Abstract
Background
Over the past several decades, rural and indigenous populations in Latin America have experienced abrupt and profound transformations in their lifestyles and economies, many having remarkable health consequences. Yet, these changes have had heterogeneous effects on the population's biology in different local contexts.
Objectives
The primary goal was to characterize the nutrition transition and biomarkers of noncommunicable diseases (NCD) risk in 2 Chilean indigenous populations that have had divergent histories of subsistence strategies (agropastoralism compared with hunter-gathering) in the last few millennia and live in contrasting environments, and to identify context-specific factors driving the nutrition and epidemiological transitions.
Methods
One-hundred-and-ninety (90 Pehuenche and 100 Atacameño) participants aged 18–87 y completed demographic, food-frequency, and physical activity questionnaires as well as measurements of some NCD risk biomarkers: blood pressure, weight, height, body fat percentage, waist circumference, blood total cholesterol, HDL cholesterol, triglycerides, and glucose. Framingham risk scores (FRSs) were calculated based on age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, smoking, diabetes status, and hypertension medication.
Results
Few differences in dietary composition and physical activity patterns were observed between the 2 populations. Multivariate analyses showed no differences between the 2 populations in any of the individual NCD risk biomarkers or FRSs after adjusting for age, sex, time since last meal, food insecurity in childhood, ultraprocessed food consumption, and physical activity.
Conclusions
Despite contrasting ecological and historical contexts, the 2 groups are converging into similar processes of market and wage-labor integration and transitioning to a Western diet high in processed and nonlocal foods, although some aspects of their “traditional” foodways are still in practice. The frequency of individuals exhibiting NCD biomarkers “at-risk” is relatively high and corresponds to other populations that have gone through nutrition transition. Furthermore, none of these biomarkers or FRSs differed between the 2 populations, suggesting a homogenization in the NCD risk factors.
Keywords: indigenous health, nutrition transition, Atacama Desert, Pehuenche, Mapuche, Atacameño, traditional food, food delocalization, dietary change
Over the past few decades, indigenous populations in Latin America have experienced major transformations in their lifestyles and economies, resulting in far-reaching consequences for their health and well-being. In this study, several biomarkers for non-communicable diseases (NCD) related to metabolic syndrome were evaluated among two indigenous populations living in highly contrasting environments (Atacama Desert and temperate rainforests in the Andes mountain slopes) who have had divergent histories of subsistence strategies (agropastoralism and hunting-gathering). Context-specific factors driving the nutrition transition in each group were identified; but, despite variation in local contexts, their diets and lifestyles are converging into similar patterns. Likewise, no significant differences in NCD risk for any of the biomarkers evaluated were found, suggesting a homogenization in the health outcomes associated with food and lifestyle changes in this region.
Introduction
Chronic noncommunicable diseases (NCD) account for >70% of deaths in adults worldwide, with cardiovascular diseases being the leading cause of NCD mortality (1). NCD, or the so-called “diseases of affluence,” were until recently associated with a nation's wealth, in contrast to infectious diseases, which had an inverse association with income across countries (2). However, although the overall trend indicates that NCD prevalence has slightly declined in the past 2 decades, developing countries have faced an increased incidence and burden of NCD risk during the same period (1, 3). The epidemiological transition from infectious to chronic diseases was particularly rapid in developing countries in Latin America that experienced accelerated economic growth in the 1990s, as is the case of Brazil, Chile, and Mexico, among others (4–6). Both low- and middle-income countries and marginalized groups in wealthy countries today bear a higher NCD burden because of further exposure to risk factors and limited access to primary care compared with their wealthier counterparts (7). NCD risk factors, such as obesity, are disproportionately more prevalent among some ethnic minorities, people of lower socioeconomic status, and rural populations in most countries in Latin America (8, 9). Among the factors explaining the abrupt shift in disease burden in low- and middle-income countries are urbanization, changes in technology that affect energy expenditure during transportation, work and leisure, and dietary changes, particularly with the consumption of highly processed foods (10).
