Abstract
Research Question: Does reproductive outcome differ among the various subgroups of poor ovarian responders according to the Bologna criteria?
Design: This was a retrospective, cohort study including poor ovarian responders according to Bologna criteria, undergoing an ICSI cycle from January 2011 until December 2017. Patients were divided into four groups: (1) age ≥ 40 years and abnormal ovarian response test, (2) age ≥ 40 years, abnormal ovarian reserve test and one previous poor response to stimulation, (3) age ≥ 40 years and one previous poor response, (4) abnormal ovarian reserve test and one previous poor response.
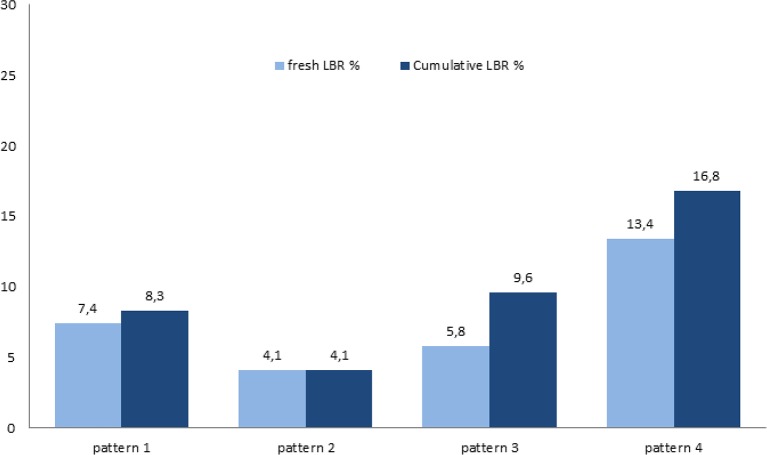
Result(s): Overall, 846 cycles in 706 Bologna poor ovarian responders were included: 310 cycles in group 1, 169 in group 2, 52 in group 3, and 315 in group 4. There were significant differences in age, antral follicle count, antimüllerian hormone, cycle cancellation rates, and number of retrieved oocytes between the four groups. Live birth and cumulative live birth rate differed significantly between groups and were highest in Group 4 [Live birth rate: 7.4% (1) vs. 4.1% (2) vs. 5.8% (3) vs. 13.4% (4), p = 0.001 and Cumulative live birth rate: 8.3% (1) vs. 4.1 % (2) vs. 9.6% (3) vs. 16.8% (4) p < 0.001]. The multivariate GEE analysis revealed that the number of MIIs and the Bologna criteria pattern were the variables which were significantly associated with cumulative live birth rate.
Conclusion(s): Poor ovarian responders represent a heterogeneous population. The young subpopulation has a better clinical prognosis in terms of fresh and cumulative live birth rate.
Keywords: Bologna criteria, poor ovarian response, poor responders, cumulative live birth rate, ICSI
Introduction
The occurrence of poor ovarian response amongst patients undergoing ovarian stimulation for in vitro fertilization (IVF) ranges from 9 to 24%, meaning that almost one in four patients harbors a poor reproductive prognosis (1–4). This category of patients commonly referred to as poor responders (PORs), remains a challenge for the fertility expert, as studies up to now have failed to identify a therapeutic approach that can modify or improve their fertility outcomes (5–7). The lack of conclusive evidence is strongly related to the vast heterogeneity of criteria that until recently were used to define PORs, which made it impossible to compare studies among them and develop valid, evidence-based management strategies (8). Indeed, until a few years ago, a myriad of vague and mostly arbitrary definitions of POR were used (9). The first attempt to overcome this hurdle, by accurately defining PORs in a standardized way, was carried out in 2011 by the European Society for Human Reproduction and Embryology (ESHRE) with the introduction of the Bologna Criteria (BC) (10). In the definition of POR by the BC, at least two of the following features must be present: advanced maternal age (≥40 years), a previous poor ovarian response with ≤ 3 oocytes retrieved after conventional stimulation and/or an abnormal ovarian reserve test (ORT) [i.e., antral follicle count (AFC) <7 or antimüllerian hormone (AMH) <1.1 ng/ml]. In the absence of advanced maternal age or abnormal ORT, a patient can be defined as POR after two episodes of poor ovarian response following maximal stimulation. The BC have thus inevitably reduced the heterogeneity previously present in the definition of POR and certainly represent a key step in framing the characteristics that better affiliate with this difficult setting of patients. However, there is evidence that even within the BC population, a significant degree of heterogeneity still persists (11). Indeed, several patterns or subgroups of PORs can be distinguished within the BC based on possible combinations of risk factors, ORT results, and IVF attempts (11–13). These subpopulations clearly encompass diverse baseline characteristics and the prognostic potential of these naturally emerging subgroups, as well as their reproductive outcomes, remain unclear and urge further investigation. These discrepancies within BC patients may explain why the few randomized controlled trials (RCTs) conducted in BC POR did not show a benefit of any treatment modality in terms of live birth rates (LBR). Furthermore, although LBR following a fresh IVF cycle is a key outcome measure for treatment success, cumulative LBR after the utilization of all fresh and frozen–thawed embryos derived from one stimulation cycle, has emerged as a more clinically meaningful outcome. In this context, the aim of our study was to evaluate cumulative LBR in different patterns of BC PORs.
