Abstract
The insect bacterium Wolbachia pipientis is being introgressed into Aedes aegypti populations as an intervention against the transmission of medically important arboviruses. Here we compare Ae. aegypti mosquitoes infected with wMelCS or wAlbB to the widely used wMel Wolbachia strain on an Australian nuclear genetic background for their susceptibility to infection by dengue virus (DENV) genotypes spanning all four serotypes. All Wolbachia-infected mosquitoes were more resistant to intrathoracic DENV challenge than their wildtype counterparts. Blocking of DENV replication was greatest by wMelCS. Conversely, wAlbB-infected mosquitoes were more susceptible to whole body infection than wMel and wMelCS. We extended these findings via mosquito oral feeding experiments, using viremic blood from 36 acute, hospitalised dengue cases in Vietnam, additionally including wMel and wildtype mosquitoes on a Vietnamese nuclear genetic background. As above, wAlbB was less effective at blocking DENV replication in the abdomen compared to wMel and wMelCS. The transmission potential of all Wolbachia-infected mosquito lines (measured by the presence/absence of infectious DENV in mosquito saliva) after 14 days, was significantly reduced compared to their wildtype counterparts, and lowest for wMelCS and wAlbB. These data support the use of wAlbB and wMelCS strains for introgression field trials and the biocontrol of DENV transmission. Furthermore, despite observing significant differences in transmission potential between wildtype mosquitoes from Australia and Vietnam, no difference was observed between wMel-infected mosquitoes from each background suggesting that Wolbachia may override any underlying variation in DENV transmission potential.
Author summary
Aedes aegypti transmit a number of medically important arboviruses, including dengue, Zika, chikungunya, Mayaro and yellow fever viruses. Over the past 50 years, the burden of Ae. aegypti-transmitted disease has significantly increased–underscoring how current methods of vector control are unable to cope with this problem. Wolbachia-based biocontrol methods show extreme promise in reducing the global burden of vector-borne disease. The wMel strain, widely being used in field trials around the world, substantially reduces the ability of Ae. aegypti mosquitoes to transmit dengue, Zika, and other viruses. Here we describe a comprehensive comparative study of the viral blocking abilities of wMel to wMelCS and wAlbB which have previously been shown to have stronger viral blocking or an expanded utility in extreme environments, respectively. Using two different methods to measure viral replication and transmission potential, we show that both strains provide improved viral protection over wMel in the lab supporting further examination in field trials. We further compare the transmission potential of wMel in two different genetic backgrounds and find that wMel provides equivalent levels of viral blocking despite differences observed in wildtype mosquitoes, suggesting that viral blocking induced by wMel may override any underlying variation for DENV transmission potential.
Introduction
Aedes aegypti can transmit a number of medically important arboviruses, including dengue, Zika, chikungunya, Mayaro and yellow fever viruses [1–5]. Together, these viruses cause significant morbidity and mortality across the world, with an estimated 100 million people experiencing a symptomatic dengue virus (DENV) infection each year [6]. Disease control largely relies on methods aimed at suppressing Ae. aegypti populations. These methods have failed to eliminate dengue as a public health problem in disease endemic countries.
The last decade has seen the field testing of multiple Wolbachia-based mosquito biocontrol methods [7,8]. The World Mosquito Program (WMP) has successfully introgressed the wMel strain of Wolbachia, originally from Drosophila melanogaster, into field populations of Ae. aegypti [9–12]. wMel has been well-characterised for its capacity to block DENV infection and replication in Ae. aegypti using different challenge methods [1,13–18]. It demonstrates very effective virus blocking in mosquitoes that have been inoculated with virus, reducing the proportion of mosquitoes that become infected and reducing the tissue viral load (in a variety of tissues) by several orders of magnitude in those that do develop infection [15–17,19]. Further, wMel almost completely reduces the transmission potential of mosquitoes orally challenged with cultured DENV spiked into animal blood [13,15,16,20].
Viremic blood meals from acute dengue patients pose a more rigorous challenge to the virus blocking phenotype mediated by wMel, with some wMel-infected mosquitoes developing infectious saliva [14,15,18]. Nonetheless, this level of wMel-imparted anti-DENV resistance is projected to result in local elimination of DENV transmission in most endemic settings [14].
Other Wolbachia strains transinfected into Ae. aegypti show promise for their enhanced viral blocking abilities or the ability to tolerate extreme environments. The Wolbachia wMelCS strain, also from D. melanogaster, is closely related to wMel [21] and has been successfully transinfected into Ae. aegypti. wMelCS was found to impart similar fitness costs and potentially increased pathogen blocking attributes on Ae. aegypti as wMel [16]. The wAlbB strain, derived from Ae. albopictus [22], has been shown to block DENV relative to Wolbachia-uninfected mosquitoes in lab-based vector competence assays [23]. Comparative studies found wAlbB was able to block DENV as effectively as wMel, and characterisation of life history traits showed wAlbB resulted in minimal host fitness costs [15,24]. Most notably, wAlbB was shown to be more tolerant of cyclical heat stress than wMel, suggesting it may be more stable in areas of high temperature [25,26]. wAlbB-infected mosquitoes have recently been released as part of a pilot field trial in Malaysia where the strain was able to successfully establish in the field and passive monitoring suggests a reduction in the incidence of dengue cases [27]. Both strains show promise for their utilisation in Wolbachia-based interventions.
In the current study, we perform side-by-side measurements of the susceptibility to DENV infection of Ae. aegypti lines (Cairns, Australia) infected with wMel, wMelCS or wAlbB, alongside their uninfected, wildtype counterparts generating one of the most comprehensive and rigorous comparisons to date. First, we perform viral challenges with DENV strains representative of each of the four serotypes circulating in Asia, via intrathoracic inoculation. Second, using data from oral blood feeding with viremic blood from Vietnamese dengue patients, we deliver physiologically relevant insights into the virus transmission potential of each of the three Wolbachia-infected strains. We additionally evaluate the effect of host nuclear background on virus susceptibility, comparing wMel and its uninfected control strain on a second genetic background (Ho Chi Minh City, Vietnam) to that from Cairns.
