Abstract
This study determined if a SystemCHANGE™ intervention was more efficacious than attention control in increasing immunosuppressive medication adherence and improving outcomes in adult kidney transplant recipients during a 6-month intervention phase and subsequent 6-month (no intervention) maintenance phase. The SystemCHANGE™ intervention taught patients to use person-level quality improvement strategies to link adherence to established daily routines, environmental cues, and supportive people. Eighty-nine patients (average age 51.8 years, 58% male, 61% African American) completed the 6-month intervention phase. Using an intent-to-treat analysis, at 6 months, medication adherence for SystemCHANGE™ (median 0.91, IQR 0.76–0.96) and attention control (median 0.67, IQR 0.52–0.72) patients differed markedly (difference in medians 0.24, 95% CI 0.13–0.30, P < .001). At the conclusion of the subsequent 6-month maintenance phase, the gap between medication adherence for SystemCHANGE™ (median 0.77, IQR 0.56–0.94) and attention control (median 0.60, IQR 0.44–0.73) patients remained large (difference in medians 0.17, 95% CI 0.06–0.33, P = .004). SystemCHANGE™ patients evidenced lower mean creatinine and BUN at 12 months and more infections at 6 and 12 months. This first fully powered RCT testing SystemCHANGE™ to improve and maintain medication adherence in kidney transplant recipients demonstrated large, clinically meaningful improvements in medication adherence.
Clinical Trial Registration: NCT02416479.
Keywords: clinical research/practice, clinical trial, health services and outcomes research, immunosuppressant, immunosuppression/immune modulation, kidney transplantation/nephrology
1 |. INTRODUCTION
The high rate of immunosuppressive medication nonadherence (MNA) in kidney transplant recipients1 is associated with poor outcomes and staggering costs of over $33 000 per patient in the 3 years posttransplant.2,3 Short-term kidney transplant outcomes have improved, yet long-term outcomes continue to languish, in part due to poor medication adherence (MA). Numerous systematic reviews and meta-analyses have reported the efficacy of MA interventions in the acute and chronically ill general population.4–11 Multicomponent interventions are associated with the greatest effect sizes; however, even with multicomponent interventions, effect sizes in meta-analyses remain small.7 A majority of interventions, when used, are guided by psychological theories that focus on enhancement of knowledge through education, attitude through counseling, and behavior through skills training.4,12 Benefits of these interventions are limited and preoccupied with intention and motivation.12,13 Most transplant intervention studies focused only on motivation and intention have equally disappointing MA results.14–21
Bronfenbrenner’s Socio-Ecological Model (SEM)23 and Deming’s Plan-Do-Check-Act (PDCA) model22 provide the foundation for the SystemCHANGE™ approach, which harnesses reliable person-centered systems that people have already established—daily routines, environment, and important others—as possible system-based solutions evaluated to support MA using person-level quality improvement strategies. The SystemCHANGE™ intervention is implemented at the individual, micro- (immediate environmental setting of family, peers, health services, workplace), meso- (interrelations between family, health care provider, employer), and exolevels (outside of the person’s immediate setting but affecting the functional setting).23
SystemCHANGE™ was developed by incorporating the PDCA cycle into Bronfenbrenner’s SEM.26 The quality improvement movement, using root-cause analysis, successfully used the PDCA cycle as a framework improving processes within an organizational system.22 The SystemCHANGE™ approach applies quality improvement methods at a personal level, not the organizational system level, using the following steps.22 In the “Plan” step, a problem such as MNA is defined and possible causes and solutions are hypothesized. During the “Do” step, MA solutions are implemented. The “Check” step evaluates the results of the plan and a decision is made about whether MA has been achieved. The “Act” step identifies what was learned in the “Check” step and further MA solutions are implemented if needed. The successful solution is then standardized. An unsuccessful change informs a new PDCA cycle.
Using the SystemCHANGE™ approach, we guided the individual to conduct a “small experiment” where we (1) assessed the medication systems (including other people important for medication taking, eg, spouses, adult children), how the systems influence medication taking, and possible solutions for improving MA; (2) implemented the proposed solutions for improving MA; (3) tracked MA data with electronic medication (EM); and (4) evaluated MA data24,25 We found a nearly fourfold greater effect size in a pilot study using the SystemCHANGE™ intervention to improve immunosuppressive MA compared to previous adherence interventions.26
The aim of this study was to ascertain whether a SystemCHANGE™ intervention was more effective than an attention control intervention in increasing immunosuppressive MA in adult kidney transplant recipients at the completion of a 6-month intervention and a subsequent 6-month maintenance (no intervention) phase. The hypothesis was that adult kidney transplant recipients who participated in the SystemCHANGE™ intervention would have a higher immune-suppressant MA rate than those in the attention control group at both time points. The exploratory aim was to determine whether the SystemCHANGE™ intervention was more effective in improving health outcomes (eg, creatinine/BUN, infections, acute and chronic rejections, kidney failure, death). An attention control intervention, which included providing patient education materials focused on health-related activities, was designed to make the interventions received by the two groups as similar as possible except for the SystemCHANGE™ intervention and to provide attention to the control group to decrease attrition.
2 |. MATERIALS AND METHODS
2.1 |. Study design
The design was a single-blinded (participants), 2-arm randomized controlled trial (RCT) using repeated measures. Table 1 delineates the study design. The research assistant (RA) collected demo-graphic information, performed cognitive screening, and provided an EM cap and bottle. During the 3-month screening phase, all participants used EM to document MA. Those who were adherent (MA rate of ≥85%) exited the study. Those with a documented MA rate of less than 85% were given the opportunity to enter into the intervention phase of the study where they were randomized to either the SystemCHANGE™ intervention (treatment) or the attention control intervention (control). All participants received standard care. Tables 1 and 2 delineate the elements of both interventions. After the 6-month intervention phase, there was a 6-month maintenance phase during which there was no intervention.
TABLE 1.