Materials and Methods
Study Design
This was a retrospective, single-center cohort study including PORs according to BC and undergoing an intracytoplasmic sperm injection (ICSI) cycle, at the Centre for Reproductive Medicine, Universitair Ziekenhuis Brussel, Belgium, from January 2011 to March 2017. This study was approved by the Ethics Committee of Brussels University Hospital (approval B.U.N. 143201938863).
Eligibility Criteria
Clinical data regarding all ICSI cycles using a fixed antagonist protocol and gonadotrophin dose of at least 300 IU per day were collected. Patients were included if they fulfilled at least two of the following criteria: advanced maternal age (≥40 years), abnormal ORT (AMH <1.1 ng/ml or AFC <7) or a previous poor ovarian response (≤ 3 oocytes with a conventional stimulation protocol). Additional inclusion criteria were: age between 18 and 43 years, body mass index (BMI) of 17–35 kg/m2, presence of both ovaries and absence of any untreated endocrine abnormality. Women were excluded if they underwent preimplantation genetic testing (PGT).
Women who fulfilled the aforementioned criteria but, for unknown reasons, still had frozen embryos remaining or who had transferred the remaining embryos to another IVF unit, while not delivering a live born following their stimulated ICSI cycle, were excluded from the analysis in order to minimize the risk of misclassification bias. All patients had a follow up of at least 2 years.
Bologna Criteria Patterns
The population of patients that fulfilled these eligibility criteria was divided into four different patterns: (1) age ≥40 years and abnormal ORT, (2) age ≥40 years, abnormal ORT and one previous poor response to stimulation, (3) age ≥40 years and one previous poor response, (4) abnormal ORT and one previous poor response to stimulation (11).
AMH Analysis
AMH testing was performed between one and 3 months prior to the start of the ART cycle. Until the 24th of April 2012, our center used the AMH Immunotech (IOT) kit (Beckman Coulter Inc., Marseilles, France). Between the 25th of April 2012 and 3rd of July 2013, the Gen II kit was used (Beckman Coulter, Inc., Chaska, Minnesota, USA); between the 4th of July 2013 and 17th of September 2014 the modified Gen II test kit was used and since the 18th of September 2014 the Elecsys platform (Roche Diagnostics International AG, Rotkreuz, Switzerland) has been used. To homogenize AMH levels across these time intervals we used published conversion formulas (14, 15).
Treatment Protocol
From day 2 or 3 of the menstrual cycle all patients received fixed daily doses of ≥300 IU of highly purified human menopausal gonadotropin (hp-hMG) or recombinant follicle stimulating hormone (FSH) until the day prior to human chorionic gonadotrophin (hCG) administration.
Pituitary suppression was achieved with daily administration of a gonadotropin releasing hormone (GnRH) antagonist starting from day 6 of stimulation onwards. Women did not use oral contraceptives or estrogen priming prior to ovarian stimulation. A blood sample to measure estradiol (E2), progesterone (P), FSH, and luteinizing hormone (LH) levels and an ultrasound was performed on Day 2 or 3 of the menstrual cycle and from day 7 or 8 of the cycle onwards. Triggering of final oocyte maturation was achieved using 5,000 IU highly purified urinary or recombinant hCG, when at least two follicles reached 17 mm in mean diameter. In case of mono-follicular development, patients were given the option to proceed to oocyte retrieval nonetheless. All patients of our cohort with monofollicular development proceeded with oocyte pick-up. Cycle was canceled if there was no follicles development after 10 days of stimulation. Cumulus-oocyte complexes (COCs) were collected by transvaginal aspiration 36 h after hCG administration, followed by insemination via ICSI (16). Luteal phase support consisted of vaginal micronized progesterone (200 mg, three times a day), initiated the day after the oocyte retrieval and continued until at least 7 weeks, in case of a positive pregnancy test.
