Abstract
Background
X-linked hypophosphatemia in children is characterized by elevated serum FGF23, hypophosphatemia, rickets, lower extremity bowing, and growth impairment. We compared the efficacy and safety of continuing conventional therapy, consisting of oral phosphate and active vitamin D, versus switching to burosumab, a fully human monoclonal antibody against FGF23, in pediatric X-linked hypophosphatemia.
Methods
In this randomised, active-controlled, open-label, phase 3 trial at 16 clinical sites, we enrolled children with X-linked hypophosphataemia aged 1–12 years. Key eligibility criteria were a total Thacher rickets severity score of at least 2·0, fasting serum phosphorus lower than 0·97 mmol/L (3·0 mg/dL), confirmed PHEX (phosphate-regulating endopeptidase homolog, X-linked) mutation or variant of unknown significance in the patient or a family member with appropriate X-linked dominant inheritance, and receipt of conventional therapy for at least 6 consecutive months for children younger than 3 years or at least 12 consecutive months for children older than 3 years. Eligible patients were randomly assigned (1:1) to receive either subcutaneous burosumab starting at 0·8 mg/kg every 2 weeks (burosumab group) or conventional therapy prescribed by investigators (conventional therapy group). Both interventions lasted 64 weeks. The primary endpoint was change in rickets severity at week 40, assessed by the Radiographic Global Impression of Change global score. All patients who received at least one dose of treatment were included in the primary and safety analyses. The trial is registered with ClinicalTrials.gov, number NCT02915705.
Findings
Recruitment took place between Aug 3, 2016, and May 8, 2017. Of 122 patients assessed, 61 were enrolled. Of these, 32 (18 girls, 14 boys) were randomly assigned to continue receiving conventional therapy and 29 (16 girls, 13 boys) to receive burosumab. For the primary endpoint at week 40, patients in the burosumab group had significantly greater improvement in Radiographic Global Impression of Change global score than did patients in the conventional therapy group (least squares mean +1·9 [SE 0·1] with burosumab vs +0·8 [0·1] with conventional therapy; difference 1·1, 95% CI 0·8–1·5; p<0·0001). Treatment-emergent adverse events considered possibly, probably, or definitely related to treatment by the investigator occurred more frequently with burosumab (17 [59%] of 29 patients in the burosumab group vs seven [22%] of 32 patients in the conventional therapy group). Three serious adverse events occurred in each group, all considered unrelated to treatment and resolved.
Interpretation
Significantly greater clinical improvements were shown in rickets severity, growth, and biochemistries among children with X-linked hypophosphataemia treated with burosumab compared with those continuing conventional therapy.
Funding
Ultragenyx Pharmaceutical Inc. and Kyowa Kirin International
Keywords: Burosumab, FGF23, X-linked hypophosphatemia, rickets, phosphate
Introduction
X-linked hypophosphatemia, caused by loss of function mutations in the PHEX gene, is the most common genetic cause of rickets.1,2 This disease is characterized by elevated blood levels of fibroblast growth factor 23 (FGF23), leading to renal phosphate wasting, and decreased serum 1,25(OH)2D. Resulting hypophosphatemia and defective bone mineralization cause rickets, osteomalacia, skeletal deformities, and short stature that persist into adulthood, along with impaired physical functioning and musculoskeletal pain.
Since the 1980s, conventional therapy for X-linked hypophosphatemia has entailed multiple daily doses of oral phosphate and one or more daily doses of active vitamin D.3,4 This therapy is associated with variable improvement in the clinical features of X-linked hypophosphatemia, while complicated by safety risks, including nephrocalcinosis and hyperparathyroidism.1,5 Conventional therapy can be burdensome, especially for children, with frequent dosing, gastrointestinal side effects, and careful repeated monitoring so that appropriate dose adjustments can be made to avoid complications.
In 2018, burosumab, a fully human monoclonal antibody against FGF23, received approval from the FDA and Health Canada, and received EMA conditional marketing approval for the treatment of X-linked hypophosphatemia (approval conditions vary).6,7 In a prior multicenter, phase 2 trial in 5–12 year-old children with X-linked hypophosphatemia (NCT02163577), burosumab normalized serum phosphorus concentrations, reduced rickets severity, and improved growth and physical functioning.8 Burosumab also increased serum phosphorus concentrations, and improved rickets and lower extremity bowing in 1–4 year-old children with X-linked hypophosphatemia in another phase 2 trial (NCT02750618).9 Both trials demonstrated an acceptable safety profile. Here, we present the results from the first active-control trial comparing the efficacy and safety of continuing conventional therapy versus switching to burosumab in 1–12 year-old children with X-linked hypophosphatemia who had previously been treated with conventional therapy.
Materials and Methods
Study Design and Procedures
UX023-CL301 is an international, randomised, active-control, open-label, parallel, phase 3 trial comparing the efficacy and safety of burosumab to conventional therapy for X-linked hypophosphatemia. Before randomisation, all patients underwent a 7-day conventional therapy washout period. Eligible patients were randomised to receive subcutaneous burosumab every two weeks (Q2W) or conventional therapy for 64 weeks at clinical sites in the US (5), Japan (3), UK (2), Canada (3), Sweden (1), Korea (1), and Australia (1). The active-control group resumed conventional therapy titrated and individualized by the investigator based on published recommendations.1,5 For children, the recommended oral phosphate dose is 20–60 mg/kg/day divided into three to five doses per day and alfacalcidol 40–60 ng/kg/day or calcitriol 20–30 ng/kg/day; depending on the formulation, the active vitamin D could be given one to three times a day. Burosumab was initiated at a dose of 0·8 mg/kg Q2W, injected subcutaneously by a healthcare professional at the study site or during a home health visit, and increased to 1·2 mg/kg Q2W if two consecutive pre-dose, fasting, serum phosphorus concentrations were below 1·03 mmol/L (3·2 mg/dL) and serum phosphorus had increased by <0·16 mmol/L (<0·5 mg/dL) from baseline on a single measurement.
The institutional review board at each participating site approved the protocol (Appendix). The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
Participants
Patients were recruited between 03 August 2016 and 08 May 2017 at clinical sites experienced with treating X-linked hypophosphatemia. Key eligibility criteria included: fasting serum phosphorus <0·97 mmol/L (<3·0 mg/dL); 1–12 years-old at the time of informed consent; confirmed PHEX mutation or variant of unknown significance in the patient or a family member with appropriate X-linked dominant inheritance; a total Thacher Rickets Severity Score of ≥2·0; and receipt of conventional therapy for ≥6 or ≥12 consecutive months for children younger or older than 3 years, respectively. Key exclusion criteria included: Tanner stage ≥4; height >50th percentile for age and sex, based on country-specific norms; use of growth hormone therapy within 12 months prior to screening; plasma parathyroid hormone >19 pmol/L (180 pg/mL); hypo- or hypercalcemia; renal ultrasound indicating nephrocalcinosis of Grade 4 (on a scale of 0–4)10; and planned orthopedic surgery. Additional inclusion/exclusion criteria are listed in the protocol (Appendix). No more than 70% representation of either sex was permitted. Parents or guardians provided written informed consent for their children to participate, and children gave written assent according to local guidelines.