SystemCHANGE™ and patient education interventions
| Intervention steps | Items for the provider to discuss with the patient | Location/timing |
|---|---|---|
| SystemCHANGE™ | ||
1. Plan step:
|
|
40 min in person during home or clinic visit for Steps 1 and 2 |
2. Do step:
|
|
|
Check step:
|
|
10 min at months 1, 2, 3, 4, 5 over the telephone |
| Patient education | ||
|
40 min in person during home or clinic visit | |
|
10 min at months 1, 2, 3, 4, 5 over the telephone | |
MEMS, Medication Event Monitoring System.
TABLE 2.
Design and intervention
| Randomization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||
| Screening | Intervention | Maintenance | ||||||||
| Months | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10–15 |
| Intervention group | S | S | S | V | X | X | X | X | X | M |
| MEMS use | ||||||||||
| Attention control group | S | S | S | V | Y | Y | Y | Y | Y | M |
| MEMS use | ||||||||||
Note: MEMS, Medication Event Monitoring System; S, MEMS use only; at the completion of Month 3, those who were medication adherent exited the study and those with medication nonadherence were randomized; V, In-person visit to begin either SystemCHANGE™ intervention or attention control (patient education) intervention. SystemCHANGE™ teaches patients to use person-level quality improvement strategies to link adherence to established daily routines, environmental cues and supportive people. Attention control (patient education) included discussion of first of 6 transplant health-related brochures; X, Telephone call to receive feedback (MEMS report) and discuss implemented medication adherence solution; Y, telephone call to review transplant-related education materials; M, MEMS use only; at the completion of Month 15, the MEMS were returned and the study was concluded.
2.2 |. Setting
Initially, participants were recruited from two transplant centers in the midwestern and southern United States, namely University of Missouri Healthcare in Columbia, Missouri, and the University of Tennessee Health Science Center in Memphis, Tennessee. To accrue the target sample size more quickly, three additional midwestern transplant centers were added as recruitment sites: the University of Kansas Medical Center in Kansas City, Kansas; Barnes Jewish Hospital in St. Louis, Missouri; and St. Luke’s Hospital in Kansas City, Missouri. Institutional review board (IRB) approval was obtained from the University of Missouri, University of Tennessee, and St. Luke’s Hospital. The other two centers determined that no approval was required.
2.3 |. Changes to trial design
The details of the trial design were published previously.27 Two changes to the trial design occurred. In year 2, three additional recruitment sites were added to achieve the target sample size as discussed previously. In response to the Data Safety Monitoring Board (DSMB) the study protocol was amended to include 6-month MA data for attention control intervention participants in order to identify anyone with very low MA (<0.30) and develop a plan to miti-gate any associated risks if needed. Fortunately, no attention control participants experienced a medication nonadherence level below 30%. Based on recommendations from the DSMB, participant recruitment was stopped in year 3 and target sample size was changed to 75–80 due to large effects of the SystemCHANGE™ intervention on MA. This increases the chance of a type I error.
2.4 |. Participants
A random sample of participants was obtained from the list of all eligible kidney transplant centers from the two initial participating centers. When three additional centers were added, a convenience sampling approach was used. Individuals 18 years of age or older who had received a kidney-only transplant, self-administered at least one prescribed immunosuppressive medication taken twice daily with a functioning kidney transplant, were not in the hospital, and had no diagnosis that would immediately shorten the lifespan were eligible for participation. In addition, individuals needed to have access to a telephone; the ability to speak, hear, and under- stand English; the ability to open an EM cap; and have agreement from the transplant physician and nephrologist to participate in the study. Individuals were also assessed for cognitive impairment and required to score 4 or greater on the 6-item Telephone Mental Status Screen Derived from the Mini-Mental Status Exam.28 IRB approval was obtained, and all participants provided informed consent prior to beginning the study. As directed by the individual institution’s IRB, participants provided either written or oral consent.
2.5 |. Randomization and masking
Block randomization was used with the project biostatistician pre-paring a computer-generated list of random arm assignment numbers blocked in balanced groups of four. For each arm assignment number on the list, an RA trained specifically for that arm (SystemCHANGE™ or attention control) called the first person on a list of eligible intervention participants to determine whether he/she was interested in enrolling in the next phase of the study (the intervention phase). If the participant agreed to enroll in the intervention phase, he/she was assigned to the arm for which the RA was recruiting, filling that slot on the randomization list. If the participant did not agree to enroll, the same RA called the next eligible participant to extend the invitation to enroll. This process continued until the enrollment slot was filled. Thus each eligible participant had the same chance of receiving a call from an RA enrolling for the SystemCHANGE™ arm as of receiving a call from an RA enrolling for the attention control arm. To minimize bias, patients were not given information about the nature of the two study arms, and all study personnel used the terms “SystemCHANGE™ intervention” and “Patient Education intervention” (the attention control intervention) when describing any pertinent study activities.
2.6 |. Training of RAs
Baccalaureate-prepared registered nurse RAs were trained by a SystemCHANGE™ expert using a detailed procedure manual, simulation, and role for both interventions. The expert provided the RAs with feedback on performance and retrained them as needed until they achieved 100% intervention protocol integrity.
2.7 |. Treatment fidelity
To ensure RA fidelity to both arms, a fidelity protocol checklist was used during all participant encounters to document key elements of the protocol, including number of intervention sessions, session duration, length of time between sessions, and intervention steps. Each element was rated as completed, partially completed, not completed, or N/A. Field notes were kept for every encounter (participant’s body language, environmental issues, presence of others in the home) and phone call (background noise, telephone line distortion, any difficulty hearing by RA or participant).
2.8 |. Procedures
To start the 6-month intervention phase, all participants received an in-person visit at baseline, either at their home or in the transplant clinic, followed by six telephone calls at months 1 through 6. Those randomized into the SystemCHANGE™ intervention were coached by the SystemCHANGE™ trained RA in implementing SystemCHANGE™. Study participants randomized to the attention control group received transplant patient education guided by the patient education trained RA using healthy living transplant brochures; these education sessions were provided at the same time points when the SystemCHANGE™ group received SystemCHANGE™ intervention delivery.
The maintenance phase began for both groups following completion of the intervention phase and continued for an additional 6 months. This phase was designed to examine how participants maintained MA in the absence of an intervention while continuing to use EM. All participants were provided compensation during all phases of the study up to $335. All outcomes were collected by RAs from the participants’ medical record at completion of the study.
