Abstract
Blockade of the programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) axis using antibodies against the associated receptors and ligands has yielded good clinical responses and improved overall survival in patients with non–small cell lung cancer (NSCLC). Once patients show a response to anti–PD-1/PD-L1 antibody, the median duration of response is often longer than that achieved using existing cytotoxic agents and even some molecular targeted agents. However, the response rates to these antibodies are only 15% to 20% in unselected patients with NSCLC and the cost of this therapy is high. Therefore, there is an urgent need for effective predictive biomarkers to identify patients likely to benefit. PD-L1 expression, which can be detected by immunohistochemical analysis, is a rational biomarker for selecting responders to anti–PD-1/PD-L1 antibody treatments, and this selection method has been introduced into clinical practice. However, the response rate to anti–PD-1/PD-L1 antibody in PD-L1–expressing patients with NSCLC is only 15% to 45%, response can occur in PD-L1–negative patients, and predictability based on PD-L1 expression may differ between nonsquamous NSCLC and squamous cell NSCLC. In addition, the methods of immunohistochemical analysis and evaluation of its results differ for different anti–PD-1/PD-L1 agents. This article reviews the existing data on predictive markers for the efficacy of anti–PD-1/PD-L1 antibodies in NSCLC.
Keywords: Lung cancer, Immunotherapy, PD-1, PD-L1, Biomarker
Introduction
Lung cancer is the most prevalent cancer worldwide.1 More than half of patients with lung cancer have advanced disease at the time of diagnosis, and these patients are candidates for primary systemic therapy.2–4 The prognosis of patients with lung cancer is poor, and although lung cancer is not the most frequently diagnosed cancer in the United States, it is by far the leading cause of cancer-related deaths in the United States and also worldwide.1 Therefore, advances in the treatment of lung cancer are urgently needed.
Programmed death protein 1 (PD-1) and its ligands, programmed death ligand 1 and 2 (PD-L1 and PD-L2), are immune checkpoint proteins that primarily function to limit the effector function of T cells in peripheral tissues during inflammatory responses and limit autoimmunity.5–7 However, when these proteins are expressed within the tumor microenvironment, this process represents a potent mechanism of tumor-induced immune suppression and evasion. Blockade of this pathway with antibodies against PD-1 or its ligands has yielded good clinical responses and improved overall survival (OS) in patients with lung cancer.8,9 Currently, nivolumab has been approved by the U.S. Food and Drug Administration, European Medicines Agency, and Japan for non–small cell lung cancer (NSCLC), and pembrolizumab has been approved by the U.S. Food and Drug Administration for PD-L1–positive NSCLC.10 Several other antibodies against this and other immune-modulatory targets are also under investigation (Table 1).11
Table 1.
PD-1/PD-L1 Antibodies in Clinical Development
| Target | Agent Name | Class | Phase | Company | Companion PD-L1 Detection Antibody |
|---|---|---|---|---|---|
| PD-1 | Nivolumab (BMS-936558, MDX1106) | Human IgG4 | Approved by FDA, EMA, Japan | BMS, Ono pharmaceutical | 28–8, Epitomics/Dako |
| Pembrolizumab (MK-3475, lambrolizumab) | Humanized IgG4k | Approved by FDA | Merck & Co | 22C3, Dako/Merck | |
| Pidilizumab (CT-011) | Humanized IgG1k | 2 (DLBCL) | Cure Tech | ||
| AMP-224 | Fusion protein of PD-L2 and Fc domain of human IgG | 1 | Amplimmune/GSK | ||
| AMP-514 (MEDI0680) | Humanized IgG4k | 1 | Amplimmune/GSK | ||
| PDR001 | Humanized IgG4 | 1 | Novartis | ||
| REGN2810 | Human monoclonal antibody | 1 | Regeneron | ||
| BGB A317 | Humanized monoclonal antibody | 1 | BeiGene | ||
| SHR-1210 | Monoclonal antibody | 1 | Atridia | ||
| PD-L1 | Atezolizumab (MPDL3280A) | Fully humanized IgG1 | 3 | Roche | SP142, Spring Bioscience/ Ventana/ Roche |
| Durvalumab (MEDI4736) | Human IgG1k | 3 | MedImmune/ AZ | SP263, Spring Bioscience/ Ventana/ Roche | |
| Avelumab (MSB0010718C) | Human IgG1 | 3 | Merck KGaA | Dako | |
| BMS-936559 (MDX1105) | Human IgG4 | 1 | BMS | 28–8, Epitomics/Dako |
PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; IgG, immunoglobulin G; FDA, U.S. Food and Drug Administration; EMA, European Medicines Agency; DLBCL, diffuse large B-cell lymphoma; BMS, Bristol-Myers Squibb; GSK, GlaxoSmithKline; AZ, AstraZeneca.
The response rate (RR) to anti–PD-1/PD-L1 antibodies is 13.6% to 23% and the median progression-free survival (PFS) is 2.3 to 4 months in biomarker–nonselected second-line patients with NSCLC.8–10,12–15 Because they do not appear to have clinical benefit in most patients with lung cancer and are very costly, reliable predictive markers should be established. PD-L1 expression by immunohistochemical analysis in tumor samples has been proposed as a useful predictive marker. Even though pembrolizumab has been approved for PD-L1–positive NSCLC, the RR to anti–PD-1/PD-L1 antibody in patients with NSCLC expressing PD-L1 is not as high as we are used to with mutated driver–directed therapy, at 15.6% to 48%.8–10,12–18 Moreover, predictability based on PD-L1 expression in immunohistochemical analysis may differ between nonsquamous NSCLC and squamous cell NSCLC.8,9 In addition, the field is complicated by the fact that the antibodies, staining procedures, and quantitation of PD-L1 immunohistochemical profile differ among the different companies developing anti–PD-1/PD-L1 agents. Clinical parameters, exposure history, genome complexity, and infiltrating T cells and many other potential markers are being actively studied as well in this rapidly evolving field.
The status of predictive biomarkers for anti–PD-1/PD-L1 antibodies in patients with NSCLC has not been comprehensively summarized to date; in this review, we have organized and described the existing and potential predictive biomarkers for PD-1/PD-L1 blockade in lung cancer.
Methods
We performed a literature search for studies reported from January 2012 to December 2015. Studies were identified through a computer-based search of the PubMed database and abstracts from the past meetings of the American Society of Clinical Oncology, the European Society for Medical Oncology, and the International Association for the Study of Lung Cancer. To detect studies related to predictive markers for the efficacy of anti–PD-1/PD-L1 antibodies in lung cancer, we used the terms lung cancer AND PD-1 or lung cancer AND PD-L1 in the PubMed database or looked over titles and abstracts of previously mentioned medical meetings.
Immunohistochemical Analysis of PD-L1
Technical Issues Related to PD-L1 Immunohistochemical Analysis, Heterogeneity of PD-L1 Expression, and Effect of Prior Therapy
PD-L1 has a limited number of binding sites for antibody detection using immunohistochemical analysis. The protein contains only two small hydrophilic regions, making immunohistochemical approaches in formalin-fixed, paraffin-embedded specimens relatively difficult.19,20 Therefore, unlike therapeutic PD-L1 antibodies, antibodies used for immunohistochemical analysis typically bind to PD-L1 at structurally unique sites. Unlike genetic markers, PD-L1 is a protein expression marker, and in addition to assay variabilities, it is subject to true changes with time, treatment exposure, and other therapies such as radiation.
