Abstract
Using anions to induce molecular structure is a rapidly growing area of dynamic and switchable supramolecular chemistry. The emphasis of this review is on helical anion foldamers in solution and many of the beautiful complexes are accentuated by their crystal structures. Anion foldamers are single- or multi-strand complexes—often helical—that incorporate one or more anions. The review begins by discussing foldamer structure and nomenclature and follows with discourse on the anions which are employed. Recent advances in functional foldamers that bind a single anion are examined: including induced chirality, stimuli-responsive dynamics, fluorescence changes, organocatalysis, anion transport and halogen bonding. The review then inspects multi-anion foldamers and this section is organized by the number of strands within the foldamer—from single to triple-strand foldamers. Finally, the review is punctuated by recent hydrogen and halogen bonding triple-strand anion foldamers.
1. Introduction
Contemplate the roles of helices and anions in our bodies. They engender the molecular structure that is vital for processes such as information storage, cell signaling, catalysis and specific binding. By efficiently synthesizing oligomers and polymers that fold into functional shapes, nature creates and maintains molecular machinery in the form of proteins, DNA and RNA. In general, molecular folding occurs by maximizing favorable noncovalent and covalent interactions, minimizing unfavorable ones, and the entropic compensation concomitant with hydrophobic/solvophobic collapse.1 As a result, these biomolecules are rigid enough for high-affinity, specific binding while exhibiting enough flexibility to optimally accommodate guests within “active sites.” Remarkably, the vast nuances of these folding processes are encoded in the linear sequence of the monomers. Deciphering this molecular coding—which has been parameterized over 4.5 billion years of evolution—provides the information needed to develop complex molecular machinery.
In contrast to nature, the synthetic chemist is not bound by a finite set of building blocks. Thus, the motivation to study synthetic foldamers is to augment and complement the incredible complexity of nature to develop entirely new materials. In the words of Samuel Gellman, “the realization of the potential of folding polymers may be limited more by the human imagination than by physical barriers.”2
1.1. Foldamer Structure
First coined by Gellman in the mid-1990s,3 “foldamers” are a class of synthetic oligomers that adopt well-ordered protein-like structures, which are stabilized by noncovalent interactions between non-adjacent monomers within a strand. Foldamers dynamically fold and unfold in solution; therefore, molecules that are conformationally locked, such as helicenes, are not foldamers.1
Foldamers commonly form a helical shape, as it is arguably the most efficient way to induce enthalpically favorable and tightly packed secondary structure. Therefore, foldamers are regularly used to study molecular recognition and self-organization. Nature frequently adopts this shape to develop highly complex molecular machines, and there is great interest in developing strategies to produce synthetic counterparts. In fact, the importance of helical structure was first highlighted in guestless foldamers and these systems will be briefly discussed here. Amino-acid foldamers are de novo, regular-repeating, bioinspired structures that chemically resemble proteins. These amino acid oligomers utilize functional group shape, solvophobic effects, electrostatic complementarity, and hydrogen bonding interactions to dictate folding.4 However, chemists are not limited to α-amino acids and frequently utilize β-, γ-, and δ-amino acids, or combinations, to deliver unique properties.4–7 For example, the backbone of a β-amino-acid foldamer can be more conformationally flexible and in some cases more thermodynamically stable than an α-amino-acid counterpart.8 Innovations like this have allowed chemists to expand the repertoire of secondary and tertiary structure.9 Furthermore, the rules required to program these structures are most developed for the β-amino-acid foldamers.4 For instance, the stereochemical patterning approach has yielded good agreement between predicted and realized structures.10
Diverging from natural peptides, but still utilizing amide linkages (due to their ease of synthesis and hydrogen bonding capability),11 is the aromatic oligoamide subclass of helical foldamers. These synthetic systems appropriate m- or p-amide-linked aromatic rings—typically benzenes, pyridines, and/or quinolines12—whose rigidity limits the space of accessible conformations.13 Introverted or extroverted hydrogen-bond acceptors (pyridine/quinolone-nitrogen lone pairs, methoxy or ether groups, carbonyl oxygens, phenoxide salts, or even halogens12,14) often decorate the arenes to: 1) establish strong intramolecular three-centered, amide hydrogen bonding that restricts Ar–CONHAr- and Ar–NHCOAr-bond rotation and 2) favor either syn or anti coplanar aryl- and amide-group conformations.11,15 Additionally, sterically bulky side chains too large to occupy the helical cavity can be appended to the backbone to encourage correct folding. These electrostatic, steric, and solvophobic interactions also provide preorganization to the oligomeric skeleton, offsetting the entropic cost of folding.8 As a result, aromatic-oligoamide foldamers adopt predictable helical curvatures. Tuning the ratio and placement of m- or p-amide linkages allows for the dimensions of a foldamer to be adjusted. Consequently, this class of abiotic foldamers can be adapted for guest-inclusion, which can provide further host-conformational stability. Lastly, there are notable examples of aromatic-oligoamide multi-strand foldamers1,12 and even helix bundles,16 highlighting the programmability and stability of these guestless foldamers.
1.2. Anion-induced Foldamers
Foldamer designs also use noncovalent interactions with ions as powerful directors of self-assembly. While metal and cation-based foldamers have enjoyed a rich history of development, related anion-based structures have lagged.1,2,17 Anion-coordination chemistry, first christened by Jean-Marie Lehn in 1978, is a relatively young field of research when compared to transition-metal or cation coordination chemistry, and presents unique challenges.18 The inherent properties of anions: their diverse topologies, pH dependence, and high free energies of solvation as compared to similarly-sized cations, make them more difficult to study. Additionally, electrostatic interactions between anions and ligands are largely noncovalent. Overcoming the significant entropic cost of complexing one or more ligands and anions using noncovalent interactions alone, is extremely difficult. Indeed, no orbital theories have been thoroughly established. Instead, geometrical patterns of binding and coordination number can be explained largely by the noncovalent interactions between donor-ligands, anion topology (spherical, linear, trigonal planar, or tetrahedral), and design of the host receptor.19
Given the importance of helical twist and the ubiquity and structural diversity of anions, it is not surprising that anion-directed assembly of helical structure is emerging as an exciting way to develop functional anion-responsive supramolecules. Anion foldamers have arisen as a truly unprecedented group of biomimetic oligomers that provide key atomic-scale mechanistic insight related to the structure and dynamics of biological systems.
1.3. Foldamer Nomenclature
Foldamer research has been called “the synthetic construction and functional exploitation of chain molecules with a conformational preference” by Huc and Hecht in their 2007 textbook.20 The defining characteristics of this generalized definition include a vast variety of supramolecular structures containing one or more molecules. For the purpose of easily identifying anion foldamers in this review, we propose an “m,n-foldamer” naming scheme, where m = is a letter corresponding to the number of strands, and n = the number of guests (Figure 1). For clarity, the number of strands will be represented as “s, d, t or q” corresponding to 1, 2, 3 or 4 strands, respectively. For example, a single-strand single-anion foldamer would be called a “s,1-anion foldamer” (Figure 1c), and a two-strand one-anion foldamer would be called a d,1-anion foldamer (Figure 1e). This naming scheme functions for guestless foldamers (Figure 1a), as well as cationic or neutral guest foldamers (e.g. s,1-chloroform foldamer, or s,1-water foldamer). Foldamers containing more than one guest, like ditopic foldamers, can also be represented by identifying the total number and type of guests. (e.g. s,1-ammonium 2-chloride foldamer). Foldamers with five or greater strands could be notated with their latin prefix. For example, a five-strand guestless foldamer would be a penta-foldamer. This simple nomenclature allows the reader to quickly understand the composition of the guest and foldamer complex.
Figure 1.
Cartoon representation of foldamer nomenclature. (a) a single-strand guestless foldamer (s-foldamer), (b) a two-strand guestless foldamer (d-foldamer), (c) a single-strand foldamer with a single guest (s,1-foldamer), (d) a two-strand foldamer with a single guest (d,1-foldamer), (e) a single-strand foldamer with two guests (s,2-foldamer), and (f) a two-strand foldamer with two guests (d,2-foldamer). Guests (anions in this review) are represented as green circles, and each strand as a blue or black line.
The terminology “anion helicate,” has been used to describe helical foldamers which contain more than one anion guest and these can be considered a subclass of anion foldamers.21–24 “Helicate” was a term devised by Jean-Marie Lehn in 198725 to evoke a helical di- or oligonuclear metallosupramolecule with one or more oligomeric donor ligands. Like helical foldamers, their structure makes them ideal for studying self-assembly and molecular recognition. Today, the defining characteristics of helicates are changing, as scientists have created oligomeric donor ligands that bind multiple, non-transition metal guests, like anions. While we define anion foldamers in this review as single- or multi-strand helical complexes that encapsulates one or more anions, others may designate any multi-anion, single- or multi-strand anion complex as an anion helicate (Figure 1 e–f).
1.4. Scope of the Review
This comprehensive review concentrates on the development of anion foldamers in solution
The critical need to define and organize this emerging class of supramolecular structure is underscored by: 1) the importance and ubiquity of helices and anions, 2) ill-defined use of foldamer terminology, 3) the need for unification, as many of these structures have developed through various fields, and 4) the lack of current reviews on the subject. Yet, the reader can find several excellent reviews written on anion coordination and self-assembly19,26–32 where a few of these reviews have touched on examples of anion foldamers. Surprisingly, only one seminal review, written by Jeong and coworkers, has focused solely on anion foldamers.17 However, no comprehensive reviews have been written that cover both single- and multi-anion foldamers. Given the conceptual and structural similarities between them, we set out to provide a complete review on the subject.
For clarity, and to emphasize the terminology, this review distinguishes single-anion foldamers from multi-anion foldamers. Multi-anion foldamers are generally more difficult to create than single-anion foldamers due to entropic considerations and intracavity anion-anion repulsion, and therefore deserve separate consideration. Ditopic (cation-anion),29,30 distorted macrocyclic,31 and polymeric,32,33 foldamers will be mostly untreated here, and we refer the reader to the excellent review articles written on these topics.1,2,4,12 Several solid-state examples of anion foldamers can be found in the literature, and many of these structures form the basis for figures found in this review. However, due to the challenges associated with anion directed assembly, solution study of anion foldamers is far less common. To highlight this, the review focuses on the study of these unique structures in solution. Additionally, the single-anion foldamer section is organized by the primary focus of the reported study (e.g. anion binding, chirality, stimuli responsivity, fluorescence change, organocatalytic properties, anion transport or halogen bonding behavior). We recognize this is not a perfect classification. Nevertheless, this organization allows the interested reader to quickly locate a foldameric system by the number of strands, the number of guests or a property of interest. Lastly, given the rarity of multi-anion foldamers, we have categorized this section simply by the number of strands in the structure.
2. Anion-Foldamers in Solution
As alluded to earlier, some backbone curvatures afford helical voids. One of the examples of a natural pore-containing oligopeptide is the antibiotic gramicidin, which folds into a β-helix (4 Å pore). Nature abhors a vacuum or at least more than 45 % of one according to Julius Rebek, Jr.13 Thus, these voids are usually occupied by solvent or complementary guests. Examples of neutral guest molecules include diols, amino-alcohols, saccharides, organic acids, rod-like molecules like decanediol, etc. Chiral guests give rise to chiroptical properties that can be monitored using CD spectroscopy. Most commonly, functionality induced hydrogen bonding and solvophobic interactions drive encapsulation on the interior, and in contrast to some of the more rigid foldameric hosts without hydrogen-bonds mentioned earlier, these anion-binding foldameric containers are adaptable (induced fit).33
2.1. Single-Strand Anion Foldamers (s,1-anion foldamers)
2.1.1. Anion Binding
Anion binding is obviously an important property of anion foldamers. As such, numerous groups have detailed the anion binding properties of many classes of foldamers. In this section, we discuss the solution studies associated with anion binding and classify each foldamer based on the primary type of anions studied. As noncovalent interactions are the primary contact with the anion, the medium in which they occur plays an important role. Generally, nonpolar environments enhance intermolecular foldamer contacts and the noncovalent contacts between the foldamer and the anion. As a result, many foldamer systems take advantage of hydrophobic collapse in polar solvents to induce folding which can encapsulate the guest and enhance anion binding. However, exceptions are known, and solvent discussions are provided for each individual example.
2.1.1.1. Halide Anions
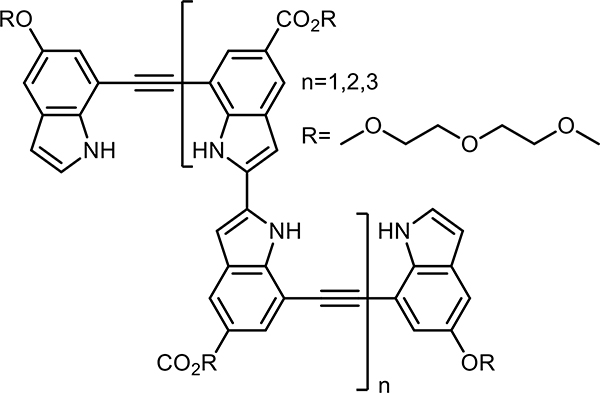
The development of anion-binding foldamers began with binding monoatomic halides. The first reported example of an anion foldamer (s,1-chloride foldamer), developed by Kyu-Sung Jeong and coworkers showcased an oligoindole-ethynylene backbone which adopted a helical conformation in solution via indole–NH hydrogen bonding to Cl− (Figure 2)34 Upon adding Cl− to a tetra-, hexa-, and octamer in CD3CN, downfield shifting of the indole–NH signals was witnessed, characteristic of hydrogen bonding. Upfield shifting of several aromatic–CH signals on only the hexa- and octamer (which were long enough to helically fold) was evidence of ring-shielding effects from aromatic stacking. In addition, NOE correlations between protons that were brought into close proximity due to helical folding were seen. These NOEs disappeared in the absence of Cl−, establishing the anion-switchability of the helical self-assembly. Cl− affinity for the octamer (1:1 binding model) was appreciable (Ka > 107 M−1 in MeCN; 2.3 × 104 M−1 in 10 % v/v H2O-MeCN), as determined by UV-Vis titration experiments. The latter association constant is impressively high considering the competitive media used, underscoring the viability of using molecular folding to create binding sites that are secluded from bulk solvent.
Figure 2.
Representative oligoindole-ethynylene foldameric backbone of a s,1-chloride foldamer developed by Jeong and coworkers that utilizes indole NH hydrogen bonds to bind to Cl−.
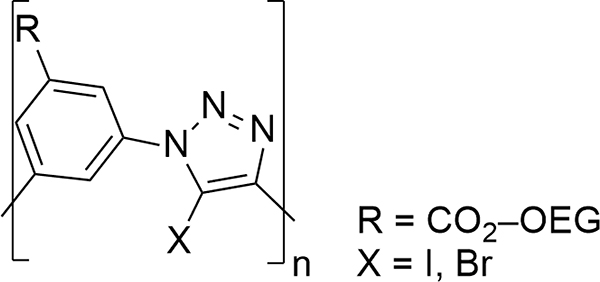
Another early example of an anion foldamer was developed by Stephen Craig and coworkers, who produced a phenylene-1,2,3-triazole-based ligand (Figure 3).35 Cu(I)-catalyzed Huisgen 1,3-dipolar cycloadditions of azides to alkynes (click chemistry) afforded 1,4-diaryl-1,2,3-triazole-containing nonamers (which is the minimum number for a helical turn) with acceptable yields. 1H 2D NOESY experiments confirmed the formation of a helical s,1-chloride foldamer in acetone-d6. Subsequent 1H NMR titration experiments measured strong binding in solution (Ka = 1.7 × 104 M−1, 1:1 binding model acetone-d6). Downfield shifting of the introverted phenylene and triazole protons suggested CH hydrogen bonding within the helical cavity. The same foldamer bound Br− with slightly lower affinity, but the binding constant dropped two orders of magnitude in the case of I−.
Figure 3.
Representative phenylene-1,2,3,-triazole foldameric backbone. Hydrogen-bond donors ortho to the “R” substituent may be appended to encourage intramolecular preorginization to create a s,1-chloride foldamer.
Craig and coworkers proposed a binding model in which an anion’s solvation sphere is replaced by the functional groups of a binding pocket.36 Furthermore, they argued that the flexibility of a foldamer can facilitate more optimal hydrogen bonding within this site than that afforded by a rigid macrocycle. Notwithstanding, macrocyclic phenylene-1,2,3-triazoles studied by Amar Flood and others typically bound halide ions more tightly than their foldameric counterparts. In addition to prepaid entropy, the authors hypothesized that the high-energy unbound state of a macrocycle (due to repulsive triazole dipoles) amounted to 5–6 kcal/mol. In contrast, an unbound foldamer can relax into a more stable “anti” arrangement of dipoles. Presumably, pre-assembled foldamers embody every productive quality described above: prepaid entropy and optimal electrostatic interactions via induced fit.
