Abstract
Purpose
Clinically relevant pharmacokinetic interactions exist between gastric acid-reducing agents and certain weakly basic drugs that rely on acidic environments for optimal oral absorption. In this study, we examine whether the administration of betaine hydrochloride under fed conditions can enhance the absorption of atazanavir, an HIV-1 protease inhibitor, during pharmacologically-induced hypochlorhydria.
Methods
In this randomized, single-dose, 3 period, crossover study healthy volunteers received ritonavir-boosted atazanavir (atazanavir/ritonavir 300/100 mg) alone, following pretreatment with the proton pump inhibitor rabeprazole (20 mg twice daily), and with 1500 mg of betaine HCl after rabeprazole pretreatment. Atazanavir was administered with a light meal and gastric pH was monitored using the Heidelberg Capsule.
Results
Pretreatment with rabeprazole resulted in significant reductions in atazanavir Cmax (p<0.01) and AUC0-last (p<0.001) (71 and 70%, respectively), and modest decreases in ritonavir Cmax and AUClast (p<0.01) (40% and 41%, respectively). The addition of betaine HCl restored 13% of ATV Cmax and 12% of AUClast lost due to rabeprazole.
Conclusions
The co-administration of rabeprazole with atazanavir resulted in significant decreases in atazanavir exposure. The addition of betaine HCl did not sufficiently mitigate the loss of ATV exposure observed during RAB-induced hypochlorhydria. Meal effects lead to a marked difference in the outcome of betaine HCl on atazanavir exposure than we previously reported for dasatanib under fasting conditions.
Keywords: absorption, atazanavir, food effects, PPIs, weakly basic drugs
INTRODUCTION
A significant body of clinical data supports the use of human immunodeficiency virus (HIV) oral protease inhibitors (PIs) as integral components in combination antiretroviral therapy (ART; (1)). Since the approval of the first PI in 1995, advances in dosing, tolerability and efficacy of drugs in the class, have contributed to their widespread use. Despite their overall effectiveness, metabolic side effects and clinically relevant drug-drug-interactions continue to be limitations of use (1-3). Interactions arising from the concomitant use of gastric acid reducing agents (ARAs) with certain PIs have drawn considerable interest due to the potential for marked reductions in PI exposure and compromised in vivo activity.
Atazanavir (Reyataz®) is a potent and selective PI indicated for use in combination with other antiretrovirals for the treatment of HIV-1 infection in adults and pediatric patients over three months of age. In adults, atazanavir is administered once daily with food as a 300 mg tablet taken with 100 mg of ritonavir (atazanavir/ritonavir 300/100 mg) or, more recently, as a fixed-dose combination with 150 mg of cobicistat (Evotaz®; (4, 5)). Coadministration of atazanavir with ritonavir or cobicistat (potent CYP3A4 and P-glycoprotein (P-gp) inhibitors), exploits a favorable drug-drug-interaction, effectively enhancing the systemic exposure of atazanavir (6). Alternatively in treatment-naïve patients, atazanavir may be given at the 400 mg dose strength without a pharmacokinetic boosting agent.
Similar to the other drugs in this class, atazanavir is a weak base that exhibits pH-dependent aqueous solubility over the physiologic pH range. On the basis of its poor solubility and extensive metabolism, it is classified as a Biopharmaceutical Drug Disposition Classification System (BDDCS) class 2 drug (7, 8). The pharmacokinetics of atazanavir is complex, highly variable, and its absorption is altered by the presence of food, gastric ARAs, and substrates and/or inhibitors of drug metabolizing enzymes and transporters (8-12). The in vitro solubility profile of atazanavir and available clinical data suggest that elevations in gastric pH by ARAs can alter drug absorption resulting in lower systemic exposures.
Interactions between ARAs and weakly basic drugs , particularly in the therapeutic areas of HIV and oncology, have been widely studied due to the prevalence of ARA use in these populations, as well as the potential for undesirable consequences (3, 13-15). The clinical relevance of such an interaction depends on the level of impact to drug absorption as well as the therapeutic index of the victim drug. ARA class, dose and time of administration relative to the victim drug can all alter the magnitude of the interaction. PPIs are the most potent ARA class and serve as a “worst-case-scenario” in the evaluation of an interaction as they exert their pharmacologic effect through irreversible binding to active gastric proton pumps (H+/K+ -ATPase) at the secretory surface of gastric parietal cells.
Previous pharmacokinetic analyses with atazanavir have shown that the concurrent use of high doses of PPIs result in substantial reductions to atazanavir absorption. In the absence of a PK boosting agent, atazanavir exposures were reduced > 90% in healthy volunteers pretreated with high doses of the PPI omeprazole (40 mg once daily), compared to atazanavir alone (400mg once daily; (10)). Exposure reductions of 72-76% were observed when the same dose of omeprazole was coadministered with ritonavir-boosted atazanavir, compared to boosted-atazanavir alone (9). In both examples, atazanavir was co-dosed with a light meal. A lower dose of omeprazole (20 mg once daily) resulted in more modest reductions in boosted atazanavir exposures (39-46%; (12)). As such, the current FDA label for atazanavir allows for the use of low doses of PPIs (comparable to omeprazole 20 mg once daily) in treatment-naïve patients using a time-staggered approach (administration of the two agents is separated by 12 hours). The use of PPIs in treatment-experienced patients and/or those taking unboosted regimens is not recommended (4).