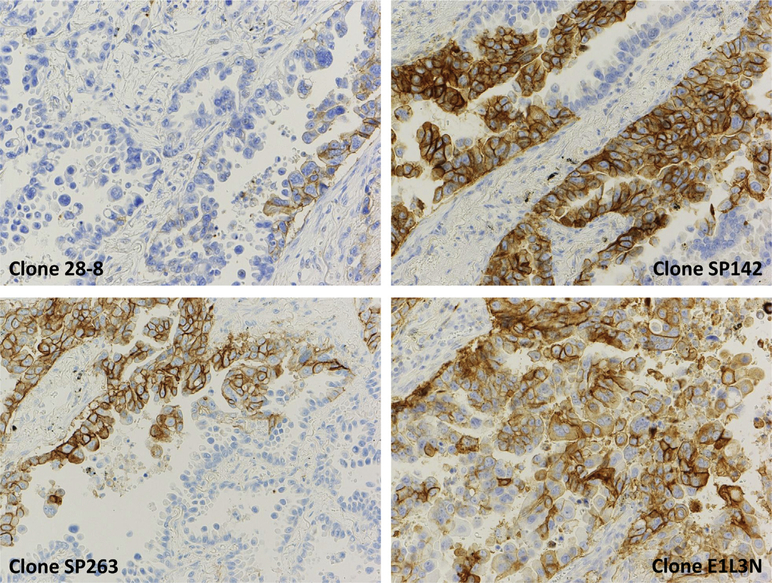
As shown in Table 1, each companion diagnostic of PD-L1 immunohistochemical analysis has been developed to correspond to each company’s anti–PD-1/PD-L1 agents. Figure 1 shows examples of PD-L1 immunohistochemical analysis using 28–8 (Dako, Carpentaria, CA), SP142 (Spring Bioscience, Pleasanton, CA), SP263 (Spring Bioscience), and E1L3N (Cell Signaling Technology, Danvers, MA) antibodies. E1L3N has not been used in any of the clinical trials. The antibodies used for PD-L1 detection differ among these diagnostic tests, and some tests evaluate the percentage of tumor cells (TCs) stained, whereas others evaluate not only TCs stained but also tumor-infiltrating immune cells (ICs). Moreover, the cutoff points for a positive result or scoring system differ among diagnostic tests. These differences limit the interpretation and comparison of clinical trial biomarker data across trials. In addition, the difference not only in antibodies for staining PD-L1 but also in staining techniques (e.g., manual versus automated techniques) will affect test results.
Figure 1.
Examples of programmed death protein 1 (PD-L1) immunohistochemical analysis using 28–8 (Dako, Carpentaria, CA), SP142 (Spring Bioscience, Pleasanton, CA), SP263 (Spring Bioscience), and E1L3N (Cell Signaling Technology, Danvers, MA). Courtesy of Dr. Yasushi Yatabe, Aichi Cancer Center.
In 80 surgically resected patients with stage II NSCLC, PD-L1 expression was evaluated using three different antibodies: 28–8, SP142, and E1L3N. Staining of any intensity in at least 1% of TCs in a sample was considered a positive result. Thirty-six percent of cases (29 of 80) were positive when SP142 was used, 24% (19 of 80) when E1L3N was used, and 34% (27 of 80) when 28–8 was used. The three antibodies showed concordant results in only 76% of cases (61 of 80).21
PD-L1 expression was measured with SP142 and E1L3N in 49 NSCLC cases in which chromogenic immunohistochemical analysis and quantitative immunofluorescence were used to assess heterogeneity and concordance of assays. These PD-L1 antibodies showed poor concordance (Cohen κ range 0.124–0.340) when conventional chromogenic immunohistochemical analysis was used, and they showed intraassay heterogeneity (SP142 coefficient of variation 12.17%–109.61% and E1L3N coefficient of variation 6.75%–75.24%) and significant interassay discordance (26.6%) when quantitative immunofluorescence was used.22 The International Association for the Study of Lung Cancer (IASLC) is currently conducting a comparison of the different assays in a large number of patients that is eventually to be correlated with immunotherapy outcomes, and the results of this comparison are eagerly awaited.
PD-L1 expression was assessed by immunohistochemical analysis using the SP142 antibody in 25 patients with NSCLC and matched pair samples (14 synchronous primary tumor and metastasis pairs and 11 metachronous primary tumor and metastasis pairs). PD-L1 status in the TCs or ICs with a 5% cutoff remained unchanged in all paired samples, and in the TCs or ICs with a 1% cutoff remained unchanged in 80% of pairs (11 of 14 synchronous pairs and 9 of 11 metachronous pairs).23 In another study, PD-L1 expression was assessed using the 28–8 antibody. Samples were categorized as positive when TC membranes were stained to any intensity in 5% or more of TCs. In 57 paired primary tumor and metastasis samples, discordance with negative primary and positive metastasis was observed in 12% of samples (7 of 57), whereas 11% (6 of 57) were positive in primary tumor and negative in metastasis. When cutoff levels were set to 1% and 50%, the concordance rates were 63% and 89%, respectively.24
PD-L1 expression in immunohistochemical analysis was compared between pre– and post–epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitor biopsy specimens to evaluate whether these targeted therapies can affect PD-L1 expression. In 58 paired biopsy specimens from patients with epidermal growth factor receptor gene (EGFR) mutations, the PD-L1 expression levels varied between biopsy specimens in 13 pairs (22%). In eight paired biopsy specimens of anaplastic lymphoma kinase gene (ALK)-positive patients, the PD-L1 expression levels varied between biopsy specimens in two pairs (25%).25 In 95 paired archival and fresh tumor samples in the phase 2 study of atezolizumab (FIR trial), PD-L1 expression was assessed using the SP142 antibody separately in TC and IC compartments. The concordance rate of PD-L1 expression between fresh and archival tissue at the TC3 (at least 50% PD-L1–positive cells) or IC3 (at least 10% PD-L1–positive cells) cutoff was 88% when the same method of tissue procurement was used (resection or biopsy). The concordance rate was 65% when different methods of tissue procurement were used.26
In summary, the concordance rates of PD-L1 positivity in different antibodies ranged from 73.4% to 76%, and the concordance rates of PD-L1 positivity between primary tumor and metastatic tumor ranged from 63% to 100%. PD-L1 immunohistochemical status changed during the course of treatment in 12% to 35% of the patients.