Randomisation
Patients were block randomised 1:1 to treatment groups by an Interactive Web Response System (Bioclinica). Randomisation was stratified by rickets severity (total Thacher Rickets Severity Score ≤2·5 vs >2·5), age (<5 vs ≥5 years), and region (Japan vs the rest of world) to ensure balanced treatment allocation in Japan and meet regulatory submission guidelines.
Outcomes
The primary outcome was change in rickets severity at Week 40 as assessed using the Radiographic Global Impression of Change, compared between treatment groups; this outcome was also assessed at Week 64. This assessment provides one score for the change between baseline and Week 40 (or Week 64); hence there is no baseline score. For this qualitative assessment of rickets, three uniformly trained pediatric radiologists independently identified skeletal abnormalities (including physeal/metaphyseal lucency, separation, fraying, and concavity) in baseline wrist and knee radiographs. These radiologists then determined the degree to which these abnormalities appeared better, worse, or unchanged in post-baseline radiographs.11,12 Patient identifiers, treatment, and treatment duration were unknown to the readers, but knowledge of the radiograph sequence was necessary for the assessment. The Radiographic Global Impression of Change is a 7-point ordinal scale: −3 (severe worsening), −2 (moderate worsening), −1 (minimal worsening), 0 (unchanged), +1 (minimal healing), +2 (substantial healing), and +3 (complete healing). These radiologists provided a wrist, knee, and a global score; the latter reflected the overall impression of change in both the wrist and knee radiographs, but was determined separately from the wrist and knee scores. Each final score (wrist, knee, global) was the mean from all three readings. A key secondary endpoint was the lower limb deformity score at Week 64, determined using the Radiographic Global Impression of Change 7-point ordinal scale, adapted to assess leg bowing (genu varum) and knock knees (genu valgum) in standing radiographs.
Rickets severity was also assessed by the Thacher Rickets Severity Score, a validated measure that assigns a total score ranging from 0 (no rickets) to 10 (severe rickets) based on the sum of scores of the more severely affected wrist (0–4) and knee (0–6).13 This assessment provides independent scores for each radiograph (ie Baseline, Week 40, and Week 64). Tom Thacher, MD, performed this assessment and was blinded to treatment, treatment duration, and radiograph sequence. Rickets Severity Score was assessed at Baseline, Week 40, and Week 64. Change from baseline to Weeks 40 and 64 in serum alkaline phosphatase activity, a biochemical measure of rickets severity, was also a key secondary endpoint.2
Another key secondary endpoint was the change from baseline to Week 64 in recumbent length/standing height Z-score determined using age and sex matched normative data from the US Center for Disease Control and Prevention.14 To smoothly transition from recumbent length to standing height, 0·8 cm was subtracted from recumbent length before pooling data with standing height.14 Length/height measurements were made in triplicate per visit and averaged to reduce error. Growth velocity was analyzed in children who had pre-study growth records available. Baseline growth velocity, expressed as centimeters per year, was calculated using pre-study data from within 1.5 years prior to baseline for children with available records. Week 64 growth velocity was calculated using the baseline and Week 64 measurements. A growth velocity Z-score was also calculated based on Tanner’s standard, accounting for age and sex.15 The mid-point of the patient’s age interval was used to locate the closest reference age provided by the Tanner Standard (e.g. the mid-point of the age interval for baseline was between the earliest growth measurement available within 1·5 years prior to enrollment and baseline, while the mid-point of the age interval for Week 64 was between baseline and Week 64). Children with a mid-point age under 2·25-years were excluded, because younger ages are not available in Tanner’s standard.
Fasting laboratory tests were obtained in the morning prior to dosing of conventional therapy or burosumab. Fasting serum phosphorus, 1,25(OH)2D, urinary phosphorus, and TmP/GFR (the tubular maximum for phosphate reabsorption per glomerular filtration rate) were assessed throughout the study; the timing of all assessments can be found in the protocol (Appendix).
The six-minute Walk Test (6MWT) was administered to assess mobility in patients ≥5 years-old, reported as the percentage of predicted values for a normal population, matched for age and sex.16 Patient-reported outcomes, including pain and fatigue, were assessed using age-appropriate measures, resulting in a smaller number of subjects analyzed per assessment; this data will be submitted for publication in a subsequent manuscript.
To assess safety, all adverse events were tabulated, and a comprehensive metabolic panel was performed including assessment of serum calcium, plasma intact parathyroid hormone, and urine calcium excretion at regular intervals (Appendix). Presence of nephrocalcinosis was assessed by a reader blinded to treatment using renal ultrasonography using an ordinal scale ranging from normal (0) to stone formation (4).10 Physical examination, including assessment of body weight and vital signs, was regularly performed and a record of concomitant medications was collected throughout the study. Quantification of anti-burosumab antibodies was performed (Toray Industries, Tokyo, Japan).
Statistical Analysis
This study (UX023-CL301; NCT02915705) was powered to compare the effect of burosumab on improvement of rickets using the Radiographic Global Impression of Change global score at Week 40 against conventional therapy assuming a mean global score of 1·80 and 1·40, respectively, and a common standard deviation of 0·50. Given these assumptions, a sample size of 60 (30 per group) provided approximately 80% power to detect a difference in the mean global score at Week 40 between groups using a 2-sample t-test with a 2-sided alpha level of 0·05.
All patients who received ≥1 dose of treatment were included in the analysis. Clinical outcomes are presented as mean, least squares (LS) mean, LS mean change from baseline, or LS mean difference between groups with standard error (SE) or deviation (SD), or two-sided 95% confidence interval (CI). Statistical tests were conducted as two-sided hypothesis tests performed at the 5% level of significance. Analysis of covariance (ANCOVA) was used for the Week 40 primary analysis, including the Radiographic Global Impression of Change global score and total Rickets Severity Score, using treatment group and baseline age stratification factor as independent variables and baseline total Rickets Severity Score as a continuous covariate. The Radiographic Global Impression of Change, by definition, does not have a baseline value. Logistic regression was used to compare the number of patients within each group who scored at least substantial healing of rickets (Radiographic Global Impression of Change ≥2). The generalized estimating equations (GEE) model with exchangeable covariance structure was used to analyze all clinical outcomes with repeated measures. For the GEE model, categorical variables include treatment group, study visit and interaction between treatment group and study visit, and baseline stratification factors and covariates. For length/height Z-score, baseline age and height/length Z-score were included as continuous covariates.
An independent data monitoring committee was established to monitor patient safety throughout the trial. Requests for individual de-identified participant data will be reviewed for ≥12 months after study completion to researchers providing methodologically sound proposals that are in accordance with our data sharing policy listed on Ultragenyx Pharmaceutical Inc. website (https://www.ultragenyx.com/pipeline/clinical-trial-transparency/).