2.9 |. SystemCHANGE™ intervention
The SystemCHANGE™ intervention supports patient-designed, RA interventionist-guided, small experiments using Deming’s Plan-Do-Check-Act cycle to redesign the personal environmental system and daily health behavior routines. The SystemCHANGE™ intervention began with an in-person visit by the RA where the participant was guided to assess his or her individual systems including important others who shape medication taking, how the personal systems and others influence medication taking routines, and the individual’s plan for a small “experiment(s)” focused on using the environment for improving MA. The proposed individual systems’ solutions to improve adherence was then implemented, adherence data were tracked using EM, and monthly adherence data were evaluated with the support of the RA. The participant was implementing the small “experiment”.
Providing MA feedback is the “Check” step of the SystemCHANGE™ intervention. Feedback reports graphically displayed comparisons of actual medication taking time with desired time (goal time set by participants). Graphical feedback is used by participants to evaluate if and when the selected personal system solution is working as a strategy to improve adherence. If the solution is working, then graphs reflect consistency in the time medications are taken compared with the goal time. If the graphs do not reflect this consistency, then participants are encouraged to select another system solution from the list generated at the first session. Further details on the SystemCHANGE™ intervention have been previously published.27,29
At the completion of each month during the 6-month intervention phase, participants were mailed their EM report and contacted by the RA to evaluate their MA, in addition to the effecttiveness of the implemented solutions. The participants reflected on what they were learning about their medication taking, how the implemented solution was changing their MA, and any other changes to medication taking routines that might be needed. If the MA score remained stagnant or decreased, the RA would encourage the participant to implement a new solution and evaluate its effectiveness the following month. If MA continued to improve, or was >85%, the participant was encouraged to continue the same solution.
2.10 |. Attention control intervention
To increase the retention of the attention control group in the study, both the SystemCHANGE™ and attention control interventions were designed to deliver exactly the same amount of time and attention to both groups. The 6-month attention control intervention involved an in-person visit where the first of six educational bro- chures, developed by the International Transplant Nurses Society addressing healthy living in transplant recipients, was reviewed.30 Each subsequent month for 6 months, the RA contacted the participant to discuss one of the materials. The duration of these interactions was designed to be equivalent to the SystemCHANGE™ intervention. Attention control participants continued to use the EM system during this phase.
2.11 |. Maintenance phase
Both groups entered a 6-month maintenance phase after the active interventions. During this phase, both groups continued using the EM cap and bottle but did not receive any interaction with the RA other than their monthly receipt of the study stipend.
2.12 |. Outcomes
The primary outcome was the average 6-month immunosuppressive MA rate defined as doses taken on time/total doses as measured by the Medication Event Monitoring System SmartCap® (MEMSCap™). Adherence at 12 months was a secondary outcome. The MA rate calculation method has been previously described but is briefly described here.32 If the dose of the medication is taken within a 3-hour window (±1.5 hours of the prescribed time) a “0.50” is assigned; if the dose is not taken within the 3-hour window but is taken within a 12-hour window (±6 hours of the prescribed time) a “0.25” is assigned, and if the dose is not taken within a 12-hour window (±6 hours of the prescribed time, ie, if the dose was missed) a 0 is assigned (p. 526). Perfect adherence is 1.00 and complete nonadherence is 0.00. Exploratory outcomes were creatinine/BUN, infections, acute and chronic rejections, kidney failure, and death. Safety and adverse events were assessed in an ongoing fashion by the Primary Investigators and a DSMB, which met biannually. Perceived health status, which reflects people’s overall perception of their health, including both physical and psychological dimensions, was measured by one question, “How is your health in general?” Respondents selected excellent, very good, good, fair, poor, or very poor. The question has good reliability and validity.33
2.13 |. Statistical analysis
Sample size and power calculations were based on comparing expected immunosuppressive MA rates of participants in each group at the completion of the 6-month intervention.27 We expected a mean MA difference of 0.10 based on our pilot study findings and the literature and assumed an effect size (standardized mean difference) of 0.70 based on a conservative estimate from our pilot work. Based on an alpha of 0.05, 86 participants (43 per arm) provided 90% power to detect this effect with a two-sample t-test.
Statistical analyses were carried out using CRAN R (RStudio 1.0.136) and SAS 9.4. The primary analysis was conducted using an intention-to-treat approach. Participants who dropped out for any reason (eg, death, stroke, started dialysis, could not follow protocol) prior to the 6-month time point were assigned an adherence score of 0. We compared the 6-month adherence rates for the two groups first using a two-sided Wilcoxon-Mann-Whitney test, then with a linear regression model that included participant race (white, nonwhite), marital status (married, not married), perceived health score, and perceived social support as covariates. Adherence rates at 12 months were compared in the same way.
In a secondary analysis, we assessed the MA patterns in both the SystemCHANGE™ and attention control groups to determine when the intervention became effective (eg, what “dose” is needed) and examine the pattern of decay in MA over time, throughout both the intervention and maintenance phases, for both groups. Using a mixed model with a random participant intercept to account for clustering, we modeled monthly MA as a function of group, a linear time slope, and a group-by-slope interaction.
Exploratory outcomes (eg, creatinine/BUN, infections, acute and chronic rejections, kidney failure, death) were analyzed using frequencies and means. Creatinine and BUN were categorized into low, high, or normal ranges.
2.14 |. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3 |. RESULTS
Patients were enrolled between January 12, 2015 and April 14, 2017. Of 1096 persons contacted for participation, 580 (52.9%) agreed to participate and 438 (75.5%) completed screening. Figure 1 delineates the flow of participants in the study and reasons for follow-up losses. Of those who completed screening, 281 (64.2%) were adherent and excluded from the study. In total, 156 (35.6%) patients were nonadherent and assessed for eligibility for the intervention phase of the study. A total of 130 participants were randomized, 65 into SystemCHANGE™ and 65 into attention control. After randomization, 25 participants in SystemCHANGE™ and 16 in the attention control groups declined to participate in the study. Consequently, 89 participants were enrolled in the study. During the 6-month intervention phase, 5 withdrew from the study (3 from SystemCHANGE™ and 2 from the attention control intervention), yielding a sample of 84 (94% retention) for the 6-month intervention phase analysis. The 6-month maintenance phase was completed by 73 participants (82% retention).