PD-L1 Expression as a Prognostic Marker in NSCLC
Before a marker is labeled predictive, the effect of the marker as a prognostic marker must be taken into account. In a meta-analysis of six studies including 1157 patients with NSCLC, high PD-L1 expression was correlated with poor prognosis in terms of OS.27 In contrast, PD-L1 expression was analyzed using the 22C3 antibody (Dako) in 678 patients with stage I through stage III NSCLC. When a cutoff value of at least 50% was used, cells showing positive membrane staining and patients with high PD-L1 expression showed significantly longer OS.28 In 50 patients with stage III NSCLC who were treated by definitive chemoradiotherapy, PD-L1 expression was assessed using the E1L3N antibody, and PD-L1 positivity was associated with poorer OS and PFS.29 Thus, the prognostic implications of PD-L1 are still uncertain.
PD-L1 Expression as a Predictive Marker of Nivolumab in NSCLC
In the phase 3 study comparing nivolumab with docetaxel for patients with previously treated squamous cell NSCLC (CheckMate 017), the nivolumab group showed longer OS (median 9.2 versus 6.0 months, hazard ratio [HR] = 0.59, 95% confidence interval [CI]: 0.44–0.79, p < 0.001), longer PFS (median 3.5 versus 2.8 months, HR = 0.62, 95% CI: 0.47–0.81, p < 0.001), and a higher RR (20% versus 9%, p = 0.008).8 Expression of PD-L1 protein was evaluated retrospectively in pretreatment (archival or recent) tumor biopsy specimens using an anti–PD-L1 antibody (28–8). Samples were categorized as positive when staining of the TC membrane (at any intensity) was observed at the prespecified expression levels of 1%, 5%, or 10% of cells in a section that included at least 100 TCs that could be evaluated. Of 272 patients who underwent randomization, 225 showed quantifiable PD-L1 expression. Across the expression levels (1%, 5%, and 10%), PD-L1 expression was not predictive of response, PFS, or OS (Table 2).
Table 2.
PD-L1 Expression in Immunohistochemical Analysis as a Predictive Marker for PD-1/PD-L1 Blockade
| Study Name (Phase) | Histologic Diagnosis | Line | Agent/Dose | N | PD-L1 Expression and No. patients | OS in overall patients (mo) | PD-L1 Expression and OS | PFS in overall patients (mo) | PD-L1 Expression and PFS | RR in overall patients (%) | PD-L1 Expression and RR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate0178 (phase 3) | SqNSCLC | Second | Nivolumab 3 mg/kg every 2 wk (vs. docetaxel) |
135 (137) | <1% | 54 | 9.2 (0.59a) | <1% | 0.58a | 3.5 (0.62a) | <1% | 0.66a | 20% | <1% | 17% |
| ≥1% | 63 | ≥1% | 0.69a | ≥1% | 0.67a | ≥1% | 17% | ||||||||
| <5% | 75 | <5% | 0.70a | <5% | 0.75a | <5% | 15% | ||||||||
| ≥5% | 42 | ≥5% | 0.53a | ≥5% | 0.54a | ≥5% | 21% | ||||||||
| <10% | 81 | <10%o | 0.70a | <10%o | 0.70a | <10%o | 16% | ||||||||
| ≥10% | 36 | ≥10% | 0.50a | ≥10% | 0.58a | ≥10% | 19% | ||||||||
| CheckMate0579 (phase 3) | Non-SqNSCLC | Second | Nivolumab 3 mg/kg every 2 wk (vs. docetaxel) |
292 (290) | <1% | 108 | 12.2 (0.73a) | <1% | 0.90a | 2.3 (0.92a) | <1% | 1.19a | 19% | <1% | 9% |
| ≥1% | 123 | ≥1% | 0.59a | ≥1% | 0.70a | ≥1% | 31% | ||||||||
| <5% | 136 | <5% | 1.01a | <5% | 1.31a | <5% | 10% | ||||||||
| ≥5% | 95 | ≥5% | 0.43a | ≥5% | 0.54a | ≥5% | 36% | ||||||||
| <10% | 145 | <10% | 1.00a | <10% | 1.24a | <10% | 11% | ||||||||
| ≥10% | 86 | ≥10% | 0.40a | ≥10% | 0.52a | ≥10% | 37% | ||||||||
| KEYNOTE00110 (phase 1) | NSCLC | Any | Pembrolizumab 2 mg/kg every 3 wk 10 mg/kg every 3 wk 10mg /kg every 2 wk |
495 | <1% | 28 | 12 | <1% | 10.4 | 3.7 | <1% | 4.0 | 19.4% | <1% | 10.7% |
| 1%–49% | 103 | 1%–49% | 10.6 | 1%–49% | 4.1 | 1%–49% | 16.5% | ||||||||
| ≥50% | 73 | ≥50% | NR | ≥50% | 6.4 | ≥50% | 45.2% | ||||||||
| KEYNOTE01018 (phase 3) | PD-L1+NSCLC | Second + | Pembrolizumab 2 mg/kg every 3 wk (vs. docetaxel) |
344 (343) | ≥1% | 344 | 10.4 (0.71a) | ≥1% | 0.71a | 3.9 (0.88a) | ≥1% | 0.88a | 18% | ≥1% | 18% |
| ≥50% | 205 | ≥50% | 0.54a | ≥50% | 0.59a | ≥50% | 30% | ||||||||
| KEYNOTE01018 (phase 3) | PD-L1+NSCLC | Second + | Pembrolizumab 10 mg/kg every 3 wk (vs. docetaxel) |
346 (343) | ≥1% | 346 | 12.7 (0.61a) | ≥1% | 0.61a | 4.0 (0.79a) | ≥1% | 0.79a | 18% | ≥1% | 18% |
| ≥50% | 195 | ≥50% | 0.50a | ≥50% | 0.59a | ≥50% | 29% | ||||||||
| Phase 1 study of atezolizumab12 | NSCLC | Any | Atezolizumab 0.01–20 mg/kg every 3 wk |
88 | TC0-2 and IC0-2 | 58 | 16 | TC0-2 and IC0-2 | 16 | 4 | TC0-2 and IC0-2 | 4 | 23% | TC0-2 and IC0-2 | 16% |
| TC3 or IC3 | 21 | TC3 or IC3 | 18 | TC3 or IC3 | 4 | TC3 or IC3 | 48% | ||||||||
| POPLAR13 (phase 2) | NSCLC | Second | Atezolizumab 1200 mg every 3 wk (vs. docetaxel) |
144 | TC0 and IC0 | 32% | 12.6 (0.73a) | TC0 and IC0 | 1.04a | 2.7 (0.94a) | TC0 and IC0 | 1.12a | 15% | TC0 and IC0 | 8% |
| TC1-3 or IC1-3 | 68% | TC1-3 or IC1-3 | 0.59a | TC1-3 or IC1-3 | 0.85a | TC1-3 or IC1-3 | 18% | ||||||||
| TC2-3 or IC2-3 | 37% | TC2-3 or IC2-3 | 0.54a | TC2-3 or IC2-3 | 0.72a | TC2-3 or IC2-3 | 22% | ||||||||
