Abstract
Neural stem cells (NSCs) are crucial for development, regeneration, and repair of the nervous system. Most NSCs in mammalian adult brains are quiescent, but in response to extrinsic stimuli, they can exit from quiescence and become reactivated to give rise to new neurons. The delicate balance between NSC quiescence and activation is important for adult neurogenesis and NSC maintenance. However, how NSCs transit between quiescence and activation remains largely elusive. Here, we discuss our current understanding of the molecular mechanisms underlying the reactivation of quiescent NSCs. We review recent advances on signaling pathways originated from the NSC niche and their crosstalk in regulating NSC reactivation. We also highlight new intrinsic paradigms that control NSC reactivation in Drosophila and mammalian systems. We also discuss emerging evidence on modeling human neurodevelopmental disorders using NSCs.
Introduction
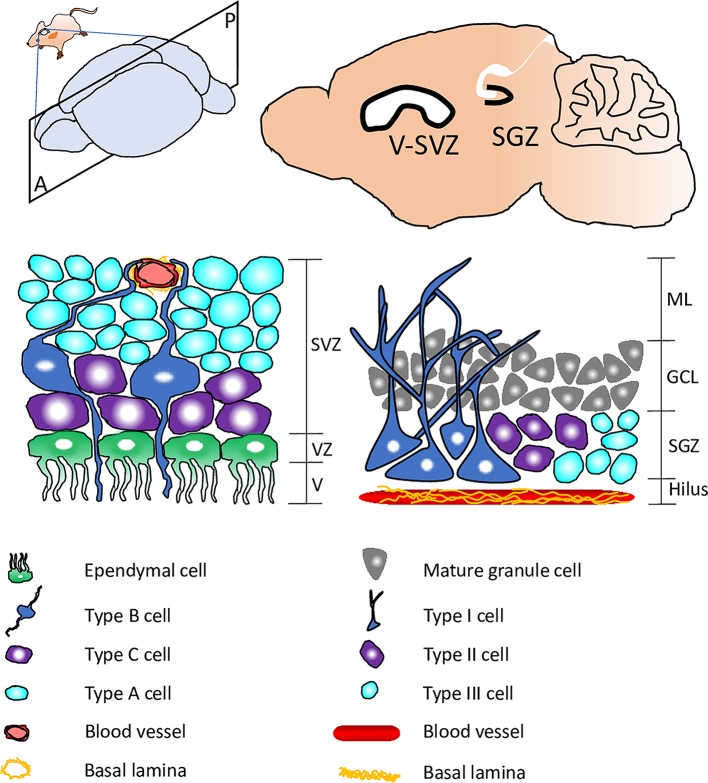
The ability of stem cells to switch between quiescence and proliferation is crucial for tissue homeostasis and regeneration. Most neural stem cells (NSCs) in the mammalian adult brain exist in quiescence, a mitotic-dormant state, without undergoing proliferation or differentiation [1]. In response to physiological stimuli such as the presence of nutrients and physical exercise, quiescent NSCs can exit from quiescence and become reactivated to generate new neurons [2]. Conversely, stress, anxiety, and old age reduce the proliferation capability of NSCs [3]. Failure in NSC reactivation is thought to result in cognitive decline during old age [4]. In the mammalian adult brain, radial glial cells (type B) are NSCs that reside within the ventricular–subventricular zone (V–SVZ)/subependymal zone (SEZ) in the walls of the lateral ventricles, while radial glial cells (type I) are NSCs located in the subgranular zone (SGZ) of the hippocampal dentate gyrus (Fig 1) [5, 6].
Fig 1. Schematic representation showing neurogenic niches within the mammalian adult brain.
Top: a sagittal section of the mouse brain with neurogenic niches SGZ and V–SVZ highlighted. Bottom: schematics showing quiescent NSCs (type B in SVZ; type I in SGZ) and their surrounding cellular and molecular components within the V–SVZ (left) and SGZ (right). GCL, granule cell layer; ML, molecular layer; NSC, neural stem cell; SGZ, subgranular zone; SVZ, subventricular zone; V, ventricular space; VZ, ventricular zone.
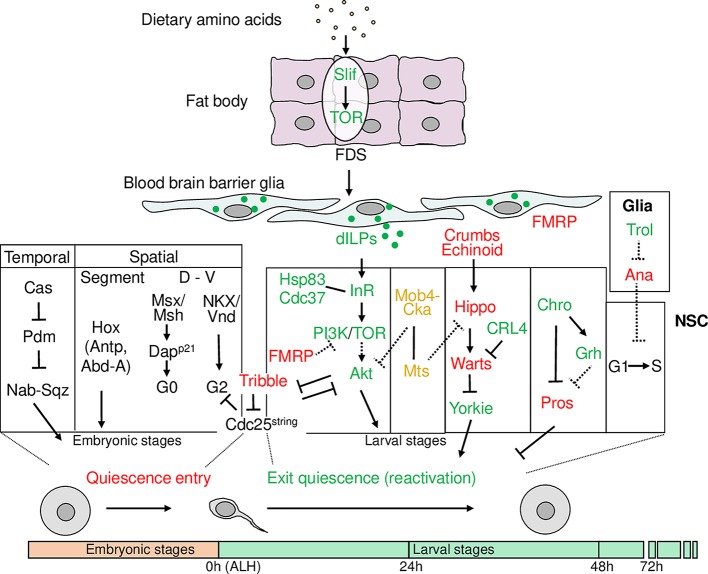
NSCs in invertebrates such as Drosophila melanogaster also switch between a reversible transition between quiescence and reactivation [7–10]. Drosophila NSCs, also known as neuroblasts, enter quiescence for about 24 hours between embryogenic and postembryonic neurogenesis [7–10] (Fig 2). Because embryonic NSCs shrink their cell size following each cell division, by the end of the embryonic stage, the diameter of NSCs is decreased from approximately 10–14 μm to approximately 3–4 μm [7, 8]. Most NSCs in the abdominal regions of the ventral nerve cord (VNC) undergo apoptosis [11], while NSCs in the brain hemispheres and the thoracic VNC enter quiescence and subsequently exit quiescence during larval stages [8, 12, 13]. When larval NSCs exit quiescence, they undergo cell growth to reach the cell diameter of about approximately 7 μm before their first cell division in larval stages [14, 15].
Fig 2. Schematic representation showing various factors within Drosophila fat body, BBB glia, and NSCs that regulate Drosophila NSC quiescence entry and reactivation.
Factors promoting NSC reactivation are in green, while factors maintaining NSC quiescence or preventing reactivation are in red. Abd-A, Abdominal-A; ALH, after larval hatching; Ana, Anachronism; Antp, Antennapedia; BBB, blood–brain barrier; Cas, Caster; Cdc37, Cell division cycle 37; Chro, Chromator; Cka, Connector of kinase to AP-1; CRL4, Cullin-RING ligase 4; Dapp21, Dacapo (ortholog of p21CIP/p27KIP1/p57KIP2 family); dILPs, insulin/IGF-like peptides; D–V, dorsal to ventral; FDS, fat-body–derived signal; FMRP, Fragile X mental retardation protein; Grh, Grainy head; Hsp83, Heat shock protein 83; InR, Insulin receptor; Mob4, Monopolar spindle-one-binder family member 4; Msx/Msh, Muscle segment homeobox (ortholog of MSX1/2/3); Mts, Microtubule star; Nab, NGFI-A-binding protein; NKX/Vnd, Ventral nervous system defective (ortholog of NKX family); NSC, neural stem cell; Pdm, Pou-domain proteins Pdm1 and 2; PI3K, Phosphatidylinositol 3-kinase; Pros, Prospero; Slif, Slimfast; Sqz, Squeeze; TOR, Target-of-Rapamycin; Trol, Terribly reduced optic lobes.
Drosophila larval NSCs exit quiescence (reactivate) in response to feeding upon larval hatching [8, 12, 13]. The crucial dietary components for NSC reactivation are amino acids, but not nucleotide precursors, lipids, or vitamins [14]. However, none of the 11 essential amino acids alone in the food is sufficient for NSC reactivation, underscoring the importance of protein synthesis [14]. The signaling relay from the presence of dietary amino acids to the brain is controlled by an endocrine organ named the fat body, a functional equivalent of the mammalian liver and white fat [14, 16, 17]. The fat body senses circulating amino acids by the cationic amino-acid transporter Slimfast (Slif), leading to the activation of the Target-of-Rapamycin (TOR) pathway, which induces an unknown fat-body–derived signal (FDS) [16, 18]. The FDS is thought to reach the brain, stimulating NSC reactivation [14] (Fig 2). While extrinsic niche-derived cues allow NSCs to reactivate in respond to changes in the external environment such as the presence of nutrition, exercise, drug administration, or injury, intrinsic mechanisms represent another facet of control that is dependent on nuclear factors and cell-cycle regulators within NSCs during their reactivation.
Signaling integration in the CNS barriers regulates the activation of NSCs
The blood–brain barrier (BBB) forms an insulation barrier to restrict free crossing of substances from the blood and protects the central nervous system (CNS) from toxins, inflammation, and pathogens while providing a microenvironment for neuroglia signaling [19] (Fig 3). The integrity of the mammalian BBB is primarily attributed to CNS endothelial cells that vascularize the brain [20]. These endothelial cells are connected by specialized intercellular tight junctions that have an important barrier function: to restrict paracellular permeability [21]. This permeability may be influenced by calcium oscillations of CNS endothelial cells [22]. In addition to their function as a barrier, CNS endothelial cells also supply the brain with essential nutrients by producing nutrient transporters such as glucose carrier, amino-acid carriers, and major facilitator domain containing 2A (Mfsd2a), a lysolipid transporter for docosahexaenoic acid (DHA) [23–25]. Mutations in human MFSD2A result in severe microcephaly syndrome, a neurodevelopmental disorder [26, 27]. Endothelial cells in the V–SVZ secrete factors that have an opposing effect in activating or maintaining quiescence of NSCs [28, 29]. To activate NSCs, betacellulin acts on epidermal growth factor receptor (EGFR), activating the extracellular signal-regulated kinase (ERK)/AKT, also known as protein kinase B (PKB), pathway to enter a proliferative stage [28]. On the other hand, neurotrophin 3 (NT-3) up-regulates endothelial isoform of nitrous oxide synthase (eNOS) that promotes NSC quiescence in a nitrous oxide (NO)-dependent manner [29]. In vitro study has, however, demonstrated a dose-dependent effect of NO in the balance of NSC quiescence–activation, with low concentration resulting in an increase in cell proliferation, while high concentration resulted in a decrease in cell proliferation [30]. Further investigations into the mode of NO regulation within the BBB niche will shed light on the dynamic regulation of NSC activation under homeostatic conditions and in response to external insult.