Embryo Transfer
On day 3 or 5 after oocyte retrieval an ultrasound-guided fresh embryo transfer (ET) was performed. The day of the transfer was chosen in accordance with our internal policy; specifically, when at least 4 embryos of top quality (at least 7 cells with maximum 10% fragmentation) or good quality (at least 6 cells with maximum 20% fragmentation) were present on Day 3, embryo culture was extended to Day 5, followed by fresh ET on Day 5. Otherwise, ET took place on Day 3. The maximum number of embryos transferred in the fresh cycle was limited to three.
Cryopreservation
Vitrification of supernumerary good quality embryos was performed on Day 3 or Day 5 using closed blastocyst vitrification high security straws combined with dimethylsulphoxide and ethylene glycol bis (succinimidyl succinate) as the cryoprotectants (17). Good-quality Day 3 embryos were defined as embryos that reached the 6-cell stage with <20% fragmentation. Good-quality Day 5 embryos were defined as having trophectoderm and inner cell mass quality scores of at least AB, BA, or BB (18).
Frozen–Thawed Embryo Transfer
Frozen ET, following warming of vitrified embryos, was performed either in a natural cycle, with or without hCG triggering, or in an artificial cycle, as previously described elsewhere (19). The decision regarding the type of preparation for the frozen ET cycle was made by the physician, based on the menstrual cycle pattern of the patient. The number of embryos transferred in the frozen-thawed cycles was one or two in compliance with Belgian regulatory guidelines and according to the patients' individual preference.
Primary Outcome
The primary outcome was cumulative LBR defined as the number of deliveries with at least one live birth (>22 weeks of gestation) resulting from one initiated or aspirated ART cycle, including all cycles in which fresh and/or frozen embryos were transferred, until one delivery with a live birth occurred or until all embryos were used, whichever occurred first (20).
Secondary Outcomes
The secondary outcomes included biochemical pregnancy rate (BPR, a pregnancy diagnosed only by the detection of hCG in serum or urine and that does not develop into a clinical pregnancy), clinical pregnancy rate (CPR, a pregnancy diagnosed by ultrasonographic visualization of one or more gestational sacs), ongoing pregnancy rate (OPR, diagnosed by ultrasonographic visualization of an intrauterine sac with embryonic pole demonstrating cardiac activity at 10 weeks of gestation), and LBR following fresh ET (delivery of a live born after 22 weeks of gestation, following the fresh IVF/ICSI cycle only) (20).
Statistical Analysis
Outcomes were analyzed by BC pattern. In order to ascertain patients' baseline characteristics and important aspects of the treatment, continuous data are presented as mean ± standard deviation (SD) and median-interquartile range (IQR), while categorical data are described by number of cases, including numerator and denominator, and percentages. Categorical data and continuous data that did not show a normal distribution were analyzed by Pearson's χ2-test/Fisher exact test or Kruskal–Wallis test as appropriate.
Furthermore, in order to assess the association between cumulative LBR and BC patterns after adjustment for relevant confounders, multivariable regression models with estimation by generalized estimating equations (GEE) were used in order to account for inclusion of multiple cycles of a patient. The potential predictors considered for the analysis were BMI, number of metaphase II (MIIs) oocytes and day/number of embryos transferred in the fresh cycle.
All statistical tests used a two-tailed α of 0.05. All analyses were performed using STATA 13.0 (StataCorp. Stata Statistical Software: Release 13. College Station, TX, USA).
Results
Baseline Characteristics
Overall, data from 846 cycles in 706 Bologna PORs were included in the analysis and divided into four different patterns: 310 cycles in pattern 1, 169 in pattern 2, 52 in pattern 3, and 315 in pattern 4. Patients' baseline characteristics among the four groups were similar regarding BMI and basal FSH. There were significant differences in female age (41.3 ± 1.2 vs. 41.4 ± 1.1 vs. 41.3 ± 1.0 vs. 35.3 ± 3.5, respectively, for the four patterns, P < 0.001), AFC (4.6 ± 2.7 vs. 4.4 ± 2.6 vs. 8.8 ± 4.2 vs. 4.6 ± 2.7, respectively, P < 0.001), and AMH (0.57 ± 0.29 vs. 0.54 ± 0.28 vs. 1.7 ± 0.6 vs. 0.5 ± 0.3, respectively, P < 0.001), among the four patterns. The baseline characteristics of patients are summarized in (Table 1).