Mitigation strategies targeting transient gastric reacidification with oral acidic solutions to improve the extent of absorption of weakly basic drugs with pH-dependent solubility have been previously reported. The systemic exposure of the antifungal agent posaconazole was increased >70% when administered with Coca-Cola (pH = 2.5), compared to administration with water (16). Ray et al. reported that atazanavir concentrations three hours post-dose increased in four of six HIV patients following administration of 400 mg atazanavir with cola, relative to water (17). Acidic solutions have also been investigated as a means to overcome exposure loss during PPI-induced hypochlorhydria (fasting gastric pH >4). Studies investigating the use of Coca-Cola as a means to increase the extent of atazanavir, posaconazle and ketoconazole absorption during PPI use have been reported with varying success. In the case of posaconazole and ketoconazole, administration with cola improved the systemic absorption of both agents in healthy volunteers, but was unable to completely compensate for the loss of exposure during PPI-induced hypochlorhydria. Administration of boosted and unboosted atazanavir with cola and a light meal to healthy volunteers following pretreatment with omeprazole had no appreciable impact on atazanavir exposure (9, 10, 16, 18).
Another mitigation strategy involves the use of solid reacidifying agents, such as betaine hydrochloride (BHCl). BHCl is a nutraceutical available over-the-counter and is commonly used as a digestive aid. When taken orally, BHCl rapidly dissociates into free betaine and hydrochloric acid. Recently, our laboratory demonstrated that BHCl can rapidly and transiently reacidfy gastric pH in healthy volunteers pretreated with the PPI rabeprazole (19). Furthermore, we have also shown that a single 1500 mg oral dose of BHCl was sufficient to mitigate the reduced exposure of dasatinib, another weakly basic drug with pH-dependent solubility, in healthy subjects pretreated with rabeprazole (20). Based on these data, we hypothesize that the concomitant use of BHCl with atazanavir may be a more effective mitigation strategy compared to Coca-Cola since in the same volume of water (250 mL), BHCl has a greater buffering capacity and provides a higher equivalent of acid (in the form of H+ ions).
Here we examine whether BHCl can mitigate marked reductions in atazanavir exposure during rabeprazole-induced hypochlorhydria under fed conditions.
METHODS
A total of eight healthy, non-smoking volunteers between the protocol allowed ages of 18-65 were enrolled in this three-period crossover study. Subject demographics are summarized in Table 1. The mean age and body mass index (BMI) ±SD were 37 ±16 years and 24 ±2.5 kg/m2, respectively. Eligibility was determined by medical history review, physical examination, 12-lead electrocardiogram and clinical laboratory evaluations. Baseline gastric pH measurements were determined during screening visits through the use of the Heidelberg pH Diagnostic System (Heidelberg Medical Inc., Mineral Bluff, GA) to confirm normochlorhydria (fasting gastric pH <4), as previously described (19). Female subjects who were surgically sterile or post-menopausal (no history of menorrhea in the past 12 months) were eligible to participate. Individuals with a history of gastrointestinal disease and those taking concomitant medications two weeks prior to enrollment were excluded.
Table I.
Enrolled Subject Demographics
| Total N | 8 |
|---|---|
| Male | 7 |
| Female | 1 |
| Race/Ethnicity | |
| Caucasian | 4 |
| Asian | 2 |
| African American | 1 |
| Hispanic | 1 |
| Age (years) | |
| Mean ± SD | 37±16 |
| Range | 22-59 |
| Body Mass Index | |
| Mean ± SD | 24±2.5 |
| Range | 21-28 |
Participants provided written informed consent to participate in the study. The study was approved by the Committee on Human Research of the University of California, San Francisco and registered on the US National Institutes of Health Clinical Trials Database ( NCT01759875; https://clinicaltrials.gov/ct2/show/NCT01759875).
Study Design
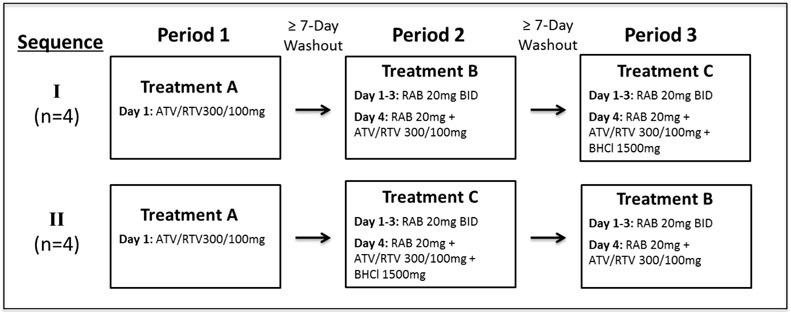
This was an open-label, randomized, three-treatment, cross-over study conducted at the Clinical & Translational Science Institute’s Clinical Research Center at the University of California, San Francisco. All subjects fasted overnight prior to receiving atazanavir in each treatment and consumed a standardized light meal (336 kcal, 5.1 g fat, 9.3 g protein) ten minutes prior to each dose of atazanavir. Gastric pH was monitored using the Heidelberg pH capsule on the morning of each study day until three hours post-atazanavir dosing. Ten minutes after the light meal participants first received treatment A, which was a single oral dose of atazanavir/ritonavir 300/100 mg with 250 mL of water. For treatments B and C, participants were pretreated with rabeprazole 20 mg twice daily with food for three days. On pharmacokinetic sampling days, an additional 20 mg dose of rabeprazole was administered with four ounces of low fat yogurt at least 2 hours prior to atazanavir. When gastric pH remained above pH 3.8 for at least 10 min, subjects received their light meal and then 10 minutes later either atazanavir/ritonavir 300/100 mg alone (Treatment B), or 1500 mg of BHCl followed by atazanavir/ritonavir 300/100 mg five minutes after BHCl administration (Treatment C; Fig. 1). Participants were block randomized (4 subjects per block) for the order in which they would receive treatments B and C. Treatment periods were separated by at least a seven-day washout.
Fig 1.