Results
Intrathoracic injections with DENV
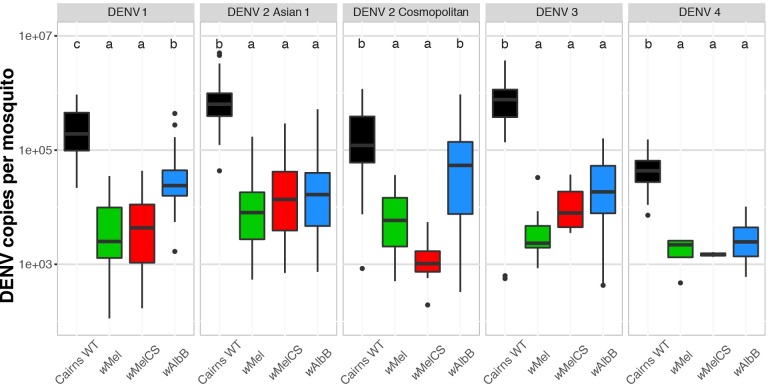
We generated Ae. aegypti mosquito lines with wMel, wMelCS, or wAlbB infections in a genetically similar nuclear background (Cairns, Australia). We compared their susceptibility to DENV infection to that of wildtype (WT) mosquitoes after intrathoracic injection. Mosquitoes were injected with all four DENV serotypes, including two different genotypes of DENV-2, Asian 1 and Cosmopolitan, at concentrations that generally resulted in ~100% infection prevalence amongst WT mosquitoes. We observed that wMelCS provided equivalent protection as wMel for all viruses tested. While we did not observe a significant difference in the DENV viral load in wMelCS mosquitoes compared to wMel for any virus tested (Kruskal-Wallis test, p > 0.05, Fig 1), we observed reductions in the number of mosquitoes acquiring DENV infections (although only for DENV-1 challenge was this statistically significant; see Table 1, Fisher’s exact test, p < 0.05). These data suggest wMelCS may provide some additional protection over wMel at the level of DENV-1 infection prevalence after intrathoracic injection.
Fig 1. wMel, wMelCS, and wAlbB all provide viral blocking following intrathoracic DENV infection.
DENV-1-4 were intrathoracically injected in 6–7 day-old wMel-, wMelCS-, or wAlbB-infected mosquitoes as well as wildtype (WT) controls. After a 7-day incubation, RNA was extracted and DENV genomic copies quantified by qRT-PCR. Data are shown as the median DENV genomic copies per mosquito (thick line) ± interquartile ranges (box), extended by the whiskers indicating 1.5× the interquartile range, with dots indicating outliers. N for strain-virus combination are shown in Table 1. Using a Kruskal-Wallis test with Dunn’s correction, the letters indicate statistically significant differences between groups.
Table 1. DENV infection prevalence in Ae. aegypti strains with Wolbachia infections 6–7 days after intrathoracic injection of DENV-1-4.
Significant reductions in the number of infected wMelCS mosquitoes relative to wMel is indicated by #, Fisher’s Exact Test. Significant increases in the number of infected wAlbB mosquitoes relative to wMel is indicated by ‡, Fisher’s exact test.
| Percentage of mosquitoes PCR-positive for DENV (N) | ||||
|---|---|---|---|---|
| DENV Strain | Cairns wMel | Cairns wMelCS | Cairns wAlbB | Cairns WT |
| DENV-1 | 94 (48) | 51 (47) # | 98 (47) | 100 (48) |
| DENV-2 Asian 1 | 77 (48) | 65 (46) | 70 (47) | 100 (48) |
| DENV-2 Cosmopolitan | 40 (47) | 30 (47) | 58 (48) | 66 (47) |
| DENV-3 | 25 (48) | 15 (47) | 70 (47)‡ | 100 (47) |
| DENV-4 | 9 (46) | 4 (47) | 31 (48)‡ | 100 (47) |
wAlbB blocked DENV replication relative to Cairns WT, but generally provided less protection than either wMel or wMelCS (Fig 1). For DENV-1 and DENV-2 (Cosmopolitan genotype) wAlbB-infected mosquitoes had a higher DENV viral load than wMel or wMelCS-infected mosquitoes (Kruskal-Wallis Test, p < 0.05). Additionally, wAlbB mosquitoes had significantly higher infection prevalence compared to wMel when challenged with DENV-3 and DENV-4 (see Table 1, Fisher’s exact test, p < 0.05).
Challenge with acute viremic blood from dengue cases
We used patient-derived blood meals to further discriminate between the effects of wMel, wMelCS, wAlbB on the Cairns nuclear background (as above), as well as wMel and WT mosquitoes on a Ho Chi Minh City (HCM) nuclear background [18]. The inclusion of these two additional lines enabled us to investigate the influence of mosquito nuclear genetic background on virus susceptibility. All six lines were exposed in parallel to 36 blood meals derived from acute dengue cases, with each of the four DENV serotypes represented. S2 Table depicts the flow of human and mosquito samples, data collection and analysis.