In another study, Jeong measured the chain length-dependent affinities of Cl− with a series of lengthening indolocarbazole foldamers, from monomer up to tetramer. (Figure 4)37 Association constants steadily rose from monomer to dimer to trimer, 51-fold (ΔKa= 549 M−1) from monomer to dimer, 66-fold (ΔKa=36,440 M−1) from dimer to trimer, but only 4-fold (ΔKa=103,000 M−1) from trimer to tetramer in 4:1 v/v DMSO/MeOH. Therefore, that one additional indolocarbazole unit did not contribute effectively to binding. Gas phase modeling supported that six internal NHs in the tetramer formed strong hydrogen bonds to Cl−, having bond distances of 2.5Å ± 0.2, but that the terminal NHs were too far away (3.1 Å) to be forming strong hydrogen bonds. Next, a comparison of the binding affinity of Cl− between the indolocarbazole trimer and Jeong’s biindolyl foldamer trimer was investigated. The two NHs in the indolocarbazole units are fixed in a same-side cis orientation, giving preorganization to the Cl− binding event. However, the biindolyl trimer NHs can exist trans of each other to minimize dipole-dipole repulsion by rotation of the single bonds between the indole functional groups. Then, Cl− binding could switch the NHs to the cis orientation. The Ka of the biindoyl trimer in 1:1 v/v DMSO-d6/MeOH-d3 at 24 ± 1 °C was 160 M−1, ~230-fold weaker than the indocarbazole trimer (37,000 M−1). Therefore, the indocarbazole s,1-chloride foldamers strong binding affinity is a result of preorganization and ideal binding moieties.
Figure 4.
(a) Representation of Jeong’s preorganized indolcarbazole foldameric backbone. (b) Representation of a biindolyl foldamer also developed in Jeong’s group.
Together, Jeong, Jiang and coworkers have worked towards establishing structure-activity relationships by varying the hydrogen bond donor, binding-cavity geometry, chain length, and degree of preorganization. In a recent investigation, Jiang and coworkers explored the impact of terminal functional groups on folding.38 To this end, phenylene-1,2,3-triazole pentamers terminated by methyl-ester and amide-linked N-butyl, N-benzyl, and N-pyrenylmethyl groups were synthesized. Due to aggregation of the foldamers in pure CDCl3, a mixed solvent system of 3:17 v/v DMSO-d6-CDCl3 was selected. No significant Cl−, Br−, or I− binding was detected by 1H NMR spectroscopy in the case of the methyl-ester derivative. In contrast, the N-butyl derivative, which possessed two amide hydrogen-bond donors, afforded s,1-halide foldamers (Ka = 90, 153, and 142 M−1, for Cl−, Br−, I−, respectively; 1:1 binding model). The N-benzyl groups negatively impacted halide-ion affinity due to steric clashing. However, the N-pyrenylmethyl groups slightly enhanced association because of favorable π-π stacking. These studies nicely illustrate the subtle factors that influence structure-activity relationships between oligomer primary sequence and anion binding.
Some foldamers exhibit binding to both halides and oxoanions, and we highlight two such examples here. A 15-mer phenylene-1,2,3-triazole with three interspersed ethynylene spacers was tested by Jiang and coworkers for halide-ion (Cl−, Br−, and I−) and oxoanion (NO2−, H2PO4−, HSO4−, and CH3COO−) affinity.39 In 1:9 v/v DMSO-THF, the association constants were within an order of magnitude (Ka = ~106 M−1, 1:1 binding model) as determined by UV-Vis spectroscopic titrations. Based on DFT-minimized Cl− and SO42− complexes, the flexibility of the ethynylene spacers most likely accounted for the low selectivity of the receptor. Bipyridyl-bisurea and 1,10-phenanthroline-bisurea foldamers were synthesized by Darren Johnson and Michael Haley and coworkers to chelate anions in 10 % v/v DMSO-d6-CDCl3 (Figure 5).40 1H NMR titrations were carried out with Cl−, Br−, I−, and H2PO4− by fitting the changes in urea–NH chemical shifts to a 1:1 binding model. The 1,10-phenanthroline-bisurea demonstrated a modest selectivity for Cl− (Ka = 2.6 ×102 M−1) over the larger halide ions (Ka = 6.0 × 101 M−1 for Br−). However, the truncated control molecule bearing only one urea unit bound halide ions weakly and indiscriminately (Ka = ~101 M−1 for all three). The 1,10-phenanthroline-bisurea ligand formed stable complexes with H2PO4− (Ka = 4.6 ×104 M−1) in 10 % DMSO-CHCl3, as determined by UV-Vis titrations. H2PO4− affinity for the bipyridyl-bisurea ligand was higher (Ka = 7.8 ×104 M−1) due to the superior flexibility of the host backbone.41 In an X-ray crystal structure, two MeOH molecules reside within the foldameric binding pocket (Figure 6). Interestingly, each methanol–OH hydrogen bonds to a single phenanthroline–N, while each urea unit hydrogen bonds to a separate methanolic oxygen.
Figure 5.
(a) X-ray crystal structure of a 1,10-phenanthroline-bisurea oligomer fashioned by Johnson, Haley and coworkers. (b) Representation of the same foldamer.
Figure 6.
X-ray crystal structure of an ethyl-linked three-indolocarbazole foldamer encapsulating SO42− synthesized by Jeong and coworkers (see Figure 4 for the general structure).
2.1.1.2. Oxoanions
Polyatomic oxoanions represent a unique series of biologically and environmentally relevant species that interest many chemists. Recognizing these charge-diffuse, and sometime basic, anions offers unique challenges. As such, developing binding pockets designed for these anions is necessary for strong binding. For example, oxoanions have high hydration energies, making binding challenging in competitive solvents. Foldamer chemists have creatively approached these challenges by preparing conformationally flexible hosts that fold to produce secluded binding pockets suitable for anion-binding. Examples of foldamers binding more complex oxoanions is discussed in the section on stimuli-responsive foldamers.
Jeong and coworkers synthesized a series of ethynylene oligomers containing one to five diphenylurea units. Two terminal dimethylcarbinol protecting groups provided additional hydrogen donors.42 Association constants measured in 15–40% CD3OH/ DMSO-d6 mixtures for Cl− increased with increasing chain length up to the three-diphenylurea, but plateaued between chain lengths three and four (Ka = 1340 and 1350 M−1 respectively). Incredibly, no plateauing was observed for SO42− up to a chain length of four (Ka = 2230 M−1 at 3 and >1 × 104 M−1 at four) respectively. These results illustrate the difficulty in targeting anions, as they encompass a large range of attributes like size, topology, charge, chirality, pKa etc.).
Jeong and coworkers produced a three-indolocarbazole s,1-sulfate foldamer, which was fitted with two terminal alkynyl dimethylcarbinol protecting groups to provide additional hydrogen-bond donors.43 Upon adding SO42− to the receptor in 1:1:8 v/v/v CD3OD-CD2Cl2-CD3CN, characteristic upfield shifts of terminal arene protons were seen by 1H NMR spectroscopy. 1H 2D NOESY NMR confirmed helical stacking of these arenes. The foldamer was found to be selective for SO42− (Ka = 640,000 M−1, 1:1 binding model) by two orders of magnitude above the next-best guest, Cl− as determined by fluorescence spectroscopy in 10 % v/v MeOH/MeCN. In the solid state, SO42− is held within the helical cavity of the foldamer by eight hydrogen bonds (six indolocarbazole–NH and two dimethylcarbinol–OH, (Figure 6).
In addition to hydrogen bonding interactions, π-π stacking helps to stabilize the helical conformation—a common theme for foldamers. SO42− selectivity was attributed to the dimethylcarbinol hydrogen-bond donors, which could not reach Cl−. By inserting butadiynyl spacers between the indolocarbazoles, the expanded three-indolocarbazole foldamer exhibited inferior SO42− binding. However, superior H2PO4− binding was observed (Ka = 261,000 M−1 vs. 3,600 M−1 for the ethynylene-spacer derivative, 1:1 binding model, 10 % v/v MeOH-MeCN).44
In a manner reminiscent of how oligopyridines have been elegantly used to chelate transition metals, Biao Wu and coworkers have developed oligourea receptors to target anions. In an early example, an o-phenylene-bridged four-urea s,1-sulfate foldamer was fashioned in competitive media.45 An X-ray crystal structure of the p-nitrophenyl-capped oligomer binding SO42− was obtained. Eight hydrogen bonds in a pseudo-square-planar coordination geometry (when each urea is considered as a monodentate coordination vector; Figure 7). Binding studies in 0, 10, and 25 % H2O-DMSO (assessed by UV-Vis spectroscopy) revealed that the foldamer could be further enhanced by replacing the naphthyl-capped oligomer exhibited superior water-resistant SO42− binding over its p-nitrophenyl derivative. The Log Ka (1:1 binding model) for the naphthyl capped derivative in 25 % H2O-DMSO was 4.87.
Figure 7.
(a) X-ray crystal structure of an o-phenylene-bridged s,1-sulfate foldamer developed by Wu and coworkers that chelates SO42− by eight NH hydrogen bonds in a pseudo-square-planar geometry. Replacing the p-nitrophenyl cap with a napthalene cap further increased sulfate binding in mixed H2O-DMSO sytems. (b) Representation of the general structure of a bisurea foldameric backbone.
Binding SO42− in aqueous solution is challenging in part because of it’s large hydration energy (Gh = −1,080 KJ mol−1). Nevertheless, its biological and environmental relevance makes developing strong, water soluble, and selective sensors important. For this task, Bowman-James and coworkers created 2,6-dicarboxamide “pincer-based” amide and urea-based s,1-sulfate foldamers, which take advantage of the chelate effect for anion host design.46 Noteably, these foldamers were selective for SO42− by establishing multiple hydrogen bond donors preorganized for tetrahedral anions. It was found that the urea systems bound stronger than the amide ones, overcoming the hydration energy in up to 1:1 water-mixed DMSO systems. The longest foldamer, containing eight urea-based binding sites, formed a complex binding a singular SO42− anion via six hydrogen bonds. (Figure 8).
Figure 8.
X-ray crystal structure of an oligourea s,1-sulfate foldamer developed by Bowman-James and coworkers (see Figure 7 for the general structure).
Jeong prepared an indolocarbazole dimer, consisting of eight hydrogen bond donors, four indole NHs and four urea NHs, which cap the foldamer.47 This indolocarbazole foldamer was found to fold via intramolecular hydrogen bonds and dipole-dipole interactions between indolocarbazole NH units and carbonyl groups. Identified through 2D-ROESY experiments, NOE correlations between NH and CH indolocarbazole signals were a result of helical folding. The dimer was found to be selective for oxoanions, particularly SO42−, which had a Ka of 71,000 M−1 in 5 % CD3OH/CD2Cl2, almost 5-fold larger than CH3COO− (15,000 M−1), and 15.7-fold larger than Cl− (4,500 M−1). Likely, SO42− was an ideal fit for this foldamer, as NMR reveals downfield shifting for all NH signals, whereas upon the addition of Cl−, a much weaker guest, downfield shifts for only the indolocarbazole NHs were observed.
Guichard and coworkers investigated the anion-binding properties of oligourea foldamers containing a peptide sidechain of valine, alanine, and leucine which fold without guest by forming hydrogen bonds between urea groups, while leaving terminal ureas free for guest binding.48 (Figure 9a) Guichard first examined Cl− recognition of the oligourea foldamer. Using 1H NMR in DMSO-d6/CD3CN 5:95 v/v, the addition of Cl− produced a downfield shift at the terminal NHs (Δδ 1.11 and 0.75 ppm). Job plot analysis revealed a 1:1 binding mode, (Ka= 1,700–2,900 M−1). Signals near the terminal shifted the second-most (Δδ 0.28 and 0.31 ppm), a likely result of electronic changes affecting this urea when Cl− was bound. Ureas further down the chain, or at the peptide side chain, did not significantly shift, which suggested that the folded helical conformation was maintained, and these ureas were not participating in binding. Next, Guichard screened anion activity. In the same solvent, CH3COO− bound more strongly than Cl− (Ka= 3,300–3,700 M−1) but maintained a similar 1:1 binding mode. However, H2PO4− caused a strong downfield shift of the first and second ureas. Job plot analysis suggested a 1:2 complex with two H2PO4− anions, thus selectively forming a s,2-dihydrogen phosphate foldamer in the presence of this anion.
Figure 9.
Representative structures of Guichards oligourea foldamers: (a) p-bromophenyl capped, (b) t-butyl capped, and (c) 1H-indol-7-yl-urea capped.
The length of the foldamer was also an important determinate of its folding and anion binding. A comparison between this hexamer and a nonamer with an additional Val-Ala-Leu repeat unit revealed that a s,1-chloride foldamer formed. Likely, the chain length of oligourea foldamers did not modify the folding orientation. But how did the second urea linkage affect the anion binding process? By replacing the 4-bromophenyl-urea group with a tert-butyl carbamate group, Guichard and coworkers investigated how the removal of the terminal NH affected binding (Figure 9b). Upon titration with tetrabutylammonium acetate (TBA+CH3COO−), binding was an order of magnitude smaller than the hexamer (Ka= 4,500 M−1 and 22,200 M−1 respectively). However, the terminal site could be modified to improve binding. This time, the 4-bromophenyl-urea moiety was replaced with 1H-indol-7-yl-urea, a group known to selectively bind oxoanions. Again, in a solution of CD3CN containing 5 % DMSO-d6, titrated with TBA+Cl−, downfield shift of the first three urea signals was significant, suggesting a binding contribution from multiple urea groups. The modified site produced a binding Ka > 104 M−1, approximately 4–5-fold stronger than the original hexamer in the same solvent. This oligomer also formed a s,1-acetate foldamers (Ka= 3,800 M−1) in DMSO-d6 (Figure 9c). Therefore, modifications to cap ureas significantly altered anion binding affinity in oligoureas.
Chmielewski and coworkers created a linear, uncharged hydrogen bonding oligomer which folds selectively around SO42− and PhCOO− guests.49 It consists of a bis-diamidocarbazole backbone, which wraps assymetrically around tetrahedral SO42− and PhCOO− in a 1:1 complex. In contrast, Cl− bound only weakly to a single carbazole unit. Additionally, the ligand was slectively formed s,1-sulfate foldamers over other oxoanions. In a 9:1 DMSO-d6:D2O mixture, SO42− was bound 30 times more strongly (Ka=105.47 M− 1:1 binding mode) than H2PO4−, and 360 times more strongly than PhCOO−. Other highly basic oxoanions like HPO42−, PO43− and CO32− deprotonated the oligomer, which consequently provided another route to s,1-sulfate foldamer selectivity. It was theorized that the tight binding of the foldamer to SO42− was a result of its three dimensional structure, which shielded SO42− from solvent. Increasing the water content to the limit of the foldamer’s solubility at NMR concentrations (25 % D2O:75 % DMSO-d6) resulted in surprsingly high association (Ka=103.73 M−1), presumably aided by hydrophobic collapse of the foldamer. Additionally, the carbazole groups acted as fluorophores which were proximally activated during the binding event. SO42− binding induced the largest fluoresence increase, producing a way to sense SO42−.
2.1.1.3. Binding in Water
Anion binding in aqueous systems is especially challenging, both because specialized functionality is required to solubilize large organic molecules in water, and large hydration penalties must be paid to overcome the stability of anionic species solvated by water. However, foldamers can be uniquely employed to take advantage of favorable entropic gains upon hydrophobic collapse in aqueous solvents. Several synthetic foldamers possess internal cavities capable of sequestering anionic guests from bulk solution, even in pure water.
To enhance the water solubility of their foldamers, Jeong and coworkers functionalized an oligoindolocarbazole-ethynylene backbone with sodium carboxylates.50 In D2O, the three-indolocarbazole adopted a collapsed form, as indicated by the upfield shifts (0.4–1.0 ppm) relative to the mono-indolcarbazole of the benzoate hydrogens on the terminal of the foldamer. These data suggested that the longer oligomer adopted a partially folded conformation in water without guest. Upon adding NaCl, further upfield shifting of several benzoate hydrogens evidenced s,1-chlroide foldamer creation. This folded conformation was corroborated by a 1H 2D ROESY experiment. The Ka of the Cl− adduct in D2O was 65 M−1 (1:1 binding model). This binding constant was impressive given the significant penalty associated with dehydrating Cl− (~81 kcal/mol). The seminal work of Jeong and coworkers has helped to establish the power of foldamer-based anion recognition in pure water.