Clinical study design. ATV, atazanavir; RTV, ritonavir; RAB, rabeprazole; BHCl, betaine hydrochloride; BID, twice daily.
Venous blood samples for PK assessment were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 22 hours, relative to atazanavir dosing. An additional pre-rabeprazole sample was drawn during treatments B and C. Blood samples were centrifuged within 30 minutes of collection at 4°C. Plasma was subsequently separated and stored in aliquots at −80°C until bioanalysis. Plasma concentrations of atazanavir and ritonavir were measured using a validated LC-MS/MS method (API 3000, MDS Sciex, Thornhill, Ontario) by Tandem Labs, as described below.
Outcome Measures
The primary outcome measures for this study included the maximum plasma concentration (Cmax) and area under the plasma concentration-time curve from time zero to 22 hours (AUClast) of atazanavir for each treatment. Secondary outcomes included ritonavir Cmax and AUClast, as well as atazanavir and ritonavir Tmax.
Pharmacokinetic Analysis
Atazanavir and ritonavir pharmacokinetic parameters for each period were calculated from plasma concentrations using non-compartmental analyses in Phoenix Winnonlin 5 (Pharsight®, Sunnvale, CA). Cmax and Tmax were estimated from the observed data. Atazanavir and ritonavir AUCs were calculated using the linear trapezoid method.
Statistical Analysis
A sample size of eight was calculated to detect a 40% change in atazanavir AUClast with 80% power and a two-sided α = 0.05, based on an observed standard deviation of the difference of 75%. Statistical results were performed using GraphPad Prism 5.02 (La Jolla, CA). A repeated measures analysis of variance (ANOVA) with the Tukey’s test for multiple comparisons was used to determine statistical significance across all treatments for atazanavir and ritonavir pharmacokinetic parameters except Tmax. Logarithmic transformation of all pharmacokinetic parameters (except Tmax) were performed prior to statistical analyses. Additionally, the geometric mean ratios and 90% confidence intervals of atazanavir and ritonavir Cmax and AUClast were calculated for all treatment comparisons.
Quantitative Determination of Atazanavir and Ritonavir
Plasma samples were pretreated by a solid phase extraction procedure and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS ) method (21). An API 3000 was used to simultaneously detect atazanavir and ritonavir positive ions formed by Turboionspray™ ionization in the multiple reaction monitoring (MRM) mode. The lower limit of quantitation (LLOQ) of the assay in human plasma were 10.0 and 5.00 ng/mL for atazanavir and ritonavir, respectively, while the upper limit of quantitation were 10,000 and 5,000 ng/mL, respectively. The accuracy and precision of the LC-MS/MS method for atazanavir and ritonavir in human plasma were determined by analyzing low, medium, high and dilution quality control samples. The intra-assay precision for atazanavir was within 8.7% and the inter-assay precision was within 6.5%. The mean % deviation was within ±10.0%. The intra-assay precision for ritonavir was within 9.1% and the inter-assay precision was within 10.2%. The mean % deviation was within ±3.8%.
RESULTS
Study Demographics and Safety
A total of eight subjects (seven males and one female) received study medication and completed all treatments. One subject was excluded from pharmacokinetic analysis because atazanavir concentration data suggested that plasma samples for treatments A and B were switched prior to bioanalysis, but could not be confirmed. All study medications were well-tolerated and no adverse events were reported due to study drugs or Heidelberg pH Diagnostic System use.
Atazanavir Pharmacokinetics
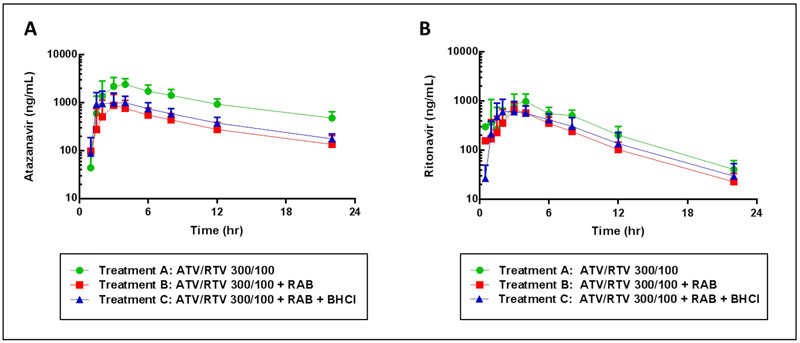
Fig. 2a shows the mean atazanavir plasma concentration-time profile for each of the three treatment periods. Following pretreatment with rabeprazole (treatment B), statistically significant reductions in atazanavir Cmax (p<0.01) and AUClast (p<0.001) of approximately 70% were observed, compared to atazanavir/ritonavir 300/100 mg alone (treatment A; Table II). The addition of BHCl to rabeprazole pretreated subjects during treatment C restored approximately 13% of atazanavir Cmax and 12% of atazanavir AUClast lost due to pretreatment with rabeprazole, although the increases were not statistically significant. No significant differences in atazanavir Tmax were observed across treatments.
Fig 2.
Mean (SD) atazanavir (A) and ritonavir (B) plasma concentration-time profiles plotted on logarithmic scale. ATV, atazanavir; RTV, ritonavir; RAB, rabeprazole; BHCl, betaine hydrochloride.
Table II.