Role of Funding Source
The funders were responsible for the study design, management, monitoring, pharmacovigilance, statistical and data analysis, and supply of burosumab. Conventional therapy medications during the study were provided directly to the site in Korea, or reimbursed by the sponsor upon request at other sites.
Results
Recruitment occurred between 03 August 2016 and 08 May 2017. Of the 122 patients screened, 32 (18 female, 14 male) were randomised to continue receiving conventional therapy, 29 (16 females, 13 males) were randomised to switch to burosumab, and 61 (39 female, 22 male) failed to meet eligibility criteria (Figure 1). There were no discontinuations from either treatment group and all patients were included in the analysis.
Figure 1. Trial Profile.
The most common reason for screen failure was a total Rickets Severity Score <2·0 (n=55). Thirteen patients were ineligible due to a serum phosphorus concentration ≥0·97 mmol/L (≥3·0 mg/dL) and six patients failed to meet inclusion criteria due to hypocalcemia or hypercalcemia. The remaining reasons for ineligibility (occurring in less than three patients, each) were: lack of a detectable PHEX mutation or variant of unknown significance in the patient or a family member with appropriate X-linked inheritance, height ≥50% based on country-specific norms, parathyroid hormone level > >19 pmol/L (180 pg/mL), unwilling to complete all aspects of the study, unwilling to provide consent/assent, insufficient duration of prior conventional therapy, serum creatinine above the age-adjusted reference range, and serum 25(OH)D <16 ng/mL. Patients could be ineligible for more than one reason.
Consistent with enrollment criteria, despite prior receipt of conventional therapy, patients exhibited hypophosphatemia, growth impairment, and radiographic evidence of rickets at baseline (Table 1). Most patients were Tanner Stage 1 at baseline. By week 64, six conventional therapy-treated patients and four burosumab-treated patients were Tanner Stage ≥2. The majority of patients had ≥1 metaphyseal radiographic abnormality in the wrist and knee at baseline.
Table 1.
Baseline Characteristics
| Characteristic | Conventional Therapy (N=32) | Burosumab (N=29) |
|---|---|---|
| Age, years | 6·3 (3·2) | 5·8 (3·4) |
| Patients <5-years-old (stratification criteria) | 12 (38) | 14 (48) |
| Male | 14 (43·8) | 13 (44·8) |
| White | 25 (78·1) | 25 (86·2) |
| Region (stratification criteria Japan vs rest of the world) | ||
| Japan | 3 (9) | 2 (7) |
| United States | 15 (47) | 16 (55) |
| Canada | 7 (22) | 2 (7) |
| Europe | 3 (9) | 2 (7) |
| Korea | 2 (6) | 0 (0) |
| Australia | 2 (6) | 7 (24) |
| Height Z-score, mean (SD) | −2·1 (0·9) | −2·3 (1·2) |
| median [Range] | −21 [−4·7, −0·1] | −2·3 [−5·0, −0·3] |
| Weight Z-score, mean (SD) | −0·6 (0·9) | −0·9 (1·2) |
| median [Range] | −0·7 [−2·3, 1·5] | −0·8 [−3·2, 1·4] |
| Tanner Stage | ||
| 1 | 31 (97) | 27 (93) |
| 2 | 1 (3) | 2 (7) |
| Serum phosphorus, mmol/L | 0·74 (0·08) | 0·78 (0·08) |
| Serum TmP/GFR, mmol/L | 0·65 (0·11) | 0·71 (0·12) |
| Serum 1,25(OH)2D, pmol/L | 96 (36) | 110 (48) |
| Serum 25(OH)D, nmol/L | 79·38 (25·14) | 80·63 (26·15) |
| Alkaline Phosphatase, U/L | 523·4 (154·4) | 510·8 (124·9) |
| Duration of previous conventional therapy, years mean (SD) | 4·3 (3·0) | 3·3 (3·1) |
| median [Range] | 3·5 [0·8–12·0] | 2·2 [0·5–12·2] |
| Total Rickets Severity Score, mean (SD) | 3·2 (1·1) | 3·2 (1·0) |
| median [Range] | 3·0 [2·0, 6·5] | 3·0 [2·0, 6·5] |
| Patients with a Total Thacher Rickets Severity Score >2·5 (stratification criteria) | 20 (63) | 19 (66) |
Data are n (%), mean (SD), and/or median [min, max]. Baseline values were assessed after a 7-day washout period, in which patients stopped treatment with conventional therapy.
Median oral phosphate dose was within the recommended range (Table 2).1,5 During the study, ten patients had no dose adjustment, three had a dose decrease, and 19 had a dose increase. Of the 19 patients with dose increases, 13 had a >20% dose increase from baseline. Most patients received oral phosphate doses four times a day.
Table 2.
Daily Doses of Oral Phosphate and Active Vitamin D in the Conventional Therapy Group
| Visit | Oral phosphate, mg/kg (N=32) | Oral calcitriol, ng/kg (N=22) | Oral alfacalcidol, ng/kg (N=9)a | |||
|---|---|---|---|---|---|---|
| Statistic | Median (Q1, Q3) | Mean (SD) (min, max) | Median (Q1, Q3) | Mean (SD) (min, max) | Median (Q1, Q3) | Mean (SD) (min, max) |
| Baseline | 32·0 (25·6, 52·9) | 36·2 (16·0) (10·4, 73·6) | 21·2 (15·9, 26·7) | 21·6 (7·4) (6·8, 34·6) | 62·3 (34·5, 104·7) | 78·1 (66·4) (17·0, 224·1) |
| Week 40 | 35·3 (28·4, 50·5) | 41·0 (20·7) (18·1, 109·5) | 22·3 (17·5, 32·7) | 26·4 (13·3) (6·8, 63·5) | 79·7 (40·2, 104·7) | 87·1 (61·4) (27·6, 224·1) |
| Week 64 | 39·3 (28·7, 52·9) | 45·8 (27·7) (18·1, 166·2) | 26·4 (17·3, 34·2) | 27·2 (13·4) (6·8, 63·5) | 79·8 (40·2, 104·7) | 86·5 (59·6) (27·6, 217·4) |
Previously published recommended dosing ranges: Oral phosphate 20–60 mg/kg/day divided into three to five doses per day, alfacalcidol 40–60 ng/kg/day or calcitriol 20 to 30 ng/kg/day taken once or divided into two or three doses per day.1,5
One patient was not included in the oral alfacalcidol results because this patient received eldecalcitol (19.5 ng/kg/day) at baseline and switched to alfacalcidol at Week 32 (11.2 ng/kg/day, with no dose adjustments thereafter).
Median daily doses of active vitamin D were generally in the recommended range (Table 2).1,5 Of the 22 patients receiving calcitriol, nine had no adjustment, two had a dose decrease, and 11 had a dose increase. Of the nine patients receiving alfacalcidol, four had no dose adjustment, one had a dose decrease, and four had an overall dose increase. Of the 15 patients who had a dose increase in active vitamin D, 13 had a >20% dose increase from baseline.