Hua Jiang and group synthesized cationic phenylene-1,2,3-triazole oligomers with water-soluble side chains (quaternary ammonium salts).51 Like m-arylene-ethynylene foldamers, these phenylene-1,2,3-triazoles existed as random coils in nonpolar solvents like MeOH but adopted helical conformations in water (even without a guest). This behavior was confirmed by the marked 1H NMR upshifts of aromatic protons with increasing D2O content. When the solvent reached 80 % D2O-CD3CN, broadening of the resonances was also noticed, which evidenced aggregation. UV-Vis spectroscopic experiments at lower concentrations of ligand revealed a hypochromic response with increasing H2O content, which was indicative of π-π stacking. When plotting the degree of foldedness vs. % H2O, cooperative, sigmoidal relationships were observed for the longer oligomers, whereas a more linear trend was evident for the shorter chains. The chiral derivative—with a terminally-appended (S)-arylethylamido group—exhibited negative CD responses with increasing H2O content. DLS and CD spectroscopy allowed for the characterization of higher-order helical columns that formed in aqueous media. In 75 % H2O-MeOH, Cl− and to a lesser degree F− induced hypochromic changes in the UV-Vis spectra, suggesting enhancement of the folded state. Additionally, the binding of anions retarded higher-order aggregation, possibly due to anion-anion repulsion.
2.1.1.4. Larger anions
To accommodate larger anionic guests, specialized foldamers with expanded binding pockets have been created. Xin Zhao, Zhan-Ting Li and coworkers have developed aromatic-oligoamide foldamers with expanded helical cavities that enwrap organic anions. To bind benzene-1,3,5-tricarboxylate, Zhao and Z.-T. Li and coworkers designed aromatic oligoamides with alternating benzene and naphthalene units (Figure 10).52
Figure 10.
Representative structure of oligomers with alternating benzene and naphthalene units created by Zhao, Li, and coworkers.
Interestingly, the free heptamer and anion complex exchanged slowly on the NMR timescale in DMSO-d6. When more than one equivalent of benzene-1,3,5-tricarboxylate guest was added, free host signals could no longer be detected, which suggested tight binding. Based on the marked downfield shifting of numerous amide–NH and CH protons, it was deduced that strong intermolecular hydrogen bonding occurred in solution. In contrast to the pentamer, the majority of the heptamer terminal naphthalene protons experienced upshifts, consistent with helical folding. The nonamer exhibited similar behavior in solution. Additionally, 1H 2D NOESY spectroscopy confirmed the helical folding of both oligomers around their guest as evidenced by intra- and intermolecular NOEs. The nonamer formed s,1-benzene-1,3,5-tricarboxylate foldamers strongly in DMSO (Ka = 5.5 ×106 M−1, 1:1 binding model), as determined by UV-Vis spectroscopy. The heptamer, in contrast, performed inferiorly in terms of guest binding by an order of magnitude.
Zhao, Li, and coworkers next created m-substituted-benzamides—a tri-, pent-, and heptamer—to bind mono-, di-, and tricarboxylate anions.53 In DMSO-d6, downfield shifting of the amide–NH protons on the pent- and heptamer upon adding benzene-1,3,5-tricarboxylate was noted, which was consistent with strong hydrogen bonding in solution. 2D NOESY NMR experiments evinced both intra- and intermolecular through-space interactions for the complexes involving both ligands, confirming s,1-benzene-1,3,5-tricarboxylate foldamer formation. However, the association constants were low (Ka = ~102 M−1 for both complexes, 1:1 binding model), which suggested poor host-guest complementarity. Screening the heptamer against mono-, di-, and tri- benzene carboxylates with varying substitution patterns did not afford higher affinities. Noteworthy, however, was the chiral induction afforded by L- and D-glutamate, as confirmed by CD spectroscopy in CHCl3.
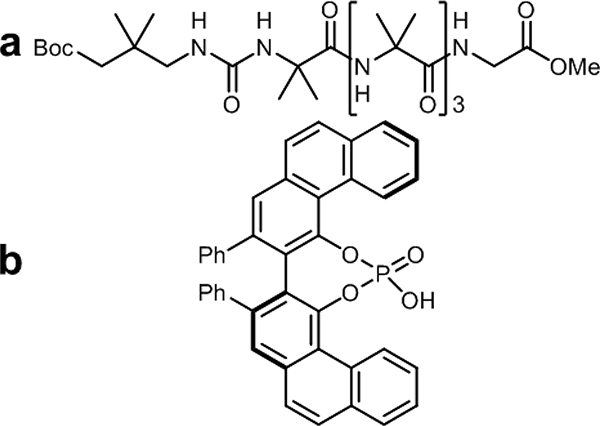
Larger guests are also able to be chiral. In a different report, Clayden and coworkers explored selective binding of chiral organophosphates with their achiral amide foldamers.54 To study chiral foldamer formation, four amide foldamers were capped with ureas containing 13C-labeled methyl groups. So long as the methyl groups did not participate in binding, the ratio of the methyl signals could be measured as proportional to the ratio of the two screw-sense conformations. Indeed, these labeled methyl groups had identical chemical shifts when both screw-sense conformations were equivalent but became asynchronous when unequal ratios of conformers were present. Each foldamer was mixed with a threefold excess of phosphoric acid in THF-d8 and proton sponge (1,8-bis(dimethylamino)naphthalene) was added to produce PO4− in situ. 13C NMR with increasing amounts of proton sponge revealed the resulting change in synchronicity in each foldamer. The urea capped oligomer produced a small asynchronous signal in the absence of base, but upon addition of up to three equivalents of proton sponge, all four produced an asynchronous signal. The Boc capped substituted oligomer, which produced the largest Δδ (28 % helical excess) was chosen for further experiments (Figure 11). Changing the solvent from THF-d8 to CD3CN decreased the helical excess to 16 %, reducing screw sense preference in a more polar solvent system. Clayden determined that the concentration of the foldamer-phosphate mixture influences screw-sense preference. Interestingly, when foldamer concentrations were above 2 mM in THF-d8 no conformational change occurred, but below 2 mM, screw-sense preference decreased as concentration decreased.
Figure 11.
(a) Representative structure of achiral urea-capped foldamer backbone developed by Clayden and coworkers. (b) A chiral phosphoric acid used to provide screw-sense preference for the helical conformation.
To further influence how a phosphate anion induces screw-sense preference, four organophosphoric acids were added. Their ability to induce conformational change was monitored again via Δδ. (R)-VAPOL-derived acid (Figure 11b) resulted in the highest induced conformational preference, likely due to steric interactions between the biaryl unit and the foldamer. In the case of all phosphoric acids used, the Δδ increased upon addition of base or phosphoric acid guest up to 32 % helical excess, and this excess was reversable and reproducible upon dilution and changing the concentration of the anion. To determine helical handedness, the foldamer was modified with enantioselectively labeled Me groups. A pro-R Me group labeled 75 % 13C and the pro-S group labeled 25 % 13C were appended, and by measuring the major and minor peaks in the 13C NMR spectrum, it was determined that acid (Figure 11b) induced a right-handed helix, while the (S)-VAPOL-derived stereoisomer induced a left-handed helix in excess.
2.1.2. Chirality
Without a means to bias the population, helical oligomers are typically racemic in solution (an equal ratio of P and M enantiomers). To influence the relative populations, chemists use chiral guests or append chiral groups to their oligomers. Utilizing the latter approach, Jeong and coworkers appended (1S)- or (1R)-phenylethylamido groups to the termini of their oligoindole-ethynylene foldamer (Figure 2).55 Prior to adding anions, almost no CD signal was detected in CH2Cl2. However, upon adding Cl− to the (1S)-phenylethylamido-functionalized oligomer, strong and positive CD signals corresponding to the absorption wavelengths of benzoate and biindole functional groups were seen. This spectroscopic response intensified with increasing Cl− concentration. Repeating the experiment with the (1R)-phenylethylamido derivative resulted in an identical CD response but with the opposite Cotton effect.
By appending terminal amides to an indolocarbazole dimer spaced by butadiynyl linkers, intramolecular hydrogen bonding between the indolocarbazole–NHs and the amide oxygens was realized.56 Attachment of (S)-arylethylamido groups to the oligomer termini led to the preferential formation of left-handed (M) s,1-chloride foldamers, as measured by CD spectroscopy in CH2Cl2, MeCN, acetone, and DMSO. As the solvent polarity increased, the CD-signal intensity decreased (especially in DMSO). Polar media effectively disrupted intramolecular hydrogen bonding and folding sans guest. Attachment of (R)-arylethylamido groups resulted in the same CD features with opposite Cotton effects. Interestingly, when the left-handed isomer was mixed with SO42− (õne equivalent) in CH2Cl2, a total switch of helical sense was witnessed. However, when SO42− was added to the guestless right-handed foldamer, its helical sense did not change. In the X-ray crystal structure of the s,1-sulfate foldamer (Figure 12), SO42− was held by four indolocarbazole–NHs and two amide–NHs in an overall pseudo-square-planar coordination geometry (if each donor unit is considered as a monodentate coordination vector). These studies introduced a powerful way to realize anion-switchable chirality.
Figure 12.
X-ray crystal structure of a two-indolocarbazole s,1-sulfate foldamer seperated by butadiynyl linkers and capped with (S)-arylethylamido groups synthesized by Jeong and coworkers (see Figure 2 for the general foldameric backbone structure).
The folding and chiroptical properties of a three-indolocarbazole-ethynylene oligomer bearing terminal amide-linked (S)-arylethylamido groups was investigated by Jeong and coworkers57 In nonpolar solvents, strong negative Cotton effects in the CD spectra were evident, whereas in polar solvents (acetone, MeCN, and DMSO) these signals were abolished. Similar polar-solvent-induced disruptions of folding sans guest were seen with the butadiynyl-linked three-indolocarbazole. Interestingly, adding anions of appropriate size (Cl−, Br−, or CH3COO− in the present study) resulted in inversions of the CD spectra in CH2Cl2. Based on an X-ray crystal structure of the Cl− complex, helical folding was determined to be left-handed (Figure 13). All six indolocarbazole–NHs hydrogen bond to a single, intracavity Cl−. These results suggest that both the helicity and chirality of this class of foldamers is highly solvent- and guest-responsive.
Figure 13.
X-ray crystal structure, obtained by Jeong and coworkers, of a left-handed three-indolocarbazole-ethynylene s,1-chloride foldamer with two terminal amide-linked (S)-arylethylamido groups (see Figure 2 for the general structure).
Jeong further developed his oligoindole foldamers by modifying them with chiral (S,S) or (R,R) 1-phenylethylamido groups.58 Each biindole unit adopted a trans conformation between the ring, which developed into a zig-zag-like conformation. Upon the addition of Cl−, these units folded into a helix with four indoles per turn. NMR and CD demonstrated the chirality of the helix, which was found to form only one helical isomer. Two of the three indole NH signals shifted downfield (Δ ppm= 2.5, 0.4 ppm for the longer foldamer in acetone-d6), which signified hydrogen bonding to the Cl− guest. However, terminal indoles did not appear to be involved in hydrogen bonding. By creating a similar s,1-chloride foldamer with the (R,R)-configuration of the 1-phenylethylamido groups, the CD spectra in 1:1 v/v CH3CN/CH2Cl2 developed the opposite Cotton effect. Therefore, the chirality of the phenylethylamido unit transfers to the folding of the helix, biasing the formation of a single isomer. These studies are leading the pursuit of other foldamers whose chirality can be reversibly controlled by chemical stimulus.
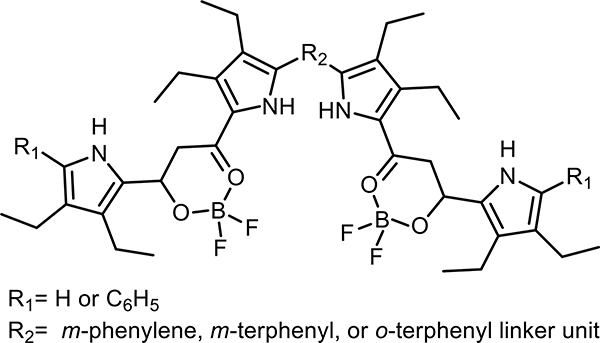
Hiromitsu Maeda, Jiang, and coworkers have developed both versatile mono- and poly-anion foldamers. Maeda and coworkers introduced a tractable strategy for chiral induction using chiral countercations.59 These π-conjugated salts (binaphthylammonium Cl− and Br−) induced the chiral folding of boron-difluoride complexes of 1,3-dipyrrolyl-1,3-propanedione oligomers (s,1-binaphthylammonium 1-halide foldamers, Figure 14). In the presence of the (R,R) countercation, the four-pyrrole oligomer in CH2Cl2 generated Cotton effects associated with the excitonic interaction between the two receptor arms connected by the m-phenylene linker. Time-dependent DFT suggested that the M-type diastereomeric ion-pair formed preferentially. The foldameric complex was also characterized using 1H NMR spectroscopy. With 1.5 equivalents of (R,R)-binaphthylammonium Cl− at −50 °C, two sets of resonances corresponding to slow-exchanging M and P helices (50:32 ratio, respectively) could be seen. In one of the few kinetic studies of an anion foldamer, EXSY NMR was utilized to determine a rate constant of 3.8 s−1 for the M-to-P conversion.
Figure 14.
Representative structure of boron-difluoride complexes of 1,3-dipyrrolyl-1,3-propanedione oligomers developed by Maeda and coworkers.
The same dipyrrolyldiketone ligands with either an m-terphenyl or o-terphenyl linker were synthesized to target L-amino acid anions.60 Both foldamers formed helical complexes with Cl− or CH3COO− at low temperatures, as confirmed by 1H NMR and ROESY NMR spectroscopy in CD2Cl2. Additionally, both foldamers with addition of anionic L-phenylalanine produced enhanced Cotton effects in CH2Cl2 at 20 °C, indicative of chiral induction. Anionic D-phenylalanine rendered the opposite CD patterns. With two of the few foldamers designed to target chiral anions, Maeda and coworkers demonstrated the potent chiroptical properties of these synthetic systems.
2.1.3. Stimuli-responsive
In nature, the activity of biopolymers is modulated through allosteric regulation, phosphorylation, and other post-translational modifications and protein-protein interactions. To this end, the development of foldamers whose form and function is controlled in a stimuli-responsive manner is a new and exciting area of development. To date, few synthetic foldamers and anion helices have stimuli-responsive properties. However, given the exciting promise that stimuli-responsive foldamers can externally regulate function, this is an area of research that is sure to see amazing developments in the future. In this section, we highlight the early progress that has been made on stimuli-response foldamers.
2.1.3.1. Light
Photochemistry offers a trackable and exciting way to control foldamer structure in a spatial and temporal manner. To this end, Jiang and coworkers developed a light-switchable phenyl-1,2,3-triazole s,1-halide foldamer, whose affinity for anions could be modulated through reversible photoisomerization of the ligand.61 Two phenyl-1,2,3-triazole units were attached to an azobenzene core (Figure 15). The trans azo linker encouraged an overall extended helical conformation (corroborated by 1H NOESY NMR in acetone-d6). The cis azo conformation was activated by UV irradiation (365 nm), resulting in a constricted, scissor-like conformation of the ligand. By storing the cis ligand in the dark for 10 days, the trans conformation was restored. Interestingly, the cis ligand bound anions more strongly than the trans (four-fold greater affinity in the case of Cl−, Ka,cis = 290 M−1 in acetone-d6, 1:1 binding model). This was likely because the inner cavity of the cis conformer was smaller than trans, which allowed it to bind more tightly to smaller anions.
Figure 15.
Representative structure of a photoswitchable phenyl-triazole foldamer backbone developed by Jiang. The photoactive azo group enables light induced conformational changes of these s,1-halide foldamers.
Flood and lab have contributed much to our understanding of phenylene-1,2,3-triazole foldamers. In an effort to create bioinspired supramolecules whose active/inactive conformations are reversible and stimuli-responsive, Flood and coworkers synthesized a chiral phenylene-1,2,3-triazole nine-mer terminated by two azobenzene groups to enable cis/trans photoisomerization (see Figure 3 for the general structure without azo groups).62 Placing the azobenzenes at the termini of the foldamer rather than the center was a unique approach. In the more thermodynamically favorable trans form, the azobenzene units were coplanar with the rest of the oligomeric backbone. By design, the cis form disrupts coplanarity, π-π stacking, and helical folding. Photoisomerization of the two azobenzenes introduced three possible isomers: trans-trans, trans-cis, and cis-cis. When exposed to visible light in MeCN, the photostationary-states were roughly 67:30:3 % (trans-trans, trans-cis, and cis-cis, respectively), as determined by RP-HPLC. In contrast, when the oligomers were exposed to UV light (365 nm), the ratios changed to 0:33:66 %. In the dark, the oligomers (predominately in the trans-trans form) bound Cl− with an Ka of 3,000 M−1 (based on UV-Vis titrations in CHCl3). After exposure to 365-nm UV light, the Ka dropped appreciably to 380 M−1. Exposure to 436-nm visible light restored the predominately all-trans isomer and its original Cl− affinity. In parallel, conductivity experiments with equimolar concentrations of the foldamer and Cl− (1 mM) were conducted. The free Cl− concentration was estimated to be 0.23 mM in the presence of the predominately all-trans photostationary state. Upon exposure to 365-nm UV light, the free Cl− concentration increased to 0.56 mM. Moreover, a concomitant increase in conductivity was observed (128 to 135 μS cm−1). Exposing the solution to visible light resulted in a conductivity decrease to almost the original level. This process could be repeated multiple times, illustrating the ability of photoresponsive foldamers to control Cl− concentrations in bulk solution. In another study, the same nine-mer sans azobenzene terminal groups bound Cl− less strongly than its macrocyclic counterpart in CDCl3.63
To improve the overall difference in Cl− binding upon irradiation, Flood and coworkers incorporated a β-sheet-like hydrogen bonding array to interlock the folded helical backbone.64 UV-Vis titrations in 50 % v/v MeCN-THF revealed that the 13-mer without the array exhibited only a 17-fold difference in binding upon UV irradiation. Incorporating the peptide-like array to the oligomeric backbone resulted in an impressive 84-fold difference, illustrating the creativity and power of this approach.