| TC3 or IC3 | 16% | TC3 or IC3 | 0.49a | TC3 or IC3 | 0.60a | TC3 or IC3 | 38% | ||||||||
| FIR17 (phase 2) | PD-L1 + NSCLC | First | Atezolizumab 1200 mg every 3 wk |
31 | TC2-3 or IC2-3 | 31 | NR | TC2-3 or IC2-3 | NR | 4.5 | TC2-3 or IC2-3 | 4.5 | 26% | TC2-3 or IC2-3 | 26% |
| TC3 or IC3 | 7 | TC3 or IC3 | NR | TC3 or IC3 | 5.4 | TC3 or IC3 | 29% | ||||||||
| FIR (phase 2) | PD-L1 + NSCLC | Second, no brain Mets | Atezolizumab 1200 mg every 3 wk |
92 | TC2-3 and IC2-3 | 92 | 10.6 | TC2-3 and IC2-3 | 10.6 | 2.7 | TC2-3 and IC2-3 | 2.7 | 16% | TC2-3 and IC2-3 | 16% |
| TC3 or IC3 | 38 | TC3 or IC3 | NR | TC3 or IC3 | 4.1 | TC3 or IC3 | 24% | ||||||||
| FIR (phase 2) | PD-L1 + NSCLC | Second, brain Mets treated | Atezolizumab 1200 mg every 3 wk |
13 | TC2-3 and IC2-3 | 13 | 6.8 | TC2-3 and IC2-3 | 6.8 | 2.5 | TC2-3 and IC2-3 | 2.5 | 23% | TC2-3 and IC2-3 | 23% |
| TC3 or IC3 | 8 | TC3 or IC3 | 7.0 | TC3 or IC3 | 2.3 | TC3 or IC3 | 25% | ||||||||
| BIRCH16 (phase 2) | PD-L1 + NSCLC | First | Atezolizumab 1200 mg every 3 wk |
139 | TC2-3 and IC2-3 | 139 | 82%b | TC2-3 and IC2-3 | 82%b | 5.5 | TC2-3 and IC2-3 | 5.5 | 19% | TC2-3 and IC2-3 | 19% |
| TC3 or IC3 | 65 | TC3 or IC3 | 79%b | TC3 or IC3 | 5.5 | TC3 or IC3 | 26% | ||||||||
| BIRCH (phase 2) | PD-L1 + NSCLC | Second | Atezolizumab 1200 mg every 3 wk |
267 | TC2-3 and IC2-3 | 267 | 76%b | TC2-3 and IC2-3 | 76%b | 2.8 | TC2-3 and IC2-3 | 2.8 | 17% | TC2-3 and IC2-3 | 17% |
| TC3 or IC3 | 122 | TC3 or IC3 | 80%b | TC3 or IC3 | 4.1 | TC3 or IC3 | 24% | ||||||||
| BIRCH (phase 2) | PD-L1 + NSCLC | Third + | Atezolizumab 1200 mg every 3 wk |
253 | TC2-3 and IC2-3 | 253 | 71%b | TC2-3 and IC2-3 | 71%b | 2.8 | TC2-3 and IC2-3 | 2.8 | 17% | TC2-3 and IC2-3 | 17% |
| TC3 or IC3 | 115 | TC3 or IC3 | 75%b | TC3 or IC3 | 4.2 | TC3 or IC3 | 27% | ||||||||
| Phase 1b study of avelumab14 | NSCLC | Second + | Avelumab 10 mg/ kg every 2 wk | 184 | <1% | 20 | 8.4 | <1% | 4.6 | 11.6wc | <1% | 5.9wc | 13.6% | <1% | 10.0% |
| ≥1% | 122 | ≥1% | 8.9 | ≥1% | 12.0wc | ≥1% | 15.6% | ||||||||
| Phase 1/2 study of durvalumab15 | NSCLC | Second + | Durvalumab 10 mg/kg every 2 wk | 200 | <25% | 92 | 16% | <25% | 5% | ||||||
| ≥25% | 84 | ≥25% | 27% | ||||||||||||
Indicating hazard ratio compared with in control arm.
Six-month overall survival rate.
Week.
PD-L1, programmed death ligand 1; PD-1, programmed death protein 1; OS, overall survival; PFS, progression-free survival; RR, response rate; NSCLC, non-small cell lung cancer; SqNSCLC squamous cell non-small cell lung cancer; Non-SqNSCLC, nonsquamous cell non-small cell lung cancer; TC, tumor cell; IC, tumor-infiltrating immune cell; NR, not reached; Mets, metastases.
In a parallel phase 3 study comparing nivolumab with docetaxel for patients with previously treated nonsquamous NSCLC (CheckMate 057), nivolumab also showed a longer OS (median 12.2 versus 9.4 months, HR = 0.73, 95% CI: 0.59–0.89, p = 0.002) and a higher RR (19% versus 12%, p = 0.02). PFS did not favor nivolumab over docetaxel (median 2.3 versus 4.2 months), and the Kaplan-Meier curves of PFS crossed each other.9 Expression of PD-L1 protein was evaluated retrospectively in pretreatment (archival or recent) tumor biopsy specimens using the same method as the CheckMate 017 study. Of 582 patients who underwent randomization, 455 showed quantifiable PD-L1 expression. In contrast to the data on squamous lung cancer, the test for interactions showed a strong predictive association between PD-L1 expression (≥1%, ≥5%, and ≥10%) and response, PFS, and OS. Nivolumab was associated with longer OS and PFS, as well as with higher RR, than was docetaxel at PD-L1 expression levels of at least 1%, at least 5%, and at least 10% (see Table 2).
PD-L1 Expression as a Predictive Marker of Pembrolizumab in NSCLC
In the phase 1 study, 495 patients with NSCLC received 2 mg/kg pembrolizumab every 3 weeks, 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks, and the association between PD-L1 expression and clinical benefit was evaluated (KEYNOTE-001).10 The RR was 19.4% and the median PFS and OS were 3.7 and 12.0 months, respectively. The anti–PD-L1 antibody 22C3 was used for immunohistochemical analysis, and PD-L1 expression was evaluated in neoplastic and intercalated mononuclear inflammatory cells within tumors. Receiver operating characteristic analysis using the outcome of investigator-assessed immune-related response criteria was performed to define the PD-L1 cut point. PD-L1 expression of at least 50% in neoplastic cells was selected as the cutoff; among the patients, the RR, median PFS, and median OS were 45.2%, 6.3 months, and not reached, respectively (see Table 2). On the basis of this result, pembrolizumab was approved for patients with advanced NSCLC whose disease had progressed after other treatments and who showed PD-L1 expression at least 50% in TCs in the PD-L1 IHC 22C3 pharmDx test (Dako).