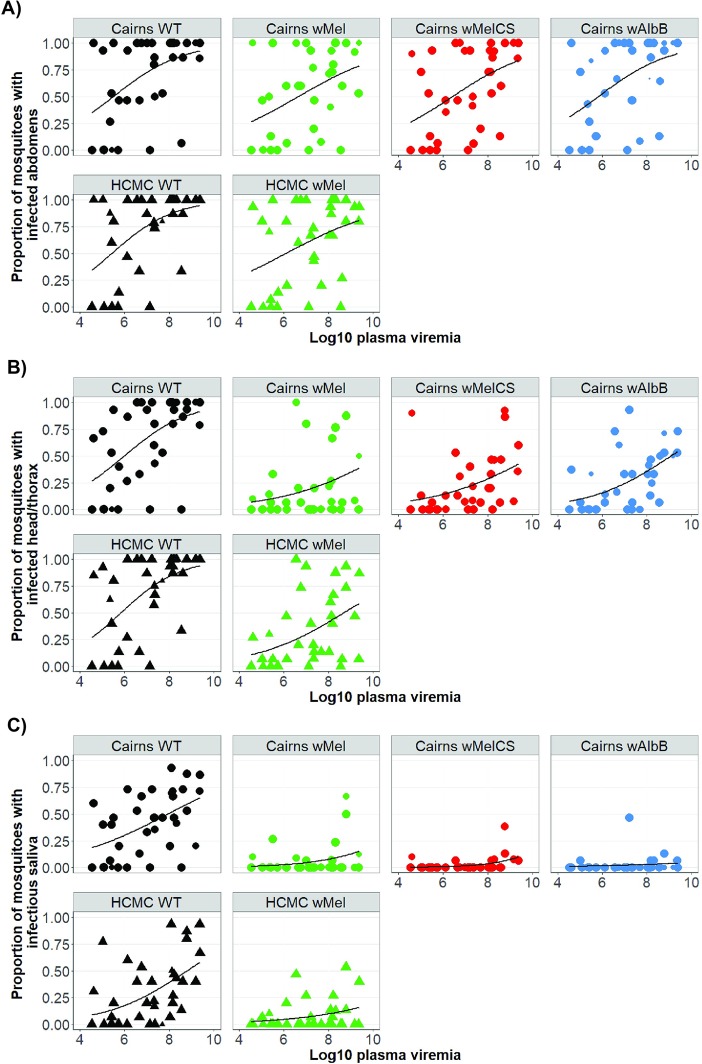
In the 36 patient-derived blood meals, DENV-2 and DENV-4 were most prevalent (n = 14 and 13 respectively), followed by DENV-1 (n = 8) and DENV-3 (n = 1). S1 Fig illustrates the range of blood plasma viremias observed in the study. Across the 36 blood feeding events, between 54 and 72% of the mosquitoes from each strain developed an abdomen infection (Table 2). Although the magnitude of the effect was not large, all Wolbachia strains decreased DENV infection prevalence, compared to Cairns WT mosquitoes (Fig 2A, S3A Table)
Table 2. Prevalence of DENV infection in the abdomen tissue, head/thorax tissue and saliva-inoculated mosquitoes, measured as a proxy for midgut infection, dissemination and transmission potential, respectively.
Mosquitoes had been fed on blood from 36 independent acutely infected dengue patients, and were harvested for collection 14 days after exposure. The number of mosquitoes harvested indicates the total sample size for each strain.
| Percentage of mosquitoes PCR-positive for DENV | ||||
|---|---|---|---|---|
| Host background-Wolbachia strain | # mosquitoes harvested | Abdomen tissue |
Head/thorax tissue |
Saliva-inoculated mosquitoes |
| Cairns WT | 523 | 70.7 | 65.4 | 42.3 |
| Cairns wMel | 494 | 54.5 | 20.0 | 5.7 |
| Cairns wMelCS | 521 | 57.2 | 22.1 | 2.7 |
| Cairns wAlbB | 483 | 64.4 | 26.3 | 2.7 |
| HCM WT | 507 | 71.8 | 67.7 | 31.2 |
| HCM wMel | 529 | 60.7 | 33.3 | 7.9 |
Fig 2. Proportion of mosquitoes from each of the six lines from each cohort with evidence of viral RNA in each tissue tested, after feeding indirectly on patient-derived viremic blood meals.
Each dot represents the proportion of each cohort that is infected, plotted as a function of log10 plasma viremia (RNA copies per milliliter). The size of the dot represents the number of mosquitoes tested in each cohort, up to a maximum of 15. Data are stratified by the Wolbachia infection status (where “WT” = uninfected, and the host genetic background of the mosquitoes). Figures show percentage of mosquitoes with an infection in A) abdomen tissue; B) head/thorax tissue; and C) naïve mosquitoes that were inoculated with saliva collected from index mosquitoes.
In the head/thorax tissues, the prevalence of DENV infection dropped to approximately half of that seen in the abdomen tissue for all Wolbachia-infected strains, after 14 days (Table 2). There was no difference in the prevalence of DENV infected head/thorax tissues from HCM or Cairns WT mosquitoes (Fig 2B).
Finally, we measured the transmission potential of orally-fed mosquitoes by collecting saliva from each harvested female and testing for DENV infection in the pool of recipient mosquitoes into which the saliva sample was inoculated (Fig 2C). Overall, DENV transmission potential was lowest for wMelCS (14/521 mosquitoes; 2.69%) and wAlbB (13/483; 2.69%). The highest prevalence of transmission was observed in the Cairns WT mosquitoes (221/523 = 42.26%) (Table 2). In the adjusted marginal logistic regression (S3C Table), significant reductions in odds of transmission potential for each of the Wolbachia strains relative to WT were observed, with the smallest odds for both Cairns wMelCS and wAlbB (OR = 0.019, 95% CI = 0.01–0.03, p < 0.001 exactly for both strains). The respective odds for Cairns wMel mosquitoes was only slightly higher (OR = 0.044, 95% CI = 0.03–0.07, p < 0.001).
Exploratory subgroup analysis
Using the saliva data, we performed subgroup analyses with only the three Wolbachia strains introgressed on the Cairns background, to investigate relative strength of the alternate Wolbachia strains. Both wMelCS (OR = 0.41, 95% CI = 0.20–0.83, p = 0.014) and wAlbB (OR = 0.42, 95% CI = 0.20–0.88, p = 0.021) induced greater blocking than wMel, which was used as the reference in this model (S2 Fig). We also investigated the effect of host genetic background, and whether wMel’s effectiveness differed between mosquitoes from different origins. In comparing HCM WT mosquitoes with their Cairns counterpart, we found mosquitoes from HCM had lower odds of transmission (OR = 0.499, 95% CI = 0.367–0.681, p < 0.001), with the infection prevalence for Cairns WT mosquitoes 42%, compared to only 31% for HCM WT. Between wMel strains from Cairns and HCM however, there was no observable difference in transmission potential (OR = 1.44, 95% CI = 0.826–2.516, p = 0.198). Among all mosquitoes tested, infectious saliva was detected in 5.6% of mosquitoes with Cairns wMel background and 7.9% for HCM wMel.