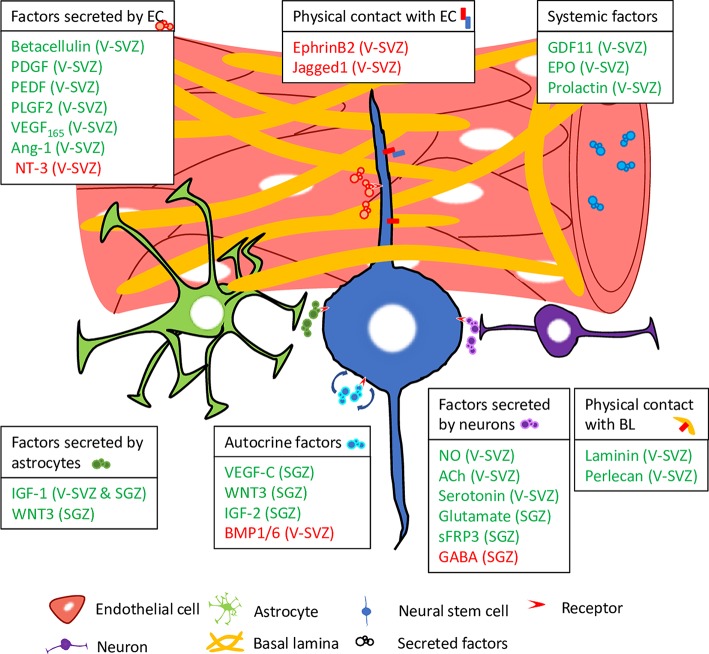
Fig 3. Schematic representation showing various factors within the neurogenic niche and from systemic circulation, as well as physical contacts with the microenvironment that regulate the balance between quiescence and reactivation of murine NSCs.
Factors highlighted in green promote reactivation, while factors highlighted in red promote quiescence. Ach, acetylcholine; Ang-1, angiopoietin-1; BL, basal lamina; BMP1/6, bone morphogenetic protein 1/6; EC, endothelial cell; EPO, erythropoietin; GABA, gamma aminobutyric acid; GDF11, growth differentiation factor 11; IGF-1/2, insulin-like growth factor-1/2; NO, nitrous oxide; NT-3, neurotrophin 3; PDGF, platelet-derived growth factor; PEDF, pigment epithelium-derived growth factor; PLGF2, placenta-derived growth factor 2; sFRP3, secreted frizzled-related protein 3; SGZ, subgranular zone; VEGF-C, vascular endothelial growth factor-C; V–SVZ, ventricular–subventricular zone; WNT3, Wnt family member 3.
Systemic signals delivered via the vasculature to the BBB niche have been implicated in the regulation of NSC activation. Using a mouse heterochronic parabiosis model by surgically joining pairs of animals, systemic factors derived from young mice, specifically identified to include growth differentiation factor 11 (GDF11), are shown to drive vascular remodeling and activation of NSC proliferation in the V–SVZ of aged mice [31]. Conversely, systemic factors derived from aged mice have an attenuating effect on NSC proliferation in the SGZ [32, 33]. In the V–SVZ, hormones such as erythropoietin and prolactin have positive effects in activating the proliferation program in quiescent NSCs [34, 35]. However, prolactin has a negligible effect on NSCs residing in the SGZ, suggesting a differential effect of hormones and possibly other soluble factors that depends on spatial cues and niche characteristics [34].
Besides endothelial cells, the brain microenvironment also contains brain pericytes, neurons, and astrocytic glia that influence barrier properties [36]. Astrocytes that extend cellular processes ensheathe the blood vessels by contacting and surrounding CNS endothelial cells through the endfeet of their basal processes [19]. Astrocytes regulate the permeability of the BBB and secrete factors such as transforming growth factor beta (TGF-β), glial-cell–derived neurotrophic factor (GDNF), and basic fibroblast growth factor (FGF) that regulate BBB development [25].
The Drosophila brain is separated from the blood-like hemolymph by the functional analogue of BBB [37]. The Drosophila BBB in larval stages is composed of 2 types of surface glia named perineural glia (PG) and subperineural glia (SPG) [38, 39]. The PG characterized with a stellate appearance are located at the outer layer, while the SPG with sheet-like morphology are located immediately beneath the PG [40–42]. The SPG are the major BBB layer because they form septate junctions at the lateral borders between the SPG cells. The BBB glia provide an important niche for the regulation of NSC quiescence and reactivation via various signaling pathways.
The insulin pathway promotes NSC reactivation
In response to nutritional input, insulin/insulin-like growth factor (IGF) signaling (IIS) controls growth, metabolism, and longevity [43]. The function of IIS in growth is evolutionarily conserved in Drosophila, in which there are a single insulin/IGF receptor (dInR) and 8 insulin/IGF-like peptides (dILPs 1–8) [44, 45]. There are at least 2 source of dILPs in the Drosophila larval brain, the specialized neurosecretory cells named insulin-producing cells (IPCs) and a set of surface glia overlying the NSCs [15, 44]. Functioning analogous to β cells of the vertebrate endocrine pancreas, IPCs produce and secrete dILP1, 2, 3, and 5 into the hemolymph and act systemically to regulate larval growth and lipid metabolism [46, 47]. By contrast, surface glia in the larval brain are dispensable for systemic growth but essential for NSC reactivation [15, 18]. During NSC reactivation in the Drosophila VNC, the production of dILP2 and dILP6 increases [15]. The dILP2 and dILP6 are found to be produced in a subset of PG glia that have a stellate morphology and are located between the NSCs and basement membrane [15, 18]. dILP3 expression was found in some glia and neurons in the CNS in the early second-instar stage [18], suggesting that dILP3 may play a role at later developmental stages following NSC reactivation. Overexpression of dILP2 or dILP6 in glia is sufficient for NSC reactivation in the absence of dietary amino acids without apparently altering larval growth [15, 18]. On the contrary, none of the dILPs 1–7, upon overexpression in IPCs, could reactivate NSCs under nutrient restriction conditions [18]. Therefore, Drosophila NSCs respond to a local source of dILPs from glial cells, but not the systemic source from IPCs, to exit quiescence. Presumably, the mitogen from the fat body stimulates the glial cells to produce and secrete dILP2 and dILP6 [15, 18, 48]. The identity of this fat-body–derived mitogen—growth factors, hormones, or signaling molecules—remains elusive. The dILPs secreted from the glial cells act locally by directly activating the insulin receptor (InR)/phosphatidylinositol 3-kinase (PI3K)/Akt pathway as well as the TOR pathway in underlying NSCs [15, 18] (Fig 2). As a result, protein biosynthesis begins, and NSCs re-enter the cell cycle through inhibition of the Forkhead box O (FOXO) transcription factor [15, 18].
NSC reactivation occurs relatively synchronously, in about 24 hours, in all neurogenic regions of the Drosophila CNS [15]. This is achieved by the function of gap junctions in the BBB glia that couple metabolic signal with synchronized calcium pulses and insulin secretion [49]. The gap junction is a transmembrane channel formed by docking of connexin hexamers from adjacent cells [50]. Gap junction proteins are required in the BBB glia for the secretion of dILP6 and subsequent coordinated calcium oscillations of SPG [49]. The inositol-triphosphate (IP3) binds to its receptor Ins3PR, a calcium channel in the endoplasmic reticulum (ER), and releases calcium from intracellular stores in glial cells to trigger NSC reactivation [49]. It is important to note that depletion of gap junction proteins does not cause the leakage of BBB because the septate junctions of the BBB glia appear to be intact [49].
Analogous to Drosophila BBB glia, in the mammalian adult hippocampus, astrocytic glia function as a niche to induce neurogenesis by promoting proliferation of NSCs and neuronal fate commitment [51]. This was first demonstrated by coculturing of adult NSCs with primary hippocampal astrocytes, which is sufficient to promote neurogenesis [51]. Astrocytes produce IGF-1, which promotes NSC proliferation in mammalian adult brains [52]. IGFs, namely InR, IGF-1 receptor (IGF-1R), and IGF-2R, are also abundantly expressed in the mammalian brain [53, 54]. IGF-1 is expressed in astrocytes, neurons, and NSCs in the hippocampus and the V–SVZ, and its expression in the brain is much higher than in systemic circulation during neurogenesis [55–58]. Locally overexpressed or directly infused IGF-1 can trigger NSC proliferation without leading to an increase of IGF-1 level in the circulation [59, 60], suggesting that locally expressed (paracrine or autocrine) IGF-1 is crucial for regulating NSC proliferation. This mitogenic role of IGF-1 leads to the activation of mammalian target of rapamycin complex 1 (mTORC1) and inhibition of FOXO via the PI3K/Akt pathway [61–64]. Both IGF-1 and the PI3K/Akt pathway promote cell-cycle progression [65, 66]. IGF-2 also promotes the proliferation of NSCs via Akt signaling, and it is highly expressed in NSCs in the hippocampal dentate gyrus [67]. Thus, the InR/PI3K/Akt pathway appears to be a common theme in promoting NSC reactivation in both flies and mammalian NSCs.
Dysregulation of critical components in the PI3K/Akt pathway has been implicated in neurodevelopmental disorders [68]. Three common mutations of IGF-1R and deletion of the chromosome region containing AKT3 have been identified in patients with primary microcephaly [69, 70], suggesting that IGF-1R mutations and AKT3 deletion may contribute to this neurodevelopmental disorder. Removing downstream effectors of the PI3K/Akt pathway in vivo, e.g., phosphoinositide-dependent kinase 1 (Pdk1), mTOR, and raptor, has also been found to cause microcephaly [71–75]. Conversely, mutations activating PI3KCA and AKT3, the predominant AKT isoform in mouse brain cortex and hippocampus, have been linked to clinical manifestations of a spectrum of enlarged brain malformations, e.g., macrocephaly, dysplastic megalencephaly, and hemimegalencephaly [72, 76, 77]. Similarly, the loss of phosphatase and tensin homolog (PTEN), a PI3K antagonist, results in increased cell proliferation and reduced cell death, which contributes to macrocephaly [78]. Subsequent study demonstrates that conditional deletion of PTEN up-regulates the reactivation of NSCs in the SGZ [79].
Other signaling pathways and proteins from glia control NSC proliferation
One of the earliest evidence on the involvement of glia in NSC reactivation was from a study on Drosophila DE-Cadherin, a cell adhesion molecule, which acts in glia cells to promote the proliferation of NSCs [80]. Besides the InR/PI3K/Akt pathway, several major evolutionarily conserved signaling cascades also regulate NSC reactivation. The TGF-β/BMP (bone morphogenetic protein) pathway plays crucial roles during various cellular processes such as cell growth and differentiation. The BMP signaling pathway promotes NSC proliferation in Drosophila because Glass bottom boat (Gbb), a BMP homolog that is expressed in NSCs, acts as an autocrine proliferation factor in NSCs [81]. Dally-like (Dlp), a heparan sulfate proteoglycan protein on the cell surface and in the extracellular matrix, functions as a coreceptor for Gbb in PG to promote NSC proliferation [81]. Interestingly, NSC-expressing Gbb also provides a paracrine signal for the survival of PG, suggesting that a bidirectional communication between NSCs and the BBB glia influences the development of both cell types [81]. In the mammalian adult brain, the BMP pathway blocks neurogenesis and directs glial differentiation of NSCs [82]. The BMP target inhibitor of differentiation 2 (ID2), its ligand BMP1, BMP6, and BMP receptor BMPR1B are all expressed in quiescent NSCs, suggesting that BMP regulates NSC proliferation in an autocrine manner [83]. BMP maintains NSC quiescence, as the loss of BMP signaling via selective ablation of upstream BMPR-IA receptor leads to a transient increase in NSC proliferation, followed by depletion of stem cell pool in the long term [84]. The inhibition effect on neurogenesis by the BMP pathway can be antagonized by Noggin through paracrine secretion by ependymal cells adjacent to the V–SVZ [82]. Similarly, Noggin expression is found in the adult dentate gyrus, antagonizing the BMP pathway to promote the proliferation of NSCs [85]. Interestingly, BMP signaling has a surprising divergent effect in promoting proliferation and quiescence in NSCs of Drosophila and adult mice, respectively. Nevertheless, because studies in Drosophila were carried out in third-instar larval brains, it remains to be determined whether BMP signaling is required for NSC reactivation in early stages and/or maintenance of NSC proliferation at later stages.