Based on the total number of planned dosing days (448 per patient), compliance with conventional therapy was >95% for all patients. Ten patients (31%) missed 1–2 full day(s) of dosing, and three (9%) patients missed 3–7 full days of dosing (non-consecutive). The main reasons reported for missed doses were an adverse event (illness), running out of medication, and noncompliance.
All burosumab-treated patients received all planned doses, except three patients who each missed one dose. In the burosumab group, 21 (72%) patients remained at a dose of 0·8 mg/kg and eight (28%) patients had dose increases to 1·2 mg/kg due to serum phosphorus below the normal range. Dose changes for either burosumab or conventional therapy occurred at various time points throughout the study.
For the primary endpoint, the burosumab group had significantly greater improvement in Radiographic Global Impression of Change global score than the conventional therapy group at Week 40 (LS mean ± SE +1·9 ± 0·1 versus +0·8 ± 0·1; difference 1·1, 95% CI 0·8, 1·5; p<0·0001) (Figure 2A). At Week 64, both groups continued to show improvement in the global score, with burosumab maintaining significantly greater improvement over conventional therapy (LS mean ± SE +2·1 ± 0·1 versus +1·0 ± 0·1; difference 1·0, 95% CI 0·8, 1·4; p<0.0001). A significantly greater number of burosumab-treated patients achieved substantial healing of rickets, defined as a Radiographic Global Impression of Change global score of ≥+2·0, than conventional therapy-treated patients at Week 40 (72% vs 6%; odds ratio 39; 95% CI 7, 212; p<0·0001); at Week 64, these percentages increased to 87% and 17% of patients, respectively (odds ratio 34; 95% CI 6, 206; p=0·0002).
Figure 2. Improvement in Rickets Severity (Primary Endpoint) and Lower Limb Bowing.
Data in panels A, B, and C are mean ± standard deviation. *p<0·05, ****p<0·0001 based on the comparison between treatment groups in the LS mean change from baseline using the ANCOVA model at Week 40 for Radiographic Global Impression of Change Global Score (A) and Week 40 Rickets Severity Score assessments (B); and using the GEE model for alkaline phosphatase assessments (C) and Week 64 rickets assessments (A, B). Radiographs in figure D show improvement in rickets with burosumab in a 4-year-old girl who previously received conventional therapy for approximately 26 months. aThe upper limit of normal for alkaline phosphatase varies by sex and age: females – 1–4 years-old 317 U/L, 4–7 years-old 297 U/L, 7–10 years-old 325 U/L, 10–15 years-old 300 U/L; Males – 1–4 years-old 383 U/L, 4–7 years-old 345 U/L, 7–10 years-old 309 U/L, 10–15 years-old 385 U/L (provided by Covance laboratories).
The decrease in Total Rickets Severity Score was nearly three times greater in the burosumab group compared with the conventional therapy group at Week 40 (LS mean change ± SE −2·0 ± 0·1 versus −0·7 ± 0·1; difference −1·3, 95% CI −1·7, −0·9; p<0·0001) (Figure 2B). Greater improvements with burosumab were maintained at Week 64 (LS mean change ± SE −2·2 ± 0·1 versus −1·0 ± 0·2; difference −1·2, 95% CI −1·6, −0·8; p<0·0001). The magnitude of improvement was similar for the separate wrist and knees scores, using both measures of rickets severity. Burosumab also demonstrated greater improvement compared to conventional therapy for the separate wrist and knee assessments (Supplemental Table 1). Figure 2D shows radiographs from a 4-year-old girl receiving burosumab.
The burosumab group demonstrated a significantly greater decrease than the conventional therapy group in alkaline phosphatase activity as early as Week 16 (mean [SD] −18% [11%] versus 0% [21%]; p<0·0001), at Week 40 (−24% [14%] versus −7% [17%]; p<0·0001), and at Week 64 (−33% [13%] versus −5% [21%]; p<0·0001) (Figure 2C). While both groups showed improvement in the lower limb deformity score, the improvement with burosumab was significantly greater than with conventional therapy (Week 64 LS mean ± SE +1·3 ± 0·2 versus +0·3 ± 0·1; difference 1·0, 95% CI 0·6, 1·4; p<0·0001) (Figure 2A).
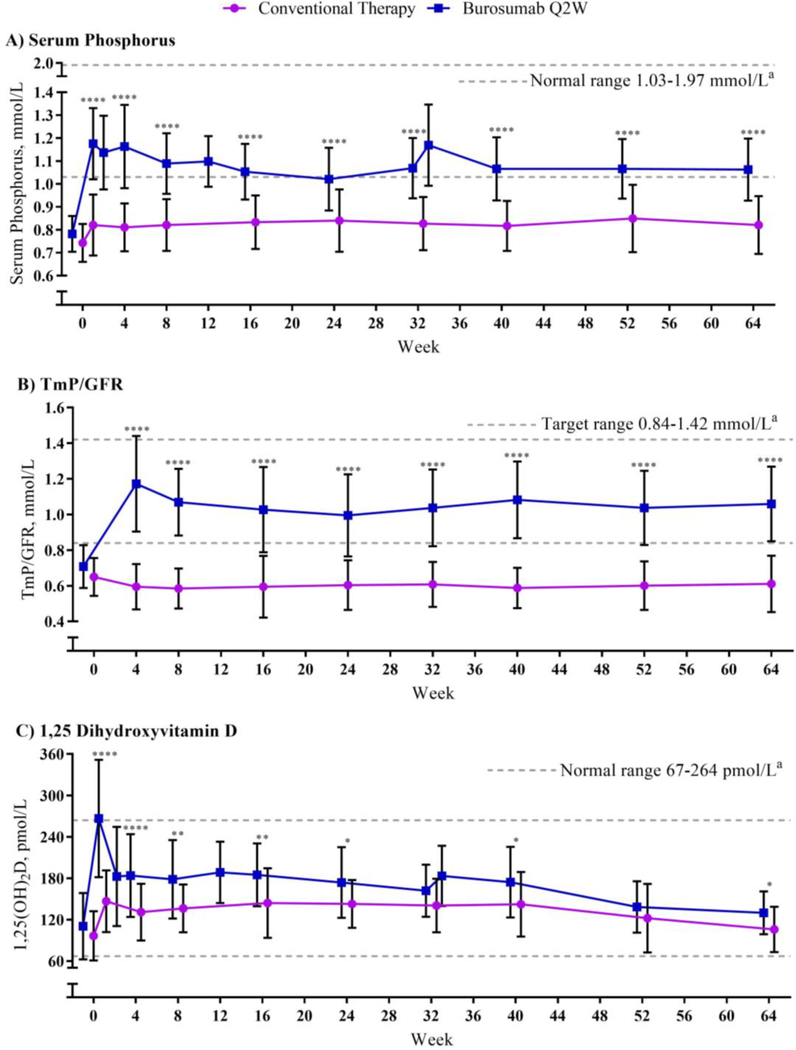
While the increase from baseline in fasting serum phosphorus with conventional therapy was minimal, the burosumab group demonstrated a mean increase in fasting serum phosphorus to within the low end of the normal range at the first post-baseline assessment (Figures 3A, S1).17 The increase in fasting serum phosphorus with burosumab was significantly greater compared with conventional therapy both at Week 40 (LS mean change ± SE 0·29 ± 0·03 versus 0·06 ± 0·02 mmol/L [0·92 ± 0·08 versus 0·20 ± 0·06 mg/dL]; p<0·0001) and Week 64 (0·29 ± 0·03 versus 0·07 ± 0·02 mmol/L [0·91 ± 0·08 versus 0·21 ± 0·06 mg/dL]; p<0·0001). The conventional therapy group demonstrated minimal change in TmP/GFR, with an LS mean change ± SE of −0·05 ± 0·02 mmol/L (−0·16 ± 0·05 mg/dL) at Week 40. In contrast, TmP/GFR in the burosumab group demonstrated an increase at the first post-baseline assessment (Week 4), with an LS mean change ± SE of 0·38 ± 0·04 pmol/L (1·19 ± 0·11 mg/dL) at Week 40 (difference between treatments p<0.0001) (Figure 3B). At Week 64, TmP/GFR in the burosumab group remained increased, while TmP/GFR in the conventional therapy group remained unchanged (LS mean change 0·37 ± 0·04 versus −0·03 ± 0·02 mmol/L [1·16 ± 0·13 versus −0·09 ± 0·07 mg/dL]; difference p<0.0001). The 24-hour urine phosphorus excretion expressed as a ratio to creatinine increased with conventional therapy, but did not change with burosumab (Figure S4).