MID50 predictions
Using the data from the blood feeding experiments, we predicted the required concentrations of virus in the plasma, measured as RNA copies/mL, to infect 50% of mosquitoes (MID50) from each strain, after 14 days (S3 Fig). We considered each of the three tissue types in our predictions (abdomen tissue, head/thorax tissue, and saliva). Estimates of the MID50 for abdomen tissue of the six strains were fairly similar, ranging from 5.5–6.7 log10 viral RNA copies/mL. For detection of virus in saliva, the predicted MID50 estimates varied greatly, from 7.9 to 16.4 log10 viral RNA copies/mL (S4 Table). Because of the wide range of predicted values, with more extreme values associated with each of the Wolbachia-infected mosquitoes, we further calculated the MID10 and MID90, to provide alternative points of comparison. The predicted virus concentrations for the MID10 to achieve infectious saliva of Wolbachia-infected strains ranged between 8 logs of virus (for HCM wMel) up to 11 logs of virus (for Cairns wAlbB). In order to detect virus in the saliva in 90% of each of the Wolbachia-infected strains (MID90), our model predicts viral concentrations of between 14 logs of virus (Cairns wMelCS) and 21.9 logs of virus (for Cairns wAlbB) would be needed in the patient-derived blood meal. This is compared to an MID90 of <12.5 logs for the WT strains from Cairns and HCM.
Reduction of viral transmission afforded by Wolbachia strains
We performed pairwise calculations to quantify the magnitude of the effect of each Wolbachia strain on virus transmission, between individual cohorts of Wolbachia-carrying mosquitoes, relative to their WT controls (Fig 3). Differences in DENV infection frequencies in the abdomen tissue between strains were small, but started to emerge between paired cohorts of Wolbachia-infected and WT mosquitoes in the head/thorax tissue. Large reductions in transmission potential are induced by all Wolbachia strains, but are most prominent in the wMelCS and wAlbB lines.
Fig 3. Wolbachia-mediated percentage change in infection prevalence of mosquitoes in the wMel, wAlbB and wMelCS strains on the Cairns and HCM genetic backgrounds.
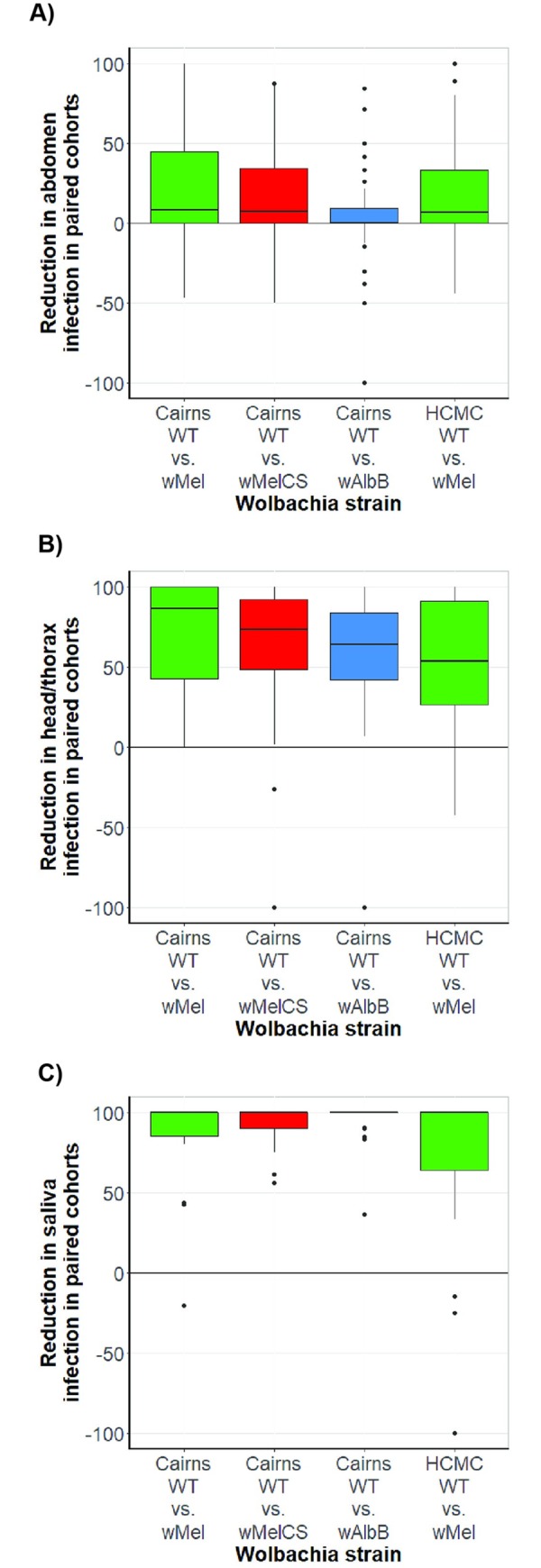
The boxplot depicts the percentage change (medians and interquartile range) in DENV infection prevalence in abdomen, head/thorax, or saliva, between paired WT and Wolbachia-infected mosquito cohorts. The y-axis represents the percentage change in infection prevalence, ranging from 100 (meaning all mosquitoes containing Wolbachia are DENV-uninfected, and any number of WT mosquitoes are DENV-infected), through 0 (reflecting equal DENV infection prevalence between the paired cohorts), to -100 (reflecting all wildtype mosquitoes being DENV-uninfected, and any number of Wolbachia-infected mosquitoes are infected with DENV). Data is stratified by host Wolbachia infection and mosquito genetic background. A) abdomen infection; B) head/thorax infection; C) saliva infection, measured by inoculation of saliva into naïve mosquitoes.
Discussion
Here we provide the first side-by-side comparisons of the pathogen blocking attributes of wMel, wMelCS and wAlbB in stably transinfected Ae. aegypti. Relative to the wMel infection in the Cairns background, both wMelCS and wAlbB provided very small, but measurable, further reductions in the DENV transmission potential of mosquitoes after feeding on viremic blood meals from dengue patients. Subgroup analyses investigating differences between mosquito genetic backgrounds detected reduced virus transmission potential in WT mosquitoes from HCM, compared to those from Cairns. A lack of detectable difference between wMel-infected mosquitoes from these same origins suggests Wolbachia may override underlying variation in infection and transmission potential seen between WT populations. Parallel virus injection experiments support the observation that wMelCS provides enhanced protection compared to wMel, albeit with a degree of serotype specificity. wAlbB in these experiments blocked infection to an intermediate degree only, similar to that seen with abdomen infections in the oral feeding experiments. Mechanistically, this might indicate differences in efficacy between strains and tissue type, and warrants further investigation.
Wolbachia strain performance compared to past studies
While Fraser, et al. [16] noted a better relative performance of wMelCS over wMel when mosquitoes were challenged with virus by intrathoracic injection, blood feeding studies with blood spiked with cultured virus, noted no difference in the relative performance of wMel and wMelCS. Although more costly and difficult to perform, we achieved increased discretionary power using viremic blood meals from dengue patients; at 14 days post-exposure, DENV transmission potential was less in wMelCS mosquito cohorts than in wMel cohorts.