Another heparan sulfate proteoglycan protein named Terribly reduced optic lobes (Trol), the Drosophila perlecan homolog, is also required for G1/S transition during NSC reactivation [86, 87]. Trol is expressed in a subset of dorsal midline glial cells of the CNS [86], suggesting that it functions non-cell–autonomously for NSC proliferation. It is believed that Trol promotes NSC reactivation through antagonizing the NSC reactivation inhibitor Anachronism (Ana) [86] (Fig 2). In addition, Trol interacts with both FGF-2 and Hedgehog (HH) in larval protein extracts [88]. The low affinity binding between heparan sulfate proteoglycan proteins and FGFs is required for the binding of FGFs to their high affinity receptors [89]. Subsequent study in mice recapitulated the conserved role of perlecan in mediating FGF-2 signaling that promotes V–SVZ NSC proliferation [90]. In adult mammalian brains, the mitogens epidermal growth factor (EGF) and FGF-2 promote NSC proliferation [91, 92]. The activity of FGF-2 can be modulated by its low affinity receptor heparin, which either activates or inhibits the mitogenic activity of FGF-2 on NSCs, likely depending on the structure, composition, or expression level of heparin on the cell surface [93, 94].
The basal lamina, a layer of extracellular matrix (ECM) secreted by the CNS endothelial cells, provides an important molecular signature for adult mammalian NSCs to modulate its activation of proliferation program [95]. During development and postnatal stages, a failure in deposition of ECM proteins, including Laminin-α4, surrounding the vasculature leads to detachment of the critical linkage between endothelial cells and V–SVZ NSCs [96]. Compared to those at quiescent stage, activated NSCs in the V–SVZ have increased expression of certain ECM receptors such as laminin receptor α6β1-integrin and syndecan-1 [97–99]. The activation of NSCs in the V–SVZ are further enhanced by their ability to bind to laminin in the vascular niche via an up-regulation of EGFR and α6 integrin by stromal-derived factor 1 (SDF1) [100]. Further elucidations on the role of ECM and other BBB niche cells such as pericytes in modulating NSC activation may provide insights into generating improved regenerative therapeutics for brain trauma and cerebral ischemia.
Drosophila Hh signaling activates NSC division in a Trol-dependent manner [88]. Mammalian Sonic hedgehog (Shh) regulates adult NSC proliferation in rat hippocampus and the V–SVZ [101, 102]. Quiescent NSCs in the V–SVZ and SGZ respond to Shh and are able to self-renew and expand the NSC population for about 1 year in vivo [103]. In the mammalian adult hippocampal SGZ, Wnt3 is expressed in astrocytes, and its overexpression is sufficient to increase neurogenesis by controlling the neuronal fate commitment and proliferation of neural precursor cells and neuroblasts, while inhibition of Wnt signaling reduces adult neurogenesis [104]. Disrupted In Schizophrenia 1 (DISC1), which is deficient in patients with schizophrenia, depression, and bipolar disorder, is expressed in adult mouse NSCs and regulates their proliferation [105]. DISC1 directly associates and inhibits glycogen synthase kinase 3 beta (GSK3β) activity, leading to a reduction of β-catenin phosphorylation and its stabilization [105]. In support of these findings in mouse NSCs, altered WNT signaling has been identified in human NSCs derived from human induced pluripotent stem cells from schizophrenia patients [106].
In summary, in both Drosophila and mammalian NSC niches, ECM plays an important role in regulating the balance of quiescence and proliferation. Not only does it serve as a physical support for NSC anchorage, it also acts as a depositing scaffold for various secreted factors by niche cells and from systemic circulation. Nevertheless, the components of ECM in both Drosophila and mammalian systems remain poorly defined, making in-depth characterization of NSC–ECM interaction difficult. Because mesenchymal stem cell quiescence–proliferation decision was shown to be dependent on substrate stiffness [107], further investigation on the mechanobiology of ECM in regulating NSC quiescence, i.e., elasticity, stiffness, microtopography, etc., is warranted.
The inhibitory role of the CNS barrier in NSC reactivation
On the flip side, the Drosophila glia niche also provides inhibitory factors that maintain NSC quiescence. Glial cells secrete the glycoprotein Ana to prevent NSCs from entering S-phase, therefore maintaining NSC quiescence [108]. Glia are also required for the activation of the evolutionarily conserved Hippo pathway that keeps NSCs in quiescence [109]. First identified in Drosophila, the Hippo pathway plays a conserved role in tumorigenesis, organ development, and stem cell maintenance [110]. In the absence of dietary amino acids, 2 intercellular transmembrane proteins Crumbs and Echinoid are expressed in both NSCs and their glial cell niche [109]. This intercellular interaction of Crumbs and Echinoid activates the Hippo pathway composed of Tao-1, Hippo, Salvador, and large tumor suppressor (Lats)/Warts in Drosophila NSCs [109, 111–113]. This growth-repressive kinase cascade ultimately phosphorylates the transcriptional coactivator Yorkie, resulting in its cytoplasmic retention [109, 114]. In the presence of dietary amino acids, Echinoid was down-regulated mainly in glia over time, and Crumbs is lost in both glia and NSCs, leading to the inactivation of the Hippo pathway and, in turn, translocation of Yorkie into the nucleus to activate downstream targets such as bantam microRNA, ultimately triggering NSC reactivation [109]. Although loss of the Hippo pathway causes premature NSC reactivation on the fed condition, it is unable to overcome the requirement of dietary amino acids [109]. It is unknown how the nutritional status alters the expression of Crumbs and Echinoid in the brain. Possibly the protein turnover or the intracellular trafficking of Crumbs and Echinoid is controlled in response to nutrition.
Notch signaling has been implicated in the maintenance of mammalian NSC quiescence because Notch downstream effector Hes family bHLH transcription factor 1 (HES1) and HES5 inhibit neuronal differentiation [115]. The physical contact maintained between NSCs in the V–SVZ and endothelial cells allows ligands ephrinB2 and Jagged1, which are expressed by endothelial cells, to trigger ephrine (Eph) and Notch signaling in the NSCs to maintain quiescence [116]. This was achieved synergistically by inhibiting proliferation through Eph signaling and blocking differentiation via Notch signaling. Inhibition of Notch signaling results in a transient increase in NSC proliferation, followed by stem-cell–pool depletion in the long term [117–119]. Notch ligand delta-like protein 1 (Dll1), which is expressed in activated NSCs and subsequently segregated into the daughter cell undergoing differentiation after asymmetric division, maintains quiescence of adjacent NSCs, suggesting a feedback loop for NSC maintenance between sister cells [120]. Patients with deleterious mutations in the DLL1 gene are found to have developmental delay, intellectual disability, and brain malformations [121].
In a pioneer study conducted by Palmer and colleagues, they demonstrated that proliferating NSCs within the SGZ can be found close to angiogenic capillary tips, suggesting a possible role for angiogenic regulators in NSC activation [122]. A subsequent study on platelet-derived growth factor (PDGF) in the V–SVZ, a well-known angiogenic factor, in which its in vivo introduction led to formation of hyperplastic nodules containing highly proliferating NSCs, strongly suggests its role in activating NSC proliferation [123]. This was followed by the elucidation of pigment epithelium-derived factor (PEDF), angiopoietin-1 (Ang-1), and vascular endothelial growth factor 165 (VEGF165), all of which were found to be involved in activating NSC proliferation in the V–SVZ [124–126]. In addition, placenta-derived growth factor 2 (PLGF2), an important player in endothelial stimulation and pathological angiogenesis, activates NSCs in the V–SVZ niche by interacting with vascular endothelial growth factor 1 (VEGFR1) [127]. Similarly in the SGZ, VEGF-C interacts with VEGFR3, which activates quiescent NSCs through the ERK/AKT pathway [128]. It is interesting to note that while PDGF, Ang-1, VEGF, and PLGF2 are proangiogenic, PEDF is instead antiangiogenic. Such opposing regulations of angiogenesis that converge in activating NSCs suggest that dynamic neurogenesis occurs in both physiological and pathological situations. Future studies on the synergistic effect of these angiogenic regulators on NSC activation in conjunction with angiogenic sprouting or remodeling in the BBB niche are warranted.
Remodeling of cortex glia during NSC reactivation
In the Drosophila larval CNS, each NSC and its progeny are individually surrounded by cortex glial membrane to form the NSC lineage within the chamber [129]. How can the BBB glial signals reach NSCs if NSC lineages are enclosed by the cortex glia? Speder and Brand showed that in early larval stages, NSCs are not covered by the cortex glial membrane, allowing direct contact between the BBB glia and NSCs [130]. Once NSCs reactivation is completed by 48 hours after larval hatching, the cortex glia chambers are closed at around the same time [130]. The development of cortex glial chambers is also dependent on nutrition—essential amino acids [130]. NSC reactivation drives the formation of cortex glial chambers in both fed and nutritional restriction conditions [130]. The intact cortex glial chambers are crucial for maintaining the survival of newborn neurons, but not NSC survival or proliferation [130].
The role of neurons in NSC reactivation
Innervation of stem cell niches in the V–SVZ and SGZ by projections from proximal and distal neurons have distinct effect in regulating NSC reactivation. In the adult dentate gyrus, the cellular processes of NSCs wrap around the cell bodies of granule neurons and touch and/or ensheathe putative glutamatergic synapses likely formed between mossy cells (MCs), a major type of excitatory neurons, and mature granule cells [131]. Indeed, granule neurons release secreted frizzled-related protein 3 (sFRP3) to maintain NSC quiescence in the SGZ [132]. The differential activation of MCs regulates the balance of quiescence and reactivation of NSCs within the SGZ, in which the direct MC–NSC glutamatergic pathway favors reactivation and indirect MC–interneuron–NSC gamma aminobutyric acid (GABA)ergic pathway favors quiescence [133–135]. In the V–SVZ, a new population of choline acetyltransferase (ChAT)+ neurons release acetylcholine to stimulate NSC proliferation [136]. In addition, serotonergic axons originated from neurons in the raphe nuclei exert a positive effect on the proliferation of NSCs in the V–SVZ [137]. Nitrergic neurons located in close proximity to the adult V–SVZ regulate NSC proliferation in a negative manner [138]. In Drosophila, quiescent NSCs extend their primary cellular extension into the neuropil [8, 15], raising the intriguing possibility that neurons may also function as a niche to regulate Drosophila NSC reactivation.