Flood and coworkers further utilized the aryl-triazole based system to study allosteric regulation.65 In biology, allosteric control is a common method of regulating the activity of enzymes. However, regulation of foldamer function is still in its infancy. Already, the structure of foldamers is affected by various stimuli like binding, temperature, and solvent, among others. In this work, a covalently linked azobenzene acted as a photoswitch, which changed the cis/trans conformation of the foldamer to promote random coil or helical structure. They initially began this study to discern the affect that anion size had on binding affinity, but amazingly found that the anion size controlled the formation of a s or d,1-anion foldamer. Smaller anions < 4.5 Å3 Cl−, Br−, NO2−, and NO3− formed s,1-anion foldamers, but anions > 4.5 Å3 SCN−, BF4−, ClO4−, ReO4−, PF6−, and SbF6− formed d,1-anion foldamers. Therefore, anion size determined foldamer type. However, it was realized by CD spectroscopy and 1H NMR that the larger anions created a chiroptical function between a chiral d,1-anion foldamer and racemic s,1-anion foldamer formation. The formation was still solvent dependent. Moderately polar solvents like THF, CHCl3 and CH2Cl2 created no CD signal. However, upon the addition of MeCN CD signal increased. Anions which favored s,1-anion foldamers also produced no CD response, like Cl−, which destroyed CD response upon the addition of just one equivalent. Five equivalents of perchlorate, on the other hand, induced little loss in CD signal intensity. It was hypothesized that CH hydrogen bonding to Cl− disrupted the weak interactions that create the chiral d,1-anion foldamer. Furthermore, the expansion of the helical pore necessary for larger anions in conjunction with a decrease π-stacking in the s,1-anion foldamer promoted multi strand formation. The d,1-anion foldamer was additionally stabilized by reduced torsion angle compared to its s,1-anion foldamer counterpart, and increased π-stacking between the two strands overwhelms the entropic penalty.
2.1.3.2. Acid/Base
As a chemical stimulus, acid/base chemistry is an attractive route to modulate structure. The typically fast reaction kinetics, reversibility and ubiquity are factors leading to its regular occurrence in the literature. For example, using the ethynylene-linked three-indolocarbazole with two terminal dimethylcarbinol protecting groups, Jeong and coworkers next targeted chiral organic anions to induce enantiomeric folding (see Figure 4 for the general structure).66 In CH2Cl2, the guestless oligomer was CD silent, and the addition of SO42− did not produce a CD signal. However, with addition of (R)-10-camphorsulfonate, (s-1-(R)-10-camphorsulfonate foldamer formation) strong CD signals with a positive Cotton effect (attributed to the exciton coupling of indolocarbazole chromophores) were observed. Complete inversion of the CD spectrum resulted when (S)-10-camphorsulfonate was added to the same oligomer (s-1-(S)-10-camphorsulfonate foldamer formation). Thus, by adding either the (R) or (S) organic anion, biased formation of the corresponding diastereomeric helical complex could be achieved. On-off acid-base controlled switching was accomplished by adding adenosine 3’,5’-cyclic monophosphate to the oligomer, which induced a CD signal. Through protonation of the chiral guest with trifluoracetic acid, anion binding became negligible, and the CD signal was turned off. Adding a base, 1,4-diazabicyclo[2.2.2]octane, to solution resulted in almost complete recovery of the CD signal. This cycle could be repeated many times with nearly the same result.
In a separate study, Jiang and coworkers designed a phenylene-1,2,3-triazole with a central resorcinol group to serve as a switch regulator(Figure 16).67 To preorganize the ligand, extroverted acyl-amino groups were appended to the oligomeric backbone. Deprotonation of the resorcinol–OHs (pKa = 9.44) led to the rearrangement of the hydrogen bonding network along the backbone of the oligomer. Specifically, triazole intramolecular hydrogen bonding to the central resorcinolate deactivated these two hydrogen-bond donors, inducing an open “W” conformation of the ligand.
Figure 16.
Representative structure of pH induced switching of phenylene-1,2,3,-triazole developed by Jiang and coworkers.
Thus, the authors could induce the “W” conformation with two ligand equivalents of basic 1,8-diazabicyclo[5.4.0]undec-7-ene (Figure 16b) and restore the helical conformation with picric acid (Figure 16a). In the presence of Cl− in 3:47 v/v DMSO-d6-CDCl3, intracavity protons downshifted, whereas exterior aryl protons barely shifted. Terminal aryl protons, however, shifted upfield because of ring-current effects. This anion-induced folding was confirmed by 1H NOESY NMR spectroscopy, and a 1:1 Ka of 8.1 × 104 M−1 was determined for the s,1-chloride foldamer. In stark contrast, Cl− affinity for the deprotonated, “W” s,1-chloride foldamer was 260-fold lower (Ka = 308 M−1, 1:1 binding model). Interconversion of the isomers proved facile even in the presence of Cl−.
By incorporating two pyridinium units into a 3,5-bis(triazole)-pyridine motif, Jiang and coworkers introduced charge-assisted CH hydrogen-bond donors to their foldamer.68 This strategy allowed the authors to achieve appreciable halide-ion affinity in competitive aqueous media. 1H NMR titrations of their nonamer with Cl−, Br−, and I− in 3:47 v/v D2O-pyridine-d5 afforded impressive and similar binding constants (Ka = 4.62 × 104, 6.99 × 104, 3.32 × 104, M−1 respectively, 1:1 binding model).
2.1.4. Fluorescence Change
Often, foldamers are constructed with rigid, highly conjugated molecules, resulting in unique spectrophotometric properties. Conveniently, Jeong’s oligoindole-ethynylenes proved strongly fluorescent in the absence of anion. Adding Cl− to foldamers of sufficient length (hex-, oct-, and decamers) in 20 % v/v MeOH-CHCl3 led to large hypochromic and bathochromic shifts of the emission bands, likely arising from intramolecular excimer formation in the aromatic arrays.69 For the shorter tetramer, the emission band was unperturbed by the addition of Cl−. Aside from possessing strong Cl− affinity (as established earlier), the decamer also bound F− quite strongly in 20 % v/v MeOH-CH2Cl2 (Ka = 1.2 × 106 M−1, 1:1 binding model)
2.1.5. Organocatalysis
The field of green chemistry has promoted organocatalysts to provide sustainable routes to specific drugs and other materials. Commonly, chemists take inspiration from biology to design these new catalysts. Helical chirality and selective anion binding are two strategies utilized by nature to achieve enantioselective chemical transformations. However, the efficient transfer of chirality from a helical organocatalyst is rare. To this end, Mancheño and coworkers synthesized phenelene-1,2,3,-triazole nonamer, which included a trans-1,2-diaminocyclohexyl core unit to preorganize the helical scaffold and bias one-handed folding.70 The (R,R) and (S,S) catalysts accelerated enantioselective dearomatization of quinolines (96:4 and 4:96 e.r., respectively) via C2-selective nucleophilic addition of silyl ketene acetals. Mechanistically, the Cl− complexation of a preformed N-acylquinolinium salt helped bring the catalyst and substrate in close proximity, whereby substrate interaction with the M or P helical backbone prior to nucleophilic attack resulted in efficient chiral transfer (Figure 17).
Figure 17.
Cartoon representation of steroselective catalysis utilizing Mancheño and coworkers anion-acceptor triazole nonamer. Reprinted (Adapted or Reprinted in part) with permission from Zurro, M.; Asmus, S.; Beckendorf, S.; Mück-Lichtenfeld, C.; Mancheño, O. G. Chiral Helical Oligotriazoles: New Class of Anion-Binding Catalysts for the Asymmetric Dearomatization of Electron-Deficient N – Heteroarenes. J. Am. Chem. Soc. 2014, 136 (40), 13999–14002. Copyright 2014 American Chemical Society
Mancheño and Theresa Fischer continued to use this foldamer to catalyze enantioselective dearomatization reactions of pyridines.71 The dearomatization of 2-picoline in the presence of the (R,R) and (S,S) catalysts provided high selectivity. (97:3, 49:6 e.r., respectively). Additional reactions with other pyridines resulted in similarly high yields and enantioselectivity, outperforming some squaramide and thiourea catalysts. In one such case, the dearomatization of 1,2-dihydroxypyridines resulted in an almost perfect e.r. of 99:1. Mancheño and Fischer saw similar enantioselectivity in Reissert-type dearomatization of isoquinoline derivatives (86:14 e.r)72 and diazarenes (92:8)73, insinuating a broad range of heterocycle reactivity, and regioselectivity, even with multiple reactive sites. It was determined that solvent and acylating agent were also determining factors of enantioselectivities. Given the efficiency of the helix to transmit chiral information, this new and exciting application of synthetic anion foldamers bodes well for the development of other anion foldamer catalysts in the future.
2.1.6. Anion Transporting Foldamers
For a molecule to function as an anion transporter it must move a polar anion across a lipophilic membrane. The unique ability of anion foldamers to induce secondary structure and shield polar functionality engenders suitable properties for anion transport. For example, anion foldamers typically bind anions within their interiors. In doing so, the polar anion is shielded from the surrounding environment and nonpolar functional groups can be projected toward the surrounding nonpolar portion of the lipid in the bilayer. In general, appreciable anion binding is a beneficial trait for anion transport. However, suggestive transport data by Matile and lab has shown that in some systems, stronger anion binding can reduce transport. Therefore, there may be an important balance between binding thermodynamics and kinetics.74 Cleary, further study of anion foldamer transporters are needed to better understand anion transport in foldamer systems. Measuring binding affinity can provide complementary information to anion transport assays and both can be used to further understand anion transport in anion foldamers. To date, smaller pseudofoldamers have been created which show that foldamers are good candidates for anion transport75, but so far, only two anion transporting foldamers have been made.
Enhancing preorganization and improving folding can impart anion transport function. Jiang and coworkers used intramolecular hydrogen bonding to preorganize the backbone of their earlier systems. Installing amide functionalities on aromatic rings that are adjacent to the triazole rings allowed for intramolecular hydrogen bonding to the N2 and N3 triazole nitrogens (Figure 18).76 The induced partial or total preorganization resulted in enhanced folding. The fully preorganized aryl-triazole s,1-anion foldamer bound Cl−, Br− and I− (Ka = 757, 367, and 134 M−1 respectively, in CD2Cl2) significantly greater than a similar foldamer with two fewer preorganizing amide N-H hydrogen bonds. (Ka = 91, 109, and 86 M−1 respectively, in CD2Cl2). The eight-fold greater binding was a result of increased preorganization that reduced the entropic penalty in binding. Interestingly, the semi-preorganized structure allowed for enough flexibility to bind to Br− more strongly than the other halides.
Figure 18.
X-ray crystal structure of an aryltriazole foldamer backbone developed by Jiang where preorganization is induced by amide NH hydrogen bonds to the triazole acceptors (see Figure 3 for the general structure).
Lucigenin Cl− selective assays were used to determine the anion transport ability in these systems. Lucigenin is a chemiluminescent compound used in this experiment whose fluorescence is quenched by Cl−. Valinomycin is also used in this experiment and acts as a cation uniporter to assist the foldamer in a K+/ Cl− symport mechanism. The fully preorganized foldamer had a 65% greater normalized fluorescence change than the non-preorganized version as determined from the Lucigenin transport assays with and without the valinomycin, signifying that it transported Cl− at a greater rate. Furthermore, bilayer lipid membrane conductance studies revealed that this foldamer acts via an anion carrier mechanism and does not form a channel within the bilayer.
Taking inspiration from other ortho-phenylene bisurea systems, Davis and coworkers created a tetraurea foldamer that functioned as an anion transporter (Figure 19).77 1H NMR titrations were used to assess the ability of these molecules to bind chloride. TBA+ Cl− binding studies in 95% DMSO-d6 with 0.5 % H2O suggested 1:1 binding with a Ka = 8.7 × 102 M−1. A bisurea small molecule precursor also bound Cl− with a comparable magnitude (Figure 19b). The similar binding of the foldamer and the bisurea was attributed to competitive intramolecular hydrogen bonding interactions in the tetraurea foldamer, which was observed in the unbound state. Lucigenin transport assays on this foldamer in POPC and cholesterol liposomes at a 1:1000 ratio showed that this molecule acts as a Cl− transporter. Interestingly, the smaller molecule (Figure 19b) also showed similar but greater transport (~37% vs. ~45% decrease in normalized fluorescence for the small molecule and CF3 functionalized foldamer respectively), suggesting some correlation between binding and transport in these systems.
Figure 19.
Representative structure of (a) anion transporting tetra-urea foldamer backbone and (b) bisurea small molecule receptor created by Davis and lab.
2.1.7. Halogen Bonding Foldamers
The previously discussed anion foldamers utilized hydrogen bonding to chelate anions within their helical cavities. In contrast, despite its strong directionality and unique electronics, halogen bonding78,79,88–92,80–87 has been utilized only sparingly to create anion foldamers. The first solution-phase example of a helical foldamer that included a halogen bond donor was developed by Antonio Caballero and Pedro Molina.93 Two iodo-1,2,3-triazolium halogen-bond donors were connected by a naphthalene-2,7-diol core. To serve as a spectroscopic handle and encourage π-π stacking, the oligomer was capped with photoactive, terminal pyrene units. Subsequently, fluorescence titrations with hydrogen pyrophosphate and H2PO4− afforded impressive binding constants in acetone (Ka ≥ 106 M−1, 1:1 binding model). As compared to the proteo-control molecule, the halogen bonding oligomer bound H2PO4− an order of magnitude stronger. Moreover, in 9:1 v/v CD3CN/CD3OD, the halogen bonding oligomer bound hydrogen pyrophosphate five-fold better than the proteo-control molecule, as determined by 1H NMR titrations. Moreover, this convenient “turn-on” fluorescence chemosensor was selective for hydrogen pyrophosphate.
The second example of a solution-phase foldamer with halogen bond donors was created by Paul Beer and coworkers. Phenylene-iodo-1,2,3-triazole foldamers were synthesized with four convergent halogen bond donors. The anthracene-capped ligand bound I− noticeably in 1:1 v/v CDCl3-acetone-d6 (Ka = 2,712 M−1, 1:1 binding model) as ascertained by 1H NMR titration experiments. An X-ray crystal structure of the complex was obtained (Figure 20), and due to the size of the iodide, the four halogen bond donors converged to point perpendicular from the backbone plane and bind the guest. Interestingly, the anthracene terminal groups are not π-stacked. Concomitantly, no excimer emission was seen during fluorescence spectroscopic titrations.94 Given the challenge associated with bringing four large iodine halogen bond donors to bind on a single anion, this is a remarkable feat.
Figure 20.
X-ray crystal structure of a phenylene-iodo-1,2,3-triazole foldamer with bound I− created by Beer and coworkers.
2.2. Double-Strand Anion Foldamers (d,1-anion foldamers)
Multi-strand foldamers add an extra layer of complexity to foldameric structure. Significant entropy must be overcome when multiple oligomers both associate and fold, like in DNA or multimeric proteins. These systems provide insight into developing complex structure and unique binding pockets that reach toward the complexity of nature. Both hydrogen bonding and halogen bonding has been used to coax oligomers into double-strand anion foldamers (d,1-anion foldamers) and here we present the few examples that have been studied in solution.
Continuing their work on tetradentate iodotriazole halogen bonding anion receptors, Beer and coworkers developed additional halogen (Figure 21a) and chalcogen (Figure 21b) bonding neutral oligomers.95 These σ-donor hosts created a hydrophobic binding pocket that is selective for less hydrated anions. The halogen bonding scaffold was appended with ester coupled triethylene glycol groups para to the triazole rings to enhance water solubility. Amide or esters were coupled with triethylene glycol groups ortho to the triazoles to sterically reduce bond rotation away from the interior of the foldamer, inducing preorganization. ITC binding experiments revealed that I− bound more tightly through multidentate cooperative interactions in pure water (up to K2 = 3.53 × 106 M−1 with a 2:1 host:guest binding mode) than easier to dehydrate ions like SCN− or ClO4−. Beer also substituted the halogen bond donors for chalcogen bond donors (Te). The chalcogen bonding oligomer showed similar binding but less selectivity for I− (K2= 7.30 × 104 with a 2:1 host:guest binding mode). However, this was the first example of an all-chalcogen bonding anion receptor in water. Beer and coworkers explored adding various fluorescent groups to the termini of the foldamer. For example, a 4-aminonaphthaleneide group transformed the foldamer into a fluorescent “turn on” I− sensor. Other fluorophores did not induce a discernable fluorescence change but did alter binding affinity.