Table 1.
Baseline characteristics of patients.
|
Pattern 1 Age ≥ 40 y AMH < 1.1 (n = 310) |
Pattern 2 Age ≥ 40 y AMH < 1.1 PS ≤ 3 COCs (n = 169) |
Pattern 3 Age ≥ 40 y PS ≤ 3 COCs (n = 52) |
Pattern 4 Age < 40 y AMH < 1.1 PS ≤ 3 COCs (n = 315) |
P-value | |
|---|---|---|---|---|---|
| Age (years) | 41 (40–42) 41.3 ± 1.2 |
41 (41–42) 41.4 ± 1.1 |
41 (40–42) 41.3 ± 1.0 |
36 (33–38) 35.3 ± 3.5 |
<0.001a |
| BMI (kg/m2) | 24 (22–28) 25.1 ± 4.4 |
24 (22–28) 24.9 ± 4.3 |
24 (21–27) 24.2 ± 4.6 |
24 (22–28) 25.2 ± 4.7 |
0.483a |
| Basal FSH | 10.1 (8.0–12.8) 10.8 ± 4.0 |
10.3 (7.7–13.3) 10.8 ± 4.6 |
9.6 (7.6–11.4) 9.6 ± 3.2 |
9.8 (7.4–12.4) 10.5 ± 4.4 |
0.389a |
| AMH (ng/ml) | 0.55 (0.30–0.80) 0.57 ± 0.29 |
0.47 (0.29–0.78) 0.54 ± 0.28 |
1.5 (1.3–1.9) 1.7 ± 0.6 |
0.4 (0.3–0.7) 0.5 ± 0.3 |
<0.001a |
| AFC | 4 (2–6) 4.6 ± 2.7 |
4 (2–6) 4.4 ± 2.6 |
8 (5–12) 8.8 ± 4.2 |
4 (3–6) 4.6 ± 2.7 |
<0.001a |
| PS attempts (n) | 1 (0–2) | 2 (1–4) | 2 (1–4) | 2 (1–3) | <0.001a |
PS, previous stimulation; COC, cumulus-oocyte complex; BMI, body mass index; FSH, follicle stimulating hormone; AMH, antimüllerian hormone; AFC, antral follicle count.
Kruskal–Wallis test. Values are mean (SD) and median (IQR).
Ovarian Response and Characteristics of Embryo Development
Among the different subgroups, the number of COCs retrieved was significantly different (3.5 ± 2.3 vs. 2.9 ± 1.8 vs. 3.8 ± 1.6 vs. 2.7 ± 1.7 for pattern 1, 2, 3, 4, respectively, P < 0.001). Similarly, Implantation rates were significantly different: 54 (17%) vs. 26 (11%) vs. 7 (8%) vs. 50 (22%) for pattern 1, 2, 3, 4, respectively, P = 0.001. Cycle cancellation rates differed also significantly between groups (25.3% vs. 21.9% vs. 17.3 vs. 31.4%, P = 0.041). The number of embryos transferred was higher in pattern 4 compared to the other patterns (1.4 ± 2.1 vs. 1.3 ± 0.9 vs. 1.6 ± 1.0 vs. 2.2 ± 1.5, P < 0.001). Most ETs (94%) took place on Day 3. These results are presented in (Table 2).
Table 2.
Cycle characteristics of controlled ovarian stimulation.