Intrinsic mechanisms controlling NSC reactivation
Intrinsic mechanisms in certain stem cell subpopulations could play a dominating role in regulating their behavior. Indeed, NSCs from different spatial niches within the V–SVZ reactivate to give rise to neurons that are phenotypically reminiscent of their site of origin even when transplanted heterotopically [139]. Such an intrinsic response is governed by regulators that are often transcription factors, epigenetic modifications, and cell-cycle regulators.
Controlling NSC reactivation by regulators of the InR/PI3K/Akt, BMP, and Hippo pathways
With the discoveries on the roles of various signaling pathways in NSCs and their niche, recent studies have identified regulators of these signaling pathways that are critical for NSC quiescence and reactivation. Heat shock protein 83 (Hsp83), a Hsp90 family molecular chaperone, is an intrinsic regulator of the dInR pathway during NSC reactivation [140]. Hsp83, together with its cochaperone Cell division cycle 37 (Cdc37), facilitates the activation of dInR and promotes NSC reactivation intrinsically [140]. Hsp83 likely binds to dInR in NSCs in a near-native state poised for activation by binding of dILPs [140]. In the presence of dietary amino acids, the expression of hsp83 is dramatically up-regulated, which serves as an additional mechanism for activation of the dInR pathway in NSCs in response to nutritional stimuli [140]. The interaction between Hsp90 and InR is conserved in mammalian systems. In human fibroblasts, Hsp90 promotes insulin signaling in mitogenesis through interaction with intracellular InR β subunit [141]. In mammals, the expression level of Hsp90 in the brain is the highest among all tissues [142]. Hsp90’s clients include α-synuclein in Parkinson's disease and tau in Alzheimer's disease, and therefore, it is heavily implicated in neurodegenerative diseases [143].
Fragile X mental retardation protein (FMRP) is an RNA-binding protein, and its deficiency causes Fragile X syndrome, the most common genetic form of intellectual disability (ID) and autism spectrum disorders (ASDs). Drosophila FMRP is expressed in both NSCs and glial cells, and it prevents NSC reactivation by inhibiting the InR/PI3K/Akt pathway in NSCs and an unknown mechanism in the glia [144, 145]. Like its Drosophila homolog, mammalian fragile X-related protein 2 (FXR2P) inhibits NSC proliferation in the adult hippocampus by up-regulation of BMP signaling [146]. Mammalian FMRP and FXR1P and FXR2P, 2 other proteins from the same family, play distinct regulatory roles in adult neurogenesis, including NSC proliferation, transition from NSCs to intermediate progenitor cells, and neuronal maturation [146–148].
The protein turnover of Lats/Warts, a core protein kinase in the Hippo pathway, is regulated by a Cullin-really interesting new gene (RING) ligase named CRL4Mahjong, an evolutionarily conserved E3 ubiquitin ligase composed of Cullin4 (Cul4), DNA damage-binding protein 1 (DDB1), regulator of cullins-1 (Roc1), and a substrate receptor named Mahjong [149]. Both DDB1 and Mahjong are up-regulated in reactivated NSCs compared with quiescent NSCs and are required for NSC reactivation [149]. Depletion of ddb1 or mahjong in NSCs leads to delayed NSC reactivation and a microcephaly-like phenotype [149]. CRL4Mahjong targets Warts for ubiquitination and degradation, therefore releasing Yorkie into the nucleus to trigger NSC reactivation [149]. The interaction between CRL4 and Warts/Lats is conserved because in human cancer cells, CRL4 E3 ligase activity is increased, leading to the ubiquitination and down-regulation of Lats1/2 [150].
The role of mammalian Hippo pathway and CRL4 complex in NSC reactivation is largely unknown. Upon BMP4-induced mouse adult NSC quiescence, WW and C2 containing domain 2 (WWC2) (Kibra homolog), Lats2 (Warts homolog), and Crumbs2 (Crumbs homolog) are up-regulated [151]. Whether Crumbs activates the hippo pathway to maintain NSC quiescence in mammalian adult brains remains to be determined. Rat Cul4B is highly expressed in mitotic NSCs and its knockdown arrests primary NSCs at G2/M transition [152]. Analogous to the microcephaly-like brains observed in Drosophila ddb1 mutants, in the mouse developing brain, a CNS-specific depletion of DDB1 leads to decreased NSC proliferation and the formation of smaller brains [153]. In zebrafish, the CRL4 complex with a substrate receptor named cereblon (CRBN) controls NSC proliferation and brain size [154, 155]. Zebrafish ddb1- or CRBN-depleted embryos develop smaller brains with a reduction of the number of proliferating cells [154, 155]. Variants of human Cul4B are associated with neurodevelopmental disorders, including X-linked ID, mental retardation, and cortical malformations [156–159].
The opposing roles of the InR/PI3K/Akt and Hippo pathways are coordinated by members of the conserved striatin-interacting phosphatase and kinase (STRIPAK) complex [160]. STRIPAK members are found to have differential expression in quiescent and reactivating NSCs through a transcriptional profiling [160]. microtubule star (mts), the catalytic subunit of protein phosphatase 2A (PP2A), maintains NSC quiescence primarily by inactivating Akt [160]. Two other components of STRIPAK, named monopolar spindle-one-binder family member 4 (mob4) and connector of kinase to AP-1 (cka), promote NSC reactivation by facilitating the association between Mts and Hippo, presumably resulting in the dephosphorylation and inactivation of Hippo [160]. Therefore, the STRIPAK members first turn off the InR/PI3K/Akt pathway to maintain NSC quiescence and subsequently turn off the Hippo pathway to promote NSC reactivation. Interestingly, Cerebral cavernous malformation 3 (Ccm3), a STRIPAK component, is expressed in the CNS BBB and modulates the organization and function of the BBB [161, 162].
Adenomatous polyposis coli (APC) family proteins APC1 and APC2, negative regulators of the Wingless/Wnt pathway, play a redundant role in Drosophila larval NSC reactivation, but loss of both APC1 and APC2 did not seem to result in any accumulation of β-catenin (Armadillo) in NSCs [163]. Whether the Wingless pathway is involved during NSC quiescence and reactivation awaits further investigation.
Transcriptional and epigenetic regulations of NSC reactivation
At the end of embryogenesis, exit of proliferation of Drosophila NSCs is controlled by combined functions between temporal transcriptional factors and spatial regulators such as Hox proteins [12] (Fig 2). Temporal transcriptional factors Pou-domain proteins Pdm1 and Pdm2 (Pdm) prevent NSC quiescence through down-regulation of Nab, as Nab normally induces NSC quiescence with its co-factor Squeeze [12]. Another temporal transcriptional factor, Castor, promotes quiescence by inhibiting Pdm [12] (Fig 2). Differential expression of Hox genes Antennapedia (Antp) and Abdominal-A (Abd-A) is responsible for the different timing of entry into quiescence in different segments [12].
Homeodomain transcription factor Prospero (Pros) is well-known for its role in neural differentiation by directly repressing progenitor and cell-cycle genes [164, 165]. Pros is also capable of driving proliferating NSCs into quiescence when transiently expressed in NSCs [166]. The levels of Pros in the nucleus distinguish Drosophila NSC fates: absence for self-renewal/proliferation, low for quiescence, and high for differentiation [166]. Pros is repressed by spindle matrix proteins composed of Chromator (Chro)/chromo domain protein interacting with Z4 (Chriz), Megator, and enhanced adult sensory threshold (East) that function intrinsically in NSCs to promote NSC reactivation [167] (Fig 2). Chro also promotes the expression of grainy head, which indirectly represses pros expression in NSCs [167]. Chro appears to function downstream of the InR/PI3K pathway during NSC reactivation, although it remains unknown whether Chro is a direct target of the InR/PI3K pathway [167].
Several transcription factors play counterbalancing roles in the regulation of mammalian NSC quiescence and reactivation [168–171]. Achaete-scute homolog 1/mammalian achaete scute homolog 1 (ASCL1/MASH1), a proneural basic helix–loop–helix transcription factor, promotes the activation of quiescent NSCs in both the adult V–SVZ and hippocampus [172]. The expression of Ascl1 in NSCs can be induced by neurogenic stimuli or inactivation of the Notch signaling pathway [172]. Oscillatory or sustained expression of Ascl1 regulated by its repressor HES1, a downstream effector of Notch signaling, determines whether NSCs commit to a renewal or differentiation program, respectively [173, 174]. The ASCL1 protein level is negatively regulated by an E3-ubiquitin ligase, HECT, UBA, and WWE domain-containing 1 (HUWE1), and inhibitor of DNA binding 4 (ID4), which reverses proliferating NSCs back into the quiescent stage [175, 176]. Mutations in the human HUWE1 gene have been linked to X-linked ID [177, 178]. Genetic-screened homeobox 2 (GSX2), a homeodomain transcription factor, and tailless homolog (TLX), an orphan nuclear receptor, also play a critical role in promoting activation of subpopulation of V–SVZ NSCs [170, 179]. On the other hand, repressor element 1-silencing transcription factor (REST) and FoxO transcription factors are required for the maintenance of quiescent NSCs [171, 180–182].
Epigenetic regulations such as chromatin remodeling and histone modifications also play critical roles in regulating NSC behaviors by modulating gene expression in a long-lasting manner without altering genomic sequence [183]. B lymphoma Mo-MLV insertion region 1 homolog (BMI1), a core component of chromatin remodeling complex named polycomb repressive complex 1 (PRC1), controls mammalian NSC proliferation by repressing a cyclin-dependent kinase (CDK) inhibitor p16INK4a [184]. In contrast, chromatin remodeling factor chromodomain-helicase-DNA–binding protein 7 (CHD7) maintains NSC quiescence through repressing the transcription of cyclins and CDKs and promoting the expression of Notch downstream effector Hes5 [185]. Histone H2AX phosphorylation, following GABAA receptor activation, limits V–SVZ NSC proliferation and self-renewal [186]. Histone deacetylase 3 (HDAC3) is important for NSC proliferation by regulating G2/M progression through stabilization of CDK1 [187]. Enhancer of zeste homolog 2 (EZH2), a subunit of PRC2, represses gene expression through H3K27 methylation and promotes NSC proliferation through regulating the PTEN/Akt/mTOR pathway [188].
Molecular signatures, heterogeneity, and cell-cycle regulation of quiescent NSCs
Quiescence of stem cells has long been thought as a dormant state, passively waiting for activating signals [189]. Increasing evidence has changed this long-held paradigm and indicates that quiescence is actively maintained [83, 190]. This active maintenance of quiescence state in NSCs serves as a reserved pool of stem cells that can replace damaged stem cells for long-term somatic cell generation, insulating against risks of stem cell depletion and accumulation of tumorigenic mutations after multiple rounds of cell division [191]. Quiescent NSCs have unique molecular signatures that are distinct from those of proliferative NSCs. Transcriptomics with temporal analysis of molecular interplay during the transitioning of quiescence to activated stage in the SGZ and V–SVZ are revealed by using bulk and single-cell RNA sequencing (RNA-seq) [83, 99, 192, 193]. Quiescent NSCs in both niches have enriched expression of genes involved in cell–cell adhesion and cell–microenvironment interaction, suggesting that intrinsic and extrinsic signals are actively involved in maintaining stem cell quiescence [99, 193].