In the phase 2/3 study that compared the efficacy and safety among 2 mg/kg every three weeks pembrolizumab, 10 mg/kg every 3 weeks pembrolizumab and docetaxel for PD-L1 expressed (≥1% of TCs) advanced NSCLC that progressed after platinum-doublet chemotherapy (KEYNOTE-010), PD-L1 expression was assessed using the 22C3 antibody in TCs as a tumor proportion score.18 Pembrolizumab, 2 and 10 mg/kg every 3 weeks, significantly prolonged OS compared with docetaxel (median 10.4 versus 12.7 versus 8.5 months; for 2 mg/kg versus docetaxel, HR = 0.71, 95% CI: 0.58–0.88, p = 0.0008; for 10 mg/kg versus docetaxel, HR = 0.61, 95% CI: 0.49–0.75, p < 0.0001). Pembrolizumab, 2 and 10 mg/kg every 3 weeks, also showed higher RRs (18% versus 18% versus 9%; p = 0.0005 for 2 mg/kg versus docetaxel; p = 0.0002 for 10 mg/kg versus docetaxel), although the difference in PFS was not significant between pembrolizumab, 2 mg/kg every 3 weeks, and docetaxel (median 3.9 versus 4.0 versus 4.0 months, respectively; for 2 mg/kg versus docetaxel, HR = 0.88, 95% CI: 0.74–1.05, p = 0.07; for 10 mg/kg versus docetaxel, HR = 0.79, 95% CI: 0.66–0.94, p = 0.004). The HRs in OS and PFS were lower, and RRs were higher when they were analyzed only in patients with a tumor proportion score of at least 50% (see Table 2).
PD-L1 Expression as a Predictive Marker of Atezolizumab in NSCLC
In contrast to the biomarkers for nivolumab and pembrolizumab, PD-L1 expression in studies atezolizumab has been evaluated using an anti–PD-L1 antibody (SP142) separately in TC and IC compartments. In the first published report of a phase 1 study,30 the expression of PD-L1 in TC/IC was categorized as follows: TC3/IC3, at least 10% PD-L1–positive cells; TC2/IC2, at least 5% PD-L1–positive cells; TC1/IC1, at least 1% PD-L1–positive cells; and TC0/IC0, less than 1% PD-L1–positive cells. The association between the response to atezolizumab and PD-L1 expression in ICs reached statistical significance (p = 0.015), whereas the association with PD-L1 expression in TCs did not (p = 0.920). However, in an updated report of the phase 1 study,12 the threshold of TC3 was changed from at least 10% PD-L1–positive cells to at least 50% PD-L1–positive cells. The RR was 48% in patients with NSCLC in the PD-L1 expression categories TC3 or IC3 (see Table 2).
In the randomized phase 2 trial comparing atezolizumab with docetaxel for patients with previously treated NSCLC (POPLAR), atezolizumab showed a longer OS (median 12.6 versus 9.7 months; HR = 0.73, 95% CI: 0.53–0.99, p = 0.040), although PFS and RR did not differ between the two arms (median PFS 2.7 versus 3.0 months, RR 15% versus 15%).13 PD-L1 expression was evaluated using the same method as in the updated report of the phase 1 study.12 Higher PD-L1 expression in TCs/ICs was associated with improved RR, PFS, and OS (see Table 2). Moreover, both TCs and ICs were independently predictive of improvement in OS. The HR of OS in TC1–3 and IC0 was 0.37 (95% CI: 0.12–1.13) and 0.63 (95% CI: 0.36–1.12) in IC1–3 and TC0.
In the phase 2 trial of atezolizumab for locally advanced or metastatic PD-L1–selected (TC2–3 or IC2–3) NSCLC (FIR), patients were divided into three cohorts.17 Cohort 1 included 31 evaluable patients without brain metastases who had received atezolizumab as a first-line treatment, cohort 2 included 92 evaluable patients without brain metastases who had received atezolizumab as a second- or later-line treatment, and cohort 3 included 13 evaluable patients with treated brain metastases who had received atezolizumab as a second- or later-line treatment. Higher PD-L1 expression (TC3 or IC3) was associated with higher RR, longer PFS, and longer OS (see Table 2). In another phase 2 trial of atezolizumab for locally advanced or metastatic PD-L1–selected NSCLC (BIRCH), patients were divided into three cohorts.16 Cohort 1 included 139 evaluable patients who received atezolizumab as a first-line treatment, cohort 2 included 267 evaluable patients who received atezolizumab as a second-line treatment, and cohort 3 included 253 evaluable patients who received atezolizumab as a third- or later-line treatment. Higher PD-L1 expression (TC3 or IC3) was associated with higher RR in all cohorts, and longer PFS in the second-line and third- or later-line cohorts (see Table 2).
PD-L1 Expression as a Predictive Marker of Avelumab in NSCLC
In the phase 1b study, 184 evaluable patients with NSCLC received avelumab.14 The RR, median PFS, and median OS were 13.6%, 11.6 weeks, and 8.4 months, respectively. PD-L1 expression was evaluated, and patients with at least 1% of TCs staining for PD-L1 were classified as PD-L1 positive. Tumor expression of PD-L1 was associated with a higher RR and longer PFS and OS than with PD-L1–negative tumors (see Table 2).
PD-L1 Expression as a Predictive Marker of Durvalumab in NSCLC
In the phase 1/2 study, 200 evaluable patients with NSCLC received durvalumab.15 The RR was 16%. PD-L1 expression was evaluated using an anti–PD-L1 antibody (SP263) in TCs, and patients with at least 25% of TCs stained for PD-L1 at any intensity were classified as PD-L1 positive. PD-L1–positive patients were correlated with a higher RR in this study (see Table 2).