FIGURE 1.

Participant flow diagram
Table 3 delineates baseline sample demographics of patients randomized into the interventions (n = 89). The average age was 51.8 years (standard deviation [SD] 10.5) with 58.4% (n = 52) male and nearly two-thirds African American (61%, n = 54). Marital status differed somewhat between the two groups and was included as a covariate in the regression analysis. Baseline MA scores were comparable between the two groups.
TABLE 3.
Patient demographics at baseline
| Characteristics | Total sample (n = 89) |
SystemCHANGE™ (n = 45) |
Attention-control (n = 44) |
|---|---|---|---|
| Age M (SD) | 51.8 (10.5) | 53.0 (11.2) | 50.7 (9.7) |
| Gender | |||
| Male, n (%) | 52 (58.4) | 30 (66.67) | 22 (50) |
| Ethnicity, n (%) | |||
| Caucasian | 34 (38) | 16 (33) | 19 (43) |
| African American | 54 (61) | 31 (65) | 25 (57) |
| Other | 1 (1) | 1 (2) | |
| Education level, n (%) | |||
| College/some college | 59 (66) | 28 (62) | 31 (70) |
| High school/some high school | 30 (34) | 17 (38) | 13 (30) |
| Marital status, n (%) | |||
| Married | 51 (48) | 20 (44) | 31 (70) |
| Divorced | 18 (20) | 12 (27) | 6 (14) |
| Never married | 16 (17) | 9 (20) | 7 (16) |
| Living with someone | 2 (2) | 2 (4) | |
| Widowed | 2 (3) | 2 (4) | |
| Employment disabled, n (%) | |||
| Disabled | 32 (36) | 15 (33) | 17 (39) |
| Full time | 30 (34) | 15 (33) | 15 (34) |
| Part time | 9 (10) | 2 (4) | 7 (16) |
| Unemployed | 7 (8) | 4 (9) | 3 (7) |
| Retired | 11 (12) | 9 (20) | 2 (5) |
| Etiology, n (%) | |||
| Hypertension | 32 (36) | 20 (44) | 12 (28) |
| Diabetes mellitus | 11 (12) | 5 (11) | 6 (14) |
| Polycystic kidney disease | 10 (11) | 5 (11) | 5 (11) |
| Other | 36 (40) | 15 (33) | 21 (48) |
| Number of kidney transplants, n (%) | |||
| 0 | 75 (84) | 37 (82) | 38 (86) |
| 1 | 12 (13) | 6 (13) | 6 (14) |
| >1 | 2 (2) | 2 (4) | |
| Type of current transplant cadaveric, n (%) | 64 (72) | 33 (73) | 31 (70) |
| Uses pillbox, n (%) | 69 (75) | 39 (82) | 30 (68) |
| Number of current prescribed meds, M (SD) | 9.04 (4.5) | 9.22 (4.2) | 8.86 (4.8) |
| Number of over-the-counter medication, M (SD) | 2.08 (1.7) | 2.07 (1.7) | 2.09 (1.6) |
| Medication adherence score | 0.67 (0.15) | 0.67 (0.18) | 0.68 (0.12) |
| Moderate medication adherence (70%−84%) | 0.77 (0.04) | 0.78 (0.04) | 0.77 (0.05) |
| Low medication adherence (<70%) | 0.55 (0.15) | 0.52 (0.20) | 0.57 (0.08) |
SD, standard deviation. [Correction added on 15 November 2019, after first online publication: The fourth column heading was corrected to Attention-control (n=44)].
Table 4 compares SystemCHANGE™ and attention control intervention MA scores at the 6-month intervention and 6-month maintenance phases. Using an intent-to-treat analysis, at the completion of the 6-month intervention phase, mean MA for SystemCHANGE™ (median 0.91, interquartile range [IQR] 0.76–0.96) and attention control (median 0.67, IQR 0.52–0.72) patients differed markedly (difference in medians 0.24, 95% confidence interval [CI] 0.13–0.30, P < .001). Similar results were found at the conclusion of the 6-month maintenance phase, with MA for SystemCHANGE™ (median 0.77, IQR 0.56–0.94) and attention control (median 0.60, IQR 0.44–0.73) patients remaining meaningfully different (difference in medians 0.17, 95% CI 0.06–0.33, P = .004). In a “completers” analysis, where only those with MA data at the end of the 6-month intervention phase were compared, MA for SystemCHANGE™ (median 0.93, IQR 0.78–0.96) and attention control (median 0.68, IQR 0.55–0.79) patients again differed widely (difference in medians 0.25, 95% CI 0.14–0.30, P < .001). Likewise, in a “completers” analysis at the end of the 12-month maintenance phase, MA for SystemCHANGE™ (median 0.77, IQR 0.56–0.94) and attention control (median 0.60, IQR 0.44–0.73) patients remained substantially different (difference in medians 0.17, 95% CI 0.06–0.33, P < .001).
TABLE 4.
Intention-to-treat comparison of SystemCHANGE™ and attention control medication adherence scores at baseline, 6 mo (end of 6 mo intervention phase) and 12 mo (end of 6 mo maintenance phase)
| SystemCHANGE™ | Attention control | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Median (IQR) | N | Mean (SD) | Median (IQR) | Pa | |
| Baseline | 45 | 0.67 (0.18) | 0.73 (0.65, 0.79) | 44 | 0.68 (0.12) | 0.71 (0.61, 0.77) | NA |
| 6 mo | 45 | 0.81 (0.25) | 0.91 (0.76, 0.96) | 44 | 0.64 (0.24) | 0.67 (0.52, 0.79) | <.001 |
| 12 mo | 45 | 0.65 (0.37) | 0.77 (0.56, 0.94) | 44 | 0.53 (0.29) | 0.60 (0.44, 0.73) | .004 |
IQR, interquartile range; SD, standard deviation.
P value for two-sided Wilcoxon-Mann-Whitney test.