Figure 21.
(a) Representative structure of phenylene-1,2,3,-triazole halogen and (b) chalcogen bonding foldamer backbone develeped by Beer and lab.
To develop double-strand foldamers, Jiang and coworkers added two terminal 1,8-naphthalimides appended to a phenylene-1,2,3-triazole five-mer. These functional groups assisted with π-π stacking and served as a spectroscopic handle.96 1H NMR titrations in THF-d8 suggested the initial formation of a d,1-chloride foldamer based on the pattern of chemical shifting of several phenylene protons (upfield until 0.5 equivalents of Cl− were added then downfield). The duplex could only be assembled at NMR concentrations of ligand (~0.5 mM); moreover, the d,1-chloride foldamer was somewhat unstable (K2 < 100 M− with a 2:1 binding mode). UV-Vis and/or fluorescence titration experiments in THF fitted to a 1:1 binding model afforded significant binding (Ka = ~106 M−1 for Cl−, Br−, and I−). Interestingly, the helical receptor did not selectively bind anions. In support of anion-induced folding, an excimer emission arising from stacked naphthalimides centered at 480 nm was observed. Using NMR concentrations of ligand, 1H 2D NOESY spectroscopy also confirmed compact helical folding upon adding anions.
Another interesting example comes from the Flood group, who has been leading the study of anion based double-strand foldamers. By extending their previous backbone to a 15-mer (see Figure 3 for the general structure) with six intramolecular hydrogen bonding amide groups, Flood and coworkers probed the effect of bulk H2O concentration on Cl− affinity.97 Based on broadened 1H NMR signals and CD features in the absence of Cl−, the authors deduced that the foldamer was at least partially preorganized in pure MeCN. Interestingly, the addition of Cl− produced another rare example of a double-strand anion foldamer, which was in equilibrium with a s,1-chloride foldamer and free host. Quantitative UV-Vis titrations were conducted to measure Cl− affinity in pure MeCN, 25 % v/v MeCN-H2O, and 50 % v/v MeCN-H2O (the limit of the 15-mer’s solubility). Unsurprisingly, the overall Cl− affinity of the 15-mer dropped by a factor of 13 when the H2O concentration was increased from 0 to 25 %. However, at 50 %, the overall association doubled as compared to that in 25 % v/v MeCN-H2O. In addition, the d,1-chloride foldamer formed preferentially in solution with increasing H2O composition. In 100 % MeCN, the d,1-chloride foldamer outcompeted the s,1-chloride foldamer when > 0.5 equivalents of Cl− were titrated. These data demonstrate the influence of the hydrophobic effect, which enhanced Cl− affinity and promoted duplex self-assembly. Van ‘t Hoff and ITC analyses revealed that in 50 % H2O the Cl− binding was enthalpically dominated. Nevertheless, duplex formation came at no entropic cost, which suggests π-π stacking served to offset this penalty. Overall, the high Cl− affinity that the foldamer exhibited in 50 % v/v MeCN-H2O (K1 = 2.3 × 105 M−1, K2 = 3.8 ×107 M−1; 2:1 host-guest binding model) was an impressive feat. Unfortunately, only the s,1-chloride foldamer could be crystallized (Figure 22). Within the helical cavity, Cl− is held by all six 1,2,3-triazole−CH hydrogen-bond donors in a distorted octahedral coordination geometry. Weaker phenylene–CH hydrogen bonds are also evident. Additionally, a Na+ is chelated by the oxygens of two acyclic oligoether groups located outside of the helical cavity (not shown).
Figure 22.
X-ray crystal structure of a phenylene-1,2,3-triazole foldamer binding intracavity Cl− developed by Flood and coworkers (some functional groups removed for clarity, see Figure 3 for the general structure).
Zhu and coworkers developed an amide-linked phenylene-1,2,3-triazole oligomer with a terminal photoactive pyrene unit (Figure 23).98 This ligand in the presence of less than half an equivalent of SO42− in 0.5 % DMSO-d6-acetone-d6 at −30 °C formed a d,1-sulfate foldamer, which was characterized by a 1H 2D NOESY NMR spectroscopy. The NOEs were consistent with a d,1-sulfate foldamer, as were the characteristic shifts of key aromatic signals (upfield-then-downfield with an inflection at 0.5 equivalents of SO42−). > 0.5 equivalents of guest favored the s,1-sulfate foldamer.
Figure 23.
Representative structure of an amide-linked phenylene-1,2,3-triazole backbone constructed by Zhu and coworkers.
In a follow-up study, Li, Zhu, and coworkers created new amide-linked phenylene-1,2,3-triazole derivatives.99 To one terminus of a three-triazole ligand a photoactive pyrene was appended. Less than half an equivalent of SO42− induced d,1-sulfate foldamer formation in CD2Cl2. In 1H NMR, several aromatic signals initially moved upfield in response to intermolecular π-π stacking but subsequently moved to their original positions when > 0.5 equivalents of guest were present. This characteristic pattern in shifting was consistent with the formation of a double anion foldamer. Li and coworkers then modified their foldamer to bias single strand binding. When a terminal amide-linked N-phenyl group was appended to the ligand, three amide–NHs, three triazole–CHs, and two phenylene–CHs from one strand could converge on a single SO42− anion in CD2Cl2. Accordingly, all eight of these protons shifted downfield upon addition of SO42−. However, most of the terminal, N-phenyl and pyrene protons shifted upfield, which evidenced π-π stacking. This helical 1:1 binding conformation was confirmed by 1H 2D NOESY NMR spectroscopy. Moreover, the association constant for the SO42− adduct was determined by 1H NMR titrations (Ka = 1,300 M−1, 1:1 binding model in CD2Cl2). The relatively few examples of higher-order anion foldamers studied in solution underscores the added challenges and complexity associated with these supramolecular structures. Clearly, future examples will only help establish this important class of structure.
3. Multi-Anion Foldamers in Solution
Due to the inherent challenges in anion self-assembly coupled with the enthalpic penalty associated with simultaneously forcing anions into close proximity and eliciting columbic repulsion, multi-anion foldamers are extremely rare. Hydrogen bonds and π-π stacking again play a prominent role in the assembly of these foldamers. However, other noncovalent interactions, like halogen bonding, are increasingly being used to enforce self-assembly. These examples will be discussed as well. Throughout this review, the dearth of solution data will be augmented by structural discussion permitted by X-ray crystal structures of these beautiful complexes.
3.1. Single-Strand Multi-Anion Foldamers (s,n>1-anion foldamers)
We begin with solution-phase single-strand multi-anion foldamers. Using strong hydrogen bonding urea groups Wu has produced an impressive array of anion foldamers that have been structurally characterized in the solid-sate and in solution. Wu and coworkers synthesized a series of o-phenylene-bridged oligoureas with increasing chain length (from three to six urea), capped with p-nitrophenyl groups.100 Four new s,2-anion foldamers and one isomer were characterized in the solid state. In an X-ray crystal structure of the four-urea oligomer, the Cl− anions sit above and below the helical planes (Figure 24a). Each Cl− is held by urea–NH hydrogen bonds with an overall bent coordination geometry (when each urea is considered as a monodentate coordination vector). Due to rotation about the phenylene–urea bonds, the urea donors point in an up-down-up-down pattern, so that the first and third ureas chelate one Cl−, while the second and fourth chelate the other Cl−. Taken together, the binding cavity is arranged in a square-like configuration.
Figure 24.
(a) X-ray crystal structure of an o-phenylene-bridged four-urea s,2-chloride foldamer. (b) X-ray crystal structure of an o-phenylene-bridged six-urea s,2-chloride foldamer. (c) X-ray crystal structure of a o-phenylene bridged 1-naphthyl-terminated four-urea s,2-chloride foldamer. (d) X-ray crystal structure of a 1-anthracenyl-capped five-urea s,2-chloride foldamer. All structures were developed by Wu and coworkers (see Figure 7 for the general structure).
Impressively, the Cl−-Cl− distance is only 3.6 Å, which must be stabilized by hydrogen bonding interactions to overcome the severe electrostatic repulsion. In the case of the five-urea oligomer, the Cl−-Cl− distance widens (3.8 Å) in response to the slightly larger helical cavity. The six-urea ligand houses two Cl− anions that are 3.9 Å apart. The first two urea donors bind the first Cl− in plane with the helical turn. The third, fourth, and fifth ureas chelate the second Cl−. Interestingly, the sixth urea flips to align itself with the helical axis and hydrogen bonds to the second urea oxygen. Its terminal p-nitrophenyl is rotated orthogonally from the helical-turn plane. In the X-ray crystal structure of the six-urea isomer, the Cl−-Cl− distance grows to 4.0 Å (Figure 24b). The second urea points along the helical axis and hydrogen bonds the sixth urea oxygen. DFT calculations revealed that the six-urea isomers have similar energies (within 2.0 kcal mol−1). Possibly, a urea moiety in each structure aligns itself with the helical axis in order to increase helical pitch, thus, relieving Cl−-Cl− repulsion. The foldamers were also studied in solution. Qualitative 1H NMR titrations were performed in CDCl3, and the patterns in chemical shifting upon adding Cl− were consistent with helical folding. Additionally, 2D NOESY NMR spectroscopy confirmed structural congruence between the solution-phase and solid-state data. Lastly, UV-Vis titrations in 0.5 % v/v DMSO-CHCl3 revealed two-step changes in the difference spectra, which provided evidence for 1:2 host-guest binding.
Wu and coworkers synthesized a similar series of o-phenylene-bridged oligoureas (four-, five-, and six-urea) but with fluorescent 1-naphthyl or 1-anthracenyl terminal groups.101 Six new Cl− complexes were elucidated by single-crystal X-ray diffraction. Almost all ligands racemically formed s,2-chloride foldamers in a helical conformation. However, the 1-naphthyl five-urea derivative, which included two TBA cations in the unit cell, was completely M resolved. The 1-naphthyl four-urea cocrystallized with two Cl− anions in much the same way as the p-nitrophenyl derivative. Interestingly, the naphthyl units are not π-stacked, but the naphthyl−CH hydrogen bonds to the first urea oxygen and weakly to the Cl− (Figure 24c). Each Cl− is bound by urea−NH hydrogen bonds from alternating units. The greater Cl−-Cl− distance of 3.9 Å suggests that sterically bulky groups help encourage cavity expansion. The even bulkier 1-anthracenyl groups appended to the four-urea allow for a greater expansion (Cl−-Cl− distance is 4.0 Å, not shown).
Additionally, 1-anthranceyl protons form CH hydrogen bonds with each Cl−, stabilizing this expanded conformation. In the case of the 1-anthracenyl-capped five-urea, both 1-anthracenyl-urea units nearly align themselves with the helical axis, which allows these groups to hydrogen bond (Figure 24d). The foldamers were also studied in solution. Cl− affinity in DMSO-d6 was ascertained with 1H NMR titrations (Ka = ~102 M−1 for all ligands, 1:1 binding model as determined by Job plots—a method used to determine the binding stoichiometry of a host-guest system). However, s,2-anion foldamers also were characterized by ESI-HRMS in CHCl3 solutions, suggesting that the foldamers self-assembled in less competitive media.
Jiang and coworkers constructed a series of anion-switchable amide-linked phenylene-1,2,3-triazoles designed to fold into helical conformations around halide ions102 (see Figure 2 for general structure). Upon titrating Cl−/Br− to a hexamer in pyridine-d5, triazole–CH and amide–NH protons shifted downfield as expected, indicative of intermolecular hydrogen bonding. Moreover, association constants for Cl− and Br− were calculated (Ka = 540 and 83 M−1, respectively; 1:1 binding model). A one-turn helical complex was deduced by 2D NOESY NMR spectroscopy. In comparison, the 12-mer was expected to fold around its guests in two turns. Upon titrating Cl−, the amide–NH protons initially upshifted when less than 1.6 equivalents of guest were present. When > 1.6 equivalents of guest were present, these resonances shifted downfield. From this pattern in chemical shifting, the authors surmised a 1:2 host-guest stoichiometry. Accordingly, the binding isotherms fit well to a 1:2 host-guest model, affording noteworthy association constants (K1 = 4.9 ×102 and K2 = 13 M−1) in competitive media. The weaker second association suggested the process was not cooperative, consistent with the electrostatic repulsion between intracavity guests. Two Br− anions were also accommodated by the 12-mer with lower affinity. Interestingly, quantitative 2D NOESY NMR evidenced a deformation of the host to increase its helical pitch upon adding excess Cl−, presumably to relieve electrostatic repulsion. The 18-mer was designed to form three helical turns around halide ions. 1:4 v/v DMSO-d6/pyridine-d5 was utilized to prevent aggregation of these longer oligomers. In this more competitive solvent system, the 18-mer bound Cl− two-fold more strongly than the 12-mer; additionally, the second association was almost 32-fold stronger. These data strongly suggest that the longer oligomer better alleviated the charge repulsion between bound guests. Notably, these s,2-anion foldamers were the first to be characterized in solution.
Biopolymers frequently form ditopic complexes that result in various “turn-on” functional states. Synthetic analogues are scarce, which motivated Jiang and coworkers to create a foldamer that encapsulated Cl− and β-D-glucopyranoside simultaneously.103 To accomplish this task, the researchers synthesized benzoylbenzohydrazide pentamers capped with either dimethoxyphenyl or pyrene units (Figure 25). To help preorganize the ligands, hydrazide–NH···oxygen hydrogen bonds were incorporated along the backbone. Additionally, multiple hydrogen bond donors/acceptors could point inwardly to complement both anions and saccharides. When studying the dimethoxyphenyl derivative in CDCl3 by 1H NMR spectroscopy, the hydrazide–NH protons initially shifted upfield with less than five equivalents of Cl−, then experienced a chemical shift inversion. This pattern in chemical shifting was consistent with a 1:2 host-guest stoichiometry, corroborated by Job plots. Additionally, 1H 2D NOESY NMR spectroscopy confirmed helical folding of the ligand around Cl−. Providing further evidence, adding Cl− to the pyrene-capped ligand generated a broad excimer emission (centered at ~480 nm) due to the association of an excited-state dimer. Cl− and β-D-glucopyranoside affinities were initially determined separately by 1H NMR titrations. Cl− complexation by both oligomers was modest (Ka = 101–102 M−1, 1:1 binding model) in CDCl3. β-D-glucopyranoside affinity for both ligands was comparable (Ka = 102 M−1, 1:1 binding model). Moreover, addition of the saccharide to the pyrene-capped oligomer caused homologous changes in the emission spectra, indicating a folded host-guest complex. To deduce the synergistic effect of adding both guests to the dimethoxyphenyl-capped derivative simultaneously, CD spectroscopy was utilized. A strong CD signal was created only in the presence of both guests (20 equivalents each). Addition of either guest without the other resulted in a weak or nonexistent CD signal.
Figure 25.
Representative structure of benzoylbenzohydrazide s,2-chloride β-D-glucopyranoside foldamer backbone developed by Jiang and coworkers.
Maeda and coworkers have created versatile single and multi-strand anion foldamers. As with previous foldamers, boron-difluoride complexes of 1,3-dipyrrolyl-1,3-propanediones were synthesized. In the present study a sept-, non-, and 15-mer were created.104 Remarkably, septamer-1:1, 15-mer-1:2, and nonamer-2:2 host-guest complexes were characterized in the solid state. The stunning d,2-chloride foldamer produced by the nonamer possesses a Cl− channel lined with eight pyrrole–NH hydrogen-bond donors. Each of the two Cl−s are bound in a distorted tetrahedral coordination geometry. However, only 1:1 host-guest complexes were observed when studying the sept- and nonamers in solution. In contrast, the 15-mer with two equivalents of Cl− in CD2Cl2 at −50 °C formed a s,2-chloride foldamer, in agreement with its X-ray structure (Figure 26). This was the second example of a solution-persistent s,2-anion foldamer. In the solid state, each of the two Cl− anions are bound by four pyrrole–NH hydrogen bonds in a pseudo-square-planar coordination geometry, and the intracavity Cl−-Cl− distance is 4.6 Å. Additionally, 1,3-propanedione α-hydrogen-CH hydrogen bonding is observed. The formation of this Cl− foldamer in solution was confirmed by the downfield shifted signals in the NMR which was consistent with the X-ray crystal structure. In addition, COSY and ROESY NMR experiments supported the formation of the foldamer. To ascertain Cl−, Br−, and I− affinity UV-Vis titrations were conducted in CH2Cl2. In the case of the 15-mer, Cl− binding was extremely strong (K1 = 1.2 ×108 and K2 = 3,200 M−1, 1:2 host-guest binding model) and uncooperative. Finally, UV-Vis stopped-flow spectroscopy was utilized to assess the kinetics of s,1-foldamer self-assembly. At this concentration, the kinetics of s,2-foldamer self-assembly could not be assessed. Interestingly, folding rates slowed with increasing chain length. This represents one of the rare examples of anion foldamer kinetics being studied.