PD-L1 Expression as a Predictive Marker in SCLC
In the phase 1/2 trial of nivolumab with or without ipilimumab for recurrent small cell lung cancer (SCLC) (CheckMate 032), 40 patients treated with nivolumab, 3 mg/kg every 2 weeks, and 46 patients treated with nivolumab, 1 mg/kg, plus ipilimumab, 1 or 3 mg/kg every 3 weeks, were assessed for efficacy. The RRs were 18% in the nivolumab-alone arm, and 17% in the with-ipilimumab arm. Responses were observed in patients regardless of PD-L1 expression.31
In the phase 1b trial of pembrolizumab (KEYNOTE-028), 24 patients with SCLC after failure of standard chemotherapy and with PD-L1 expression in at least 1% of cells in tumor nests or PD-L1–positive bands in stroma using the 22C3 antibody were treated with pembrolizumab, 10 mg/kg every 2 weeks. The RR and median PFS were 29.2% and 1.8 months, respectively. There was no obvious correlation between the proportion of PD-L1–expressing cells and response, and only a small minority of patients with SCLC were able to get a reliable result owing to tissue and timing limitations.32
Interferon-γ
Interferon-γ (IFN-γ) is an important regulator of immunity that is produced predominantly by natural killer and natural killer T cells as part of the innate immune response, and by activated T cells once antigenspecific immunity develops. Expression of PD-L1 as an adaptive response to endogenous antitumor immunity can occur because PD-L1 is induced on most TCs in response to interferons, predominantly IFNγ.33 In the phase 1/2 study of durvalumab, frozen tumor samples with sufficient mRNA quality were profiled with 100 preselected immune activation genes using Fluidigm Biomark (Fluidigm, South San Francisco, CA).15 IFN-γ expression showed the highest correlation with response, and the RR was 33% (14 of 43) and 8% (6 of 79) in IFN-γ–positive and IFN-γ–negative patients, respectively. Moreover, combined IFN-γ–positive and PD-L1–positive patients showed the highest RR (46% [10 of 22]). In a phase 1 study of atezolizumab, gene expression analysis was also performed using the Bio-Mark HD real-time PCR Platform (Fluidigm).30 In patients with melanoma, pretreated tumors in responding patients showed elevated expression of IFN-γ as well as IFN-γ–inducible genes. However, these associations were weaker in patients with NSCLC. Immune-related gene expression patterns were evaluated in patients with melanoma who had received pembrolizumab in KEYNOTE-001. Samples from 19 and 62 patients were analyzed using the NanoString platform as a discovery set and validation set, respectively. The 10-gene “preliminary IFN-γ” signature correlated with RR (p = 0.047) and PFS (p = 0.016).34
Nonsynonymous Mutation Burden and Neoantigens
In a phase 1 study of nivolumab and BMS-936559, these inhibitors showed promising efficacy toward melanoma and NSCLC compared with toward other malignancies.35,36 These two tumors generally contain high numbers of somatic mutations as a result of exposure to ultraviolet radiation or cigarette smoke.37 A proposed explanation for the activity of the T-cell checkpoint blockade in tumors such as melanoma and NSCLC is the boosting of T-cell reactivity against “neoantigens,” which are T-cell epitopes that are newly formed as a consequence of tumor-specific mutations.38 The association between the mutational landscape of NSCLC determined by whole exome sequencing and the response to pembrolizumab was investigated in 16 discovery cohort patients and 18 validation cohort patients.39 In the discovery cohort, the confirmed RR was higher in patients with a high nonsynonymous burden (defined as an above the median burden of the cohort [209]) than in those with a low mutation burden (below the median) (RR 63% versus 0%, Fisher’s exact p = 0.03). PFS was also longer in patients with a high nonsynonymous burden (median 14.5 versus 3.7 months, log-rank p = 0.01, HR = 0.19, 95% CI: 0.05–0.70). In the validation cohort, the confirmed RR was higher in patients with a high nonsynonymous burden (>200) than in those with a low mutation burden (<200), although there was no significant difference between groups (RR 56% versus 22%, Fisher’s exact p = 0.33). PFS was significantly longer in patients with high nonsynonymous burden (median not reached versus 3.4 months, log-rank p = 0.006; HR = 0.15, 95% CI: 0.04–0.59).
Consistent with these data are the recent data on responses in patients with colon cancer with mismatch repair (MMR)-deficient tumors. DNA mismatch repair deficiency causes large numbers of mutations, particularly in regions of repetitive DNA sequences, a phenomenon known as microsatellite instability.40 MMR deficiency can arise as a consequence of inheritance of an inactive allele of one of the mismatch repair genes, such as mutL homolog gene (MLH1), mutS homolog 2 gene (MSH2), mutS homolog 6 gene (MSH6), and PMS1 homolog 2, mismatch repair system component gene (PMS2), with subsequent loss of the remaining wild-type copy, a genetic disorder known as Lynch syndrome. In contrast, sporadic inactivation of MMR genes occurs in colorectal cancer at frequencies of 4% to 20%.38,40 In a phase 2 study evaluating the efficacy of pembrolizumab among 41 patients with advanced metastatic carcinoma with or without MMR deficiency, immune-related RR, PFS, and OS were significantly greater in MMR-deficient colorectal cancer.41 Whole exome sequencing revealed a larger number of somatic mutations per tumor in MMR-deficient tumors than in mismatch repair–proficient tumors (mean 1782 versus 73, p = 0.007). This number is even higher than the average seen in lung tumors from heavy smokers. This finding also supports the idea that mutation-associated neoantigen recognition is an important component of the endogenous antitumor immune response and may be a predictive marker of PD-1/PD-L1 blockade.
Correlations with Smoking, EGFR and KRAS Mutations, and ALK Fusions
There is an association between Kirsten rat sarcoma viral oncogene homolog gene (KRAS) mutations and smoking, whereas there is an opposite correlation between EGFR mutations and smoking.42 The average mutation frequency was found to be more than 10-fold higher in smokers than in never-smokers (on the basis of whole exome sequencing).43 In the subgroup analysis of CheckMate057, patients with wild-type EGFR/KRAS mutations showed a greater benefit from nivolumab in terms of PFS and OS (Table 3).9 In the subgroup analysis of KEYNOTE-010, patients with wild-type EGFR also showed a greater benefit from pembrolizumab in terms of OS than in patients with EGFR mutations (see Table 3).18 In a study investigating the association between the mutational landscape and the response to pembrolizumab, KRAS mutations were found in seven of 14 patients with a partial response or stable response lasting more than 6 months, whereas these mutations were found in one of the other 17 patients. However, the results of subgroup analyses in other studies did not reveal an independent correlation between EGFR/KRAS mutations and the response to anti–PD-1 antibody (see Table 3).44,45
Table 3.
Association between EGFR Mutation/KRAS Mutation/ALK Fusion Gene and Efficacy of Anti-PD-1/PD-L1 Antibody
| Study Name (Phase) | Histologic Diagnosis | Line | Agent/Dose | N | Genetic Alteration and No. Patients | OS in overall patients (mo) | Genetic Alteration and OS | PFS in overall patients (mo) | Genetic Alteration and PFS | RR in overall patients (%) | Genetic Alteration and RR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate00344 (phase 1) | NSCLC | Second + | Nivolumab 1 mg/kg every 2 wk 3 mg/kg every 2 wk 10 mg/kg every 2 wk |
129 | EGFR mutant | 12 | 17% | EGFR mutant | 16.7% | ||||||
| EGFR wild | 56 | EGFR wild | 19.6% | ||||||||||||
| KRAS mutant | 21 | KRAS mutant | 14.3% | ||||||||||||
| KRAS wild | 36 | KRAS wild | 25.0% | ||||||||||||
| CheckMate0579 (phase 3) | Non-SqNSCLC | Second | Nivolumab 3 mg/kg every 2 wk (vs. docetaxel) |
292 (290) | EGFR mutant | 82 | 12.2 (0.73a) | EGFR mutant | 1.18a | 2.3 (0.92a) | EGFR mutant | 1.46a | |||
| EGFR wild | 340 | EGFR wild | 0.66a | EGFR wild | 0.83a | ||||||||||
| KRAS mutant | 62 | KRAS mutant | 0.52a | KRAS mutant | 0.82a | ||||||||||
| KRAS wild | 123 | KRAS wild | 0.98a | KRAS wild | 1.52a | ||||||||||
| ALK positive | 0 | ALK positive | – | ALK positive | – | ||||||||||
| ALK negative | 243 | ALK negative | 0.71a | ALK negative | 0.81a | ||||||||||
| KEYNOTE01018 (phase 3) | PD-L1 + NSCLC | Second + | Pembrolizumab 2 mg/kg every 3 wk 10 mg/kg every 3 wk (vs. docetaxel) |
690 (343) | EGFR mutant | 86 | 0.67a | EGFR mutant | 0.88a | ||||||
| EGFR wild | 875 | EGFR wild | 0.66a | ||||||||||||
| Phase 3/4 study of nivolumab45 | NSCLC | Second + | Nivolumab 3 mg/kg every 2 wk | 531 | EGFR mutant | 55 | 12% | EGFR mutant | 16% | ||||||
| EGFR wild | 300 | EGFR wild | 11% | ||||||||||||
| ALK positive | 12 | ALK positive | 8% | ||||||||||||
| ALK négative | 299 | ALK negative | 12% | ||||||||||||
Indicating hazard ratio compared with in the control arm.