[Correction added on 15 November 2019, after first online publication: The mean and median values of Baseline have been corrected].
Table 5 delineates the regression model results for adherence at 6 and 12 months. Controlling for marital status, race, and perceived health, membership in the intervention group was associated with an estimated 20% higher MA at 6 months and 16% higher at 12 months (P < .001).
TABLE 5.
Regression model results for adherence at 6 and 12 mo
| 6-mo model | 12-mo model | |||||
|---|---|---|---|---|---|---|
| Explanatory variable | Beta (95% CI) | SE | P | Beta (95% CI) | SE | P |
| Intercept | 0.76 (0.56–0.95) | 0.099 | <.001 | 0.55 (0.28–0.82) | 0.14 | <.001 |
| Intervention group | 0.20 (0.12–0.27) | 0.039 | <.001 | 0.16 (0.06–0.26) | 0.05 | <.001 |
| Married | 0.01 (−0.07 to 0.10) | 0.043 | .77 | 0.05 (−0.06 to 0.17) | 0.06 | .33 |
| White | −0.04 (−0.12 to 0.04) | 0.04 | .34 | −0.01 (−0.12 to 0.10) | 0.06 | .82 |
| Perceived health | −0.01 (−0.03 to 0.01) | 0.01 | .32 | 0.009 (−0.02 to 0.04) | 0.02 | .57 |
| Social support appraisal | −0.007 (−0.04 to 0.04) | 0.02 | .71 | 0.02 (−0.03 to 0.07) | 0.03 | .35 |
Figure 2 depicts the pattern of MA over the course of the study including both intervention and maintenance phases using a random intercept model. Initially, a model with both a random intercept and random time slope were both fit, but the random time slope was deemed unnecessary due to its small variance. A group-by-time interaction was considered, but due to a strong similarity in the de- caying trends in the SystemCHANGE™ and attention control groups (SC slope −0.0056; PE slope −0.0056), the interaction term was dropped.
FIGURE 2.
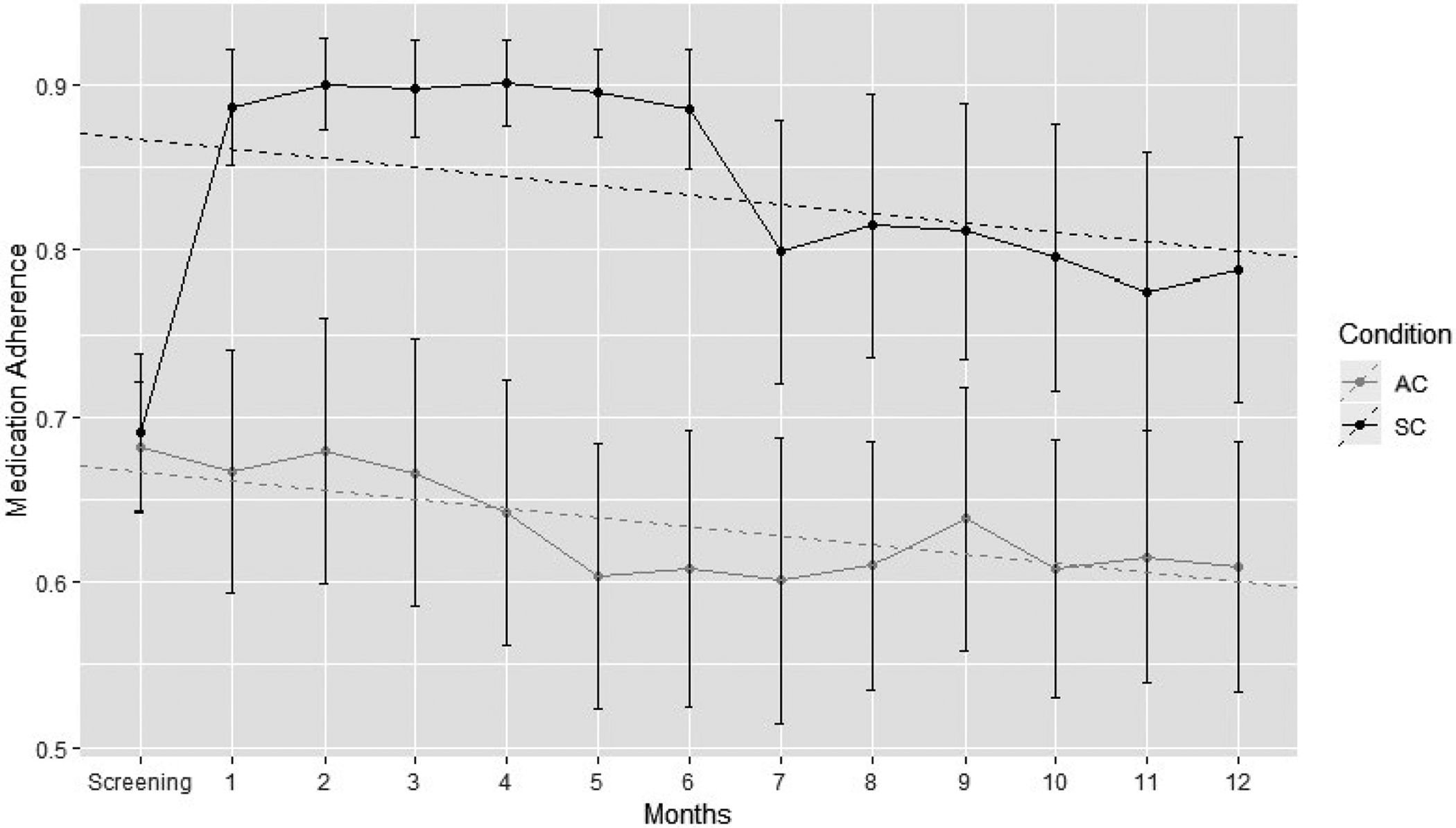
Random intercept model of SystemCHANGE™ and patient education interventions. AC, attention control group; SC, SystemCHANGE™ group
Figure 3 shows the means and frequencies for creatinine, BUN, and infections. Only 3 acute and chronic rejections, no kidney failures, and one death occurred during the study so these data are not presented. No adverse events were reported during this study.