Figure 26.
X-ray crystal structure of a 15-mer s,2-chloride foldamer composed of boron-difluoride complexes of 1,3-dipyrrolyl-1,3-propanedione oligomers synthesized by Maeda and coworkers.
By interspersing pyridine units between indolocarbazole moieties, Jeong and coworkers created new foldamers with strongly fluorescent turn-on properties in the presence of SO42−, F−, and other anions.105 The pyridine lone pairs pointed inward and served as hydrogen-bond acceptors upon folding. An X-ray crystal structure highlights the penchant of these foldamers to bind water molecules within their helical cavities (Figure 27). This foldamer could fold into a neutral s,2-water foldamer in wet nonpolar solvents (CHCl3, CH2Cl2, and toluene) but reverted to a random coil in polar solvents (acetone and DMSO). In the former solvents, the foldamer was essentially nonfluorescent due to the stacking of its indolocarbazoles and pyridines. However, in the denatured state, the ligand became strongly fluorescent, evidencing the disruption of π-π stacking in competitive media. Additionally, both acetone and DMSO were too large to fit within the helical cavity, further encouraging a random-coil conformation. Although this is an example of a neutral guest foldamer, anions also disrupted helix formation, which promoted strong turn-on fluorescence. In water-saturated CH2Cl2, SO42− and F− produced the highest-intensity fluorescence. It was surmised that anion and pyridine-lone-pair repulsion was largely responsible for anion-induced unfolding. In support of this hypothesis, protonation of the introverted pyridines with perchloric acid led to the formation of a helical SO42− adduct in wet CH2Cl2.
Figure 27.
X-ray crystal structure of a fluoresence turn-on s,2-water foldamer developed by Jeong and coworkers (see Figure 4 for the general indolocarbazole structure). Anions induced un-folding of the complex.
3.2. Double-Strand Multi-Anion Foldamers (d,n>1-anion foldamers)
The first helical anion complex of any kind was a d,2-sulfate foldamer discovered by Javier de Mendoza and lab in 1996.24 Utilizing enantiomerically pure bicyclic guanidiniums spaced by dimethyl-sulfide linkers, the authors created double-strand foldamers that encapsulated SO42− in solution (Figure 28). When SO42− was added to the dimer ligands (and other derivatives) in CDCl3, strong downfield shifts of the guanidinium–NH protons were observed by 1H NMR spectroscopy. A 2D ROESY experiment confirmed intermolecular ROEs, consistent with d,2-sulfate foldamer formation. As bicyclic guanidiniums themselves possess stereocenters, (R,R) or (S,S), the resultant foldamers were stereospecific. CD-spectroscopic studies in MeCN revealed that the enantiomers gave rise to mirror-image spectra. The higher ellipticities in the presence of SO42− evidenced anion-induced helicity.
Figure 28.
Representative structure of bicyclic guanidinium oligomers (two-mer) composed by de Mendoza and coworkers.
The first examples of multi-strand halide-ion foldamers were developed by Maeda and coworkers.106 Solution-persistent d,2-chloride and bromide foldamers were assembled using boron-difluoride 1,3-dipyrrolyl-1,3-propanediones linked by phenylene-diethynylene spacers (Figure 29). The octamer and decamer in CDCl3 at −50 °C formed d,2-chloride foldamers upon adding approximately one equivalent of Cl−. The slow-exchanging species (free ligand, s,2, and d,2-foldamer) were distinguishable by 1H NMR spectroscopy. Addition of excess Cl− destabilized the d,2-chloride foldamer in favor of the single helices. Impressively, the 10-mer d,1-chloride foldamer also formed at room temperature. Double-strand foldamer self-assembly was corroborated by DOSY NMR spectroscopy. d,1-bromide foldamers were also formed under similar conditions. Given their small size, low charge, and variable coordination preference, halide ions are extremely challenging targets. Thus, Maeda and coworkers are pioneers of halide-ion-templated foldamer self-assembly.
Figure 29.
X-ray crystal structure of boron-difluoride 1,3-dipyrrolyl-1,3-propanedione ligand binding Cl− in the solid state. The oliogomers form d,2-chloride foldamers in solution and were developed by Maeda and lab.
Foldamers and helices able to complex anions in broad ranges of environments are especially rare. Preorganization through metal-ligand interactions is a powerful tool for assembly, as metal-ligand bonding is robust in many solvents. Jiang and coworkers developed a ruthenium (II) complex of oligo(bipyridine-phenyl triazole) receptors and tested their formation in a variety of solvents (acetone, DMSO, and 5 % H2O: acetone).107
ROESY NMR confirmed that the trimethylsilyl capped receptor (Figure 30a) exists as a random-coiled oligomer in acetone until addition of five equivalents of TBA+Cl− induced folding in a sandwich-like 2:1 orientation around a singular Cl−, Br− I−, or NO3− in acetone. Titrations in 5 % v/v H2O: acetone revealed weaker binding but did not alter the binding mode. In the competitive hydrogen bonding solvent DMSO, the shorter trimethylsilyl capped oligomer complexed anions in a 1:1 binding mode, while the longer oligomer (Figure 30b) formed a d,1-chloride foldamer. This represents a novel double-foldamer binding approach, which could have lesser solvent dependency than other foldamers.
Figure 30.
Representative oligo(bipyridine-phenyl triazole) foldamers (a) and (b) created by Jiang and coworkers.
When foldamers need to support multiple anions, multiple strong hydrogen and halogen bonds can be used to offset charge-charge repulsion. Utilizing single-site substitutions on the backbone of C2 symmetric aryl-triazole foldamers, Flood explored the conditions in which a stimuli-responsive double – single foldmer conversion between s,1-chloride foldamer and d,2-chloride foldamer complexes formed.108 (Figure 31) These 13-subunit foldamers include six alternating triazole and seven aryl groups in an alternating aryl-triazole sequence. In an MeCN and CHCl3 solution, increasing MeCN promotes the formation of the d,2-chloride foldamer, likely because of hydrophobic π-stacking. Thermally, the s,1-chloride foldamer is preferred at elevated temperature. However, formation of the d,2-chloride foldamer could be preserved at elevated temperatures in polar solvents. This illustrates the balance between the enthalpic stabilization of greater π-stacking and hydrogen bond interactions against the entropic penalty of higher structure formation.
Figure 31.
(a) Representative structure of building blocks A and P used to create a (b) aryl-triazole foldamer backbone developed by Flood and coworkers.
To further investigate the driving force for double-strand foldamer formation, Flood and coworkers simply altered the sequence of the oligomer.108 The bisamide phenylene (A) and pyridyl (P) groups provided complimentary stabilization when alternated because of dipole-dipole interactions, but the interaction was relatively destabilizing with like contacts such as AA or PP (Figure 31) Meanwhile, guest interactions were enhanced by A residues and impaired with P. The original PAP sequence was edited to create an APA, AAA, and PPP oligomers.
Binding affinities showed that PAP bound Cl− the weakest (Ka = 105 M−1 1:1 binding mode) and AAA the strongest (Ka =107 M−1 1:1 binding mode). However, double-strand formation occurred most readily with the APA oligomers, and the least with the PAP. If dipole-dipole stacking dominated, both APA and PAP should be more stabilized than AAA or PPP oligomers. Rather, anion stabilization remained the dominate factor, as APA oligomers stabilized binding to two Cl− anions by two bisamide phenylene groups. Therefore, by placing multiple favorable contacts near the end of the sequence, stabilization of multiple anions was preferred.
3.3. Triple-Strand Multi-Anion Foldamers (t,n>1-anion foldamers)
Given the complexity of anion triple-strand multi-anion foldamers, it is not surprising to note that there are only very few examples reported to date. The development of triple-strand multi-anion foldamers has primary come independently from the likes of Wu and Berryman. In the following sections we will describe in detail this emerging class of supramolecules.
3.3.1. Hydrogen Bonding Triple-Strand Multi-Anion Foldamers
As can be noted from this review, hydrogen bonding interactions are the dominant noncovalent force used to interact with anions within foldamers. It is not surprising to note that most examples of triple-strand multi-anion foldamers employ strong hydrogen bond donors and highly charged polyatomic oxoanions.
The exemplary work of Wu and coworkers has greatly contributed to the field of multi-strand anion foldamers as well as our understanding of anion coordination in general. Their first report of a multi-strand anion foldamer in 2011 was also the first example of a t,2-phosphate foldamer.22 Again, Wu and coworkers utilized o-phenylene-bridged bisurea oligomers but targeted larger anions in the present work. Inspired by the odd-even rule of M2L3 helicates developed by Albrecht and coworkers,109,110 an ethylene spacer (even number of carbons) was utilized to link two bisurea subunits. A beautiful X-ray crystal structure was obtained in which three tetraurea ligands enwrap two intracavity PO43− anions (Figure 32a). Each PO43− is held by six ureas (through 12 urea–NH hydrogen bonds) originating from three separate ligands. Each edge of a PO43− tetrahedron is bound by one urea with an overall pseudo-octahedral coordination geometry (if each urea is considered as a monodentate coordination vector). Hence, the bisurea is analogous to a bipyridine moiety. At its termini, the foldamer is stabilized by nearly-orthogonal CH··π interactions. At the midpoint of the triplex, the ethylene linkers taper so that the structure resembles an hourglass. The t,2-phosphate foldamer was studied in solution using 1H NMR spectroscopy in 5 % v/v D2O-DMSO-d6. Upon titrating more than 0.66 equivalents of PO43−, the ligand resonances were well-resolved and consistent with the solid-state structure. Moreover, marked downfield chemical shifts of the urea–NH protons indicated strong hydrogen bonding in solution. In contrast, terminal p-nitrophenyl protons shifted upfield strongly due to ring-current shielding effects. 2D NOESY and DOSY NMR experiments also corroborated the proposed structure. Interestingly, upon titrating SO42−, the 1H NMR spectroscopic changes were more consistent with a 1:1 complex, likely due to the lower charge density of SO42−.
Figure 32.
(a) X-ray crystal structure of an o-phenylene-bridged tetraurea t,2-phosphate foldamer. (b) X-ray crystal structure of a 4,4’methylenebis(phenyl)-linked o-phenylene-bridged tetraurea t,2-phosphate foldamer. (c) X-ray crystal structure of a 4,4’methylenebis(phenyl)-linked o-phenylene-bridged bis(urea) t,2-phosphate foldamer. All structures were created by Wu and coworkers (see Figure 7 for the general structure).
In a follow-up paper, Wu and coworkers explored the effect of spacer length/rigidity in forming foldamers, mono-bridged structures, or mesocates110 Using the same o-phenylene-bridged bisurea linked by a p-xylylene spacer, an elongated t,2-phosphate foldamer was synthesized. Unfortunately, only a preliminary X-ray crystal structure of the complex was obtained. Using the same functional groups linked by a phenylene spacer, Wu and coworkers created yet another t,2-phosphate foldamer.111 Impressively, this foldamer reversibly converted to an A4L6 tetrahedral cage as a function of peripheral templation and solvent.
In an effort to create a highly selective choline binding site within the linker region of the t,2-phosphate foldamer, Wu and coworkers utilized the same o-phenylene-bridged bisureas linked by a 4,4’-methylenebis(phenyl) spacer.112 The resulting aromatic box was electron-rich, providing a suitable binding site for complementary cations. In a magnificent X-ray crystal structure, three intertwining ligands are held together by two terminal PO43− anions. As before, each PO43− is bound by six ureas in a pseudo-octahedral coordination geometry (Figure 32b). Interestingly, unlike the first t,2-phosphate foldamer, the new complex lacks molecular C3 symmetry. Remarkably, within the aromatic box, a tetramethylammonium (TMA) countercation is encapsulated—stabilized by multiple cation-π interactions afforded by six aromatic rings (average N···centroid distance is 4.5 Å) as well as intracavity ion-pairing. This t,1-tetramethylammonium, 2-phosphate foldamer could also bind biologically relevant cations like choline. Upon mixing one equivalent of choline with ligand, chemical upshifts of the choline protons in 1.5 % D2O-acetone-d6 indicated guest encapsulation within the aromatic box. This binding arrangement was confirmed by 1H 2D NOESY and DOSY NMR as well as HRMS experiments. Acetylcholine also proved to be a suitable guest for the foldamer but was bound 20-fold less strongly. Through fluorescence displacement titrations (using a 4-(4′-dimethylamino)styryl-1-methylpyridinium probe), a selectivity value of 15 was obtained (chlorine:acetylcholine). Mechanistically, choline selectivity emerged from a dual-site binding motif: TMA headgroup encapsulation as well as hydroxyl-tail hydrogen bonding. In the next study, it was discovered that the hydroxyl tail likely hydrogen bonded a PO43− oxygen.
Next, Wu and coworkers studied seven chiral quaternary ammonium cations, which were used to induce one-handed triple-strand foldamer complexation.113 The same t,1-tetramethylammonium, 2-phosphate foldamer used previously to bind choline was repurposed for the present studies. Crystallization of the ligand with racemic α-methylcholine resulted in equal populations of M- and P-foldamers. Enantioselective encapsulation of the (R)- or (S)-enantiomer by an M- or P-foldamer, respectively, was observed in the solid state. As expected, the trimethylammonium headgroup is held within the aromatic box by numerous cation-π interactions. Additionally, the hydroxyl group is located within the foldamer and hydrogen bonds to a PO43− oxygen (Figure 32c). As evidenced by the upfield shifts of their trimethylammonium headgroup protons, all seven of the targeted guests were encapsulated by the PO43− triple-strand foldamer in solution (CD3CN). Monitored by CD spectroscopy, the addition of chiral, non-racemic guests to the triple-strand foldamer resulted in enhanced populations of M or P helices. CD spectroscopic titrations also afforded binding constants in MeCN. Notably, both α- and β-methylcholine were bound by the foldamer with Ka in the ~106 M−1 range (1:1 binding model). Taken together, this ditopic triple-strand foldamer, which employs hydrogen bonding, solvophobic interactions, ion pairing, and cation-π interactions, is a unique and exciting supramolecular receptor that stands at the forefront of the field.
3.3.2. Halogen Bonding Triple-Strand Multi-Anion Foldamers
Despite the rich examples of foldamers that bind anions through primarily hydrogen bonding interactions, there are compelling reasons to employ other noncovalent interactions. The halogen bond is one such interaction where stabilization energies can be comparable to hydrogen bonds, yet even greater directionality and polarizability can be attained. Berryman and coworkers was the first to use halogen bonds to create higher-order anion foldamers.114 Prior to the work presented herein, there had not been any reports of a multi-strand I− foldamer nor of a triple-strand Br− foldamer. This is unsurprising given the challenges associated with coordinating multiple ligands around a small anion with low charge. To develop a robust halide-ion triple foldamer that self-assembles even at elevated temperatures, halogen-bonding m-arylene-ethynylene oligomers were synthesized. As the synthesis of the eventual nonamer target was expected to be challenging, a trimer was first constructed, and preliminary anion binding studies were carried out in solution and the solid state. In a separate investigation by Berryman and coworkers, a closely related trimer bound Cl−, Br−, and I− modestly in 2:3 v/v CDCl3-CH3NO2 (K1 = 2,630, 4,690, 4,380 M−1, respectively; 1:1 host-guest binding model).115 Noteworthy was the preference of the receptor for the larger halide ions. Impressively, the oligomer also chelated a large and charge-diffuse oxoanion, ReO4−, in solution and the solid state. Utilizing and expanding this design, an oligomer with three 4-iodopyridinium halogen-bond donors with two 1-tert-butyl-3,5-diethynylbenzene groups acting as spacers and capped with two 4-methoxytolan groups was created. Introverted halogen bond donors had never been attached to an m-arylene-ethynylene backbone up to this point. The tricationic arylethynyl nonamer enhanced halogen bond strength and amazingly formed t,2-bromide and t,2-iodide foldamers (helicates) in DMF/MeCN mixtures. The supramolecular complex resembled a tubular channel lined with nine halogen bond donors. (Figure 33). Remarkably, the foldamer remained stable at temperatures up to 68 °C (the limit of the NMR probe) as well as in 1:1 v/v D2O/DMF-d7 solution for at least twenty days, despite the chemical instability of 4-iodopyridiniums, which when unfolded were readily hydrolized with residual water in a matter of hours.
Figure 33.
(a) Crystal structure of the halogen bonding t,2-bromide foldamer with encapsulated Br−. (b) Crystal structure of halogen bonding t,2-iodide foldamer with encapsulated I−. Both structures were developed by Berryman and coworkers.
Kinetic and mechanistic studies are rare for any foldamer, let alone higher order foldamers. Studies of this unique structure using 1H 2D EXSY NMR revealed long lifetimes for ligand exchange and an associative process, which remained on the order of seconds even at elevated temperatures.21 Stop-flow experiments showed that anion exchange from I− to Br− occurred rapidly, faster than the millisecond timescale. Therefore, this helicate was kinetically stable and maintained its structure while accommodating rapid anion exchange. Exchange experiments using 1H qNMR showed that larger halides Br− and I− were similarly stable, but pore size and halogen bond induced binding was ill suited for Cl−, resulting in selective triple helicate formation with larger halides.