Atazanavir Pharmacokinetics with and Without Gastric pH Modulators
| Geometric Mean Ratios (90% CIs) | ||||||
|---|---|---|---|---|---|---|
| PK Parameter | ATV/RTV (Treatment A) |
ATV/RTV + RAB (Treatment B) |
ATV/RTV + RAB + BHCl (Treatment C) |
B vs A | C vs B | C vs A |
| Cmax (ng/mL) | 2,920 ± 917 | 933 ± 545 | 1,270 ± 524 | 0.291 (0.183-0.462) | 1.44 (0.909-2.29) | 0.420 (0.265-0.666) |
| AUC0-last (ng*hr/mL) | 235,000 ± 6,690 | 7,410 ± 3,580 | 10,100 ± 3,790 | 0.300 (0.206-0.439) | 1.41 (0.967-2.06) | 0.424 (0.290-0.620) |
| Tmax (hr) | 3.0 (2.0-4.0) | 3.0 (2.0-3.0) | 3.0 ± (1.5-6.0) | N/A | N/A | N/A |
Pharmacokinetic parameter data presented as mean ± SD. Tmax is expressed as median (range). CI, confidence interval; PK, pharmacokinetic; ATV, atazanavir; RTV, ritonavir; RAB, rabeprazole; BHCl, betaine hydrochloride; Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC0-last, area under the plasma concentration-time curve from time zero to 22 hours.
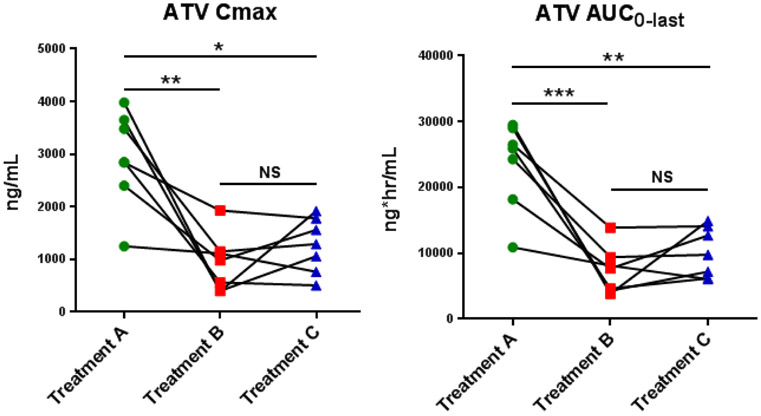
To further quantify the effect of rabeprazole and betaine HCl on atazanavir exposures on an individual basis, spaghetti plots of individual atazanavir Cmax and AUClast values by treatment were prepared (Fig. 3). Atazanavir Cmax and AUClast were reduced in all subjects following pretreatment with rabeprazole (Treatment B), compared to the control (Treatment A). Five of the seven subjects experienced greater than a 55% decline in Cmax and AUClast following pretreatment with rabeprazole, with three experiencing greater than 80% declines in both parameters. The remaining two subjects experienced more modest decreases (11-48%) in atazanavir AUClast and Cmax. Interindividual differences in gastric pH profiles did not appear to be correlated with Cmax within each treatment.
Fig 3.
Individual atazanavir (ATV) area under the concentration time curve (AUC) and maximum concentration (Cmax) values for administration of ATV/RTV alone (Treatment A), pretreatment with rabeprazole (Treatment B) and the addition of betaine HCl (Treatment C).
Considerable overlap in individual atazanavir Cmax and AUClast parameters was observed between treatments B and C. With the addition of BHCl (Treatment C), three subjects experienced unexpected further declines in atazanavir exposure. Interestingly, two of these subjects were also least sensitive to pretreatment with rabeprazole, as measured by their lower geometric mean ratios (B-to-A) relative to the other subjects (Fig. 3). Conversely, the subject most sensitive to pretreatment with rabeprazole also exhibited the largest gains in Cmax and AUClast of 38% and 37%, respectively, with the addition of BHCl. Compared with treatment B (PPI), Cmax and AUC increased in 4 and 6 subjects, respectively, during Treatment C (PPI + BHCl). However, response to BHCl, as measured by increases in atazanavir exposure, did not appear to correlate with responses to BHCl, as measured by reductions in gastric pH.
Ritonavir Pharmacokinetics
Pretreatment with rabeprazole resulted in moderate decreases in mean ritonavir AUClast and Cmax (p<0.01) values of 42% (90% CI, 26%-54%) and 40% (90% CI, 14%-76%), respectively (Table III). Similar to atazanavir, the addition of BHCl during rabeprazole-induced hypochlorhydria restored 12% and 13% of ritonavir Cmax and AUClast, respectively, that was lost due to pretreatment with rabeprazole. Increases in exposure were not statistically significant.
Table III.
Ritonavir Pharmacokinetics with and Without Gastric pH Modulators
| Geometric Mean Ratios (90% CIs) | ||||||
|---|---|---|---|---|---|---|
| PK Parameter | ATV/RTV (Treatment A) |
ATV/RTV + RAB (Treatment B) |
ATV/RTV + RAB + BHCl (Treatment C) |
B vs A | C vs B | C vs A |
| Cmax (ng/mL) | 1,210 ± 392 | 730 ± 254 | 860 ± 272 | 0.602 (0.241-0.861) | 1.21 (0.849-1.74) | 0.731 (0.511-1.05) |
| AUC0-last (ng*hr/mL) | 7,210 ± 1,610 | 4,280 ± 1,180 | 5,180 ± 1,340 | 0.585 (0.460-0.743) |
1.22 (0.960-1.55) |
0.713 (0.562-0.905) |
| Tmax (hr) | 3.0 (1.0-8.0) | 3.0 (2.0-4.0) | 3.0 ± (1.5-6.0) | N/A | N/A | N/A |
Pharmacokinetic parameter data presented as mean ± SD. Tmax is expressed as median (range). CI, confidence interval; PK, pharmacokinetic; ATV, atazanavir; RTV, ritonavir; RAB, rabeprazole; BHCl, betaine hydrochloride; Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC0-last, area under the plasma concentration-time curve from time zero to 22 hours.