FIGURE 3.
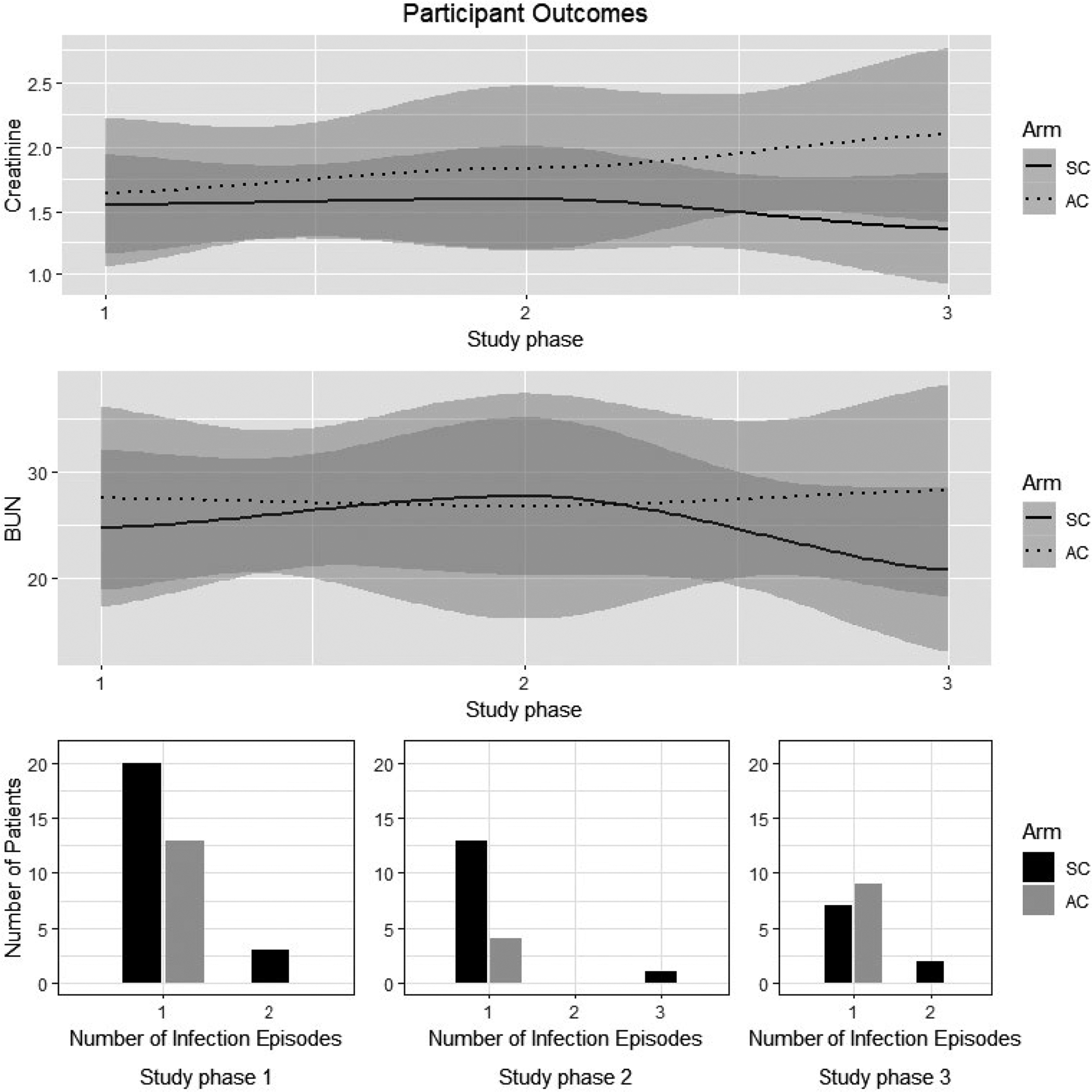
Patient outcome data. AC, attention control group; SC, SystemCHANGE™ group. Study Phase 1 was baseline; phase 2 was 6 mo and phase 3 was 12 mo. There are two depths of shaded areas in the upper figures. The 95% confidence interval bands are displayed as shaded area around each line. Lightly shaded areas pertain to the line that is closest. In both trend plots, the dotted line and the solid lines are close enough to each other such that each line’s shaded area overlaps to form the darker shaded areas
4 |. DISCUSSION
This is the first fully powered RCT designed to test the SystemCHANGE™ intervention to improve and maintain MA in kidney transplant recipients, an exemplar population of chronically ill adults. Study sample MNA rates were consistent with prior reports in the kidney transplant population.1 Immunosuppressive MA improved dramatically during the first month of the SystemCHANGE™ intervention and was sustained through the 6-month intervention. The improvement was sustained until month 12 of the maintenance phase, which has not been achieved in other MA intervention studies with kidney transplant patients.21,34
This shows the potential to deliver the intervention less frequently; perhaps once in the clinical setting followed by less frequent, periodic “boosters” delivered over the telephone or by a smartphone application though the frequency of these must be further tested, perhaps using adaptive interventions, multiphase optimization strategies (MOST), and Sequential Multiple Assignment Randomized Trial (SMART) designs.
The SystemCHANGE™ intervention is an innovative approach because it moves away from blaming the patient for poor MA. Rather than focusing on education and motivation, the SystemCHANGE™ intervention taught patients to use person-level quality improvement strategies to link adherence to established daily routines, environmental cues, and supportive people. This is consistent with other multi- component intervention studies that have improved MA in transplant recipients.35,36
Consistent with the theoretical framework, successful SystemCHANGE™ solutions involved changes in the environment that supported MA. Successful solutions involved setting cell phone alarms and placing medications next to objects in the environment of daily routines such as close to the coffee pot, television remote control, toothbrush, or car keys. Other people in the environment who may “touch” the medication taking processes were also involved in supporting the solutions. Ultimately, medications were in the right place at the right time so that medication taking was effortless.