|
Pattern 1 Age ≥ 40 y AMH < 1.1 (n = 310) |
Pattern 2 Age ≥ 40 y AMH < 1.1 PS ≤ 3 COCs (n = 169) |
Pattern 3 Age ≥ 40 y PS ≤ 3 COCs (n = 52) |
Pattern 4 Age < 40 y AMH < 1.1 PS ≤ 3 COCs (n = 315) |
P-value | |
|---|---|---|---|---|---|
| COCs | 3 (2–5) 3.5 ± 2.3 |
3 (2–4) 2.9 ± 1.8 |
4 (2–5) 3.8 ± 1.6 |
3 (1–3) 2.7 ± 1.7 |
<0.001a |
| MII | 3 (1–4) 2.9 ± 2.1 |
2 (1–4) 2.4 ± 1.6 |
3 (2–4) 3.1 ± 1.6 |
2 (1–3) 2.2 ± 1.5 |
<0.001a |
| Cycle with ET, n (%) | 230 (74) | 131 (76) | 43 (83) | 200 (64) | 0.001b |
| Number of embryos transferred | 1 (0–2) 1.4 ± 2.1 |
1 (1–2) 1.3 ± 0.9 |
2 (1–2) 1.6 ± 1.0 |
2 (1–3) 2.2 ± 1.5 |
<0.001a |
| Day of transfer, n (%) | |||||
| Day 3 | 219 (95) | 126 (96) | 43 (100) | 178 (89) | 0.005c |
| Day 5 | 11 (5) | 5 (4) | 0 (0) | 22 (11) | |
| Cycle cancelation, n (%) | 79 (25.3%) | 37 (21.9%) | 9 (17.3%) | 99 (31.4%) | 0.041b |
PS, previous stimulation; AMH, antimüllerian hormone; COC, cumulus oophorus complex; MII, metaphase II oocytes; ET, embryo transfer.
Kruskal–Wallis test. Values are mean (SD) and median (IQR).
Pearson χ2-test. Values are number (percentage).
Fisher exact test. Values are number (percentage).
Reproductive Outcomes
Reproductive outcomes are displayed in (Table 3). There was no statistically relevant difference in terms of BPR and CPR among different patterns. However, OPR (7.4% vs. 4.1% vs. 5.8% vs. 13.7%, respectively, for the four patterns, with P = 0.002), LBR (7.4% vs. 4.1% vs. 5.8% vs. 13.4%, respectively, with P = 0.001), and cumulative LBR (8.3% vs. 4.1% vs.9.6% vs. 16.8%, respectively with P < 0.001) significantly differed among the four patterns (Figure 1). In particular, the P-values for the unadjusted pairwise comparisons for cumulative LBR between patterns were as follow: pattern 1 vs. 2 (P = 0.09), pattern 3 vs. 1 (P = 0.8), pattern 4 vs. 1 (P = 0.002), pattern 3 vs. 2 (P = 0.13), pattern 4 vs. 2 (P < 0.001), pattern 4 vs. 3 (P = 0.19).
Table 3.
Reproductive outcomes.
|
Pattern 1 Age ≥ 40 y AMH < 1.1 (n = 310) |
Pattern 2 Age ≥ 40 y AMH < 1.1 PS ≤ 3 COCs (n = 169) |
Pattern 3 Age ≥ 40 y PS ≤ 3 COCs (n = 52) |
Pattern 4 Age < 40 y AMH < 1.1 PS ≤ 3 COCs (n = 315) |
P-value | |
|---|---|---|---|---|---|
| Biochemical pregnancy rate, n (%) | 60 (19.2) | 31 (18.3) | 9 (17.3) | 58 (18.4) | 0.985b |
| Clinical pregnancy rate, n (%) | 54 (17.3) | 26 (15.4) | 7 (13.5) | 50 (15.9) | 0.879b |
| Ongoing pregnancy rate, n (%) | 23 (7.4) | 7 (4.1) | 3 (5.8) | 43 (13.7) | 0.002b |
| LBR, n (%) | 23 (7.4) | 7 (4.1) | 3 (5.8) | 42 (13.4) | 0.001c |
| Cumulative LBR, n (%) | 26 (8.3) | 7 (4.1) | 5 (9.6) | 53 (16.8) | <0.001b |
PS, previous stimulation; AMH, antimüllerian hormone; COC, cumulus-oocyte complex; LBR live birth rate (following the fresh embryo transfer); cumulative LBR (definition – to clarify the table when seen isolated from the text).
Pearson χ2-test. Values are number (percentage).
Fisher exact test. Values are number (percentage).
Figure 1.
Fresh and cumulative live birth rates in different POR patterns.
Categorization of patients in subgroups based on AFC (rather than AMH) resulted in similar findings (Supplementary Table 1).
Furthermore, in order to avoid selection bias (patients repeating a second cycle may have had a better prognosis), an extra analysis was performed: main reproductive outcomes were analyzed per patient (one cycle per patient). Results remained approximately the same (Supplementary Table 2).