Intra-gastric pH Analysis
The mean gastric pH at the time of atazanavir dosing and immediately following a light meal (t = 0) did not differ significantly across the three treatments: 2.76 ±1.27, 2.36 ±0.66 and 2.89 ±1.17 (mean ± SD) for treatments A, B and C, respectively. While there was considerable overlap in pH for all treatments throughout the measurement period, subjects treated with BHCl were still able to achieve the lowest observed gastric pH in the study (0.838 ±0.391), even exceeding the lowest pH observed in the control group (1.39 ±0.906). The time to achieve this pH after BHCl administration averaged 67 ±33 minutes, which suggests a delay in the onset of BHCl reacidification in the fed state, relative to the fasting state (20). Moreover, the length of the reacidification period, as measured by the time for gastric pH to rebound to pH >4, averaged 76 ±20 minutes. It should be noted however that the majority of enrolled subjects (n = 5 of the 8 subjects) did not achieve a gastric pH > 4 by three hours post-ATV dosing.
DISCUSSION
In this study, we confirmed previous findings that atazanavir exposure is markedly reduced in the presence of high doses of PPIs, but BHCl was not able to reverse this affect. The interaction between atazanavir and ARAs is believed to result from reduced drug solubility in elevated gastric pH. Therefore, mitigation strategies aimed at transient gastric reacidification have the potential to reverse this effect. Previously, our group has shown that a single 1500 mg dose of BHCl was able to safely and significantly reverse the effect of rabeprazole-induced hypochlorhydria on the absorption of dasatinib, a BCR-ABL tyrosine kinase inhibitor, in healthy fasting volunteers (19). Here, we investigated whether the use of BHCl could similarly mitigate reductions in atazanavir exposure in healthy volunteers when administered after a light meal. Although atazanavir and dasatinib share similar physiochemical properties, recovery of atazanavir exposure with BHCl was minimal, compared with dasatinib. A closer examination of gastric pH-time profiles highlights differences arising from the presence of food not previously observed in the fasted state, which may have contributed in part to the poor recovery. Before considering the impact of BHCl on gastric pH and atazanavir bioavailability, it is important to understand the effect of rabeprazole.
Reductions in atazanavir Cmax and AUC following pretreatment with rabeprazole in this study were comparable to those previously reported in healthy volunteers taking high doses of the PPI omeprazole (9). Interestingly, despite similar reductions in atazanavir exposure, gastric pH during pretreatment with omeprazole resulted in substantially higher gastric pH around the time of atazanavir administration, compared to what we observed with rabeprazole (approximately pH 6 and pH 3, respectively). It is unclear why the differences in pH were observed as both PPIs, omeprazole and rabeprazole, were administered for multiple days to reach maximum acid suppression; however, one consideration is food. Subjects in both studies completed a standardized low fat breakfast immediately prior to receiving atazanavir. In healthy individuals with normochlorhydria, the presence of food results in transient elevations in gastric pH; however, the degree and duration of this effect is highly content-dependent. For example, meals taken with milk, which has a high buffering capacity, result in higher postprandial gastric pH, compared to meals taken with water, such as in this study (22, 23). Therefore, it is possible that differences in meal content contributed to differences in gastric pH. Despite the higher gastric pH noted with omeprazole, the pH-time profiles for the control group (no PPI) were similar between the two studies suggesting that the pH difference on PPI study days was real and unlikely due to differences in data collection or analysis technique.
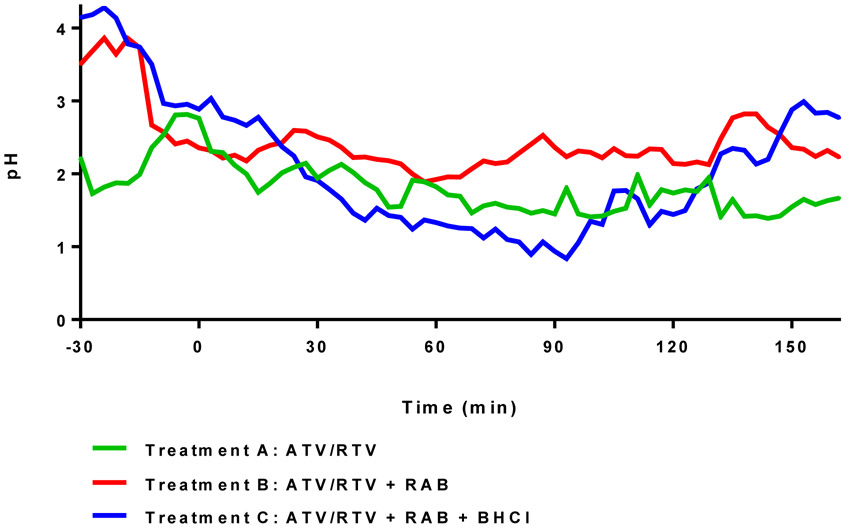
In this study, a three hour post-dose gastric pH observation window was selected as it was expected to be most critical period for atazanavir absorption based on a reported median Tmax of 2.7 hours in healthy subjects (8). During the post-dose monitoring period , gastric pH was slightly higher in treatment B (pretreatment with rabeprazole), compared with the control (treatment A), although the difference between the two treatments was smaller (Fig. 4) than what our group has previously observed in the fasted state (20). Based on atazanavir’s in vitro solubility profile, the greatest loss to solubility occurs between pH 2-4, where a decrease in solubility from 5.2 mg/mL to <0.005 mg/mL is reported (8). The gastric pH-time profiles for treatment A and B fall in the pH range of approximately 1.5 and 3 following atazanavir dosing; therefore, relatively small changes in gastric pH within this range have the potential to result in marked changes in drug solubility.
Fig 4.