Multiple, independent origins of wAlbB infections in Ae. aegypti [17,22] make the comparisons of studies more difficult as Wolbachia densities and tropism may underlie the observed differences. Previous work established that wAlbB lowers DENV infection and dissemination prevalence relative to uninfected Ae. aegypti [23]. In a comparison of Wolbachia strains, Joubert et al. [15] detected similar viral loads in whole mosquito bodies whether they carried wMel or wAlbB, after DENV-2 oral challenge (with spiked blood meals). Ant et al. [17] found that wAlbB had a higher DENV infection prevalence in abdomens than wMel, but lower infection prevalence in salivary glands, although neither was statistically significant. These latter results are consistent with our general findings that wAlbB’s inhibition of virus increases as the virus progresses through the mosquito body, surpassing wMel’s levels of viral inhibition. Time-course and anatomical investigations of both DENV and Wolbachia are needed to help understand the differences between wAlbB’s and wMel’s virus blocking attributes.
Wolbachia’s blocking effect is less pronounced when mosquitoes are challenged with DENV serotype 1, but all three Wolbachia strains induced particularly effective blocking against DENV-4, in both injection and oral challenge experiments (Fig 1 and S2 Fig). A number of studies [14,18,28,29], have noted that circulating DENV-4 appears to be less infectious to mosquitoes than other serotypes; the consistently high levels of Wolbachia blocking against DENV-4 may represent an opportunity to examine mechanisms/pathways involved in DENV interference, driven by virus genotypes and/or Wolbachia strains.
Comparison of inoculation and oral challenge approaches
Injection experiments performed here extend the results of Fraser et al. [16], through the addition of wAlbB and by employing a more expansive panel of challenge viruses to characterise the viral blocking capacity of Wolbachia strains. Our results demonstrate a reasonable correlation between estimates of whole body infection derived from intrathoracic injection of virus and a strain’s relative susceptibility to infection in the abdomen when fed on patient-derived blood. Direct injection of virus into the mosquito thorax bypasses many internal barriers that a virus must clear to be successfully transmitted, likely underestimating a strain’s blocking capacity in the reduction of virus dissemination and transmission. A more appropriate comparison of results might be the contrast between injection results (estimating Wolbachia’s ability to block virus prior to dissemination) and the infection prevalence in abdomens after patient-derived blood feeding experiments. Using this approach, we noted that similarly high abdomen infection frequencies for wAlbB, relatively to wMel and wMelCS, in both injection and oral challenge experiments (see Tables 1 and 2).
Considerations for Wolbachia deployment
Multiple studies suggest that wMel, wMelCS, and wAlbB have mild impacts on Ae. aegypti fitness [1,15,17,22,24]. Given the successful introgression of wMel and wAlbB into field populations thus far [9–12,27], we expect all three strains to perform well in the field. A major concern for Wolbachia-based interventions is the attenuation of virus blocking. Two main considerations here are the likelihood of attenuation affecting all Wolbachia strains and how alternative Wolbachia strains can be deployed to replace less effective ones.
Studies thus far have not found a single mechanism of major effect that underlies Wolbachia-mediated viral blocking. Multiple mechanisms, including competition over resources and immune priming, are suggested to contribute to blocking [30–32]. As such, attenuation of the blocking phenotype for all Wolbachia strains is unlikely. However, if a single mechanism of major effect were employed by Wolbachia strains across supergroups to interfere with virus replication and transmission, and DENV evolves to evade this mechanism, then the capacity to use replacement strains as part of a resistance management strategy is diminished. With deployment of suitable replacement strains, any extension of the strategy’s longevity allows additional time for improving and evaluating other vaccine candidates, and development of alternative vector control strategies. Efficient replacement of an existing Wolbachia strain requires the cytoplasmic incompatibility (CI) loci of the replacement strain to be uni- or bidirectionally incompatible with those of the primary strain. The observed bi-directional CI phenotype of wAlbB with both wMel and wMelCS suggests a number of options for secondary releases [15,16] should they be needed to overcome any attenuation of the desired viral blocking phenotype. Nevertheless, investigations into the mechanisms by which each strain inhibits virus infection, replication and transmission should thus be a heightened priority to help gauge the long-term stability of Wolbachia’s antiviral effect in this context.
In summary, our data support the use of wMelCS and wAlbB in first-line releases to reduce dengue burden in endemic countries, or as part of management strategies in the event of any attenuation of the virus-blocking phenotype. Given the effective performance of all strains, and that lab-based studies suggest wAlbB may have a broader optimal temperature range [25,26] there is a suite of strains feasibly available for deployment in an expanded range of environmental conditions. While our data suggest that wMelCS and wAlbB provide a small reduction in DENV transmission potential compared to wMel, the operational impact of this difference is not clear. Field investigations to monitor the establishment and stability of each strain across variable environmental conditions as well as the impact on dengue incidence are warranted to understand if these differences show any significance in the field.
Materials and methods
There were two parallel experiments conducted in this study. The injection experiments were conducted at Monash University, Melbourne, Australia, while the vector competence experiments were performed at Oxford University Clinical Research Unit (OUCRU), in Ho Chi Minh City, Vietnam.
Ethics statement
Blood feeding of mosquito colonies at Monash University on adult, human volunteers was performed in accordance with Monash University Human Research Ethics permit number CF11/0766-2011000387. Written informed consent was provided by all volunteers prior to commencement. Although ethics approval was not required for blood feeding of mosquito colonies in Vietnam, adult volunteers were still requested to sign an informed consent form prior to their participation. All patients participating in the clinical observation study in Vietnam were prospectively enrolled as part of a study approved by the ethics committees of the Hospital for Tropical Diseases (CS/NÐ/16/27), Oxford Tropical Research Ethics Committee (45–16), and University of Melbourne Human Research Ethics Committee (1648095). These approvals allowed patients admitted to the Hospital for Tropical Diseases, who were >15 years of age, had been unwell for <96 hrs, and were suspected to have dengue to provide a single venous blood sample for use in mosquito feeding. Written informed consent was prospectively obtained from all participants by qualified staff from the hospital.