4. Conclusion, Outlook, and Prospective
Anion foldamers remain an emerging area of supramolecular chemistry at the interface of chemistry, biochemistry and molecular recognition. In a relatively short period of time, supramolecular chemists have realized impressive structural diversity and defined new chemical space by incorporating abiotic functional groups within secondary structure. To date, several dynamic and stimuli-responsive properties have been established. For example, anion-switchable single-/multi-strand foldamers demonstrate that complex structure can be modulated with simple external stimuli. Additionally, incorporating photoisomerizable azo groups, these supramolecules become light responsive. Moreover, as many anions undergo acid-base chemistry at physiologically relevant pH, anion foldamers can be pH-responsive nanocomponents. Other stimuli, including chiral ligands and chiral anions, have been explored to induce helical chirality. Furthermore, anion foldamers are tractable hosts. Mimicking nature, chemists have created diverse solvent-secluded active sites capable of adjusting their dimensions in response to guests—often in aqueous or other competitive media. Through helical self-assembly around a target guest, these increasingly sophisticated and modular supramolecules can bring photoactive functional groups into contact and emit fluorescence. The diverse topology of anion-induced synthetic helical structure illustrates that chemists are beginning to master anion induced folding.
Nevertheless, many challenges within the field remain. For example, the synthesis of oligomeric strands is typically long and generally low yielding. To truly take advantage of the complexity inspired by nature, the synthesis of anion foldamers will need to become more efficient. Inspiration will likely come from fields like solid phase peptide synthesis that have demonstrated exceptional synthetic efficiency. Furthermore, incorporating dynamic covalent chemistry and/or polymerization will only serve to increase the size of possible structures. Additionally, developing new techniques to selectively assemble single or multiple strand hosts, will increase the complexity of developed systems. Finally, kinetic studies on anion foldamers are critically lacking and will be required to truly harness the programmable assembly of these structures. As new applications emerge, it will become necessary to understand how these molecules operate in solution and the solid state as well as in biological environments, like cells. In the future, the intricacy of structure and function of anion foldamers will begin to approach that of biological systems.
As we look towards the horizon, the utility of anion induced helical structure will likely include new functional materials and bioinspired machines. Synthetic ion channels, stereoselective catalysts, and transmembrane drug transporters are just a sample of promising applications. Future structures will surely take inspiration from biology, yet include chemical innovations creatively enabled by the ingenuity of supramolecular chemists. “Nanos gigantum humeris insidentes,” we continue to proceed towards Gellman’s vision: “Mastery over foldamers should provide access to a new universe of molecules that profoundly influence chemistry and society.”7
Acknowledgements
We would like to thank funding provided by the National Science Foundation (NSF) CAREER CHE-1555324, the Center for Biomolecular Structure and Dynamics CoBRE (NIH NIGMS grant P20GM103546) and the University of Montana (UM). We give a special thanks to Dan Decato for his continued feedback.
Bios
Eric A. John:
Eric A. John received his B.Sc. in Chemistry from The University of Wisconsin-Eau Claire in 2016, where he developed competitive hydrogen bonding liquid crystal systems under the supervision of Prof. Kurt N.Wiegel. He is currently working on his Ph.D. at The University of Montana under the guidance of Prof. Orion B. Berryman. Eric’s research interests include halogen bonding foldamers, synthetic anion transport, and host-guest chemistry.
Casey J. Massena:
Casey J. Massena received his Ph.D. in organic and supramolecular chemistry at the University of Montana under the guidance of Prof. Orion B. Berryman. Casey’s research interests include the design and bioconjugation of self-adjuvanting immunotherapeutics for the treatment of influenza and substance abuse.
Orion B. Berryman:
Orion B. Berryman grew up in Homer, Alaska surrounded by ocean and mountains. In 2003 he received his B.A. in Chemistry from the University of New Hampshire. He earned his Ph.D. in organic/inorganic chemistry in 2008 working with Darren W. Johnson at the University of Oregon on anion-π interactions and developing anion receptors. As an NIH postdoctoral fellow he worked with Julius Rebek Jr. at the Scripps Research Institute developing light responsive cavitands and ligands for uranyl sequestration. He joined the chemistry faculty at the University of Montana in 2012 where he received an NSF CAREER award. He is also a member of the Center for Biomolecular Structure and Dynamics and director of the Small Molecule X-ray Core at the University of Montana. His research uses halogen bonding interactions and anion binding to drive the assembly of higher-order anion foldamers.
References
- (1).Hill DJ; Mio MJ; Prince RB; Hughes TS; Moore JS A Field Guide to Foldamers. Chem. Rev. 2001, 101, 3893–4011. [DOI] [PubMed] [Google Scholar]
- (2).Gellman SH Foldamers: A Manifesto. Acc. Chem. Res. 1998, 31, 173–180. [Google Scholar]
- (3).Appella DH; Christianson LA; Karle IL; Powell DR; Gellman SH β-Peptide Foldamers: Robust Helix Formation in a New Family of β-Amino Acid Oligomers. J. Am. Chem. Soc. 1996, 118, 13071–13072. [Google Scholar]
- (4).Martinek TA; Fülöp F Peptidic Foldamers: Ramping up Diversity. Chem. Soc. Rev. 2012, 41, 687–702. [DOI] [PubMed] [Google Scholar]
- (5).Cubberley MS; Iverson BL Models of Higher-Order Structure: Foldamers and Beyond. Curr. Opin. Chem. Biol. 2001, 5, 650–653. [DOI] [PubMed] [Google Scholar]
- (6).Martinek TA; Fülöp F Side-Chain Control of β-Peptide Secondary Structures: Design Principles. Eur. J. Biochem. 2003, 270, 3657–3666. [DOI] [PubMed] [Google Scholar]
- (7).Horne WS; Gellman SH Foldamers with Heterogeneous Backbones. Acc. Chem. Res. 2008, 41, 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Guichard G; Huc I Synthetic Foldamers. Chem. Commun. 2011, 47, 5933–5941. [DOI] [PubMed] [Google Scholar]
- (9).Cheng RP Beyond de Novo Protein Design - De Novo Design of Non-Natural Folded Oligomers. Curr. Opin. Struct. Biol. 2004, 14, 512–520. [DOI] [PubMed] [Google Scholar]
- (10).Pilsl LKA; Reiser O α/β-Peptide Foldamers: State of the Art. Amino Acids 2011, 41, 709–718. [DOI] [PubMed] [Google Scholar]
- (11).Huc I Aromatic Oligoamide Foldamers. Eur. J. Org. Chem. 2004, 1, 17–29. [Google Scholar]
- (12).Zhang DW; Zhao X; Hou JL; Li ZT Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [DOI] [PubMed] [Google Scholar]
- (13).Mecozzi S; Rebek J The 55% Solution: A Formula for Molecular Recognition in the Liquid State. Chem.: Eur. J. 1998, 4, 1016–1022. [Google Scholar]
- (14).Li ZT; Hou JL; Li C; Yi HP Shape-Persistent Aromatic Amide Oligomers: New Tools for Supramolecular Chemistry. Chem.: Asian J. 2006, 1, 766–778. [DOI] [PubMed] [Google Scholar]
- (15).Zhang A; Han Y; Yamato K; Zeng XC; Gong B Aromatic Oligoureas: Enforced Folding and Assisted Cyclization. Org. Lett. 2006, 8, 803–806. [DOI] [PubMed] [Google Scholar]
- (16).De S; Chi B; Granier T; Qi T; Maurizot V; Huc I Designing Cooperatively Folded Abiotic Uni- A Nd Multimolecular Helix Bundles. Nat. Chem. 2018, 10, 51–57. [DOI] [PubMed] [Google Scholar]
- (17).Juwarker H; Jeong KS Anion-Controlled Foldamers. Chem. Soc. Rev. 2010, 39, 3664–3674. [DOI] [PubMed] [Google Scholar]
- (18).Heyer D; Lehn JM Anion Coordination Chemistry - Synthesis and Anion Binding Features of Cyclophane Type Macrobicyclic Anion Receptor Molecules. Tetrahedron Lett. 1986, 27, 5869–5872. [Google Scholar]
- (19).Anion Coordination Chemistry; Bowman-James K; Bianchi A; García-España E, Eds.; WILEY-CVH, Weinheim, Germany, 2011, 1–523. [Google Scholar]
- (20).Foldamers: Structure, Properties, and Applications; Huc I, Hecht S, Eds.; WILEY‐VCH, Verlag GmbH, 2007, 1–460. [Google Scholar]
- (21).Massena CJ; Decato DA; Berryman OB A Long-Lived Halogen-Bonding Anion Triple Helicate Accommodates Rapid Guest Exchange. Angew. Chem., Int. Ed. 2018, 57, 16109–16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Li S; Jia C; Wu B; Luo Q; Huang X; Yang Z; Li QS; Yang XJ A Triple Anion Helicate Assembled from a Bis(Biurea) Ligand and Phosphate Ions. Angew. Chem., Int. Ed. 2011, 50, 5721–5724. [DOI] [PubMed] [Google Scholar]
- (23).Liu Y; Parks FC; Zhao W; Flood AH Sequence-Controlled Stimuli-Responsive Single-Double Helix Conversion between 1:1 and 2:2 Chloride-Foldamer Complexes. J. Am. Chem. Soc. 2018, 140, 15477–15486. [DOI] [PubMed] [Google Scholar]
- (24).Sánchez-Quesada J; Giralt E; Seel C; Prados P; Dalcol I; de Mendoza J Anion Helicates: Double Strand Helical Self-Assembly of Chiral Bicyclic Guanidinium Dimers and Tetramers around Sulfate Templates. J. Am. Chem. Soc. 2002, 118, 277–278. [Google Scholar]
- (25).Moras D; Harrowfield J; Siegel J; Chevrier B; Lehn JM; Rigault A Spontaneous Assembly of Double-Stranded Helicates from Oligobipyridine Ligands and Copper(I) Cations: Structure of an Inorganic Double Helix. Proc. Natl. Acad. Sci. 2006, 84, 2565–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kubik S Anion Recognition in Water. Chem. Soc. Rev. 2010, 39, 3648–3663. [DOI] [PubMed] [Google Scholar]
- (27).Zhao J; Yang D; Yang XJ; Wu B Anion Coordination Chemistry: From Recognition to Supramolecular Assembly. Coord. Chem. Rev. 2019, 378, 415–444. [Google Scholar]
- (28).Gale PA; Howe ENW; Wu X; Spooner MJ Anion Receptor Chemistry: Highlights from 2016. Coord. Chem. Rev. 2018, 375, 333–372. [Google Scholar]
- (29).Gale PA; Caltagirone C Anion Sensing by Small Molecules and Molecular Ensembles. Chem. Soc. Rev. 2015, 44, 4212–4227. [DOI] [PubMed] [Google Scholar]
- (30).Gimeno N; Vilar R Anions as Templates in Coordination and Supramolecular Chemistry. Coord. Chem. Rev. 2006, 250, 3161–3189. [Google Scholar]
- (31).He Q; Tu P; Sessler JL Supramolecular Chemistry of Anionic Dimers, Trimers, Tetramers, and Clusters. Chem 2018, 4, 46–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Vargas-Zúñiga GI; Sessler JL Pyrrole N–H Anion Complexes. Coord. Chem. Rev. 2017, 345, 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ferrand Y; Huc I Designing Helical Molecular Capsules Based on Folded Aromatic Amide Oligomers. Acc. Chem. Res. 2018, 51, 970–977. [DOI] [PubMed] [Google Scholar]
- (34).Chang KJ; Kang BN; Lee MH; Jeong KS Oligoindole-Based Foldamers with a Helical Conformation Induced by Chloride. J. Am. Chem. Soc. 2005, 127, 12214–12215. [DOI] [PubMed] [Google Scholar]
- (35).Juwarker H; Lenhardt JM; Pham DM; Craig SL 1,2,3-Triazole CH···Cl- Contacts Guide Anion Binding and Concomitant Folding in 1,4-Diaryl Triazole Oligomers. Angew. Chem., Int. Ed. 2008, 47, 3740–3743. [DOI] [PubMed] [Google Scholar]
- (36).Juwarker H; Lenhardt JM; Castillo JC; Zhao E; Krishnamurthy S; Jamiolkowski RM; Kim KH; Craig SL Anion Binding of Short, Flexible Aryl Triazole Oligomers. J. Org. Chem. 2009, 74, 8924–8934. [DOI] [PubMed] [Google Scholar]
- (37).Suk J-M; Jeong K-S Modulation of Binding Affinities between Foldamer-Based Anion Receptors and Chloride Ion. Bull. Korean Chem. Soc. 2011, 32, 2891–2892. [Google Scholar]
- (38).Yang L; Zhao W; Che YK; Wang Y; Jiang H Influence of Terminal Substituents on the Halide Anion Binding of Foldamer-Based Receptors. Chinese Chem. Lett. 2017, 28, 1659–1662. [Google Scholar]
- (39).Li Q; Huang F; Shang J; Che Y; Wang Y; Zhao W; Jiang H Aryl-Triazole Foldamers with Ethynyl Spacers as Effective Receptors for Halides and Oxyanions. Tetrahedron Lett. 2016, 57, 1691–1694. [Google Scholar]
- (40).Gavette JV; Evoniuk CJ; Zakharov LN; Carnes ME; Haley MM; Johnson DW Exploring Anion-Induced Conformational Flexibility and Molecular Switching in a Series of Heteroaryl-Urea Receptors. Chem. Sci. 2014, 5, 2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gavette JV; Mills NS; Zakharov LN; Johnson CA; Johnson DW; Haley MM An Anion-Modulated Three-Way Supramolecular Switch That Selectively Binds Dihydrogen Phosphate, H2PO4-. Angew. Chem., Int. Ed. 2013, 52, 10270–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kim MJ; Lee HW; Moon D; Jeong KS Helically Foldable Diphenylureas as Anion Receptors: Modulation of the Binding Affinity by the Chain Length. Org. Lett. 2012, 14, 5042–5045. [DOI] [PubMed] [Google Scholar]
- (43).Kim J. Il; Juwarker H; Liu X; Lah MS; Jeong KS Selective Sulfate Binding Induces Helical Folding of an Indolocarbazole Oligomer in Solution and Solid State. Chem. Commun. 2010, 46, 764–766. [DOI] [PubMed] [Google Scholar]
- (44).Suk JM; Kim J. Il; Jeong KS An Indolocarbazole Trimer with an Expanded Cavity for Anion Binding. Chem.: Asian J 2011, 6, 1992–1995. [DOI] [PubMed] [Google Scholar]
- (45).Jia C; Wu B; Li S; Huang X; Yang XJ Tetraureas versus Triureas in Sulfate Binding. Org. Lett. 2010, 12, 5612–5615. [DOI] [PubMed] [Google Scholar]
- (46).Jia C; Wang QQ; Begum RA; Day VW; Bowman-James K Chelate Effects in Sulfate Binding by Amide/Urea-Based Ligands. Org. Biomol. Chem. 2015, 13, 6953–6957. [DOI] [PubMed] [Google Scholar]
- (47).Lee HJ; Jeon HG; Jeong KS Anion-Induced Switching of the Helical Orientation of a Chiral Indolocarbazole Dimer. Supramol. Chem. 2015, 27, 378–385. [Google Scholar]
- (48).Diemer V; Fischer L; Kauffmann B; Guichard G Anion Recognition by Aliphatic Helical Oligoureas. Chem.: Eur. J. 2016, 22, 15549. [DOI] [PubMed] [Google Scholar]