GEE Analysis for Cumulative LBR
The multivariate GEE analysis revealed that the number of MIIs (coefficient 0.03, P = 0.006) and the BC pattern [coefficients for pattern 1: (–), pattern 2: −0.04, pattern 3: 0.01, pattern 4: 0.14, P < 0.001] were the only variables which were significantly associated with cumulative LBR. Post-hoc pairwise comparisons revealed that pattern 4 was significantly associated with higher cumulative LBR compared to all the other patterns after adjustment for confounders: pattern 4 vs. 1 (P < 0.001), pattern 4 vs. 2 (P < 0.001), pattern 4 vs. 3 (P = 0.046). These findings are presented in (Table 4).
Table 4.
GEE regression analysis for cumulative LBR.
| Cumulative LBR | Coefficient | Standard error | P-value |
|---|---|---|---|
| BMI | 0.002 | 0.003 | 0.42 |
| MII | 0.03 | 0.01 | 0.006 |
| Number of embryos transferred | −0.013 | 0.02 | 0.6 |
| Day of transfer | |||
| Day 3 | 0.35 | ||
| Day 5 | −0.03 | 0.03 | |
| Pattern | |||
| Pattern 1 | – | – | |
| Pattern 2 | −0.04 | 0.04 | |
| Pattern 3 | 0.01 | 0.06 | <0.001 |
| Pattern 4 | 0.13 | 0.04 | |
LBR, live birth rate; BMI, body mass index; MII, metaphase II oocytes.
Discussion
To our knowledge, this retrospective study is the first to investigate cumulative LBR using ESHRE's different subpopulations of PORs. We stratified patients into more homogeneous sub-categories and found statistically significant differences in several baseline characteristics such as female age, AFC and AMH levels. Fresh and cumulative LBR differed significantly between the four patterns and according to our results, pattern 4, representing a younger subpopulation, with a better clinical prognosis. In other terms, we provide evidence that clinical heterogeneity, which in turn translates into different outcome prognosis, still exists within BC PORs.
The role of age as an independent predictor of reproductive outcomes has been previously highlighted, given the increase in the percentage of aneuploidy rates with age, being 30% in women younger than 35 years and rising over 90% in women older than 42 years (21, 22). In our study, despite a higher number of oocytes yielded and a lower cycle cancelation in pattern 3 (the only subgroup with normal ORT), the best clinical outcomes were observed in pattern 4, underlining the importance of age.
Following the publication of the BC in 2011, very few studies have been conducted to investigate their validity (23). Our results are in contrast with previous reports finding similar fresh LBR in different subgroups of BC PORs (13, 24). In particular, La Marca et al. (13)ĭncluded 210 PORs in a retrospective analysis and showed similar LBR ranging from 5.5 to 7.4% in all groups defined as follows: group 1 (two cycles with <4 oocytes) vs. group 2 (age > 40 years + cycle with <4 oocytes), vs. group 3 (age > 40 + abnormal markers of ovarian reserve), vs. group 4 (cycle with <4 oocytes + abnormal markers of ovarian reserve) vs. group 5 (cycle with <4 oocytes + age > 40 + abnormal markers of ovarian reserve). Similar findings were reported by Busnelli et al. (24), in a retrospective analysis of 362 women allocated to five subgroups generated using BC, specifically: (i) anamnestic risk factors and one previous poor ovarian response; (ii) anamnestic risk factors and an abnormal ORT; (iii) an abnormal ORT and one previous poor ovarian response; (iv) anamnestic risk factors, an abnormal ORT and one previous poor ovarian response; (v) two episodes of poor ovarian response after maximal stimulation. The analysis showed LBR around 6% and no differences among the various subgroups.
Several reasons may explain the discrepancy between our results and those reported by Busnelli et al. and La Marca et al. First, our sample size of 706 PORs is considerably larger, limiting the probabilities of type II error. Second, the difference in age of the younger subgroup of the previously described studies compared to our own could also be an explanation (i.e., median age was 38 ± 3.9 years vs. 35.3 ± 3.5 in La Marca et al. and our study, respectively). Additionally, the differences in the threshold used for ovarian reserve biomarkers and the gonadotropin starting dose may also account for these divergent results.
In contrast with the aforementioned findings, our results are in line with those described by Bozdag et al., who demonstrated LBR of 2.3–8.7% per started IVF cycle in BC PORs, with “young proven” PORs having the most favorable reproductive outcomes (12). Nonetheless, our primary endpoint was cumulative LBR, which is a more relevant clinical outcome, while Bozdag et al. assessed only fresh LBR (12).