Mean pH-time profiles by treatment prior to and following oral administration of ATV/RTV 300/100 mg (t=0 min). Individual 3-minute median pH values were averaged to construct each profile. A low fat meal was consumed 10 min prior to ATV/RTV dosing in treatments A and B. In Treatment C the meal was administered 10 min prior to BHCl dosing, which was followed 5 minutes later by ATV/RTV dosing. In treatments B and C the meal was administered 10 min after subjects exhibited a gastric pH of 3.8 or greater for 10 min.
The relationship between intra-gastric pH and atazanavir bioavailability has been previously explored by Eley et al. (24). Using pooled data from two ARA studies, the authors concluded that atazanavir exposures were largely unaffected at gastric pH < 4, but decreased substantially at pH > 4. Although ritonavir-boosted atazanavir regimens were investigated in both studies included in the ad hoc analysis, only data from the atazanavir 400 mg arms (without ritonavir) were presented. Here, substantial reductions in atazanavir exposure were noted in both treatments B and C, despite gastric pH recordings below 4 in the hours following atazanavir dosing (Fig. 4).
Although the pH effects on atazanavir absorption and solubility have been well documented, drug metabolizing enzymes and transporters can also impact exposure and should be considered here. Atazanavir is both a substrate and an inhibitor of CYP3A4 as well as a substrate of P-gp. Most PPIs are largely metabolized in the liver by the CYP2C19 and CYP3A4 isoforms, and as a result, can contribute to interactions with drugs metabolized by the same CYPs (25). Rabeprazole, however, is unique in that it is metabolized primarily by non-P450-mediated mechanisms (26). The selection of rabeprazole in this study, therefore, was important for isolating the effect that pH plays on drug absorption by minimizing the impact of CYP-related metabolism on study drug exposure. Measuring systemic concentrations of rabeprazole could have helped in confirming that levels were not impacted by the other study drugs.
The loss of ritonavir exposure is another consideration for the observed reductions in atazanavir exposure. Similar to atazanavir, ritonavir is an HIV PI and a weakly basic drug (pKa = 2.8) with antiviral properties at therapeutically relevant doses (300 mg twice daily). At low doses (100 mg once daily), ritonavir exerts no antiviral activity but acts as a potent inhibitor of CYP3A4 and P-gp. Coadministration of ritonavir with atazanavir, a substrate of CYP3A4 and P-gp, results in a favorable drug-drug interaction that reduces atazanavir metabolism and improves bioavailability. The interplay between apical gut efflux transporters and metabolizing enzymes located within enterocytes has been well-characterized (27, 28). Generally, drugs with high permeability rates and extensive metabolism (BDDCS Class 1 and 2) are able to enter enterocytes through passive diffusion. However, for BDDCS Class 2 compounds such as atazanavir, poor aqueous solubility increases their susceptibility to apical efflux transporter effects in the gut (29). In this study, ritonavir Cmax and AUC were both reduced by approximately 40% following pretreatment with rabeprazole, likely a result of its own pH-dependent solubility. Similar modest reductions in ritonavir exposure were also observed in the presence of high doses of the PPI omeprazole (9). The impact of a low dose of ritonavir on the pharmacokinetics of atazanavir has been characterized previously in a drug interaction study (8). In that study, the addition of 100 mg of ritonavir to 300 mg of atazanavir increased atazanavir Cmax and AUC by 86 and 238%, respectively. Therefore, reductions in ritonavir exposure in our study likely contributed to a decrease in atazanavir bioavailability; however, given that ARAs also reduce atazanavir levels in the absence of ritonavir, other factors were likely to be involved.
The effect of BHCl on the pharmacokinetics of atazanavir during rabeprazole-induced hypochlorhydria was examined in treatment C. The addition of BHCl only restored 12-13% of the mean atazanavir exposure lost due to pretreatment with rabeprazole and the increase was not statistically significant. Evaluation of pH-time profiles indicate that the buffering effect of food appeared to reduce the reacidification potential of BHCl, compared to the fasted state. Following consumption of a light meal, the mean gastric pH values for all three treatments converged around pH 2.5-3 at the time of atazanavir administration (Fig. 4). During treatment A, pH levels increased in all subjects at the start of the meal. For treatments B and C, where subjects were pretreated with rabeprazole and confirmed to have pH ≥4 prior to the start of their meal, gastric pH fell in all but one subject immediately following food intake, presumable due to the buffering effects of the meal (Fig. 4.). Overall, the presence of food in this study appeared to narrow the difference in gastric pH between treatments containing the PPI versus the control, and delay the onset of BHCl, as evidenced by a shallower decline in gastric pH following administration of BHCl, relative to the fasted state. Both of the aforementioned observations may help explain recovery differences in exposures between this study and our dasatinib study, which was conducted in a fasted state.
Oral drug absorption is complex and multifactorial. Determining the relative contribution of, and interplay between, individual factors that alter bioavailability can be challenging, particularly for a drug like atazanavir, which patients are instructed to take with food and in combination with other medications. To our knowledge, no group has investigated the relationship between gastric pH as it relates to food and atazanavir pharmacokinetics, most likely because gastric pH elevations are one of many ways food affects GI physiology and drug absorption (30). However, to minimize potential confounders in this study, all participants were administered the same standardized light meal during each treatment. Alterations in gastric acid are thought to play a role in the clinical interaction between atazanavir and ARAs. There are, however, other factors outside of the scope of this study that may account in part to lower atazanavir exposures in the presence of ARAs.