Using single-cell RNA-seq, quiescent NSCs in the V–SVZ are found to be heterogeneous and can be further subclassified into dormant state (qNSC1) and primed-quiescent state (qNSC2), with the latter being a transitory state in which genes involving protein synthesis and the cell cycle are up-regulated in preparation for subsequent reactivation [83]. A similar preactivation stage can be found in quiescent NSCs in the SGZ, in which protein translation capacity is up-regulated [193]. On the other hand, activated NSCs can be further subclassified into nondividing aNSC1 and dividing aNSC2, demonstrating that there exists a quiescent-activated continuum rather than a binary state [83]. From a metabolic perspective, the activation of quiescent NSCs involves transitioning from lipid metabolism, specifically glycolytic metabolism and fatty acid oxidation, to oxidative metabolism in the mitochondria [83, 193, 194]. While reactive oxygen species (ROS) are closely related to mitochondrial respiration, a study by Le Belle and colleagues showed that NADPH oxidase (NOX)-derived ROS enhance the shift of quiescent to proliferating NSCs as well as neurogenesis [195]. Whether mitochondrial-derived ROS play a role, if any, in enhancing this shift remains to be elucidated.
NSC heterogeneity has been the subject of immense study to categorize them into different matrices, e.g., morphology, site of origin, molecular signatures, etc., with important implications for understanding differential neurogenic capabilities among NSCs [196–198]. Within the SGZ, 2 variants of quiescent NSCs with distinct morphologies respond selectively to extrinsic stimulations, as physical exercise activates only radial NSCs, while seizure activates both radial and horizontal NSCs [199]. A recent study by Morizur and colleagues found that quiescent NSCs located in the V–SVZ display membrane receptors that are distinct from the activated NSCs and that niche signaling could be important in maintaining such a heterogenous population [99]. On the other hand, positional heterogeneity among NSCs within the V–SVZ is maintained even when they are grafted heterotopically or grown in vitro, implying that an inherent “memory” could be imprinted that may persist even when the external environment is changed [139].
It was widely believed that quiescent stem cells, including mammalian quiescent NSCs, arrest in the G0 stage. However, a recent study from Andrea Brand’s laboratory challenged this dogma by reporting that in the Drosophila VNC, the majority of quiescent NSCs (approximately 75%) arrest in the G2 stage, while the remaining approximately 25% of quiescent NSCs are in G0 [200]. An evolutionarily conserved pseudokinase, Tribbles, induces G2 NSCs to enter quiescence during late embryogenesis by targeting Cdc25String for degradation [200]. During larval stages, Tribbles maintains G2 NSC quiescence by blocking Akt activation [200]. Activating the insulin pathway by overexpressing the activated form of Akt in NSCs represses the tribbles transcription, triggering NSC reactivation [200]. Compared with G0-arrested cells, G2 quiescent cells can reactivate more quickly in response to nutritional stimulus [200]. They also have the advantage of maintaining genomic integrity via high-fidelity homologous-recombination–mediated repair in response to DNA damage [200].
Whether quiescent NSCs arrest at the G2 or G0 stage is determined by a CDK inhibitor Dacapo (Dap)/p57KIP2 at the end of embryogenesis [201]. Dap directs NSCs to enter G0 quiescence, and loss of dap resulted in NSCs switching from G0 to G2 quiescence [201]. The G2/G0 quiescent NSCs have distinct spatial distribution, with G0 NSCs primarily occupying dorsal regions of the CNS and G2 NSCs primarily occupying ventral regions [201]. However, there is no bias for G2/G0 quiescent NSCs along the anterior–posterior axis [201]. These observations pose an interesting possibility that dorsal–ventral patterning factors may influence the choice between G2 and G0 quiescence. Indeed, the dorsal patterning transcription factor Muscle segment homeobox (Msh) directly binds to the enhancer sequence of dap, which is known to be sufficient for dap expression in the embryonic CNS, to promote G0 quiescence in a subset of dorsal NSCs [201, 202]. On the contrary, the ventral patterning factor ventral nervous system defective (Vnd), which is expressed in G2 quiescent NSCs that are located ventrally, does not have a role in promoting G0 quiescence [201].
The precise modulation of proliferation program in the activation of NSCs is important in maintaining the stem cell pool and generating differentiated neurons. One of the key differentiating hallmarks of quiescent and activated NSCs is the cell-cycle activity. In the mammalian brain, CDK-inhibitory proteins (CDKIs)/kinase inhibitory proteins (KIPs), i.e. p21cip1/waf1, p27kip1, and p57kip2, play the role of a molecular brake on the cell cycle during the G1 to S transition because their reduction leads to the activation of the proliferation program [203–205]. However, persistent abrogation of p21cip1/waf1 and p57kip2 ultimately leads to NSC exhaustion and impaired neurogenesis [203, 205]. As alluded earlier, the role of Dap, the Drosophila ortholog of p57kip2, in the spatial regulation of NSC quiescence demonstrates the conserved role of CDKIs in the negative regulation of NSC activation [206]. p16INK4a, another CDKI of the CDK-inhibitory protein/inhibitory protein of CDK4 (INK4) family—the expression of which increases in age—acts as a negative regulator of NSC activation in the V–SVZ only under the presence of neurogenic stimuli such as running [207]. Further studies on the remaining yet-to-be characterized CDKIs, i.e., p15INK4b, p18INK4c, and p19INK4d, in both Drosophila and mammalian systems will shed light on the possible interplay of these CDKIs in regulating NSC quiescence. Besides CDKIs, the tumor suppressor gene p53 also acts as an additional layer of regulation on NSC proliferation because the loss of p53 leads to radical activation of quiescent NSC in the V–SVZ [208]. Given that p53 is a key regulator of a variety of cellular processes, e.g., metabolism, senescence, etc., that are intimately linked to NSC activation, a holistic approach in studying how p53 might affect downstream activation genes is warranted [209].
Conclusions and future perspectives
Drosophila represents an invaluable model system for in-depth dissection of molecular mechanisms underlying NSC quiescence and reactivation because of the conserved regulatory pathways shared with the mammalian brain and the availability of an arsenal of powerful genetic tools [13]. Future studies in mammalian systems on the conserved nature of the intrinsic regulators of NSC reactivation discovered in Drosophila will shed light on how these regulators might modulate stem cell behavior in a more complex system, with important implications in understanding neurological disorders and potential targets for therapeutic purposes. An emerging theme from the host of studies on molecular players governing NSC reactivation in Drosophila and mammalian system presented in this review is the complex, precise, and intricate balancing of quiescence and reactivation of NSCs within the neurogenic niche that allows them to respond to changes in the external environment and also the intrinsic development/aging clock in producing appropriate number of neurons while maintaining a stem cell pool for long-term neurogenesis. Thus, the dysregulation of these molecular players may result in neurodevelopmental diseases. A systems biology approach in understanding how NSCs reconcile and integrate the barrage of seemingly conflicting regulatory signals into a binary decision of quiescence or reactivation might prove useful in understanding the biology of NSC reactivation and the heterogeneity that exists within the NSC population.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang Y-Z, Plane JM, Jiang P, Zhou CJ, Deng W. Concise Review: Quiescent and Active States of Endogenous Adult Neural Stem Cells: Identification and Characterization. STEM CELLS. 2011;29(6):907–12. 10.1002/stem.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10(2):59–66. 10.1007/s12017-008-8031-4 . [DOI] [PubMed] [Google Scholar]
- 3.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20(1):1–17. Epub 2009 Sept 11. 10.1016/j.euroneuro.2009.08.003 . [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227(2):497–507. Epub 2011 Oct 18. 10.1016/j.bbr.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain. Cell. 1999;97(6):703–16. 10.1016/s0092-8674(00)80783-7 . [DOI] [PubMed] [Google Scholar]
- 6.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. Journal of Neuroscience. 2001;21(18):7153–60. 10.1523/JNEUROSCI.21-18-07153.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartenstein V, Rudloff E, Campos-Ortega JA. The pattern of proliferation of the neuroblasts in the wild-type embryo of Drosophila melanogaster. Roux's archives of developmental biology: the official organ of the EDBO. 1987;196(8):473–85. 10.1007/BF00399871 . [DOI] [PubMed] [Google Scholar]
- 8.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125(1):145–57. 10.1016/0012-1606(88)90067-x . [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Developmental Biology. 1992;149(1):134–48. 10.1016/0012-1606(92)90270-q [DOI] [PubMed] [Google Scholar]
- 10.Prokop A, Bray S, Harrison E, Technau GM. Homeotic regulation of segment-specific differences in neuroblast numbers and proliferation in the Drosophila central nervous system. Mechanisms of development. 1998;74(1–2):99–110. 10.1016/s0925-4773(98)00068-9 . [DOI] [PubMed] [Google Scholar]
- 11.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264(5159):677–83. 10.1126/science.8171319 . [DOI] [PubMed] [Google Scholar]
- 12.Tsuji T, Hasegawa E, Isshiki T. Neuroblast entry into quiescence is regulated intrinsically by the combined action of spatial Hox proteins and temporal identity factors. Development. 2008;135(23):3859–69. 10.1242/dev.025189 . [DOI] [PubMed] [Google Scholar]
- 13.Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139(23):4297–310. 10.1242/dev.080515 . [DOI] [PubMed] [Google Scholar]
- 14.Britton JS, Edgar Ba. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development (Cambridge, England). 1998;125(11):2149–58. [DOI] [PubMed] [Google Scholar]
- 15.Chell JM, Brand AH. Nutrition-Responsive Glia Control Exit of Neural Stem Cells from Quiescence. Cell. 2010;143(7):1161–73. 10.1016/j.cell.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114(6):739–49. 10.1016/s0092-8674(03)00713-x . [DOI] [PubMed] [Google Scholar]
- 17.Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10(3):199–207. 10.1016/j.cmet.2009.08.002 . [DOI] [PubMed] [Google Scholar]
- 18.Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471(7339):508–12. 10.1038/nature09867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–17. 10.1083/jcb.34.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brown NM, Pfau SJ, Gu C. Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev. 2018;32(7–8):466–78. 10.1101/gad.309823.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Bock M, Wang N, Decrock E, Bol M, Gadicherla AK, Culot M, et al. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog Neurobiol. 2013;108:1–20. 10.1016/j.pneurobio.2013.06.001 . [DOI] [PubMed] [Google Scholar]
- 23.Campos-Bedolla P, Walter FR, Veszelka S, Deli MA. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch Med Res. 2014;45(8):610–38. 10.1016/j.arcmed.2014.11.018 . [DOI] [PubMed] [Google Scholar]
- 24.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–6. 10.1038/nature13241 . [DOI] [PubMed] [Google Scholar]
- 25.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. 10.1038/nrn1824 . [DOI] [PubMed] [Google Scholar]