The BC have been criticized for several reasons including the lack of clarity in defining risk factors for poor ovarian response and the fact that they do not account for oocyte quality and other factors that can be associated with diminished ovarian reserve (11, 23, 25, 26). However, the major issue remains the persistence of heterogeneity among patients that very often present with different baseline characteristics and therefore diverse prognoses. In this context, in yet another attempt to overcome the limitations of the BC, a modified definition of impaired ovarian response has been proposed by the Poseidon Group (Patient-Oriented Strategies Encompassing Individualized Oocyte Number) (27). These novel criteria categorize women to more homogeneous subgroups according to age, ORTs and oocyte yield in previous OS cycles. However, currently, there are no data comparing the clinical relevance of the Bologna and the Poseidon criteria and further research is warranted.
The major strength of the current study relies on its large sample size and the choice of cumulative LBR as primary outcome. The inclusion of PORs according to BC is another positive appraisal point, given the recent reluctance of fertility experts in using the BC in studies regarding poor ovarian response (23). Furthermore, the exclusive use of an antagonist protocol to achieve pituitary suppression translates into increased homogeneity, which helps in drawing valid conclusions.
Nonetheless, the retrospective study design should be considered a limitation. Although a significant effort has been made to eliminate all known sources of systematic error through multivariable analysis allowing adjustment for relevant confounders, non-apparent sources of bias might still persist. In addition, given the difficulty to pool data relevant to other risk factors related to POR, we only analyzed 4 out of the possible 8 BC POR subpopulations (11). Finally, although the study design was fairly robust regarding the fresh cycles, frozen ET preparation was not consistent among the population. However, frozen cycle protocols with natural or artificial preparation have been shown to be equally effective (28–31).
In conclusion, establishing the BC represented the first real attempt of the medical community to reduce the vast heterogeneity underlying the definition of POR that greatly limited extrapolation of results from different studies and validation of conclusions. However, despite a proven applicability in subsequent research, the BC include patients with diverse baseline characteristics and therefore different clinical outcomes. The results of our study emphasize the discrepancy in clinical outcomes among the different subpopulations of PORs, with the younger subgroup having a better prognosis. Further modification of BC, perhaps by stratifying PORs in different subcategories that display more similar characteristics, may improve the shortcomings of the present definition.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Brussels University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ARo, JE, and AK were responsible for data extraction and collection. ARo and PD were responsible for the statistical analysis. ARo, EB, and PD were responsible for writing the manuscript. SS-R, MV, ARa, SM, CB, PP, AV, and HT have all contributed in the interpretation and editing of the manuscript. PD was responsible for the concept and the final revision of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Walter Meul for his invaluable help during data collection and abstraction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00208/full#supplementary-material
References
- 1.Errazuriz J, Drakopoulos P, Pening D, Racca A, Romito A, De Munck N, et al. Pituitary suppression protocol among Bologna poor responders undergoing ovarian stimulation using corifollitropin alfa: does it play any role? Reprod Biomed Online. (2019) 38:1010–7. 10.1016/j.rbmo.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 2.Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online. (2015) 30:581–92. 10.1016/j.rbmo.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Ubaldi FM, Rienzi L, Ferrero S, Baroni E, Sapienza F, Cobellis L, et al. Management of poor responders in IVF. Reprod Biomed Online. (2005) 10:235–46. 10.1016/S1472-6483(10)60946-7 [DOI] [PubMed] [Google Scholar]
- 4.Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM. What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol. (2018) 30:155–62. 10.1097/GCO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 5.Drakopoulos P, Vuong TNL, Ho NAV, Vaiarelli A, Ho MT, Blockeel C, et al. Corifollitropin alfa followed by highly purified HMG versus recombinant FSH in young poor ovarian responders: a multicentre randomized controlled clinical trial. Hum Reprod. (2017) 32:2225–33. 10.1093/humrep/dex296 [DOI] [PubMed] [Google Scholar]