The SystemCHANGE™ approach involves a key component of using MA data from EM to develop and track solution effectiveness. Though EM alone has shown marginal success in improving MA,37 when combined with SystemCHANGE™ the medication execution taking and timing details provide actionable information for improvement and effectiveness tracking.38
The MAGIC study findings are clinically meaningful. The difference between the median MA score of the SystemCHANGE™ group (0.91) and the attention control group (0.67) at 6 months is 0.24. This means for an individual with the common MNA pattern of taking the morning dose on time and the evening dose late, using our previously published approach for scoring twice daily dosed medications, this translates into taking the morning dose on time and the evening dose on time.4 Another common MNA pattern is missing medications, particularly in the evening.27,33 An improvement in MA score of 0.24 can be translated into a clinical improvement of not missing a dose that was previously missed every other day. This same clinical significance holds for the 12-month maintenance endpoint where the difference between the median MA score of the SystemCHANGE™ group (0.77) and the attention control group (0.60) at 12 months is 0.17.
The SystemCHANGE™ intervention also appeared to produce robust effects when delivered by different RAs. During the MAGIC study due to RA attrition, four different RAs delivered SystemCHANGE™ without any observable fluctuation in study participants’ involvement in SystemCHANGE™ activities. Training was provided as previously described and all SystemCHANGE™ participants received 100% of the “dose” of the SystemCHANGE™ components. Although there was variation in timing of the 6 monthly intervention “doses” that ranged from 100% at baseline to a low of 62% at month 5, this variation did not have an impact on the efficacy of the intervention. This observation further supports the consideration of an intervention with less frequent intervention and instead, periodic boosters, which enhances sustainability. The MAGIC study had very high participant retention rates for a 12-month study. There are several possible explanations. Both groups received a $20 monthly gift card throughout the phases. Additionally, the RAs developed relationships with the participants through the home visit and monthly phone calls during the intervention phase. Although payments are not clinically practical, the collaborative relationship between providers and patients can be established and maintained.
The MAGIC study sample was 61% African American, which is much higher than the overall kidney transplant population. The 2017 United Network of Organ Sharing national data indicate that only 27% of individuals receiving a kidney transplant were African American.31 In contrast, the sample was similar to national data on other demographics such as gender, and of note, nonwhite ethnicity, a risk factor for immunosuppressive MNA. Thus, generalization of the MAGIC study results is particularly important in regard to this high-risk group as well as being beneficial for individuals who may be at lower risk but still struggle with MA.1
This study was not powered for clinical outcomes; however, the trends for renal function for the SystemCHANGE™ group were in the anticipated direction, which is consistent with other studies testing MA intervention to improve outcomes.35,39 With immunosuppressive MA, recipients may be at higher risk for infections due to increased immune- suppression. This trend also seemed to be supported by our data.
The study has several limitations including the low intervention recruitment rate, the use of EM, and the amount of in-person visit time. Recruitment was planned to see how many MNA participants would accept the intervention. This step resulted in a pool of willing participants who evidenced poor MA at the same rate usually observed in our prior work and the literature. The dropout rate was nearly identical between groups, which indicates lack of participant interest in addressing MA rather than intervention intensity. The EM was used because it provides critical actionable MA details of initiation, implementation (medication taking and timing), and persistence, which guided development of successful MA SystemCHANGE™ solutions.40 The EM may have influenced MA though the first month of MA data during the screening phase was dropped to reduce the Hawthorne effect. Additionally, the attention control intervention cannot be considered standard of care. Consequently, the effect of the intervention may in fact be larger than that measured in the study when compared to standard care with no EM monitoring. To reduce in-person visit time, future alternatives should be explored including delivery by videoconferencing technology, video self-instruction, and/or lay providers, reducing clinical staff implementation burden.41
In conclusion, the SystemCHANGE™ intervention is efficacious for improving immunosuppressive MA for adult kidney transplant recipients. Improvement occurs within 2 months upon intervention initiation with sustained improvement over the 6 months of intervention and 6-month of maintenance. Outcomes also appear to trend in the direction of improvement. This robust intervention could be applicable to other patient populations and dosing regimens. However, the efficacy in other populations must be tested. The next study’s goal will be to test effectiveness of the intervention in kidney transplant recipients in the clinic setting.
ACKNOWLEDGMENTS
The authors would like to thank the following persons for their support of the MAGIC study: Drs. James Eason, Dan Brennan, Aditi Gupta, John Chen, Louise Miller, Debra Gayer, and Brittany Heady, Rebecca Cusanelli, Megan Teeter, Kamala Karri, Tracie Moore, Sarah Owens, Kathleen Engel, Susan Dodson, Teresa Berkley, Catherine Pantik, Ronnie Blackburn, and Jeremy Provance.
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 1 R01 DK093592-01A1; National Institutes of Health, Grant/Award Number: NIH T32NR015426
Abbreviations:
- DSMB
Data Safety Monitoring Board
- EM
electronic medication
- IRB
institutional review board
- MA
medication adherence
- MEMSCap™
Medication Event Monitoring System SmartCap®
- MNA
medication nonadherence
- MOST
multiphase optimization strategies
- PDCA
plan-do-check-act
- SEM
socio-ecological model
- SMART
sequential multiple assignment randomized trial.
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Because there are a limited number participants, there is the potential that they could be inadvertently identified. We will release our study data under a data-sharing agreement that provides for (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; (3) a commitment to destroying or returning the data after analyses are completed; and (4) an approved IRB from the requesting researcher.