Figure 3. Serum Phosphorus, TmP/GFR, and 1,25(OH)2D.
Data are expressed as mean ± standard deviation; some post-baseline values are slightly offset from the actual treatment week to avoid overlapping error bars. Assessments at Weeks 1, 2, and 33 only occurred in the burosumab group to measure peak dose effect. Due to the difficulty of collecting urine samples from young children, the number of patients included in TmP/GFR assessments at different time points ranged from 27–30 for conventional therapy and 22–23 for burosumab. *p<0·05, **p<0·01, ****p<0·0001 based on the comparison between treatment groups in the LS mean change from baseline using the generalized estimation equation model. aNormal (or target) ranges provided by Covance laboratories.
Serum 1,25(OH)2D in the conventional therapy group had an LS mean change ± SE of 45 ± 9 pmol/L (18 ± 4 pg/mL) at Week 40 and 3 ± 7 pmol/L (1 ± 3 pg/mL) at Week 64 (Figure 3C). Serum 1,25(OH)2D in the burosumab group increased by an LS mean ± SE of 71 ± 9 pmol/L (30 ± 4 pg/mL) at Week 40 and 24 ± 5 pmol/L (10 ± 2 pg/mL) at Week 64. The peak increase serum 1,25(OH)2D, assessed 1 week after the burosumab dose, was greater at Week 1 than at Week 33. Mean ± SE change in serum 25(OH)D at Week 40 was −2·05 ± 3·85 nmol/L (−0·82 ± 1·54 ng/mL) and −7·45 ± 4·48 nmol/L (−2·98 ± 1·79 ng/mL) for conventional therapy and burosumab, respectively; at Week 64, mean ± SE change was 2·50 ± 4·85 nmol/L (1·00 ± 1·94 ng/mL) and 1·10 ± 7·03 nmol/L (0·44 ± 2·81 ng/mL), respectively.
Burosumab was associated with a significantly greater increase than conventional therapy in length/height Z-score at Week 64 (LS mean change ± SE 0·17 ± 0·07 vs 0·02 ± 0·04; difference 0·14, 95% CI 0·0, 0·29; p=0·0490) (Figure 4A). Twenty-six patients in each group had data to compare growth velocity pre and post baseline in cm/year. Of these 22 from each group were old enough to calculate Z-score using Tanner’s standard. At baseline, mean ± SE growth velocity Z-score was −2·14 ± 1·19 (5·49 ± 1·03 cm/year) in the conventional therapy group and −1·37 ± 0·28 (6·52 ± 0·79) in the burosumab group. At Week 40, mean ± SE growth velocity Z-score was −0·37 ± 0·28 (6·27 ± 0·26 cm/year) in the conventional therapy group and 0·53 ± 0·38 (7·03 ± 0·40 cm/year) in the burosumab group. At Week 64, mean ± SE growth velocity was −0·75 ± 0·19 (5·94 ± 0·22 cm/year) in the conventional therapy group and 0·34 ± 0·31 (6·65 ± 0·29 cm/year) in the burosumab group. The difference between treatment groups at Week 64 was statistically significant (p=0·0047).
Figure 4. Height and Mobility.
Data are expressed as mean change from baseline ± standard deviation; some post-baseline values are slightly offset from the actual treatment week to avoid overlapping error bars. Recumbent length/standing height Z-score was assessed in all enrolled patients. 6MWT was assessed in patients >5 years-old and able to complete the test (conventional therapy n=20, burosumab n=13). *p<0·05 based on the comparison between treatment groups in the LS mean change from baseline using the generalized estimation equation model.
Mobility was assessed using the 6MWT in children ≥5 years of age at baseline; complete data were available for 20 conventional therapy-treated patients and 13 burosumab-treated patients. At baseline, there was a difference between treatment groups in the percent predicted distance walked, with a mean (SD) of 76% (15%) for conventional therapy and 65% (12%) for burosumab. After adjusting for this baseline difference in the GEE model, burosumab demonstrated a significantly greater improvement in distance walked than conventional therapy at Week 64 (LS mean change ± SE percentage predicted distance walked 9% ± 2% vs 2% ± 3%; difference [95% CI] 7% [0·01, 14·52]; p=0·0496) (Figure 4B).
Most patients in both treatment groups experienced at least one treatment-emergent adverse event (TEAE) (Table 3). TEAEs considered possibly, probably, or definitely related to treatment by the investigator occurred more frequently with burosumab (17 [59%] versus 7 [22%] patients); a majority of these TEAEs in the burosumab group were related to injection site reactions, were mild in severity, and resolved within a few days. Three (9%) patients in the conventional therapy group and three (10%) patients in the burosumab group had a serious TEAE, each considered unrelated to treatment and resolved. All serious TEAEs were rated mild (grade 1) or moderate (grade 2) in severity.
Table 3.