- (49).Bąk KM; Masłowska K; Chmielewski MJ Selective Turn-on Fluorescence Sensing of Sulfate in Aqueous-Organic Mixtures by an Uncharged Bis(Diamidocarbazole) Receptor. Org. Biomol. Chem. 2017, 15, 5968–5975. [DOI] [PubMed] [Google Scholar]
- (50).Suk JM; Jeong KS Indolocarbazole-Based Foldamers Capable of Binding Halides in Water. J. Am. Chem. Soc. 2008, 130, 11868–11869. [DOI] [PubMed] [Google Scholar]
- (51).Wang Y; Li F; Han Y; Wang F; Jiang H Folding and Aggregation of Cationic Oligo(Aryl-Triazole)s in Aqueous Solution. Chem.: Eur. J. 2009, 15, 9424–9433. [DOI] [PubMed] [Google Scholar]
- (52).Xu YX; Wang GT; Zhao X; Jiang XK; Li ZT Folding of Aromatic Amide-Based Oligomers Induced by Benzene-1,3,5- Tricarboxylate Anion in DMSO. J. Org. Chem. 2009, 74, 7267–7273. [DOI] [PubMed] [Google Scholar]
- (53).Shi ZM; Chen SG; Zhao X; Jiang XK; Li ZT Meta-Substituted Benzamide Oligomers That Complex Mono-, Di- and Tricarboxylates: Folding-Induced Selectivity and Chirality. Org. Biomol. Chem. 2011, 9, 8122–8129. [DOI] [PubMed] [Google Scholar]
- (54).Gratzer K; Diemer V; Clayden J Signal Transduction in Oligoamide Foldamers by Selective Non-Covalent Binding of Chiral Phosphates at a Urea Binding Site. Org. Biomol. Chem. 2017, 15, 3585–3589. [DOI] [PubMed] [Google Scholar]
- (55).Kim MC; Suk J; Kim H-J; Sim E; Lee M; Jeong K-S; Naidu VR Biased Helical Folding of Chiral Oligoindole Foldamers. Org. Lett. 2008, 10, 5373–5376. [DOI] [PubMed] [Google Scholar]
- (56).Suk JM; Naidu VR; Liu X; Lah MS; Jeong KS A Foldamer-Based Chiroptical Molecular Switch That Displays Complete Inversion of the Helical Sense upon Anion Binding. J. Am. Chem. Soc. 2011, 133, 13938–13941. [DOI] [PubMed] [Google Scholar]
- (57).Kim DA; Kang P; Choi MG; Jeong KS A Chiral Indolocarbazole Foldamer Displaying Strong Circular Dichroism Responsive to Anion Binding. Chem. Commun. 2013, 49, 9743–9745. [DOI] [PubMed] [Google Scholar]
- (58).Naidu VR; Suk JM; Lee GW; Jeong KS Chiral Oligoindole Foldamers Showing Anion-Induced Helical Bias. Bull. Korean Chem. Soc. 2009, 30, 482–485. [Google Scholar]
- (59).Kawai T; Muranaka A; Bando Y; Uchiyama M; Haketa Y; Shibaguchi H; Naito M; Takaishi K; Maeda H Asymmetric Induction in the Preparation of Helical Receptor-Anion Complexes: Ion-Pair Formation with Chiral Cations. Angew. Chem., Int. Ed. 2012, 51, 7967–7971. [DOI] [PubMed] [Google Scholar]
- (60).Maeda H; Shirai T; Bando Y; Takaishi K; Uchiyama M; Muranaka A; Kawai T; Naito M Chiroptical Control in Helical Receptor-Anion Complexes. Org. Lett. 2013, 15, 6006–6009. [DOI] [PubMed] [Google Scholar]
- (61).Wang Y; Bie F; Jiang H Controlling Binding Affinities for Anions by a Photoswitchable Foldamer. Org. Lett. 2010, 12, 3630–3633. [DOI] [PubMed] [Google Scholar]
- (62).Hua Y; Flood AH Flipping the Switch on Chloride Concentrations with a Light-Active Foldamer. J. Am. Chem. Soc. 2010, 132, 12838–12840. [DOI] [PubMed] [Google Scholar]
- (63).Hua Y; Ramabhadran RO; Karty JA; Raghavachari K; Flood AH Two Levels of Conformational Pre-Organization Consolidate Strong CH Hydrogen Bonds in Chloride-Triazolophane Complexes. Chem. Commun. 2011, 47, 5979–5981. [DOI] [PubMed] [Google Scholar]
- (64).Lee S; Hua Y; Flood AH Β-Sheet-Like Hydrogen Bonds Interlock the Helical Turns of a Photoswitchable Foldamer To Enhance the Binding and Release of Chloride. J. Org. Chem. 2014, 79, 8383–8396. [DOI] [PubMed] [Google Scholar]
- (65).Liu Y; Stutsman SR; Parks FC; Flood AH; Raghavachari K; Debnath S Allosteric Control of Photofoldamers for Selecting between Anion Regulation and Double-to-Single Helix Switching. J. Am. Chem. Soc. 2018, 140, 17711–17723. [DOI] [PubMed] [Google Scholar]
- (66).Suk JM; Kim DA; Jeong KS Helicity Control of an Indolocarbazole Foldamer by Chiral Organic Anions. Org. Lett. 2012, 14, 5018–5021. [DOI] [PubMed] [Google Scholar]
- (67).Zhao W; Wang Y; Shang J; Che Y; Jiang H Acid/Base-Mediated Uptake and Release of Halide Anions with a Preorganized Aryl-Triazole Foldamer. Chem.: Eur. J. 2015, 21, 7731–7735. [DOI] [PubMed] [Google Scholar]
- (68).Shang J; Zhao W; Li X; Wang Y; Jiang H Aryl-Triazole Foldamers Incorporating a Pyridinium Motif for Halide Anion Binding in Aqueous Media. Chem. Commun. 2016, 52, 4505–4508. [DOI] [PubMed] [Google Scholar]
- (69).Kim U. Il; Suk JM; Naidu VR; Jeong KS Folding and Anion-Binding Properties of Fluorescent Oligoindole Foldamers. Chem.: Eur. J. 2008, 14, 11406–11414. [DOI] [PubMed] [Google Scholar]
- (70).Zurro M; Asmus S; Beckendorf S; Mück-Lichtenfeld C; Mancheño OG Chiral Helical Oligotriazoles: New Class of Anion-Binding Catalysts for the Asymmetric Dearomatization of Electron-Deficient N-Heteroarenes. J. Am. Chem. Soc. 2014, 136, 13999–14002. [DOI] [PubMed] [Google Scholar]
- (71).Mancheço OG; Asmus S; Zurro M; Fischer T Highly Enantioselective Nucleophilic Dearomatization of Pyridines by Anion-Binding Catalysis. Angew. Chem., Int. Ed. 2015, 54, 8823–8827. [DOI] [PubMed] [Google Scholar]
- (72).Zurro M; Asmus S; Bamberger J; Beckendorf S; García O Chiral Triazoles in Anion-Binding Catalysis : New Entry to Enantioselective Reissert-Type Reactions. Chem.: Eur. J. 2016, 22, 3785–3793. [DOI] [PubMed] [Google Scholar]
- (73).Fischer T; Bamberger J; Mancheño OG Asymmetric Nucleophilic Dearomatization of Diazarenes by Anion-Binding Catalysis. Org. Biomol. Chem. 2016, 14, 5794–5802. [DOI] [PubMed] [Google Scholar]
- (74).Vargas Jentzsch A; Emery D; Mareda J; Metrangolo P; Resnati G; Matile S Ditopic Ion Transport Systems: Anion-π Interactions and Halogen Bonds at Work. Angew. Chem., Int. Ed. 2011, 50, 11675–11678. [DOI] [PubMed] [Google Scholar]
- (75).Choi YR; Chae MK; Kim D; Lah MS; Jeong KS Synthetic Chloride Transporters with the Binding Mode Observed in a ClC Chloride Channel. Chem. Commun. 2012, 48, 10346–10348. [DOI] [PubMed] [Google Scholar]
- (76).Si W; Zhao W; Shang J; Hou J-L; Jiang H; Che Y Preorganized Aryltriazole Foldamers as Effective Transmembrane Transporters for Chloride Anion. Org. Lett. 2014, 16, 4008–4011. [DOI] [PubMed] [Google Scholar]
- (77).Valkenier H; Dias CM; Butts CP; Davis AP A Folding Decalin Tetra-Urea for Transmembrane Anion Transport. Tetrahedron 2017, 73, 4955–4962. [Google Scholar]
- (78).Tepper R; Schubert US Halogen Bonding in Solution: Anion Recognition, Templated Self‐Assembly, and Organocatalysis. Angew. Chem., Int. Ed. 2018, 57, 6004–6016. [DOI] [PubMed] [Google Scholar]
- (79).Lim JYC; Beer PD Sigma-Hole Interactions in Anion Recognition. Chem 2018, 4, 731–783. [Google Scholar]
- (80).Costa PJ The Halogen Bond: Nature and Applications. Phys. Sci. Rev. 2017, 2, 1–16. [Google Scholar]
- (81).Bulfield D; Huber SM Halogen Bonding in Organic Synthesis and Organocatalysis. Chem.: Eur. J. 2016, 22, 14434–14450. [DOI] [PubMed] [Google Scholar]
- (82).Gilday LC; Robinson SW; Barendt TA; Langton MJ; Mullaney BR; Beer PD Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [DOI] [PubMed] [Google Scholar]
- (83).Scholfield MR; Zanden C. M. Vander; Carter M; Ho PS Halogen Bonding (X-bonding): A Biological Perspective. Protein Sci. 2013, 22, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Beale TM; Chudzinski MG; Sarwar MG; Taylor MS Halogen Bonding in Solution: Thermodynamics and Applications. Chem. Soc. Rev. 2013, 42, 1667–1680. [DOI] [PubMed] [Google Scholar]
- (85).Erdelyi M Halogen Bonding in Solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [DOI] [PubMed] [Google Scholar]
- (86).Cavallo G; Metrangolo P; Pilati T; Resnati G; Sansotera M; Terraneo G Halogen Bonding: A General Route in Anion Recognition and Coordination. Chem. Soc. Rev. 2010, 39, 3772–3783. [DOI] [PubMed] [Google Scholar]
- (87).Politzer P; Murray JS; Clark T Halogen Bonding: An Electrostatically-Driven Highly Directional Noncovalent Interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [DOI] [PubMed] [Google Scholar]
- (88).Legon AC The Halogen Bond: An Interim Perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [DOI] [PubMed] [Google Scholar]
- (89).Lauher JW; Fowler FW; Goroff NS Single-Crystal-to-Single-Crystal Topochemical Polymerizations by Design. Acc. Chem. Res. 2008, 41, 1215–1229. [DOI] [PubMed] [Google Scholar]
- (90).Politzer P; Lane P; Concha MC; Ma Y; Murray JS An Overview of Halogen Bonding. J. Mol. Model. 2007, 13, 305–311. [DOI] [PubMed] [Google Scholar]
- (91).Metrangolo P; Neukirch H; Pilati T; Resnati G Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [DOI] [PubMed] [Google Scholar]
- (92).Metrangolo P; Resnati G Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [DOI] [PubMed] [Google Scholar]
- (93).Zapata F; Caballero A; Molina P; Alkorta I; Elguero J Open Bis(Triazolium) Structural Motifs as a Benchmark to Study Combined Hydrogen- and Halogen-Bonding Interactions in Oxoanion Recognition Processes. J. Org. Chem. 2014, 79, 6959–6969. [DOI] [PubMed] [Google Scholar]
- (94).Borissov A; Lim JYC; Brown A; Christensen KE; Thompson AL; Smith MD; Beer PD Neutral Iodotriazole Foldamers as Tetradentate Halogen Bonding Anion Receptors. Chem. Commun. 2017, 53, 2483–2486. [DOI] [PubMed] [Google Scholar]
- (95).Borissov A; Marques I; Lim JYC; Félix V; Smith MD; Beer PD Anion Recognition in Water by Charge-Neutral Halogen and Chalcogen Bonding Foldamer Receptors. J. Am. Chem. Soc. 2019, 141, 4119–4129. [DOI] [PubMed] [Google Scholar]
- (96).Yang L; Wang Y; Che Y; Jiang H An Aryl-Triazole Foldamer Containing a 1,8-Naphthalimide Fluorescent Motif for Monitoring and Enhancing the Anion-Induced Folding. Org. Biomol. Chem. 2017, 15, 7747–7752. [DOI] [PubMed] [Google Scholar]
- (97).Hua Y; Liu Y; Chen CH; Flood AH Hydrophobic Collapse of Foldamer Capsules Drives Picomolar-Level Chloride Binding in Aqueous Acetonitrile Solutions. J. Am. Chem. Soc. 2013, 135, 14401–14412. [DOI] [PubMed] [Google Scholar]
- (98).Li YJ; Xu- L; Yang- WL; Liu HB; Lai SW; Che CM; Li YL. Amidetriazole: A Versatile Building Block for Construction of Oxyanion Anion Receptors. Chem.: Eur. J. 2012, 18, 4782–4790. [DOI] [PubMed] [Google Scholar]
- (99).Cao L; Jiang R; Zhu Y; Wang X; Li Y; Li Y Synthesis of 1,2,3-Triazole-4-Carboxamide-Containing Foldamers for Sulfate Recognition. European J. Org. Chem. 2014, 2014, 2687–2693. [Google Scholar]
- (100).Li S; Huang X; Yang X-J; Wu B; Jia C; Wang X Chloride Coordination by Oligoureas: From Mononuclear Crescents to Dinuclear Foldamers. Org. Lett. 2012, 14, 684–687. [DOI] [PubMed] [Google Scholar]
- (101).Yang P; Wang J; Jia C; Yang XJ; Wu B Dinuclear Chloride-Binding Foldamers Based on Fluorescent Oligoureas. Eur. J. Org. Chem. 2013, 17, 3446–3454. [Google Scholar]
- (102).Wang Y; Xiang J; Jiang H Halide-Guided Oligo(Aryl-Triazole-Amide)s Foldamers: Receptors for Multiple Halide Ions. Chem.: Eur. J. 2011, 17, 613–619. [DOI] [PubMed] [Google Scholar]
- (103).Bie F; Wang Y; Shang J; Gallagher NM; Jiang H Synergistic Recognition of Halide Anions and Saccharides by Oligohydrazide Foldamers. Eur. J. Org. Chem. 2013, 1, 8135–8144. [Google Scholar]
- (104).Haketa Y; Maeda H From Helix to Macrocycle: Anion-Driven Conformation Control of π-Conjugated Acyclic Oligopyrroles. Chem.: Eur. J. 2011, 17, 1485–1492. [DOI] [PubMed] [Google Scholar]
- (105).Jeon HG; Jang HB; Kang P; Choi YR; Kim J; Lee JH; Choi MG; Jeong KS Helical Aromatic Foldamers Functioning as a Fluorescence Turn-on Probe for Anions. Org. Lett. 2016, 18, 4404–4407. [DOI] [PubMed] [Google Scholar]
- (106).Maeda H; Kitaguchi K; Haketa Y Anion-Responsive Covalently Linked and Metal-Bridged Oligomers. Chem. Commun. 2011, 47, 9342–9344. [DOI] [PubMed] [Google Scholar]
- (107).Li X; Bie F; Zhao W; Jiang H; Wang Y; Wu L Ruthenium(II) Complexes of Aryl Triazole Foldamers as Receptors for Anions. Chem.: Eur. J. 2016, 22, 5233–5242. [DOI] [PubMed] [Google Scholar]
- (108).Liu Y; Parks FC; Zhao W; Flood AH Sequence-Controlled Stimuli-Responsive Single-Double Helix Conversion between 1:1 and 2:2 Chloride-Foldamer Complexes. J. Am. Chem. Soc. 2018, 140, 15477–15486. [DOI] [PubMed] [Google Scholar]
- (109).Albrecht M; Kotila S Formation of a “Meso-Helicate” by Self-Assembly of Three Bis(Catecholate) Ligands and Two Titanium(IV) Ions. Angew. Chem., Int. Ed. 2003, 34, 2134–2137. [Google Scholar]
- (110).Wu B; Li S; Lei Y; Hu H; Amadeu NDS; Janiak C; Mathieson JS; Long DL; Cronin L; Yang XJ The Effect of the Spacer of Bis(Biurea) Ligands on the Structure of A2L3-Type (A=anion) Phosphate Complexes. Chem.: Eur. J. 2015, 21, 2588–2593. [DOI] [PubMed] [Google Scholar]
- (111).Bai X; Jia C; Zhao Y; Yang D; Wang S-C; Li A; Chan Y-T; Wang Y-Y; Yang X-J; Wu B Peripheral Templation-Modulated Interconversion between an A4L6 Tetrahedral Anion Cage and A2L3 Triple Helicate with Guest Capture/Release. Angew. Chem., Int. Ed. 2018, 57, 1851–1855. [DOI] [PubMed] [Google Scholar]
- (112).Jia C; Zuo W; Yang D; Chen Y; Cao L; Custelcean R; Hostaš J; Hobza P; Glaser R; Wang Y-Y; et al. Selective Binding of Choline by a Phosphate-Coordination-Based Triple Helicate Featuring an Aromatic Box. Nat. Commun. 2017, 8, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Zuo W; Huang Z; Zhao Y; Xu W; Liu Z; Yang XJ; Jia C; Wu B Chirality Sensing of Choline Derivatives by a Triple Anion Helicate Cage through Induced Circular Dichroism. Chem. Commun. 2018, 54, 7378–7381. [DOI] [PubMed] [Google Scholar]
- (114).Massena CJ; Wageling NB; Decato DA; Martin Rodriguez E; Rose AM; Berryman OB A Halogen-Bond-Induced Triple Helicate Encapsulates Iodide. Angew. Chem., Int. Ed. 2016, 55, 12398–12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Riel AMS; Decato DA; Sun J; Massena CJ; Jessop MJ; Berryman OB The Intramolecular Hydrogen Bonded-Halogen Bond: A New Strategy for Preorganization and Enhanced Binding. Chem. Sci. 2018, 9, 5828–5836. [DOI] [PMC free article] [PubMed] [Google Scholar]