- 26.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47(7):809–13. 10.1038/ng.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47(7):814–7. 10.1038/ng.3313 . [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Gaviro MV, Scott CE, Sesay AK, Matheu A, Booth S, Galichet C, et al. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proceedings of the National Academy of Sciences. 2012;109(4):1317–22. 10.1073/pnas.1016199109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado AC, Ferrón SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, et al. Endothelial NT-3 Delivered by Vasculature and CSF Promotes Quiescence of Subependymal Neural Stem Cells through Nitric Oxide Induction. Neuron. 2014;83(3):572–85. 10.1016/j.neuron.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 30.Carreira BP, Morte MI, Inácio Â, Costa G, Rosmaninho-Salgado J, Agasse F, et al. Nitric Oxide Stimulates the Proliferation of Neural Stem Cells Bypassing the Epidermal Growth Factor Receptor. Stem Cells. 2010;28(7):1219–30. 10.1002/stem.444 [DOI] [PubMed] [Google Scholar]
- 31.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–4. 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LK, He Y, Park J-S, Bieri G, Snethlage CE, Lin K, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nature Medicine. 2015;21(8):932–7. 10.1038/nm.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299(5603):117–20. 10.1126/science.1076647 . [DOI] [PubMed] [Google Scholar]
- 35.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin Regulates the In Vitro and In Vivo Production of Neuronal Progenitors by Mammalian Forebrain Neural Stem Cells. Journal of Neuroscience. 2001;21(24):9733–43. 10.1523/JNEUROSCI.21-24-09733.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iadecola C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96(1):17–42. 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29(11):3538–50. 10.1523/JNEUROSCI.5564-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–58. 10.1146/annurev.neuro.30.051606.094345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28(3):587–97. 10.1523/JNEUROSCI.4367-07.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limmer S, Weiler A, Volkenhoff A, Babatz F, Klambt C. The Drosophila blood-brain barrier: development and function of a glial endothelium. Front Neurosci. 2014;8:365 10.3389/fnins.2014.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. Epub 2009 Aug 5. 10.1016/j.nbd.2009.07.030 . [DOI] [PubMed] [Google Scholar]
- 42.Freeman MR. Drosophila Central Nervous System Glia. Cold Spring Harb Perspect Biol. 2015;7(11):a020552 10.1101/cshperspect.a020552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res. 1994;42(4–5):145–51. 10.1159/000184187 . [DOI] [PubMed] [Google Scholar]
- 44.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11(4):213–21. 10.1016/s0960-9822(01)00068-9 . [DOI] [PubMed] [Google Scholar]
- 45.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336(6081):579–82. 10.1126/science.1216735 . [DOI] [PubMed] [Google Scholar]
- 46.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12(15):1293–300. 10.1016/s0960-9822(02)01043-6 . [DOI] [PubMed] [Google Scholar]
- 47.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296(5570):1118–20. 10.1126/science.1070058 . [DOI] [PubMed] [Google Scholar]
- 48.Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125(11):2149–58. . [DOI] [PubMed] [Google Scholar]
- 49.Spéder P, Brand Andrea H. Gap Junction Proteins in the Blood-Brain Barrier Control Nutrient-Dependent Reactivation of Drosophila Neural Stem Cells. Developmental Cell. 2014;30(3):309–21. 10.1016/j.devcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524(1):2–15. 10.1016/j.abb.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. 10.1038/417039a . [DOI] [PubMed] [Google Scholar]
- 52.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain research Developmental brain research. 2002;134(1–2):115–22. 10.1016/s0165-3806(02)00277-8 . [DOI] [PubMed] [Google Scholar]
- 53.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends in endocrinology and metabolism: TEM. 2005;16(2):59–65. 10.1016/j.tem.2005.01.008 . [DOI] [PubMed] [Google Scholar]
- 54.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology. 1979;105(3):666–73. 10.1210/endo-105-3-666 . [DOI] [PubMed] [Google Scholar]
- 55.Joseph D'Ercole A, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149(12):5958–62. 10.1210/en.2008-0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PF. Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2199–203. 10.1073/pnas.88.6.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O'Kusky JR, et al. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci. 2004;19(8):2056–68. 10.1111/j.0953-816X.2004.03320.x . [DOI] [PubMed] [Google Scholar]
- 58.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. European journal of pharmacology. 2004;490(1–3):25–31. 10.1016/j.ejphar.2004.02.042 . [DOI] [PubMed] [Google Scholar]
- 59.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(8):2896–903. 10.1523/JNEUROSCI.20-08-02896.2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D'Ercole AJ, Ye P, O'Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36(2–3):209–20. 10.1054/npep.2002.0893 . [DOI] [PubMed] [Google Scholar]
- 61.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, et al. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63(6):761–73. 10.1016/j.neuron.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ka M, Condorelli G, Woodgett JR, Kim WY. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141(21):4076–86. 10.1242/dev.108282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–39. 10.1016/j.stem.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540–53. 10.1016/j.stem.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Molecular and cellular neurosciences. 2003;24(1):23–40. 10.1016/s1044-7431(03)00082-4 . [DOI] [PubMed] [Google Scholar]
- 66.Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. The European journal of neuroscience. 2006;24(1):45–54. 10.1111/j.1460-9568.2006.04872.x . [DOI] [PubMed] [Google Scholar]
- 67.Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J, et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci. 2012;32(10):3376–87. 10.1523/JNEUROSCI.4248-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, et al. Brain Development and Akt Signaling: the Crossroads of Signaling Pathway and Neurodevelopmental Diseases. Journal of Molecular Neuroscience. 2017;61(3):379–84. 10.1007/s12031-016-0872-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juanes M, Guercio G, Marino R, Berensztein E, Warman DM, Ciaccio M, et al. Three novel IGF1R mutations in microcephalic patients with prenatal and postnatal growth impairment. Clinical endocrinology. 2015;82(5):704–11. 10.1111/cen.12555 . [DOI] [PubMed] [Google Scholar]
- 70.Boland E, Clayton-Smith J, Woo VG, McKee S, Manson FDC, Medne L, et al. Mapping of Deletion and Translocation Breakpoints in 1q44 Implicates the Serine/Threonine Kinase AKT3 in Postnatal Microcephaly and Agenesis of the Corpus Callosum. The American Journal of Human Genetics. 2007;81(2):292–303. 10.1086/519999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes & Development. 2009;23(14):1619–24. 10.1101/gad.1799609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/Protein Kinase Bγ in Attainment of Normal Brain Size. Molecular and Cellular Biology. 2005;25:1869–78. 10.1128/MCB.25.5.1869-1878.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tschopp O, Yang Z-Z, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, et al. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. 10.1242/dev.01864 [DOI] [PubMed] [Google Scholar]
- 74.Cloëtta D, Thomanetz V, Baranek C, Lustenberger RM, Lin S, Oliveri F, et al. Inactivation of mTORC1 in the Developing Brain Causes Microcephaly and Affects Gliogenesis. The Journal of Neuroscience. 2013;33:7799–810. 10.1523/JNEUROSCI.3294-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ka M, Condorelli G, Woodgett JR, Kim W-Y. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141:4076–86. 10.1242/dev.108282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen LA, Mirzaa GM, Ishak GE, O'Roak BJ, Hiatt JB, Roden WH, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138(6):1613–28. 10.1093/brain/awv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tokuda S, Mahaffey CL, Monks B, Faulkner CR, Birnbaum MJ, Danzer SC, et al. A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Human Molecular Genetics. 2010;20(5):988–99. 10.1093/hmg/ddq544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative Regulation of Neural Stem/Progenitor Cell Proliferation by the <em>Pten</em> Tumor Suppressor Gene in Vivo. Science. 2001;294(5549):2186 10.1126/science.1065518 [DOI] [PubMed] [Google Scholar]
- 79.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming G-l, et al. In Vivo Clonal Analysis Reveals Self-Renewing and Multipotent Adult Neural Stem Cell Characteristics. Cell. 2011;145(7):1142–55. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J Neurosci. 2003;23(8):3325–35. 10.1523/JNEUROSCI.23-08-03325.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanai MI, Kim MJ, Akiyama T, Takemura M, Wharton K, O'Connor MB, et al. Regulation of neuroblast proliferation by surface glia in the Drosophila larval brain. Sci Rep. 2018;8(1):3730 10.1038/s41598-018-22028-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–26. 10.1016/s0896-6273(00)00148-3 . [DOI] [PubMed] [Google Scholar]
- 83.Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell. 2015;17(3):329–40. 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 84.Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, et al. Signaling through BMPR-IA Regulates Quiescence and Long-Term Activity of Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell. 2010;7(1):78–89. 10.1016/j.stem.2010.04.016 . [DOI] [PubMed] [Google Scholar]
- 85.Bonaguidi MA, Peng C-Y, McGuire T, Falciglia G, Gobeske KT, Czeisler C, et al. Noggin Expands Neural Stem Cells in the Adult Hippocampus. Journal of Neuroscience. 2008;28(37):9194–204. 10.1523/JNEUROSCI.3314-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Developmental dynamics: an official publication of the American Association of Anatomists. 2002;224(4):403–12. 10.1002/dvdy.10120 . [DOI] [PubMed] [Google Scholar]
- 87.Datta S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development. 1995;121(4):1173–82. . [DOI] [PubMed] [Google Scholar]
- 88.Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, et al. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253(2):247–57. 10.1016/s0012-1606(02)00019-2 . [DOI] [PubMed] [Google Scholar]
- 89.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64(4):841–8. 10.1016/0092-8674(91)90512-w . [DOI] [PubMed] [Google Scholar]
- 90.Kerever A, Mercier F, Nonaka R, de Vega S, Oda Y, Zalc B, et al. Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Research. 2014;12(2):492–505. 10.1016/j.scr.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;18(19):7869–80. 10.1523/JNEUROSCI.18-19-07869.1998 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, et al. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19(9):3287–97. 10.1523/JNEUROSCI.19-09-03287.1999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31(3):560–73. 10.1016/j.mcn.2005.11.010 . [DOI] [PubMed] [Google Scholar]
- 94.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271(25):15292–7. 10.1074/jbc.271.25.15292 . [DOI] [PubMed] [Google Scholar]
- 95.Morante-Redolat JM, Porlan E. Neural Stem Cell Regulation by Adhesion Molecules Within the Subependymal Niche. Frontiers in Cell and Developmental Biology. 2019;7:189 10.3389/fcell.2019.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segarra M, Aburto MR, Cop F, Llaó-Cid C, Härtl R, Damm M, et al. Endothelial Dab1 signaling orchestrates neuro-glia-vessel communication in the central nervous system. Science. 2018;361(6404):eaao2861 10.1126/science.aao2861 . [DOI] [PubMed] [Google Scholar]