Recent in vitro and in situ studies have demonstrated that atazanavir permeability is sensitive to intestinal pH (31). Kis et al. reported that atazanavir permeability rates (apical-to-basolateral) increased with decreasing luminal pH over the pH range of 4.5 - 7.4. In addition, the inhibitory effect of a known P-gp inhibitor on atazanavir efflux was decreased at pH 5.5 compared to 7.4 (78% and 245%, respectively), suggesting that increases in atazanavir permeability at more acidic pHs may be due to a decrease in P-gp mediated efflux. This was a surprising observation as permeability rates for a weakly basic drug typically increase at a higher pH due to a larger proportion of the uncharged species (32). Median fasting duodenal pH in healthy volunteers is approximately 6.0 and has been reported to increase in individuals taking a PPI (16, 22). Additional clinical studies are needed to further explore the interplay between intestinal pH and atazanavir absorption in the presence and absence of food and ARAs; however, it is interesting to consider that changes in intestinal pH at the site of drug absorption may contribute in part to alterations in bioavailability.
Although our study aimed to examine the effects of gastric pH on ritonavir-boosted atazanavir pharmacokinetics, a number of considerations must be addressed. The most telling, as observed from the data presented herein, is the sheer complexity and impact of food on drug absorption. In addition to a meal’s immediate impact on gastric pH, the relative effects in the intestine, which include alteration of intestinal pH, solubilizing effects on oral dosage forms, and effects on intestinal drug metabolizing enzymes and transporters, may be of greater significance to certain drug’s dissolution and absorptive processes. Furthermore, and specifically in the case of atazanavir, the complexity surrounding bioavailability is further raised due to pharmacokinetic interactions with ritonavir that occur upon first pass, as described above. Along these lines, additional aspects of the clinical utility of BHCl in improving the absorption of weakly basic drugs given during drug-induced hypochlorhydria were also brought to light, such as the timing of the administration of BHCl relative to administration of the victim drug.
In a previously conducted pilot study, we reported that the average onset of effect of BHCl, defined as the time to achieve a pH < 3, was just over 5 minutes (19), which was subsequently used in the design of our study with dasatinib in the fasting state. However, as observed in this study, this may not necessarily be ideal under conditions in which a drug must be administered with food as gastric pH effects may become secondary to the intestinal effects on drug absorption, as described above. In this study, gastric pH begins to rise around the 90 minute time point in Treatment C, suggesting that a second dose of BHCl at or before before this point may result in additional gains to exposure since the reported atazanavir Tmax in healthy volunteers is 2.7 hours. This pH observation is consistent with the atazanavir pharmacokinetic profiles in Treatments B and C, where the greatest differences in concentrations was observed at the 1.5 hour time point (mean atazanavir concentration was 3.4 times higher in Treatment C, compared with Treatment B). Concentrations remain higher in Treatment C for subsequent time points, however, the difference between the two treatments decreases. While these observations were outside the scope of this current study, further dedicated studies to evaluate these hypotheses are warranted to broaden our understanding of the clinical utility of BHCl usage and the potential benefit it may have on patient outcomes.
The interaction between ritonavir-boosted atazanavir and PPIs has been studied previously in patients infected with HIV; however the clinical relevance is not yet fully understood because results have been inconsistent (33, 34). Although conducting this study in HIV-infected patients would have provided further insight into the clinical significance of this interaction and mitigation strategy, the choice to test this hypothesis in healthy volunteers first was made in an effort to isolate the effects of pH on the drug interaction by minimizing differences in GI physiology that have been noted in HIV patients (35), and limiting the impact additional drugs may have on pharmacokinetic outcomes. With a better understanding of the role of pH plays, future studies can assess these differences as well as their contribution to the overall clinical significance of this interaction in a patient population.
CONCLUSIONS
As expected, co-administration of the PPI rabeprazole with atazanavir/ritonavir markedly decreased the systemic concentrations of these poorly soluble, weakly basic HIV protease inhibitors. Attempts to reverse this effect through administration of the gastric reacidification agent betaine hydrochloride, previously shown to mitigate PPI-induced decreases with dasatinib, were unsuccessful here. In addition, the gastric pH changes noted for betaine hydrochloride in fasted subjects were not repeated here in subjects receiving a concomitant light meal with the parent drug. This study highlights the complexity of food effects on drug absorption and notes the necessity of further work required before the timing and expected outcome of food effects and gastric pH may be better understood.
ACKNOWLEDGEMENTS AND DISCLOSURES
Systemic concentration measurements of atazanavir and ritonavir carried out at Tandem Laboratories were supported by Bristol Myers Squibb. All other aspects of the study were funded by the Benet Fund for Excellence through contributions and through Dr. Benet’s consultations and expert witness fees and Board of Directors remunerations, all of which are made payable to the Regents of the University of California to support the research studies of the Benet Laboratory. This clinical study was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004.
ABBREVIATIONS
- ARA
Acid reducing agents
- ART
Antiretroviral therapy
- BDDCS
Biopharmaceutics Drug Disposition Classification System
- BHCl
Betaine hydrochloride
- BMI
Body mass index
- HIV
Human immune deficiency virus
- LC/MS/MS
Liquid chromatography/tandem mass spectrometry
- LLOQ
Lower limit of quantitation
- PI
Protease inhibitor
- PPI
Proton pump inhibitor
- RAB
Rabeprazole
Contributor Information
Kathleen Panter Faber, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California.
Hsin-Fang Wu, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California.
Marc R. Yago, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California
Xiaohui Xu, Bioanalytical Sciences, Bristol-Myers Squibb, Princeton, New Jersey.
Pathanjali Kadiyala, Bioanalytical Sciences, Bristol-Myers Squibb, Princeton, New Jersey.
Lynda A. Frassetto, Department of Medicine University of California San Francisco, San Francisco, California; Clinical Research Center, University of California San Francisco, San Francisco, California
Leslie Z. Benet, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California.