Safety Summary through Week 64
| Assessment | Conventional therapy N = 32 | Burosumab N = 29 |
|---|---|---|
| TEAEs | 27 (84·4) | 29 (100·0) |
| Related TEAE | 7 (21·9) | 17 (58·6) |
| Serious TEAE | 3 (9·4) | 3 (10·3) |
| Serious Related TEAE | 0 (0·0) | 0 (0·0) |
| Grade 3 or 4 TEAE | 3 (9·4) | 4 (13·8) |
| TEAE Leading to Study Discontinuation | 0 (0·0) | 0 (0·0) |
| TEAE Leading to Treatment Discontinuation | 0 (0·0) | 0 (0·0) |
| TEAE Leading to Death | 0 (0·0) | 0 (0·0) |
| Pre-defined TEAEs of Interest | ||
| Injection site reaction | 0 (0·0) | 15 (51·7) |
| Hypersensitivity | 6 (18·8) | 11 (37·9) |
| Hyperphosphatemia | 0 (0·0) | 0 (0·0) |
| Ectopic mineralization | 0 (0·0) | 0 (0·0) |
| Restless leg syndrome | 0 (0·0) | 0 (0·0) |
| TEAEs with ≥ 10% Incidence in Either Group | ||
| Pyrexia | 6 (18·8) | 16 (55·2) |
| Cough | 6 (18·8) | 15 (51·7) |
| Arthralgia | 10 (31·3) | 13 (44·8) |
| Vomiting | 8 (25·0) | 12 (41·4) |
| Nasopharyngitis | 14 (43·8) | 11 (37·9) |
| Pain in extremity | 10 (31·3) | 11 (37·9) |
| Headache | 6 (18·8) | 10 (34·5) |
| Injection site erythema | 0 (0·0) | 9 (31·0) |
| Dental caries | 2 (6·3) | 9 (31·0) |
| Tooth abscess | 3 (9·4) | 8 (27·6) |
| Injection site reaction | 0 (0·0) | 7 (24·1) |
| Rhinorrhoea | 2 (6·3) | 7 (24·1) |
| Diarrhoea | 2 (6·3) | 7 (24·1) |
| Vitamin D decrease | 1 (3·1) | 6 (20·7) |
| Constipation | 0 (0·0) | 5 (17·2) |
| Nasal congestion | 1 (3·1) | 5 (17·2) |
| Oropharyngeal pain | 1 (3·1) | 5 (17·2) |
| Vitamin D deficiency | 1 (3·1) | 5 (17·2) |
| Contusion | 0 (0·0) | 4 (13·8) |
| Ear pain | 1 (3·1) | 4 (13·8) |
| Nausea | 1 (3·1) | 4 (13·8) |
| Asthma | 1 (3·1) | 4 (13·8) |
| Seasonal allergy | 2 (6·3) | 4 (13·8) |
| Influenza | 6 (18·8) | 4 (13·8) |
| Injection site pruritus | 0 (0·0) | 3 (10·3) |
| Injection site swelling | 0 (0·0) | 3 (10·3) |
| Fall | 0 (0·0) | 3 (10·3) |
| Injection site rash | 0 (0·0) | 3 (10·3) |
| Rash | 2 (6·3) | 3 (10·3) |
| Upper respiratory tract infection | 3 (9·4) | 3 (10·3) |
| Abdominal pain upper | 3 (9·4) | 3 (10·3) |
Data are n (%). TEAE, treatment-emergent adverse event. The serious TEAE in the conventional therapy group were hospitalization or surgery for craniosynostosis, bilateral genu varum deformities, and hematuria. The serious TEAE in the burosumab group were craniosynostosis, a viral infection, and a migraine. Injection site reaction is a grouped term that includes injection site reaction, erythema, pruritus, rash, erosion, swelling, urticaria, discomfort, hypersensitivity, inflammation, and papule. Hypersensitivity is a grouped term that includes rash (generalized, erythematous, injection site), dermatitis allergic, drug eruption, and swelling face.
Three (9%) patients in the conventional therapy group and four (14%) patients in the burosumab group experienced a severe (grade 3) TEAE; only one of these events, arthralgia in the burosumab group, was considered related to treatment by the investigator and resolved within two days (Table 3). The other severe TEAEs with burosumab were dysuria, high urine ketone concentration, and gastroenteritis. In the conventional therapy group, the severe TEAEs were anaphylaxis from a nut allergy, arthralgia, and craniosynostosis (requiring surgery). All severe TEAEs resolved, except the case of arthralgia in the conventional therapy group that was noted during Week 40, which persisted at the time of this analysis. All other TEAEs were mild or moderate.
The burosumab group had a higher frequency of TEAEs of interest, pre-defined based on the known safety profile, including injection site reaction and hypersensitivity (Table 3); these events were mild to moderate in severity overall. Dental and gastrointestinal TEAEs were also more frequent in the burosumab group compared with the conventional therapy group. More patients in the burosumab group than the conventional therapy group experienced a vitamin D decrease or deficiency, as determined by the investigator. For all six patients in the burosumab group that experienced a decrease in vitamin D, decreased levels of serum 25-hydroxyvitamin D corresponded with increased levels of serum 1,25(OH)2D, likely reflecting enhanced conversion of 25-hydroxyvitamin D to 1,25(OH)2D.
There were minimal changes in plasma intact parathyroid hormone, serum calcium, urine calcium excretion, or nephrocalcinosis score in both groups (Figures S2, S3). Nephrocalcinosis scores of 1–3 were observed at baseline in 9/32 (28%) patients in the conventional therapy group and 5/29 (17%) patients in the burosumab group. At Week 64, nephrocalcinosis did not worsen or develop de novo in any patient. Of those with nephrocalcinosis at baseline, scores decreased in 7/9 (78%) patients in the conventional therapy group and 3/5 (60%) patients in the burosumab group.
Three patients tested positive for anti-burosumab antibodies at baseline, one of whom tested negative at all post-baseline assessments. Anti-drug antibody titers were low for all positive samples (<1:2, or 1:4). One patient who was positive at baseline, also tested positive for neutralizing antibody activity during post-baseline assessments. Patients who tested positive for anti-drug antibodies all showed improvements in rickets severity scores, increases in serum phosphorus, and none experienced hypersensitivity TEAEs. The remaining patients tested negative through Week 64.
Discussion
We report the first randomised, active-control, clinical trial of burosumab in children with X-linked hypophosphatemia. Switching patients from conventional therapy to burosumab normalized phosphate homeostasis and improved rickets, serum alkaline phosphatase, growth, bowing, and mobility in 1–12 year-old children with X-linked hypophosphatemia compared to continuation of conventional therapy. Although conventional therapy also demonstrated some improvement, switching to burosumab showed superior improvement for all rickets and bowing assessments at Week 40 and 64.