- 97.Shen Q, Wang Y, Kokovay E, Lin G, Chuang S-M, Goderie SK, et al. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell. 2008;3(3):289–300. 10.1016/j.stem.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, et al. Quiescence and Activation of Stem and Precursor Cell Populations in the Subependymal Zone of the Mammalian Brain Are Associated with Distinct Cellular and Extracellular Matrix Signals. Journal of Neuroscience. 2010;30(29):9771–81. 10.1523/JNEUROSCI.0700-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morizur L, Chicheportiche A, Gauthier LR, Daynac M, Boussin FD, Mouthon M-A. Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment. Stem Cell Reports. 2018;11(2):565–77. 10.1016/j.stemcr.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, et al. Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling. Cell Stem Cell. 2010;7(2):163–73. 10.1016/j.stem.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–7. Epub 2002 Dec 2. 10.1038/nn983 . [DOI] [PubMed] [Google Scholar]
- 102.Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci. 2002;16(12):2351–7. 10.1046/j.1460-9568.2002.02412.x . [DOI] [PubMed] [Google Scholar]
- 103.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–7. 10.1038/nature03994 . [DOI] [PubMed] [Google Scholar]
- 104.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–5. 10.1038/nature04108 . [DOI] [PubMed] [Google Scholar]
- 105.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–31. 10.1016/j.cell.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Topol A, Zhu S, Tran N, Simone A, Fang G, Brennand KJ. Altered WNT Signaling in Human Induced Pluripotent Stem Cell Neural Progenitor Cells Derived from Four Schizophrenia Patients. Biol Psychiatry. 2015;78(6):e29–34. 10.1016/j.biopsych.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone Marrow-Derived Human Mesenchymal Stem Cells Become Quiescent on Soft Substrates but Remain Responsive to Chemical or Mechanical Stimuli. Tissue Engineering Part A. 2008;15(1):147–54. 10.1089/ten.tea.2007.0388 [DOI] [PubMed] [Google Scholar]
- 108.Ebens AJ, Garren H, Cheyette BN, Zipursky SL. The Drosophila anachronism locus: a glycoprotein secreted by glia inhibits neuroblast proliferation. Cell. 1993;74(1):15–27. 10.1016/0092-8674(93)90291-w . [DOI] [PubMed] [Google Scholar]
- 109.Ding R, Weynans K, Bossing T, Barros CS, Berger C. The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nature communications. 2016;7:10510 10.1038/ncomms10510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pan D. The hippo signaling pathway in development and cancer. Developmental cell. 2010;19(4):491–505. 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nature reviews Cancer. 2007;7(3):182–91. 10.1038/nrc2070 . [DOI] [PubMed] [Google Scholar]
- 113.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. 10.1242/dev.045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poon CL, Mitchell KA, Kondo S, Cheng LY, Harvey KF. The Hippo Pathway Regulates Neuroblasts and Brain Size in Drosophila melanogaster. Current biology: CB. 2016;26(8):1034–42. 10.1016/j.cub.2016.02.009 . [DOI] [PubMed] [Google Scholar]
- 115.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. The EMBO Journal. 1999;18(8):2196–207. 10.1093/emboj/18.8.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nature Cell Biology. 2014;16(11):1045–56. 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Breunig JJ, Silbereis J, Vaccarino FM, Šestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20558–63. 10.1073/pnas.0710156104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ables JL, DeCarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, et al. Notch1 Is Required for Maintenance of the Reservoir of Adult Hippocampal Stem Cells. The Journal of Neuroscience. 2010;30(31):10484–92. 10.1523/JNEUROSCI.4721-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ehm O, Göritz C, Covic M, Schäffner I, Schwarz TJ, Karaca E, et al. RBPJκ-Dependent Signaling Is Essential for Long-Term Maintenance of Neural Stem Cells in the Adult Hippocampus. The Journal of Neuroscience. 2010;30(41):13794–807. 10.1523/JNEUROSCI.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawaguchi D, Furutachi S, Kawai H, Hozumi K, Gotoh Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nature Communications. 2013;4(1):1880 10.1038/ncomms2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer-Zirnsak B, Segebrecht L, Schubach M, Charles P, Alderman E, Brown K, et al. Haploinsufficiency of the Notch Ligand DLL1 Causes Variable Neurodevelopmental Disorders. The American Journal of Human Genetics. 2019;105(3):631–9. 10.1016/j.ajhg.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. Journal of Comparative Neurology. 2000;425(4):479–94. [DOI] [PubMed] [Google Scholar]
- 123.Jackson EL, García-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, et al. PDGFRα-Positive B Cells Are Neural Stem Cells in the Adult SVZ that Form Glioma-like Growths in Response to Increased PDGF Signaling. Neuron. 2006;51(2):187–99. 10.1016/j.neuron.2006.06.012 . [DOI] [PubMed] [Google Scholar]
- 124.Ramírez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, et al. Pigment epithelium–derived factor is a niche signal for neural stem cell renewal. Nature Neuroscience. 2006;9(3):331–9. 10.1038/nn1657 B45119F9-8C6D-4F0F-B8F4-7C09143E2EC6. [DOI] [PubMed] [Google Scholar]
- 125.Rosa AI, Gonçalves J, Cortes L, Bernardino L, Malva JO, Agasse F. The Angiogenic Factor Angiopoietin-1 Is a Proneurogenic Peptide on Subventricular Zone Stem/Progenitor Cells. The Journal of Neuroscience. 2010;30(13):4573 10.1523/JNEUROSCI.5597-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin R, Cai J, Kenyon L, Iozzo R, Rosenwasser R, Iacovitti L. Systemic Factors Trigger Vasculature Cells to Drive Notch Signaling and Neurogenesis in Neural Stem Cells in the Adult Brain. Stem Cells. 2018;37(3):395–406. 10.1002/stem.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crouch EE, Liu C, Silva-Vargas V, Doetsch F. Regional and Stage-Specific Effects of Prospectively Purified Vascular Cells on the Adult V-SVZ Neural Stem Cell Lineage. Journal of Neuroscience. 2015;35(11):4528–39. 10.1523/JNEUROSCI.1188-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han J, Calvo C-F, Kang TH, Baker KL, Park J-H, Parras C, et al. Vascular Endothelial Growth Factor Receptor 3 Controls Neural Stem Cell Activation in Mice and Humans. Cell Reports. 2015;10(7):1158–72. 10.1016/j.celrep.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pereanu W, Shy D, Hartenstein V. Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev Biol. 2005;283(1):191–203. 10.1016/j.ydbio.2005.04.024 . [DOI] [PubMed] [Google Scholar]
- 130.Spéder P, Brand AH. Systemic and local cues drive neural stem cell niche remodelling during neurogenesis in Drosophila. eLife. 2018;7:e30413 10.7554/eLife.30413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moss J, Gebara E, Bushong EA, Sánchez-Pascual I, O’Laoi R, El M’Ghari I, et al. Fine processes of Nestin-GFP–positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proceedings of the National Academy of Sciences. 2016;113:E2536–E45. 10.1073/pnas.1514652113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jang M-H, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, et al. Secreted Frizzled-Related Protein 3 Regulates Activity-Dependent Adult Hippocampal Neurogenesis. Cell Stem Cell. 2013;12(2):215–23. 10.1016/j.stem.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeh C-Y, Asrican B, Moss J, Quintanilla LJ, He T, Mao X, et al. Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron. 2018;99(3):493–510. 10.1016/j.neuron.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bao H, Asrican B, Li W, Gu B, Wen Z, Lim S-A, et al. Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis. Cell Stem Cell. 2017;21(5):604–17. 10.1016/j.stem.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489(7414):150–4. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paez-Gonzalez P, Asrican B, Rodriguez E, Kuo CT. Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nature Neuroscience. 2014;17(7):934–42. 10.1038/nn.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tong Cheuk K, Chen J, Cebrián-Silla A, Mirzadeh Z, Obernier K, Guinto Cristina D, et al. Axonal Control of the Adult Neural Stem Cell Niche. Cell Stem Cell. 2014;14(4):500–11. 10.1016/j.stem.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romero-Grimaldi C, Moreno-López B, Estrada C. Age-dependent effect of nitric oxide on subventricular zone and olfactory bulb neural precursor proliferation. Journal of Comparative Neurology. 2008;506(2):339–46. 10.1002/cne.21556 [DOI] [PubMed] [Google Scholar]
- 139.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic Organization of Neural Stem Cells in the Adult Brain. Science. 2007;317(5836):381–4. 10.1126/science.1144914 [DOI] [PubMed] [Google Scholar]
- 140.Huang J, Wang H. Hsp83/Hsp90 Physically Associates with Insulin Receptor to Promote Neural Stem Cell Reactivation. Stem cell reports. 2018;11(4):883–896. 10.1016/j.stemcr.2018.08.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takata Y, Imamura T, Iwata M, Usui I, Haruta T, Nandachi N, et al. Functional importance of heat shock protein 90 associated with insulin receptor on insulin-stimulated mitogenesis. Biochemical and biophysical research communications. 1997;237(2):345–7. 10.1006/bbrc.1997.7116 . [DOI] [PubMed] [Google Scholar]
- 142.Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280(6):1381–96. 10.1111/febs.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353–71. Epub 2014 Sept 25. 10.1146/annurev-pharmtox-010814-124332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Callan MA, Clements N, Ahrendt N, Zarnescu DC. Fragile X Protein is required for inhibition of insulin signaling and regulates glial-dependent neuroblast reactivation in the developing brain. Brain Res. 2012;1462:151–61. 10.1016/j.brainres.2012.03.042 . [DOI] [PubMed] [Google Scholar]
- 145.Callan MA, Cabernard C, Heck J, Luois S, Doe CQ, Zarnescu DC. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010;19(15):3068–79. 10.1093/hmg/ddq213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo W, Zhang L, Christopher DM, Teng ZQ, Fausett SR, Liu C, et al. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron. 2011;70(5):924–38. 10.1016/j.neuron.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo W, Polich ED, Su J, Gao Y, Christopher DM, Allan AM, et al. Fragile X Proteins FMRP and FXR2P Control Synaptic GluA1 Expression and Neuronal Maturation via Distinct Mechanisms. Cell Rep. 2015;11(10):1651–66. 10.1016/j.celrep.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saffary R, Xie Z. FMRP regulates the transition from radial glial cells to intermediate progenitor cells during neocortical development. J Neurosci. 2011;31(4):1427–39. 10.1523/JNEUROSCI.4854-10.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ly PT, Tan YS, Koe CT, Zhang Y, Xie G, Endow S, et al. CRL4Mahj E3 ubiquitin ligase promotes neural stem cell reactivation. PLoS Biol. 2019;17(6):e3000276 10.1371/journal.pbio.3000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26(1):48–60. 10.1016/j.ccr.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martynoga B, Mateo JL, Zhou B, Andersen J, Achimastou A, Urban N, et al. Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev. 2013;27(16):1769–86. 10.1101/gad.216804.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu HC, Enikolopov G, Chen Y. Cul4B regulates neural progenitor cell growth. BMC Neurosci. 2012;13:112 10.1186/1471-2202-13-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cang Y, Zhang J, Nicholas SA, Bastien J, Li B, Zhou P, et al. Deletion of DDB1 in mouse brain and lens leads to p53-dependent elimination of proliferating cells. Cell. 2006;127(5):929–40. 10.1016/j.cell.2006.09.045 . [DOI] [PubMed] [Google Scholar]