REFERENCES
- 1.Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; [cited 2015 October 3]. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.Josephson F Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med. 2010;268(6):530–9. [DOI] [PubMed] [Google Scholar]
- 3.Falcon RW, Kakuda TN. Drug interactions between HIV protease inhibitors and acid-reducing agents. Clin Pharmacokinet. 2008;47(2):75–89. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Atazanavir sulfate capsule, gelatin coated (Reyataz) prescribing information. 2015.
- 5.US Food and Drug Administration. Atazanavir sulfate and cobicistat (Evotaz) prescribing information. 2015.
- 6.Larson KB, Wang K, Delille C, Otofokun I, Acosta EP. Pharmacokinetic enhancers in HIV therapeutics. Clin Pharmacokinet. 2014;53(10):865–72. [DOI] [PubMed] [Google Scholar]
- 7.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Atazanavir (Reyataz) summary basis of approval. 2003.
- 9.Agarwala S, Gray K, Wang Y, Grasela D. Pharmacokinetic (PK) effect of omeprazole (OMP) on atazanavir (ATV) with ritonavir (RTV) in healthy subjects. 12th Conference on Retroviruses and Opportunistic Infections; 2005 Feb 22–25; Boston, MA. [Google Scholar]
- 10.Agarwala S, Gray K, Eley T, Wang Y, Hughes E, Grasela D. Pharmacokinetic interaction between atazanavir and omeprazole in healthy subjects. 3rd IAS Conference on HIV Pathogenesis and Treatment; 2005 Jul 24–27; Rio de Janiero, Brazil. [Google Scholar]
- 11.Wang X, Boffito M, Zhang J, Chung E, Zhu L, Wu Y, et al. Effects of the H2-receptor antagonist famotidine on the pharmacokinetics of atazanavir-ritonavir with or without tenofovir in HIV-infected patients. AIDS Patient Care STDS. 2011;25(9):509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, et al. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51(3):368–77. [DOI] [PubMed] [Google Scholar]
- 13.Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–13. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96(2):266–77. [DOI] [PubMed] [Google Scholar]
- 15.Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10(11):4055–62. [DOI] [PubMed] [Google Scholar]
- 16.Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole: monitoring of intraluminal and plasma drug concentrations. Clin Pharmacokinet. 2011;50(11):725–34. [DOI] [PubMed] [Google Scholar]
- 17.Ray JE, Marriott D, Bloch MT, McLachlan AJ. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005;60(3):291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin TW, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39(8):1671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10(11):4032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yago MR, Frymoyer A, Benet LZ, Smelick GS, Frassetto LA, Ding X, et al. The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. AAPS J. 2014;16(6):1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster A, Burzawa S, Jemal M, Loizillon E, Couerbe P, Whigan D. Quantitative determination of the HIV protease inhibitor atazanavir (BMS-232632) in human plasma by liquid chromatography-tandem mass spectrometry following automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788(2):377–86. [DOI] [PubMed] [Google Scholar]
- 22.Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–61. [DOI] [PubMed] [Google Scholar]
- 23.Malagelada JR, Longstreth GF, Summerskill WH, Go VL. Measurement of gastric functions during digestion of ordinary solid meals in man. Gastroenterology. 1976;70(2):203–10. [PubMed] [Google Scholar]
- 24.Eley T, Agarwala S, Wang R, Gray K, Chung E, Wang Y, et al. Analysis of intra-gastric pH and atazanavir bioavailability and healthy subjects. 7th International Workshop on Clinical Pharmacology of HIV Therapy; 2006 Apr 20–22; Lisbon, Portugal. [Google Scholar]
- 25.Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hepatol. 1996;8 Suppl 1:S21–5. [DOI] [PubMed] [Google Scholar]
- 26.Miura M, Satoh S, Tada H, Habuchi T, Suzuki T. Stereoselective metabolism of rabeprazole-thioether to rabeprazole by human liver microsomes. Eur J Clin Pharmacol. 2006;62(2):113–7. [DOI] [PubMed] [Google Scholar]
- 27.Cummins CL, Jacobsen W, Benet LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;300(3):1036–45. [DOI] [PubMed] [Google Scholar]
- 28.Cummins CL, Salphati L, Reid MJ, Benet LZ. In vivo modulation of intestinal CYP3A metabolism by P-glycoprotein: studies using the rat single-pass intestinal perfusion model. J Pharmacol Exp Ther. 2003;305(1):306–14. [DOI] [PubMed] [Google Scholar]
- 29.Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26(9):2039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winstanley PA, Orme ML. The effects of food on drug bioavailability. Br J Clin Pharmacol. 1989;28(6):621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kis O, Walmsley SL, Bendayan R. In Vitro and In Situ evaluation of pH-dependence of atazanavir intestinal permeability and interactions with acid-reducing agents. Pharm Res. 2014;31(9):2404–19. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Y, Benet LZ, Okochi H, Chen X. pH dependent but not P-gp dependent bidirectional transport study of S-propranolol: the importance of passive diffusion. Pharm Res. 2015;32(8):2516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guiard-Schmid JB, Poirier JM, Bonnard P, Meynard JL. Lack of interaction between atazanavir and proton pump inhibitors in HIV-infected patients treated with ritonavir-boosted atazanavir. J Acquir Immune Defic Syndr. 2006;41(3):393–4; author reply 394. [DOI] [PubMed] [Google Scholar]
- 34.Klein CE, Chiu YL, Cai Y, Beck K, King KR, Causemaker SJ, et al. Effects of acid-reducing agents on the pharmacokinetics of lopinavir/ritonavir and ritonavir-boosted atazanavir. J Clin Pharmacol. 2008;48(5):553–62. [DOI] [PubMed] [Google Scholar]
- 35.Welage LS, Carver PL, Revankar S, Pierson C, Kauffman CA. Alterations in gastric acidity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21(6):1431–8. [DOI] [PubMed] [Google Scholar]