Generation of mosquito lines
Cairns background mosquitoes
The wMel and wMelCS strains have been previously described [1,16]. To generate the wAlbB strain in an Australian Ae. aegypti genetic background, wAlbB from the WB1 strain [22] was injected into wMelF.Tet, an uninfected, genetically Australian strain. Embryonic micro-injections, creating and establishing isofemale lines were performed as previously described [16].
A wildtype (WT) colony was established by placing ovitraps in Bentley Park and Edmonton (Cairns, Australia) in September 2016. Eggs were hatched in the lab and reared to adult stage where they were sorted by species and used to establish a single colony. Eggs from the WT colony were used in experiments within three generations of colony establishment. wMel, wMelCS, and wAlbB strains were backcrossed to the Cairns WT colony for three generations to isogenize the genetic backgrounds of the three different strains.
For colony maintenance, all mosquitoes were reared as described previously, with minor differences [33]. Briefly, adult mosquitoes were maintained in a controlled temperature room at 26°C with 65% relative humidity (RH) with 12h:12h light-dark cycle and were allowed access to 10% sucrose ad libitum, as well as to human blood for reproduction.
Subcultures of these colonies were delivered to OUCRU for the blood feeding experiments. At OUCRU, colony maintenance was performed at ~28°C, 75–85% RH and 12h:12h light-dark cycle. Genetic material was obtained approximately every 3 months from the Monash colonies. Wolbachia infection status was confirmed before using the new material, as well as in each subsequent generation of maintenance, to ascertain there was no cross-contamination of Wolbachia strains between cages, or failure of maternal transmission between generations.
Ho Chi Minh City (HCM) background mosquitoes
The Ho Chi Minh City (HCM) WT mosquitoes were freshly collected from the field and then colonised in the laboratory as described in [34]. The HCM wMel line was originally produced with backcrossing of the Wolbachia infected Cairns females with HCM WT males, for five generations. From this point both the HCM WT and HCM wMel lines underwent outcrossing with 10–20% field-derived (F1) males every second generation in order to maintain genetic similarity with the local field population. As per the Cairns mosquitoes, both lines had their Wolbachia infection status confirmed by PCR each generation. The HCM WT and wMel lines were maintained in duplicate cages, of >250 females/cage, at ~28°C, 75–85% RH and a 12:12 hr light dark cycle. Mosquitoes used in blood feeding experiments were from generations 28–34 for HCM WT and 33–39 for HCM wMel.
Intrathoracic injections
Asian isolates of DENV 1–4 were obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) and from the Oxford University Clinical Research Unit, Vietnam. Virus genotype and origin are listed in S1 Table. Virus genotypes were confirmed by PCR and sequencing of the E gene. C6/36 cells were infected at a MOI of 0.1, and the cell culture supernatant was harvested 7 days later. Virus concentrations were determined by TCID50 using monoclonal antibody 4G2 (provided by Roy Hall), followed by incubation with HRP-conjugated secondary antibodies, and TMB substrate. Mosquitoes used for injection experiments were from generations G2-G5 after the completion of backcrossing for all Wolbachia infected strains, and G4-G7 for Cairns WT.
Mosquitoes were age controlled within 24 hours of one another to minimize experimental variation. For viral injections, 6–7 day old mosquitoes were intrathoracically injected with 69 nL of virus diluted in RPMI to the concentrations listed in S1 Table using a microinjector (Nanoject III, Drummond Scientific) with pulled-glass capillary needles. Mosquitoes were then incubated as per their standard rearing conditions for 7 days before collecting whole mosquitoes and testing them individually for infection status.
To quantify viral genomic copies, total RNA was extracted from mosquitoes using RNeasy 96 QIAcube HT kits (QIAGEN). DENV genome copies were quantified using pan-DENV primers that bind the DENV 3’UTR [16,35] and LightCycler Multiplex RNA Virus Master (Roche) one-step qRT-PCR mix.
Blood feeding experiments
Viremic blood meals for mosquitoes
A total of 42 venous blood meals from acute dengue cases were provided to the mosquitoes. An aliquot of the blood draw from each patient was used to determine the DENV serotype and viremia level, using a serotype-specific PCR (see Diagnostics section below). The viremia level in six blood meals was below the limit of PCR detection and were excluded from the analysis. Amongst these six feeds, we detected DENV PCR-positive mosquitoes from three of the blood meals. Applying our serotype-specific PCR on respective mosquito samples, we confirmed all three patients were infected with DENV-2. However, due to our inability to measure viremia of the original blood meal, a factor shown to increase the likelihood of subsequent virus transmission by the mosquito [34], we excluded from the analysis all six blood meals and associated mosquitoes (S2 Table).
Mosquito infection experiments
Mosquitoes 1–4 days old were exposed to dengue viremic blood using an artificial membrane feeder, for 30 mins. Up to 30 engorged mosquitoes were collected and retained for incubation, of which a maximum of 15 surviving mosquitoes were selected randomly for collection after 14 days. Abdomen, head/thorax and saliva samples were harvested, and processed as per Carrington et al. [18]. Saliva samples were inoculated into naïve WT HCM mosquitoes to confirm the presence of infectious DENV particles. Inoculated WT mosquitoes were harvested 7 days post-injection and processed [18].
PCR diagnostics
Mosquitoes were homogenized with a single bead in squash buffer (containing Tris base, EDTA, NaCl, and proteinase K), heated at 56°C for 10 min and 98°C for 15 min, before being cooled to 15°C. Samples were centrifuged for 2 min to pellet debris and 2 μL of the clarified sample was added to the PCR. PCR confirmation of mosquito tissue samples for DENV and Wolbachia infection status was performed using the combined amplification results of two PCRs for each sample. The first (duplex) one-step qRT-PCR targeted DENV using pan-serotype primers [16,35], and an Ae. aegypti internal control, RPS17. The second (multiplex) qPCR was used to confirm the infecting Wolbachia strain. This Wolbachia PCR targeted wMel, wMelPop (which also amplifies wMelCS), and wAlbB, as well as the Ae. aegypti internal control; the specific combination of positive results was used to determine the infection status. Across both PCRs, the internal control had to be positive in order to accept the results as valid.