REFERENCES
- 1.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 2.Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597–2606. 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 3.Weng FL, Chandwani S, Kurtyka KM, et al. Prevalence and correlates of medication nonadherence among kidney transplant recipients more than 6 months post-transplant: a cross-sectional study. BMC Nephrol. 2013;14:261 10.1186/1471-2369-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell CL, Conn V, Jantarakupt P. Older adult medication compliance: integrated review of randomized controlled trials. Am J Health Behav. 2006;30(6):636–650. 10.5993/ajhb.30.6.10. [DOI] [PubMed] [Google Scholar]
- 5.Roter DL, Hall JA, Merisca R, et al. Effectiveness of intervenions to improve patient compliance: a meta-analysis. Med Care. 1998;36(8):1138–1161. 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. [DOI] [PubMed] [Google Scholar]
- 7.Conn VS, Hafdahl AR, Cooper PS, et al. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49(4):447–462. 10.1093/geront/gnp037. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008(2):;Cd000011: 10.1002/14651858.cd000011.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Christensen A, Osterberg LG, Hansen EH. Electronic monitoring of patient adherence to oral antihypertensive medical treatment: a systematic review. J Hypertens. 2009;27(8):1540–1551. 10.1097/hjh.0b013e32832d50ef. [DOI] [PubMed] [Google Scholar]
- 10.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic conditions. Arch Intern Med. 2007;167:540–550. 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Ruppar TM, Delgado JM, Temple J. Medication adherence interventions for heart failure patients: a meta-analysis. Eur J Cardiovasc Nurs. 2015;14(5):395–404. 10.1177/1474515115571213. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:Cd000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive- ased behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3(8):e002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm MA, Mulloy LL, Jagadeesan M, et al. Impact of clinical pharmacy services on renal transplant patients’ compliance with immunosuppressive medications. Clin Transplant. 2001;15(5):330–336. 10.1034/j.1399-0012.2001.150505.x. [DOI] [PubMed] [Google Scholar]
- 15.Dew MA, Goycoolea JM, Harris RC, et al. An internet-based intervention to improve psychosocial outcomes in heart transplant recipients and family caregivers: development and evaluation. J Heart Lung Transplant. 2004;23(6):745–758. 10.1016/j.healun.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Hardstaff R, Green K, Talbot D. Measurement of compliance post- transplantation–the results of a 12-month study using electronic monitoring. Transplant Proc. 2003;35(2):796–797. 10.1016/s0041-1345(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 17.Dejean NB, Rostaing L, Mestre ML, et al. Educational program to reduce noncompliance after renal transplantation. Am J Transplant Suppl. 2004;4:520–521. [Google Scholar]
- 18.Klein A, Krämer I, Otto G. Impact of a pharmaceutical care program on liver transplanted patients’ compliance with immunosuppressive medication: a prospective, randomized, controlled trial using electronic monitoring. Transplantation. 2006;82(1):212 10.1097/00007890-200607152-00432. [DOI] [PubMed] [Google Scholar]
- 19.Chisholm MA, Vollenweider LJ, Mulloy LL, et al. Renal transplant patient compliance with free immunosuppressive medications. Transplantation. 2000;70(8):1240–1244. 10.1097/00007890-200010270-00020. [DOI] [PubMed] [Google Scholar]
- 20.De Geest S, Schafer-Keller P, Denhaerynck K, et al. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20(3):359–368. 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 21.De Bleser L, Matteson M, Dobbels F, et al. Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int. 2009;22(8):780–797. 10.1111/j.1432-2277.2009.81.x. [DOI] [PubMed] [Google Scholar]
- 22.Deming E, Orsini JN. The Essential Deming: Leadership Principles from the Father of Quality. New York, NY: McGraw Hill Professional; 2012. [Google Scholar]
- 23.Bronfenbrenner U. Toward an experimental ecology of human development. Am Psychol. 1977;32(7):513–531. [Google Scholar]
- 24.Alemi F, Baghi H. Self-experiments and analytical relapse prevention. Qual Manag Health Care. 2008;17(1):53–65. [DOI] [PubMed] [Google Scholar]
- 25.Alemi F, Neuhauser D, Ardito S, et al. Continuous self-improvement: systems thinking in a personal context. Jt Comm J Qual Improv. 2000;26(2):74–86. [DOI] [PubMed] [Google Scholar]
- 26.Russell CL. A clinical nurse specialist-led intervention to enhance medication adherence using the plan-do-check-act cycle for continuous self-improvement. Clin Nurse Spec. 2010;24(2):69–75. [DOI] [PubMed] [Google Scholar]
- 27.Russell CL, Moore S, Hathaway D, et al. MAGIC study: aims, design and methods using SystemCHANGE™ to improve immunosuppressive medication adherence in adult kidney transplant recipients. BMC Nephrol. 2016;17(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castanho TC, Amorim L, Zihl J, et al. Telephone-based screening tools for mild cognitive impairment and dementia in aging studies: a review of validated instruments. Front Aging Neurosci. 2014;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell CL, Miller C, Remy LM, et al. Improvement of immunosuppressive medication adherence using a SystemCHANGE intervention: case study of an older adult kidney transplant recipient. Nephrol Nurs J. 2018;45(2):171–223. [PMC free article] [PubMed] [Google Scholar]
- 30.International Transplant Nurses Society. https://itns.org. Accessed March 10, 2019.
- 31.Department of Health U.S. and Human Services. Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed March 10, 2019.
- 32.Russell CL, Conn VS, Ashbaugh C, et al. Medication adherence patterns in adult renal transplant recipients. Res Nurs Health. 2006;29(6):521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart AL, Ware JE, eds. Measuring Functioning and Well-Being The medical outcomes study approach. Durham: Duke University Press; 1992. [Google Scholar]
- 34.Berben L, Dobbels F, Kugler C, et al. Interventions used by health care professionals to enhance medication adherence in transplant patients: a survey of current clinical practice. Prog Transplant. 2011;21(4):322–331. [DOI] [PubMed] [Google Scholar]
- 35.Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: the MAESTRO- Tx trial. J Heart Lung Transplant. 2017;36(5):499–508. [DOI] [PubMed] [Google Scholar]
- 36.Foster BJ, Pai AL, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT). Am J Kidney Dis. 2018;72(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese PP, Bloom RD, Trofe-Clark J, et al. Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. Am J Kidney Dis. 2017;69(3):400–409. [DOI] [PubMed] [Google Scholar]
- 38.Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chisholm-Burns MA, Spivey CA, Graff Zivin J, Lee JK, Sredzinski E, Tolley EA. Improving outcomes of renal transplant recipients with behavioral adherence contracts: a randomized controlled trial. Am J Transplant. 2013;13(9):2364–2373. [DOI] [PubMed] [Google Scholar]
- 40.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore SM, Borawski EA, Cuttler L, et al. IMPACT: a multi-level family and school intervention targeting obesity in urban youth. Contemp Clin Trials. 2013;36(2):574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]