Burosumab demonstrated significantly greater improvement in growth compared with conventional therapy. Improvement in length/height Z-score with burosumab in this study (LS mean increase 0·17 at Week 64) mirrors the improvement observed in the prior phase 2 study, in 5–12 year-old children with X-linked hypophosphatemia (burosumab Q2W LS mean increase 0·19 at Week 64).8 In contrast, continuing conventional therapy for 64 Weeks, after already having received conventional therapy for a mean of 4.3 years, failed to further improve length/height Z-score (LS mean change 0·02). This lack of improvement is consistent with previous findings, showing that short stature persists in patients with X-linked hypophosphatemia despite receiving conventional therapy.5,18 Burosumab likely promotes growth by treating the underlying bone disease, including improving rickets, allowing for improved development of the growth plate, and straightening of the lower limbs. Though promising, longer trials are necessary to characterize the long-term growth potential of patients treated with burosumab.
We have previously reported impaired mobility in children with X-linked hypophosphatemia.8 In the current study, mean distance walked in the 6MWT at baseline was below 80% of the predicted values for a normal population in both treatment groups, consistent with clinical impairment.16 The burosumab group had significantly greater increases in the 6MWT by Week 64 compared with conventional therapy. Burosumab-associated increases in mobility were consistent with findings from the phase 2 study investigating burosumab in 5–12 year-old children with X-linked hypophosphatemia.8
By inhibiting FGF23, countering a major feature of the pathophysiology of X-linked hypophosphatemia, burosumab led to increased renal reabsorption of phosphate. Consequently, fasting serum phosphorus concentrations rose to within the normal range and remained normal throughout the trial. In contrast, conventional therapy resulted in a slight increase in fasting serum phosphorus and a slight decrease in TmP/GFR. Such findings align with previous reports suggesting that oral phosphate supplementation causes transient increases in serum phosphorus, but does not correct renal phosphate wasting.19,20 Burosumab also resulted in significantly greater increases than conventional therapy in serum 1,25(OH)2D. Although the initial dose of burosumab resulted in a peak increase of serum 1,25(OH)2D to above the upper limit of normal, subsequent doses results in smaller peaks of 1,25(OH)2D within the normal range. This pattern was consistently observed across the burosumab clinical program.
Both groups tolerated treatment well, and the safety profile of burosumab in this trial was consistent with the safety profile in the previous phase 2 pediatric studies.8,9 TEAEs were more frequent with burosumab than conventional therapy; mainly driven by TEAEs generally associated with subcutaneous injections of a protein therapeutic. Nevertheless, no patient discontinued either treatment due to TEAEs. Patients in the burosumab group transitioned from a familiar oral therapy to a new subcutaneous treatment, which may have heightened their awareness of TEAEs. In contrast, all patients treated with conventional therapy during the trial were continuing a treatment with which they were familiar. The most commonly reported TEAEs were typical of a pediatric population or frequent manifestations of X-linked hypophosphatemia. Nephrocalcinosis and hyperparathyroidism did not appear as safety concerns in this trial for either group or in the previous pediatric burosumab trials. Dental abscesses occurred more frequently in the burosumab group. Low mineralization of the dentin, abnormal pulp, and periodontitis are common abnormalities in X-linked hypophosphatemia, but severity varies among patients. The higher frequency of dental abscess in the burosumab group may be due to patient variability, or a direct dental benefit of conventional therapy. PHEX deficiency increases tissue levels of other peptides, such as osteopontin and MEPE, which are not targeted by burosumab and may contribute to the risk of tooth abscesses, independently of FGF23 or phosphate.21,22 Prior publications suggest that conventional therapy improves dentin mineralization and decreases the risk of dental abscesses and severe periodontal disease over years.23–25 However, correction of hypophosphatemia may not fully address or quickly reverse the components contributing to dental complications. Larger numbers of patients over a longer period of time will be required to shed light on the clinical significance of this observation.
One limitation of the present trial is that the treatment period was open-label. However, the primary outcome, rickets severity, was assessed by independent central radiologists who were unaware of treatment assignment and patient identifiers, and pharmacodynamic measures were assessed by a central laboratory. Another limitation is that adherence to oral phosphate and active vitamin D was not measured by drug accountability. Nevertheless, compliance was assessed by capturing the number of full missed dosing days, and mean doses of conventional therapy were within the recommended range. Lastly, this trial did not evaluate treatment in children with a baseline Rickets Severity Score <2.0, children older than 12 years at baseline, or treatment-naïve children. Previous studies in children with lower baseline rickets severity still demonstrated significant improvement with burosumab in both measures of rickets severity.8,9 The upper age limit was set at 12 years since changes over time in height and rickets severity require assessing open growth plates. It is not known whether the magnitude of benefit from burosumab would be different in patients naïve to conventional therapy, or if started in children younger than 1 year of age. We speculate that the magnitude of benefit with initiation of burosumab would vary with pubertal status, specifically the potential for improvement in final adult height, although this has not been studied. Burosumab’s impact on growth is an important direction for future research, as height Z-score often decrease during puberty in patients with X-linked hypophosphatemia receiving conventional therapy, and will be assessed in a 10-year X-linked hypophosphatemia Disease Monitoring Program (NCT03651505).18
In conclusion, in this phase 3 active-control trial, burosumab was more effective than continuing conventional therapy in improving rickets, growth, lower limb deformity, and mobility in children with X-linked hypophosphatemia. By inhibiting excess FGF23 activity and normalizing renal phosphate excretion, burosumab induced clinically meaningful improvements within 64 weeks and has the potential to prevent long-term complications associated with X-linked hypophosphatemia.
Supplementary Material
Research in Context.
Evidence before this study
X-linked hypophosphatemia, caused by loss-of-function mutations in PHEX (phosphate-regulating endopeptidase homolog, X-linked), is the most common heritable form of rickets and osteomalacia. X-linked hypophosphatemia is characterized by elevated circulating concentrations of FGF23, resulting in renal phosphate wasting, decreased 1,25(OH)2D, skeletal deformities, impaired growth, and compromised physical functioning. Since the 1980s, conventional therapy for X-linked hypophosphatemia has consisted of multiple daily doses of oral phosphate salts and one or more daily doses of active vitamin D. Although this therapy may improve rickets and skeletal deformity, the response is variable. Often rickets and short stature persist, and some patients require surgical intervention for lower extremity deformities. Also, clinical complications of conventional therapy can include nephrocalcinosis and hyperparathyroidism. Burosumab, a fully human monoclonal antibody against FGF23, received approval from the Food and Drug Administration in the US and Health Canada, and conditional marketing approval by the European Medicines Agency in 2018 for the treatment of X-linked hypophosphatemia (conditions of approval vary). Two pediatric, single-arm, phase 2, clinical trials have demonstrated that inhibition of FGF23 with burosumab restores phosphate homeostasis, and improves rickets, growth, and mobility in affected children ages 1–12 years old. Complications associated with conventional therapy such as nephrocalcinosis and hyperparathyroidism were not detected in those phase 2 pediatric trials of burosumab.