- 6.Errazuriz J, Romito A, Drakopoulos P, Frederix B, Racca A, De Munck N, et al. Cumulative live birth rates following stimulation with corifollitropin alfa compared with hp-hMG in a GnRH antagonist protocol in poor ovarian responders. Front Endocrinol. (2019) 10:175. 10.3389/fendo.2019.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polyzos NP, Corona R, Van De Vijver A, Blockeel C, Drakopoulos P, Vloeberghs V, et al. Corifollitropin alfa followed by hpHMG in GnRH agonist protocols. Two prospective feasibility studies in poor ovarian responders. Gynecol Endocrinol. (2015) 31:885–90. 10.3109/09513590.2015.1065481 [DOI] [PubMed] [Google Scholar]
- 8.Grisendi V, Mastellari E, La Marca A. Ovarian reserve markers to identify poor responders in the context of poseidon classification. Front Endocrinol. (2019) 10:281. 10.3389/fendo.2019.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. (2011) 96:1058–61.e7. 10.1016/j.fertnstert.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 10.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. Definition EwgoPOR. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. (2011) 26:1616–24. 10.1093/humrep/der092 [DOI] [PubMed] [Google Scholar]
- 11.Papathanasiou A. Implementing the ESHRE 'poor responder' criteria in research studies: methodological implications. Hum Reprod. (2014) 29:1835–8. 10.1093/humrep/deu135 [DOI] [PubMed] [Google Scholar]
- 12.Bozdag G, Polat M, Yarali I, Yarali H. Live birth rates in various subgroups of poor ovarian responders fulfilling the Bologna criteria. Reprod Biomed Online. (2017) 34:639–44. 10.1016/j.rbmo.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 13.La Marca A, Grisendi V, Giulini S, Sighinolfi G, Tirelli A, Argento C, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet. (2015) 32:931–7. 10.1007/s10815-015-0476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anckaert E, Öktem M, Thies A, Cohen-Bacrie M, Daan NM, Schiettecatte J, et al. Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem. (2016) 49:260–7. 10.1016/j.clinbiochem.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. (2010) 362:51–9. 10.1016/j.jim.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 16.Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. (2005) 83:1397–403. 10.1016/j.fertnstert.2004.10.054 [DOI] [PubMed] [Google Scholar]
- 17.Van Landuyt L, Stoop D, Verheyen G, Verpoest W, Camus M, Van de Velde H, et al. Outcome of closed blastocyst vitrification in relation to blastocyst quality: evaluation of 759 warming cycles in a single-embryo transfer policy. Hum Reprod. (2011) 26:527–34. 10.1093/humrep/deq374 [DOI] [PubMed] [Google Scholar]
- 18.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. (1999) 11:307–11. 10.1097/00001703-199906000-00013 [DOI] [PubMed] [Google Scholar]
- 19.Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. (2017) 32:2234–42. 10.1093/humrep/dex285 [DOI] [PubMed] [Google Scholar]
- 20.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, De Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. (2017) 108:393–406. 10.1016/j.fertnstert.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 21.Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi FM, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. (2017) 23:706–22. 10.1093/humupd/dmx026 [DOI] [PubMed] [Google Scholar]
- 22.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. (2014) 101:656–63.e1. 10.1016/j.fertnstert.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Boza A, Oguz SY, Misirlioglu S, Yakin K, Urman B. Utilization of the Bologna criteria: a promise unfulfilled? A review of published and unpublished/ongoing trials. Fertil Steril. (2018) 109:104–9.e2. 10.1016/j.fertnstert.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 24.Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. (2015) 30:315–22. 10.1093/humrep/deu319 [DOI] [PubMed] [Google Scholar]
- 25.Frydman R. Poor responders: still a problem. Fertil Steril. (2011) 96:1057. 10.1016/j.fertnstert.2011.09.051 [DOI] [PubMed] [Google Scholar]
- 26.Younis JS. The Bologna criteria for poor ovarian response; has the job been accomplished? Hum Reprod. (2012) 27:1874–5; author reply: 1875–6. 10.1093/humrep/des118 [DOI] [PubMed] [Google Scholar]
- 27.Poseidon G, Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. (2016) 105:1452–3. 10.1016/j.fertnstert.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. (2008) 7:CD003414. 10.1002/14651858.CD003414.pub2 [DOI] [PubMed] [Google Scholar]
- 29.Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. (2010) 20:CD006359 10.1002/14651858.CD006359.pub2 [DOI] [PubMed] [Google Scholar]
- 30.Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. (2016) 31:1483–92. 10.1093/humrep/dew120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Vijver A, Polyzos NP, Van Landuyt L, De Vos M, Camus M, Stoop D, et al. Cryopreserved embryo transfer in an artificial cycle: is GnRH agonist down-regulation necessary? Reprod Biomed Online. (2014) 29:588–94. 10.1016/j.rbmo.2014.08.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.