EGFR, epidermal growth factor receptor gene; KRAS, Kirsten rat sarcoma viral oncogene homolog gene; ALK, anaplastic lymphoma kinase gene; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; OS, overall survival; PFS, progression-free survival; RR, response rate; NSCLC, non-small cell lung cancer; Non-SqNSCLC, nonsquamous cell non-small cell lung cancer.
Other Negative Regulators
In a phase 1 study of atezolizumab,30 the expression of immune inhibitory factor genes other than programmed death ligand-1 gene (PD-L1) (programmed death ligand-2 gene [PD-L2], indoleamine 2,3-dioxygenase 1 gene [IDO1], lymphocyte activating 3 gene [LAG3], hepatitis A virus cellular receptor 2 gene [TIM3], cytotoxic T-lymphocyte associated protein 4 gene [CTLA4], CD276 gene [B7-H3], and V-set domain containing T cell activation inhibitor 1 gene [B7-H4]) was investigated, and the expression of these genes did not appear to be associated with resistance to atezolizumab. A novel immunohistochemical assay for PD-L2 was developed and applied to a total of 417 archival tumor samples, including 94 NSCLC samples.46 The extent and distribution of PD-L2 immunohistochemical labeling was significantly correlated with the extent and distribution of PD-L1 immunohistochemical labeling (range p = 0.0012 to p < 0.0001) and significantly correlated with PD-L2 mRNA quantitation by Nanostring (range p = 0.0037 to p < 0.0001). However, there were discrepancies between PD-L1 staining and PD-L2 staining in some samples. In 144 patients with head and neck squamous cell cancer who had received pembrolizumab, 200 mg every 3 weeks, PD-L2 expression was correlated with a higher RR (p = 0.072) and longer PFS (p = 0.031) after adjustment of PD-L1 expression using a logistic regression model.46
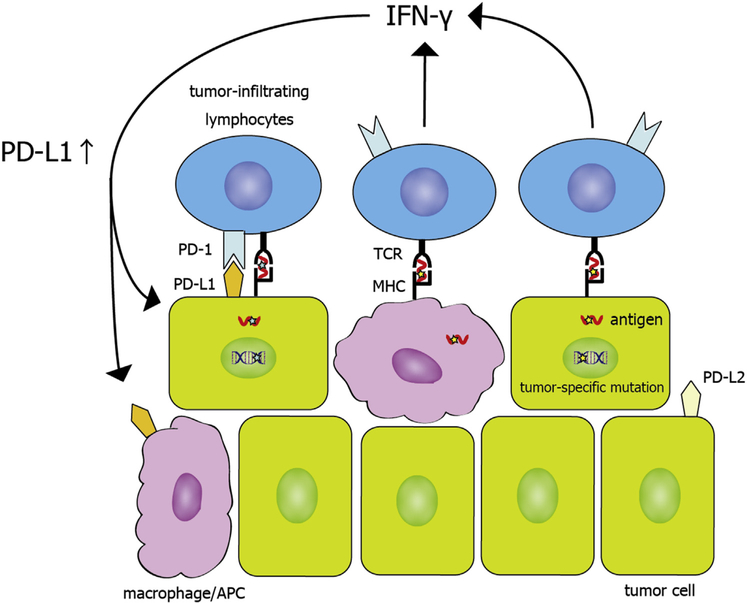
Figure 2 summarizes how the aforementioned biomarkers affect PD-1/PD-L1 mechanisms
Figure 2.
Programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) mechanisms. Tumor antigens are presented by tumor cells and antigen presenting cells (APCs), including macrophages. T cells are activated with the recognition of tumor antigens. On the other hand, upon recognition of tumor antigens, T cells produce interferon gamma (IFN-γ), which drives PD-L1 expression in the tumor microenvironment. PD-L1 binds PD-1, and this causes a suppressive signal to T cells, leading to T-cell dysfunction and tumor survival. TCR, T-cell receptor; MHC, major histocompatibility complex..
Clinical Parameters
Because anti–PD-1/PD-L1 antibodies are generally so well tolerated, it will be tempting to use them in very old patients or patients with poor performance status, who were not included in many of the trials. The importance of these factors in benefit needs to be studied. In addition, the influence of indolent or remote histories of autoimmune disorders is similarly unknown.
Summary and Future Perspectives
The development of modern cancer immunotherapy strategies has revolutionized the field of cancer treatment, with the potential of long-term disease control by activating the patients’ own immune system against cancer cells. The RR, however, has been low (averaging less than 20% in nonselected patients with lung cancer), and the costs of these antibodies are high. Therefore, patient selection by optimal predictive marker is required. Approximately 10% of patients who have low or no baseline PD-L1 expression still respond to anti–PD-1/PD-L1 antibodies. These patients should not be excluded because of the potential long-term durability of the response, which can make PD-L1 immunohistochemical status a poor biomarker. The ideal strategy may be to identify those patients who are not likely to respond to single-agent therapy and help them mount a response against their cancer by upfront combination treatments, guided (it is hoped) by biomarkers identified in the baseline tumor samples/blood samples.
Each anti–PD-1/PD-L1 agent is being developed with a different PD-L1 immunohistochemical assay; the antibody used for immunohistochemical analysis and the cutoff values differ among agents. As shown in Figure 1, stainability is different among the antibodies. Actually, the proportions of the patients with negative staining (≤1% TCs) for PD-L1 ranged from 13.7% to 19.9%.8–10,14 The difference in the stainability may result in a variety of cutoff levels according to antibodies. Standardization of the staining and scoring methods in immunohistochemical analysis is necessary before PD-L1 expression is clinically practical as a predictive marker. As PD-L1 expression can be heterogeneous and can be altered because of prior treatment, evaluation of PD-L1 expression in larger specimens and at multiple sites just before anti–PD-1/PD-L1 treatment may be ideal. This problem will be particularly acute if these agents are approved in first-line, where all of the studies used PD-L1 selection using a variety of platforms and approvals will be based on this marker.
It may be difficult to define a consistent cutoff value for PD-L1 positivity in NSCLC. In CheckMate017 and CheckMate057, cutoff values of PD-L1 were evaluated at greater or equal to 1%, greater or equal to 5%, and greater or equal to 10%. At these cutoff values, in the squamous cell NSCLC trial (CheckMate017), PD-L1 expression was not predictive of response, PFS, or OS. However, in the parallel nonsquamous cell NSCLC trial (CheckMate057), the test for interactions suggested a strong predictive association between PD-L1 expression (≥1%, ≥5%, and ≥10%) and response, PFS, and OS without much difference among them. Tumors with no expression had a survival HR indistinguishable from 1. In KEYNOTE001, in which a cutoff of at least 50% in TCs was used, the RR, PFS, and OS were more favorable in a mixture of squamous and nonsquamous PD-L1–positive patients, and pembrolizumab was approved for all patients with advanced NSCLC with the cutoff of at least 50% expression in TCs.