Added value of this study
This international, randomised, active-controlled, phase 3 trial is the first to compare the efficacy and safety of switching to burosumab versus continuing conventional therapy among 1–12 year-old children with X-linked hypophosphatemia. In this trial, burosumab resulted in greater radiographic improvement of rickets and lower extremity bowing, larger decreases in serum alkaline phosphatase activity, and greater increases in serum phosphorus, growth, and mobility than continuation of conventional therapy. That these improvements occurred in children who switched from long-standing conventional therapy to burosumab, suggests that burosumab offers a therapeutic advantage over conventional therapy. From a safety standpoint, no patient developed hyperphosphatemia or worsening nephrocalcinosis scores in either treatment group. There were no discontinuations from the study or treatment. Adverse events were more frequent with burosumab compared with conventional therapy, mostly driven by adverse events associated with injecting a subcutaneous protein therapeutic.
Implications of all the available evidence
This phase 3 trial presents the first comparison of conventional therapy and burosumab in children with X-linked hypophosphatemia, and demonstrated the superiority of burosumab over continuation of conventional therapy for several clinical outcomes, including the correction of renal phosphate wasting. By improving rickets, long bone deformities, and linear growth, burosumab offers a promising new treatment approach for children with X-linked hypophosphatemia.
Acknowledgements
The authors thank the patients, their families, and study site personnel who made the clinical trial possible: Tom Thacher, Anna Frid, Marian Hart, Valerie Wollberg, Vinieth N. Bijanki, Amy Reeves, Nasrin Khan, Lynn MacLeay, Marika Page,Vrinda Saraff, Nick Shaw, Melissa Fang, Julie Kwon, Daniel Schrader, Erfan Jaberiyanfar, Margo Black, and Michaela Durigova At Birmingham Children’s Hospital, the research was carried out at the National Institute for Health Research/Wellcome Trust Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Catherine Woods, PhD, provided medical writing support.
Declaration of Interests
The following authors served as clinical investigators for one or more studies, including this study, sponsored by Ultragenyx Pharmaceutical in partnership with Kyowa Kirin International plc: EAI, FHG, MPW, CFM, LW, ON, JHS, RP, NN, HIC, PP, ES, WH, KM, HR, GSG, AB, FP, and AAP. EAI, LW, NN, FP, and AAP have also received honoraria for serving as an advisory board member or for lectures from Ultragenyx Pharmaceutical Inc. WH has also received honoraria, consulting fees, and travel support from Ultragenyx Pharmaceutical Inc. and research funding, honoraria, and travel support from Kyowa Kirin. RP has also received personal fees from Ultragenyx Pharmaceutical Inc and Kyowa Kirin, as well as non-financial support from Kyowa Kirin. PP has also received research funding from Amgen and Shire and is currently an employee of Acendis Pharma Inc. FHG has also received research funding from Amgen and Mereo-Biopharma. MPW has received research grants from Ultragenyx Pharmaceutical Inc. GSG has received consulting fees from Ultragenyx Pharmaceutical Inc. MM, C-YC, AS, and JSM are employees and stockholders of Ultragenyx Pharmaceutical Inc. JSM also is the co-inventor of a patent application with Ultragenyx Pharmaceutical Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011; 26(7): 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nat Rev Dis Primers. 2017; 3: 17101. [DOI] [PubMed] [Google Scholar]
- 3.Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980; 303(18): 1023–31. [DOI] [PubMed] [Google Scholar]
- 4.West CD, Blanton JC, Silverman FN, Holland NH. Use of phosphate salts as an adjunct to vitamin D in the treatment of hypophosphatemic vitamin D refractory rickets. J Pediatr. 1964; 64(4): 469–77. [DOI] [PubMed] [Google Scholar]
- 5.Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014; 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb YN. Burosumab: First Global Approval. Drugs. 2018: 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Product Monograph Crysvita Burosumab Injection. https://pdf.hres.ca/dpd_pm/00048599.PDF.
- 8.Carpenter TO, Whyte MP, Imel EA, et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N Engl J Med. 2018; 378(21): 1987–98. [DOI] [PubMed] [Google Scholar]
- 9.Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019; 3(7):189–99. [DOI] [PubMed] [Google Scholar]
- 10.Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991; 325(26): 1843–8. [DOI] [PubMed] [Google Scholar]
- 11.Whyte MP, Fujita KP, Moseley S, Thompson DD, McAlister WH. Validation of a Novel Scoring System for Changes in Skeletal Manifestations of Hypophosphatasia in Newborns, Infants, and Children: The Radiographic Global Impression of Change Scale. J Bone Miner Res. 2018; 33(5): 868–74. [DOI] [PubMed] [Google Scholar]
- 12.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC. Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr. 2000; 46(3): 132–9. [DOI] [PubMed] [Google Scholar]
- 13.Thacher TD, Pettifor JM, Tebben PJ, et al. Rickets severity predicts clinical outcomes in children with X-linked hypophosphatemia: Utility of the radiographic Rickets Severity Score. Bone 2019. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ OC, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and Development In: Statistics NCfH, editor. 11 (246) ed: Department of Health and Human Services; 2002. [PubMed] [Google Scholar]
- 15.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985; 107(3): 317–29. [DOI] [PubMed] [Google Scholar]
- 16.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007; 150(4): 395–9. [DOI] [PubMed] [Google Scholar]
- 17.Lockitch G, Halstead A, Albersheim S, MacCallum C, Quigley G. Age-and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin Chem. 1988; 34(8): 1622–5. [PubMed] [Google Scholar]
- 18.Zivicnjak M, Schnabel D, Billing H, et al. Age-related stature and linear body segments in children with X-linked hypophosphatemic rickets. Pediatr Nephrol. 2011; 26(2): 223–31. [DOI] [PubMed] [Google Scholar]
- 19.Costa T, Marie PJ, Scriver CR, et al. X-linked hypophosphatemia: effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J Clin Endocrinol Metab. 1981; 52(3): 463–72. [DOI] [PubMed] [Google Scholar]
- 20.Glorieux FH, Scriver CR, Reade TM, Goldman H, Roseborough A. Use of phosphate and vitamin D to prevent dwarfism and rickets in X-linked hypophosphatemia. N Engl J Med. 1972; 287(10): 481–7. [DOI] [PubMed] [Google Scholar]
- 21.Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006; 79(5): 294–300. [DOI] [PubMed] [Google Scholar]
- 22.Salmon B, Bardet C, Coyac B, et al. Abnormal osteopontin and matrix extracellular phosphoglycoprotein localization, and odontoblast differentiation, in X-linked hypophosphatemic teeth. Connect Tissue Res. 2014; 55(sup1): 79–82. [DOI] [PubMed] [Google Scholar]
- 23.Biosse Duplan M, Coyac B, Bardet C, et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J Dent Res. 2017; 96(4): 388–95. [DOI] [PubMed] [Google Scholar]
- 24.Connor J, Olear EA, Insogna KL, et al. Conventional Therapy in Adults With X-Linked Hypophosphatemia: Effects on Enthesopathy and Dental Disease. J Clin Endocrinol Metab. 2015; 100(10): 3625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaussain Miller C, Sinding C, Septier D, Wolikow M, Goldberg M, Garabedian M. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 2007; 13(5): 482–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.