- 154.Hu Z, Holzschuh J, Driever W. Loss of DDB1 Leads to Transcriptional p53 Pathway Activation in Proliferating Cells, Cell Cycle Deregulation, and Apoptosis in Zebrafish Embryos. PLoS ONE. 2015;10(7):e0134299 10.1371/journal.pone.0134299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ando H, Sato T, Ito T, Yamamoto J, Sakamoto S, Nitta N, et al. Cereblon Control of Zebrafish Brain Size by Regulation of Neural Stem Cell Proliferation. iScience. 2019;15:95–108. 10.1016/j.isci.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vulto-van Silfhout AT, Nakagawa T, Bahi-Buisson N, Haas SA, Hu H, Bienek M, et al. Variants in CUL4B are associated with cerebral malformations. Hum Mutat. 2015;36(1):106–17. Epub 2014 Nov 11. 10.1002/humu.22718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Badura-Stronka M, Jamsheer A, Materna-Kiryluk A, Sowinska A, Kiryluk K, Budny B, et al. A novel nonsense mutation in CUL4B gene in three brothers with X-linked mental retardation syndrome. Clin Genet. 2010;77(2):141–4. Epub 2009 Dec 10. 10.1111/j.1399-0004.2009.01331.x . [DOI] [PubMed] [Google Scholar]
- 158.Tarpey PS, Raymond FL, O'Meara S, Edkins S, Teague J, Butler A, et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am J Hum Genet. 2007;80(2):345–52. 10.1086/511134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zou Y, Liu Q, Chen B, Zhang X, Guo C, Zhou H, et al. Mutation in CUL4B, which encodes a member of cullin-RING ubiquitin ligase complex, causes X-linked mental retardation. Am J Hum Genet. 2007;80(3):561–6. 10.1086/512489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gil-Ranedo J, Gonzaga E, Jaworek KJ, Berger C, Bossing T, Barros CS. STRIPAK Members Orchestrate Hippo and Insulin Receptor Signaling to Promote Neural Stem Cell Reactivation. Cell reports. 2019;27(10):2921–33 e5. 10.1016/j.celrep.2019.05.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hwang J, Pallas DC. STRIPAK complexes: structure, biological function, and involvement in human diseases. Int J Biochem Cell Biol. 2014;47:118–48. Epub 2013 Dec 11. 10.1016/j.biocel.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pagenstecher A, Stahl S, Sure U, Felbor U. A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet. 2009;18(5):911–8. Epub 2008 Dec 16. 10.1093/hmg/ddn420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Akong K, McCartney BM, Peifer M. Drosophila APC2 and APC1 have overlapping roles in the larval brain despite their distinct intracellular localizations. Dev Biol. 2002;250(1):71–90. 10.1006/dbio.2002.0777 . [DOI] [PubMed] [Google Scholar]
- 164.Chu-Lagraff Q, Wright DM, McNeil LK, Doe CQ. The prospero gene encodes a divergent homeodomain protein that controls neuronal identity in Drosophila. Development. 1991;Suppl 2:79–85. . [PubMed] [Google Scholar]
- 165.Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, et al. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11:775–89. 10.1016/j.devcel.2006.09.015 . [DOI] [PubMed] [Google Scholar]
- 166.Lai S-L, Doe CQ. Transient nuclear Prospero induces neural progenitor quiescence. eLife. 2014;3:e03363 10.7554/elife.03363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li S, Koe CT, Tay ST, Tan ALK, Zhang S, Zhang Y, et al. An intrinsic mechanism controls reactivation of neural stem cells by spindle matrix proteins. Nature Communications. 2017;8(1):122 10.1038/s41467-017-00172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.López-Juárez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, et al. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes & Development. 2013;27(11):1272–87. 10.1101/gad.217539.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Andersen J, Urbán N, Achimastou A, Ito A, Simic M, Ullom K, et al. A Transcriptional Mechanism Integrating Inputs from Extracellular Signals to Activate Hippocampal Stem Cells. Neuron. 2014;83(5):1085–97. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Niu W, Zou Y, Shen C, Zhang C-L. Activation of Postnatal Neural Stem Cells Requires Nuclear Receptor TLX. Journal of Neuroscience. 2011;31(39):13816–28. 10.1523/JNEUROSCI.1038-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mukherjee S, Brulet R, Zhang L, Hsieh J. REST regulation of gene networks in adult neural stem cells. Nature Communications. 2016;7(1):13360 EP -. 10.1038/ncomms13360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, et al. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 2014;83(5):1085–97. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342(6163):1203–8. 10.1126/science.1242366 . [DOI] [PubMed] [Google Scholar]
- 174.Sueda R, Imayoshi I, Harima Y, Kageyama R. High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes & Development. 2019;33(9–10):511–23. 10.1101/gad.323196.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Urbán N, van den Berg DLC, Forget A, Andersen J, Demmers JAA, Hunt C, et al. Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science. 2016;353(6296):292–5. 10.1126/science.aaf4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blomfield IM, Rocamonde B, Masdeu MdM, Mulugeta E, Vaga S, van den Berg DLC, et al. Id4 promotes the elimination of the pro-activation factor Ascl1 to maintain quiescence of adult hippocampal stem cells. eLife. 2019;8:e48561 10.7554/eLife.48561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moortgat S, Berland S, Aukrust I, Maystadt I, Baker L, Benoit V, et al. HUWE1 variants cause dominant X-linked intellectual disability: a clinical study of 21 patients. European Journal of Human Genetics. 2018;26(1):64–74. 10.1038/s41431-017-0038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nature Genetics. 2009;41(5):535–43. 10.1038/ng.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proceedings of the National Academy of Sciences. 2007;104:15282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gao Z, Ure K, Ding P, Nashaat M, Yuan L, Ma J, et al. The Master Negative Regulator REST/NRSF Controls Adult Neurogenesis by Restraining the Neurogenic Program in Quiescent Stem Cells. Journal of Neuroscience. 2011;31(26):9772 10.1523/JNEUROSCI.1604-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paik J-h, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs Cooperatively Regulate Diverse Pathways Governing Neural Stem Cell Homeostasis. Cell Stem Cell. 2009;5(5):540–53. 10.1016/j.stem.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Renault VM, Rafalski VA, Morgan AA, Salih DAM, Brett JO, Webb AE, et al. FoxO3 Regulates Neural Stem Cell Homeostasis. Cell Stem Cell. 2009;5(5):527–39. 10.1016/j.stem.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ma DK, Marchetto MC, Guo JU, Ming G-l, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nature Neuroscience. 2010;13(11):1338–44. 10.1038/nn.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Molofsky AV, Pardal R, Iwashita T, Park I-K, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–7. 10.1038/nature02060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jones KM, Sarić N, Russell JP, Andoniadou CL, Scambler PJ, Basson MA. CHD7 Maintains Neural Stem Cell Quiescence and Prevents Premature Stem Cell Depletion in the Adult Hippocampus. Stem Cells. 2014;33(1):196–210. 10.1002/stem.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andäng M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(14):5837–42. 10.1073/pnas.1014993108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jiang Y, Hsieh J. HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels. Proceedings of the National Academy of Sciences. 2014;111(37):13541–6. 10.1073/pnas.1411939111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, et al. Ezh2 Regulates Adult Hippocampal Neurogenesis and Memory. The Journal of Neuroscience. 2014;34(15):5184–99. 10.1523/JNEUROSCI.4129-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–40. 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nature reviews Molecular cell biology. 2013;14(6):329–40. 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li L, Clevers H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science. 2010;327(5965):542 10.1126/science.1180794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, DeLeo AM, Pastrana E, et al. Prospective Identification and Purification of Quiescent Adult Neural Stem Cells from Their In Vivo Niche. Neuron. 2014;82(3):545–59. 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015;17(3):360–72. 10.1016/j.stem.2015.07.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Knobloch M, Pilz G-A, Ghesquière B, Kovacs WJ, Wegleiter T, Moore DL, et al. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Reports. 2017;20(9):2144–55. 10.1016/j.celrep.2017.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. Proliferative Neural Stem Cells Have High Endogenous ROS Levels that Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner. Cell Stem Cell. 2011;8(1):59–71. 10.1016/j.stem.2010.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chaker Z, Codega P, Doetsch F. A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdisciplinary Reviews: Developmental Biology. 2016;5(6):640–58. 10.1002/wdev.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rushing G, Ihrie RA. Neural stem cell heterogeneity through time and space in the ventricular-subventricular zone. Frontiers in Biology. 2016;11(4):261–84. 10.1007/s11515-016-1407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adams KV, Morshead CM. Neural stem cell heterogeneity in the mammalian forebrain. Progress in Neurobiology. 2018;170:2–36. 10.1016/j.pneurobio.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 199.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, et al. Quiescent and Active Hippocampal Neural Stem Cells with Distinct Morphologies Respond Selectively to Physiological and Pathological Stimuli and Aging. Cell Stem Cell. 2010;6(5):445–56. 10.1016/j.stem.2010.03.017 . [DOI] [PubMed] [Google Scholar]
- 200.Otsuki L, Brand AH. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360(6384):99–102. 10.1126/science.aan8795 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Otsuki L, Brand AH. Dorsal-Ventral Differences in Neural Stem Cell Quiescence Are Induced by p57(KIP2)/Dacapo. Developmental cell. 2019;49(2):293–300 e3. 10.1016/j.devcel.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu TH, Li L, Vaessin H. Transcription of the Drosophila CKI gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech Dev. 2002;112(1–2):25–36. 10.1016/s0925-4773(01)00626-8 . [DOI] [PubMed] [Google Scholar]
- 203.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes & Development. 2005;19(6):756–67. 10.1101/gad.1272305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Andreu Z, Khan MA, Gómez PG, Negueruela S, Hortigüela R, Emeterio JS, et al. The Cyclin‐Dependent Kinase Inhibitor p27kip1 Regulates Radial Stem Cell Quiescence and Neurogenesis in the Adult Hippocampus. Stem Cells. 2015;33(1):219–29. 10.1002/stem.1832 [DOI] [PubMed] [Google Scholar]
- 205.Furutachi S, Matsumoto A, Nakayama KI, Gotoh Y. p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis. EMBO J. 2013;32(7):970–81. 10.1038/emboj.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Otsuki L, Brand AH. Dorsal-Ventral Differences in Neural Stem Cell Quiescence Are Induced by p57KIP2/Dacapo. Developmental Cell. 2019;49(2):293–300.e3. 10.1016/j.devcel.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Micheli L, D’Andrea G, Ceccarelli M, Ferri A, Scardigli R, Tirone F. p16Ink4a Prevents the Activation of Aged Quiescent Dentate Gyrus Stem Cells by Physical Exercise. Frontiers in Cellular Neuroscience. 2019;13:77 10.3389/fncel.2019.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. Journal of Neuroscience. 2006;26(4):1107 10.1523/JNEUROSCI.3970-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Farnebo M, Bykov VJN, Wiman KG. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochemical and Biophysical Research Communications. 2010;396(1):85–9. 10.1016/j.bbrc.2010.02.152 [DOI] [PubMed] [Google Scholar]