FTT, demonstrating the momentum that is building to update the perioperative hemodynamic management of this patient population. In fact, a recent review called upon the medical profession to collaborate and apply “enhanced recovery after surgery” protocols to the head and neck surgical population; of note, a major component of these protocols is goal-directed fluid and vasopressor administration.31
Limitations
The greatest limitation of this work was the lack of controlled postoperative care. At our institution, a separate team of surgical intensivists manage the patients with head and neck FTT in the ICU. Given the complexity of the goal-directed hemodynamic therapy algorithm and historical misgivings associated with administration of vasoactive medications in patients with FTT, we elected to limit the intervention to the clinical environment entirely under control of the authors: the intraoperative period. It is the opinion of the authors that some of the gains made by application of the goal-directed hemodynamic therapy algorithm intraoperatively may be partially diminished by postoperative practice patterns we passively witness (unguided i.v. fluid administration). By not having postoperative care controlled by the research team, it is possible that the ICU team may have treated patients inconsistently between groups; however, any such “bias” would have occurred without communication or designation from the research team as every effort was made to effectively “blind” the postoperative team to the treatment/control status of the trial. Second, discerning statistically significant differences for many other more provocative endpoints (eg, flap failure rate, direct cost, or mortality) cannot be easily achieved in a single-institution trial. We look forward to participation in further prospective multicenter trials that would provide a sample size large enough to detect differences in secondary endpoints, such as duration of mechanical ventilator support, overall length of stay, flap failure rate, and hospital costs.
CONCLUSION
The application of a goal-directed therapy algorithm using calculated assessments of circulatory volume, cardiac output, and systemic vascular resistance can shorten ICU length of stay in patients undergoing head and neck FTT. This clinical improvement occurs without increasing the incidence of medical or FTT-related complications.
Acknowledgments
Contract grant sponsor: Partial grant funding was provided by Edwards Lifesciences, LLC, Irvine, CA, grant number 13641. This project was also supported by the South Carolina Clinical and Translational Research Institute, Medical University of South Carolina’s Clinical and Translational Institute and National Institute of Health/National Center for Advancing Translational Sciences, Bethesda, MD, grant number UL1TR000062 and the National Center for Research Resources award number UL1RR029882, Charleston, SC.
Footnotes
This work was presented in part as abstracts at the annual meetings for the American Society of Anesthesiologists, San Francisco, CA, October 12–16, 2013, and the International Anesthesia Research Society, Honolulu, HI, March 21–24, 2015.
REFERENCES
- 1.Smith RB, Sniezek JC, Weed DT, Wax MK, Microvascular Surgery Subcommittee of American Academy of Otolaryngology–Head and Neck Surgery. Utilization of free tissue transfer in head and neck surgery. Otolaryngol Head Neck Surg 2007;137:182–191. [DOI] [PubMed] [Google Scholar]
- 2.Serletti JM, Moran SL. Free versus the pedicled TRAM flap: a cost comparison and outcome analysis. Plast Reconstr Surg 1997;100:1418–1424; discussion 1425–1427. [DOI] [PubMed] [Google Scholar]
- 3.Heinz TR, Cowper PA, Levin LS. Microsurgery costs and outcome. Plast Reconstr Surg 1999;104:89–96. [PubMed] [Google Scholar]
- 4.Suh JD, Sercarz JA, Abemayor E, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg 2004;130:962–966. [DOI] [PubMed] [Google Scholar]
- 5.Godden DR, Little R, Weston A, Greenstein A, Woodwards RT. Catecholamine sensitivity in the rat femoral artery after microvascular anastomosis. Microsurgery 2000;20:217–220. [DOI] [PubMed] [Google Scholar]
- 6.Monroe MM, McClelland J, Swide C, Wax MK. Vasopressor use in free tissue transfer surgery. Otolaryngol Head Neck Surg 2010;142:169–173. [DOI] [PubMed] [Google Scholar]
- 7.Booi DI. Perioperative fluid overload increases anastomosis thrombosis in the free TRAM flap used for breast reconstruction. Eur J Plast Surg 2011; 34:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandstrup B, Tønnesen H, Beier–Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo DN. Fluid overload and surgical outcome: another piece in the jigsaw. Ann Surg 2009;249:186–188. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy MC. Wet, dry or something else? Br J Anaesth 2006;97:755–757. [DOI] [PubMed] [Google Scholar]
- 11.Hand WR, McSwain JR, McEvoy MD, et al. Characteristics and intraoperative treatments associated with head and neck free tissue transfer complications and failures. Otolaryngol Head Neck Surg 2015;152:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell D, Jacob M, Hofmann–Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology 2008;109: 723–740. [DOI] [PubMed] [Google Scholar]
- 13.Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg 2014;118:1052–1061. [DOI] [PubMed] [Google Scholar]
- 15.Cannesson M Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth 2010;24:487–497. [DOI] [PubMed] [Google Scholar]
- 16.Michard F Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005;103:419–428; quiz 449–455. [DOI] [PubMed] [Google Scholar]
- 17.Navarro LH, Bloomstone JA, Auler JO Jr, et al. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioper Med (Lond) 2015;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology 2012;116:1195–1203. [DOI] [PubMed] [Google Scholar]
- 19.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000;162: 134–138. [DOI] [PubMed] [Google Scholar]
- 20.Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM classification of malignant tumours, 7th edition Oxford, UK: Wiley–Blackwell; 2010. [Google Scholar]
- 21.Salgado CJ, Chim H, Schoenoff S, Mardini S. Postoperative care and monitoring of the reconstructed head and neck patient. Semin Plast Surg 2010; 24:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley PJ. Should all head and neck cancer patients be nursed in intensive therapy units following major surgery? Curr Opin Otolaryngol Head Neck Surg 2007;15:63–67. [DOI] [PubMed] [Google Scholar]
- 23.Banic A, Krejci V, Erni D, Wheatley AM, Sigurdsson GH. Effects of sodium nitroprusside and phenylephrine on blood flow in free musculocutaneous flaps during general anesthesia. Anesthesiology 1999;90:147–155. [DOI] [PubMed] [Google Scholar]
- 24.Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002;88:65–71. [DOI] [PubMed] [Google Scholar]
- 25.Jacob M, Chappell D, Conzen P, Finsterer U, Rehm M. Blood volume is normal after pre-operative overnight fasting. Acta Anaesthesiol Scand 2008;52:522–529. [DOI] [PubMed] [Google Scholar]
- 26.Lamke LO, Nilsson GE, Reithner HL. Water loss by evaporation from the abdominal cavity during surgery. Acta Chir Scand 1977;143:279–284. [PubMed] [Google Scholar]
- 27.Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: not a benign problem. Crit Care Med 1990;18: 728–733. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald DJ. Anaesthesia for microvascular surgery. A physiological approach. Br J Anaesth 1985;57:904–912. [DOI] [PubMed] [Google Scholar]
- 29.Marsh M, Elliott S, Anand R, Brennan PA. Early postoperative care for free flap head & neck reconstructive surgery–a national survey of practice. Br J Oral Maxillofac Surg 2009;47:182–185. [DOI] [PubMed] [Google Scholar]
- 30.Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology 2007;106: 1226–1231. [DOI] [PubMed] [Google Scholar]
- 31.Bianchini C, Pelucchi S, Pastore A, Feo CV, Ciorba A. Enhanced recovery after surgery (ERAS) strategies: possible advantages also for head and neck surgery patients? Eur Arch Otorhinolaryngol 2014;271:439–443. [DOI] [PubMed] [Google Scholar]