Mosquito samples were tested by technicians blinded to the serotype and viremia information of the associated plasma sample. The samples were tested sequentially (abdomen, head/thorax and then the pooled inoculated mosquitoes). If abdomen tissue was DENV-positive, both the head/thorax and saliva samples were subsequently processed. If the abdomen was DENV-negative, then the following tissues were assumed to be uninfected, and not tested. Mosquito samples were re-tested if the PCR results were not congruent (eg: negative for DENV in the head/thorax, but positive in saliva). Samples were eventually excluded from the analysis if re-testing remained incongruent.
Mosquitoes were determined to have: a wMel infection if they were wMel(+), wMelPop(-), and wAlbB(-); a wMelCS infection if they were wMel(+), wMelPop(+), and wAlbB(-); or a wAlbB infection if they were wMel(-), wMelPop(-) and wAlbB(+). WT samples were negative for all Wolbachia targets. Any samples with combinations of results other than those listed above, or where the Wolbachia infection results between the abdomen and thorax samples did not match, were excluded from the analysis.
All primer sets have been previously described [36], except those targeting wAlbB, which were: Forward “Alb_16009_F” primer (5'-AGTAGTGCAGCGAGTCT-3'), Reverse “Alb_16009_R” primer (5'-AGTTCACTGTGCTACTTGCCA-3'), and Probe “Alb_16009_LNA500” (5'-Cyan500-TATCCCCT+ACC+A+A+AGC+AAT-BHQ1-3’). PCR-positivity for viral RNA was based on a Ct value of 35 or less for all targets.
Viral RNA was extracted from human plasma samples using MagNA 96 extraction kits (Roche). Virus sample serotyped using a validated, quantitative serotype-specific RT-PCR [37], with viremia calculated based on ratios between genome copies per mL and plaque forming units per mL, of 214:1 for DENV-1, 73:1 for DENV-2, 436:1 for DENV-3, and 101:1 for DENV-4 [37]. A subset of mosquito samples were also tested using this PCR method, when it was not possible to determine the serotype of the infecting virus using the plasma sample directly but it was observed that the mosquitoes still became infected.
Statistical analyses
For intrathoracic injection experiments, viral genomic copies per mosquito were plotted as medians (± interquartile ranges) using boxplots excluding mosquitoes negative for virus. Significant differences in viral copy numbers were determined using Kruskal-Wallis tests with Dunn’s multiple comparison correction. Significant differences in DENV infection prevalence relative to wMel were calculated using one-tailed Fisher’s exact tests.
The risk of human-to-mosquito infection was assessed using marginal logistic regression models. An unadjusted, baseline model accounting for Wolbachia strain was considered, as well as an adjusted model, accounting for Wolbachia strain, infecting virus serotype, and viremia. Infection frequencies for each cohort of mosquitoes were plotted as a function of plasma viremia, and logistic curves overlaid. For each blood meal we calculated the relative reductions in the proportion of mosquitoes that developed an infection, between each Wolbachia strain and its WT counterpart of the same host background, and plotted the medians (±IQRs) of these differences using boxplots. Finally, using marginal logistic regression modelling, we calculated the infectious dose required for 50% of the mosquitoes (Mosquito infectious dose, MID50) from each strain to a) become infected with virus, b) disseminate the virus in head/thorax tissue and c) have evidence of virus in saliva. Given the low prevalence of virus infection in strains containing Wolbachia in the head/thorax tissue and saliva, estimates of the MID50 often went beyond the range of viremias observed in the study, thus giving unacceptably large confidence intervals. Thus, we provide additional estimates of the more extreme MIDs, to achieve 10% and 90% infection as well (S4 Table).
Supporting information
(DOCX)
LOD = limit of PCR detection.
(DOCX)
Adjusted marginal logistic regression models for the risk of viral infection in the abdomen tissue (A), head/thorax tissue (B); and mosquitoes inoculated with saliva (C). The reference categories for each covariate are listed in the tables. NB: There was only a single patient blood meal containing DENV-3, therefore the confidence intervals surrounding the Odds Ratio is extremely large.
(DOCX)
The viremias required to achieve 10%, 50% and 90% of mosquitoes (the MID10, MID50 and MID90 respectively) with evidence of virus in each tissue type are calculated for each line.
(DOCX)
Viremia was measured by qRT-PCR, and reported as log10 RNA copies/mL for the 36 blood meals to which a serotype and viremia could be measured.
(TIF)
Each dot represented the proportion of each cohort that is infected, plotted as a function of log10 plasma viremia (RNA copies per milliliter), with the size of the dot indicative of the number of mosquitoes tested in each cohort, up to a maximum of 15. Data are stratified by the Wolbachia infection status, and the infecting serotype in the patient blood meal.
(TIF)
Predicted concentrations of virus leading to mosquitoes with DENV-positive (A) abdomens (representing midgut infections), (B) head/thorax (disseminated infections), and (C) infectious saliva (as measured in saliva-inoculated mosquitoes). Each point represents the predicted proportion of all mosquitoes to have DENV in the respective tissue tested, 14 days after a blood meal on a viremic blood from a dengue patient. The corresponding smoothing curves and shading (representing 95% CIs) illustrate the predicted probability based on marginal logistic regression. The point at which the smoothing curves cross the 50% on the y-axis represents the predicted concentration of virus required to infect 50% of mosquitoes (50% Mosquito Infection Dose; MID50).