The immune system is an incredibly complex and intricately regulated entity that has been tuned with multiple mechanisms of regulation. Thus, it is not surprising that clinical response to PD-1/PD-L1 blockade is not solely dependent on PD-L1 expression. Additional considerations include (1) the induction of cytotoxic T cells against tumor-specific antigens; (2) immune regulation by other mechanisms, including PD-L2, ceramide synthase 1 (LAG1), tumor necrosis factor receptor superfamily member 4 gene (OX40), adenosine, and many others; (3) the extent of cytotoxic and regulatory T-cell infiltration into the tumor microenvironment, (4) the presence and function of other immune regulatory cells such as myeloid-derived suppressor cells; and (5) the cytokine environment. This complexity is consistent with the imperfect correlation of efficacy with the PD-L1 findings described in this review article. The number of neoantigens is thought to be highly correlated with the response to the PD-1/PD-L1 blockade, but this concept would be difficult to introduce into daily clinical practice without the development of more convenient and lowercost testing methods.
Overall, PD-L1 expression has been introduced into clinical practice, but standardization of the staining and scoring methods in immunohistochemical analysis is needed. In addition, the identification or development of more sensitive and specific predictive markers is warranted to more effectively identify patients most likely to benefit and not exclude patients who could potentially benefit.
Acknowledgments
We thank Dr. Yasushi Yatabe for providing pathological images (Fig. 1). This work was supported by The Lilly Oncology Fellowship from The Japanese Respiratory Society, Alumni Scholarship from Juntendo University School of Medicine, and research fellowship from Uehara Memorial Foundation (to Dr. Shukuya).
Footnotes
Disclosure: Dr. Carbone reports personal fees from Genentech/Roch, Bristol-Myers Squibb, and Astra Zeneca, outside the submitted work. Dr. Shukuya reports personal fees from Chugai Pharmaceutical, AstraZeneca, and Ono Pharmaceutical, outside the submitted work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii56–vii64. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) version 2. 2016. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 1, 2015.
- 4.Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3488–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Trials.gov. https://clinicaltrials.gov/. Accessed December 29, 2015.
- 12.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study [abstract]. J Clin Oncol. 2015;33(suppl):8029. [Google Scholar]
- 13.Vansteenkiste J, Fehrenbacher L, Spira AI, et al. Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: Primary analyses for efficacy, safety, and predictive biomarkers from a randomized phase II study (POPLAR). Paper presented at: 2015 European Cancer Congress September 25–29, 2015; Vienna, Austria. [Google Scholar]
- 14.Gulley JL, Rajan A, Spigel DR, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or recurrent non-small-cell lung cancer progressing after platinum-based chemotherapy: A phase Ib trial. Paper presented at: 2015 European Cancer Congress September 25–29, 2015; Vienna, Austria. [Google Scholar]
- 15.Higgs BW, Robbins PB, Blake-Haskins JA, et al. High tumoral IFNγ mRNA, PD-L1 protein, and combined IFNγ mRNA/PD-L1 protein expression associates with response to durvalumab (anti-PD-L1) monotherapy in NSCLC patients. Paper presented at: 2015 European Cancer Congress September 25–29, 2015; Vienna, Austria. [Google Scholar]
- 16.Besse B, Johnson M, Jänne PA, et al. Phase II single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell cancer. Paper presented at: 2015 European Cancer Congress September 25–29, 2015; Vienna, Austria. [Google Scholar]
- 17.Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2015;33(suppl):8028. [Google Scholar]
- 18.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer. (KEYNOTE-010): a randomised controlled trial [e-pub ahead of print]. Lancet. 10.1016/S0140-6736(15)01281-7, accessed December 19, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 20.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheffield BS, Geller G, Pleasance E, et al. Predictive biomarker testing for programmed cell death 1 inhibition in non-small cell lung cancer. Presented at: 16th World Conference on Lung Cancer September 6–9, 2015; Denver, CO. [Google Scholar]
- 22.McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowanetz M, Koeppen H, Boe M, et al. Spatiotemporal effects on programmed death ligand 1 (PD-L1) expression and immunophenotype of non-small cell lung cancer (NSCLC). Presented at: 16th World Conference on Lung Cancer September 6–9, 2015; Denver, CO. [Google Scholar]
- 24.Mitchell P, Murone C, Asadi K, et al. PDL-1 Expression in NSCLC: analysis of a large early stage cohort; and concordance of expression in primary, nodes and metastasis. Presented at: 16th World Conference on Lung Cancer. September 6–9, 2015; Denver, CO. [Google Scholar]
- 25.Gainor JF, Sequist LV, Shaw AT, et al. Clinical correlation and frequency of programmed death ligand-1 (PD-L1) expression in EGFR-mutant and ALK-rearranged non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2015;33(suppl):8012. [Google Scholar]
- 26.Chaft JE, Chao B, Akerley WL, et al. Evaluation of PD-L1 expression in metachronous tumor samples and FDG-PET as a predictive biomarker in ph2 study (FIR) of atezolizumab (MPDL3280A). Presented at: 16th World Conference on Lung Cancer. ORAL02.06 September 6–9, 2015, Denver, CO. [Google Scholar]
- 27.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–456. [DOI] [PubMed] [Google Scholar]
- 28.Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. [DOI] [PubMed] [Google Scholar]
- 29.Adam J, Boros A, Lacas B, et al. Prognostic value of PDL1 expression in stage III non-small cell lung cancer (NSCLC) treated by chemo-radiotherapy (CRT). Presented at: 16th European Cancer Congress Presented on September 6–9, 2015; Denver, CO. [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonia SJ, Bendell JC, Taylor MH, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209–032 [abstract]. J Clin Oncol. 2015;33(suppl):7503. [Google Scholar]
- 32.Ott PA, Elez E, Hiret S, et al. Pembrolizumab for ED SCLC: Efficacy and relationship with PD-L1 expression. Presented at 16th World Conference on Lung Cancer September 6–9, 2015; Denver, CO. [Google Scholar]
- 33.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev. Cancer. 2012;12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature [abstract]. J Clin Oncol. 2015;33(suppl):3001. [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelderman S, Schumacher TN, Kvistborg P. Mismatch repair-deficient cancers are targets for anti-PD-1 therapy. Cancer Cell. 2015;28:11–13. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. [DOI] [PubMed] [Google Scholar]
- 39.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulogiannis G, Frayling IM, Arends MJ. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 2010;56:167–179. [DOI] [PubMed] [Google Scholar]
- 41.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer TM, McCleod M, Chandler JC, et al. An ongoing phase IIIb/IV safety trial of nivolumab (NIVO) in patients (pts) with advanced or metastatic non-small-cell lung cancer (NSCLC) who progressed after receiving 1 or more prior systemic regimens [abstract]. J Clin Oncol. 2015;33(suppl):3013. [Google Scholar]
- 46.Yearley J, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Paper presented at: 2015 European Cancer Congress September 25–29, 2015; Vienna, Austria. [Google Scholar]