(TIF)
(XLSX)
Acknowledgments
DENV isolates were obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA). We thank the patients, doctors and nursing staff of Ward D at the Hospital for Tropical Diseases for their participation in this study, and Johanna M. Duyvestyn, Etiene C. Pacidônio, and Daniela S. Gonçalves for technical assistance. Nhat Le Thanh Hoang provided valuable assistance for the statistics. Roy Hall kindly provided the 4G2 antibody used in TCID50 experiments.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by a Wellcome Trust Award No.102591/Z/13/Z to Monash University through its World Mosquito Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aeygpti populations. Nature 476: 450–455. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 2.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, et al. (2012) Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLOS Neglected Tropical Diseases 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux AB, Sinkins SP (2013) A Wolbachia wMel Transinfection in Aedes albopictus Is Not Detrimental to Host Fitness and Inhibits Chikungunya Virus. PLOS Neglected Tropical Diseases 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, et al. (2016) Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host & Microbe 19: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira TN, Rocha MN, Sucupira PHF, Carvalho FD, Moreira LA (2018) Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Scientific reports 8: 6889–6889. 10.1038/s41598-018-25236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGraw EA, O'Neill SL (2013) Beyond insecticides: new thinking on an ancient problem. Nature Reviews Microbiology 11: 181–193. 10.1038/nrmicro2968 [DOI] [PubMed] [Google Scholar]
- 8.Flores HA, O'Neill SL (2018) Controlling vector-borne diseases by releasing modified mosquitoes. Nature Reviews Microbiology 16: 508–518. 10.1038/s41579-018-0025-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 10.O'Neill S, Ryan P, Turley A, Wilson G, Retzki K, et al. (2018) Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses [version 2; referees: 2 approved]. Gates Open Research 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia GdA, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, et al. (2019) Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS neglected tropical diseases 13: e0007023–e0007023. 10.1371/journal.pntd.0007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, et al. (2019) Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates open research 3: 1547–1547. 10.12688/gatesopenres.13061.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blagrove MSC, Arias-Goeta C, Failloux AB, Sinkins SP (2012) Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proceedings of the National Academy of Sciences of the United States of America 109: 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, et al. (2015) Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Science Translational Medicine 7: 279ra237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joubert DA, WAlker T, Carrington LB, De Bruyne JT, Duong THK, et al. (2016) Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLOS Pathogens 12: e1105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, et al. (2017) Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. Plos Pathogens 13: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP (2018) The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. Plos Pathogens 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrington LB, Tran NBC, Le THN, Luong THT, Nguyen TT, et al. (2018) Field- and clinically derived estimates of Wolbachia-mediated blocking of disseminated dengue virus infection in Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences of the United States of America 115: 361–366. 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amuzu HE, McGraw EA (2016) Wolbachia-Based Dengue Virus Inhibition Is Not Tissue-Specific in Aedes aegypti. Plos Neglected Tropical Diseases 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, et al. (2014) Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLOS Neglected Tropical Diseases 8: e2688 10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, et al. (2013) Wolbachia Variants Induce Differential Protection to Viruses in Drosophila melanogaster: A Phenotypic and Phylogenomic Analysis. Plos Genetics 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi ZY, Khoo CCH, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. 10.1126/science.1117607 [DOI] [PubMed] [Google Scholar]
- 23.Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLOS Pathogens 6: e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA (2016) Fitness of wAlbB Wolbachia Infection in Aedes aegypti: Parameter Estimates in an Outcrossed Background and Potential for Population Invasion. American Journal of Tropical Medicine and Hygiene 94: 507–516. 10.4269/ajtmh.15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, et al. (2017) Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLOS Pathogens 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross PA, Ritchie SA, Axford JK, Hoffmann AA (2019) Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS neglected tropical diseases 13: e0007357–e0007357. 10.1371/journal.pntd.0007357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, et al. (2019) Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Current biology 29: 4241–4248.e4245. 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehorn J, Kien DTH, Nguyen NM, Nguyen HL, Kyrylos PP, et al. (2015) Comparative Susceptibility of Aedes albopictus and Aedes aegypti to Dengue Virus Infection After Feeding on Blood of Viremic Humans: Implications for Public Health. Journal of Infectious Diseases 212: 1182–1190. 10.1093/infdis/jiv173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, et al. (2015) Asymptomatic humans transmit dengue virus to mosquitoes. Proceedings of the National Academy of Sciences of the USA 112: 14688–14693. 10.1073/pnas.1508114112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rances E, Ye YXH, Woolfit M, McGraw EA, O'Neill SL (2012) The Relative Importance of Innate Immune Priming in Wolbachia-Mediated Dengue Interference. PLOS Pathogens 8: e1002548 10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, et al. (2013) Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLOS Pathogens 9: e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rances E, Johnson TK, Popovici J, Iturbe-Ormaetxe I, Zakir T, et al. (2013) The Toll and Imd Pathways Are Not Required for Wolbachia-Mediated Dengue Virus Interference. Journal of Virology 87: 11945–11949. 10.1128/JVI.01522-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, et al. (2009) The stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen NM, Duong THK, Vu TT, Nguyen THQ, Tran NC, et al. (2013) Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences of the United States of America 110: 9072–9077. 10.1073/pnas.1303395110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, et al. (2013) An Explosive Epidemic of DENV-3 in Cairns, Australia. PLOS ONE 8: e68137 10.1371/journal.pone.0068137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, et al. (2014) Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasites & Vectors 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hue KDT, Vu TT, Nguyen THT, Tran NBC, Huynh LAH, et al. (2011) Validation of an internatlly controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA is plasma. Journal of Virological Methods 177: 168–173. 10.1016/j.jviromet.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
LOD = limit of PCR detection.
(DOCX)
Adjusted marginal logistic regression models for the risk of viral infection in the abdomen tissue (A), head/thorax tissue (B); and mosquitoes inoculated with saliva (C). The reference categories for each covariate are listed in the tables. NB: There was only a single patient blood meal containing DENV-3, therefore the confidence intervals surrounding the Odds Ratio is extremely large.
(DOCX)
The viremias required to achieve 10%, 50% and 90% of mosquitoes (the MID10, MID50 and MID90 respectively) with evidence of virus in each tissue type are calculated for each line.
(DOCX)
Viremia was measured by qRT-PCR, and reported as log10 RNA copies/mL for the 36 blood meals to which a serotype and viremia could be measured.
(TIF)
Each dot represented the proportion of each cohort that is infected, plotted as a function of log10 plasma viremia (RNA copies per milliliter), with the size of the dot indicative of the number of mosquitoes tested in each cohort, up to a maximum of 15. Data are stratified by the Wolbachia infection status, and the infecting serotype in the patient blood meal.
(TIF)
Predicted concentrations of virus leading to mosquitoes with DENV-positive (A) abdomens (representing midgut infections), (B) head/thorax (disseminated infections), and (C) infectious saliva (as measured in saliva-inoculated mosquitoes). Each point represents the predicted proportion of all mosquitoes to have DENV in the respective tissue tested, 14 days after a blood meal on a viremic blood from a dengue patient. The corresponding smoothing curves and shading (representing 95% CIs) illustrate the predicted probability based on marginal logistic regression. The point at which the smoothing curves cross the 50% on the y-axis represents the predicted concentration of virus required to infect 50% of mosquitoes (50% Mosquito Infection Dose; MID50).
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.