Migration to urban areas accounted for most of the rise in NCD risk factors in developing countries because of the lifestyle and dietary changes associated with living in cities (10, 11); however, a recent study has shown that one of the key NCD risk factors—obesity—has dramatically increased in rural populations worldwide, even exceeding the obesity incidence seen in urban populations today and rising at a faster rate compared with urban populations in most low- and middle-income countries (8). In South America, Chile is the only country in the region where age-standardized mean BMI is already higher in rural women compared with urban women, although the trend indicates that this pattern will soon prevail in developing countries (8).
Concomitant with the epidemiological transition, low- and middle-income countries with rapidly growing economies have also experienced a nutrition transition from “traditional” foods to a Western dietary pattern (pattern 4: degenerative diseases) characterized by high intake of saturated fats, sugar, and refined carbohydrates, and low PUFAs and fiber (11). In Latin America the nutrition transition to a Western dietary pattern has been documented since the end of the 1980s, although the rate and extent at which this transition occurs varies tremendously across countries in the region (12). Likewise, comorbidities associated with the nutrition transition, such as NCD mortality, have risen concomitantly since then (13). Other behavioral characteristics related to the nutrition and epidemiological transitions, such as sedentary behavior, and tobacco and alcohol consumption, are also NCD risk factors that have increased in these populations in the last decades (7, 11).
The rapid transition from food- and nutrient-deprived environments to affluent environments can imply that individuals within a generation were regularly exposed to food insecurity and infections early in life whereas they are presented with food abundance—especially refined carbohydrate foods—as adults (14, 15). This mismatch between the poor early life conditions anticipated as a fetus and food abundance in adulthood has been found to be partly responsible for the increased prevalence of chronic diseases in developing countries (14, 16, 17). Uauy and colleagues (5, 17) have indicated that the rates at which lifestyle and dietary changes have occurred among these populations would have exposed individuals to additional NCD risk factors and therefore a higher NCD susceptibility.
A “triple evolutionary mismatch”—genetic, cultural, and developmental—has been proposed to explain the higher burden of NCD in low- and middle-income countries (15). The evolutionary genetic mismatch would explain the rise in NCD in contemporary populations as a result of our genetically based physiology, adapted to a Paleolithic hunter-gatherer lifestyle, in an unmatched environment characterized by current dietary and physical patterns. Within this framework, it is hypothesized that post–nutrition transition populations that adopted agriculture early in their history would be at lower risk of NCD due to dietary adaptations reducing the evolutionary genetic mismatch compared with populations that transitioned over just a few generations from a hunter-gatherer lifestyle to a Western dietary pattern high in refined carbohydrates and saturated fats (18–21).
In the last few decades several other NCD risk factors have been shown to have a complex interplay with health outcomes, and their effects might not be uniform across different populations and cultural contexts. For instance, socioeconomic status is positively associated with increases in BMI in low-income countries, whereas the opposite relation has been observed for high-income countries (22). Other factors exhibiting high variability in populations—and even within subgroups of a population—that have been associated with an increased risk of obesity, hypertension, and diabetes in adult life are: pathogen exposure and associated immune function during childhood (23); occupational exposures (e.g., exposure to metals, pesticides, and other hazardous chemicals) (24, 25); and environmental traits (e.g., high altitude, cold climate, arsenic in drinking water, etc.) (26–29).
To better understand the associations between long-standing subsistence strategies, nutrition transition in different cultural and environmental settings, and NCD risk–related health outcomes, this study comprised ethnographic fieldwork, oral history interviews, and collection of anthropometric and blood NCD biomarkers and dietary and physical activity data. Data were collected in 2 indigenous communities from rural areas in Chile inhabiting contrasting environments with divergent histories of subsistence strategies (hunting-gathering compared with agropastoralism). Both communities have gone through nutrition transition in the last few decades. Due to these marked differences between the 2 populations, it was expected that NCD risk biomarkers would differ between the 2 populations because each would display context-specific responses to the challenges posed by lifestyle and dietary shifts as well as market integration.
Methods
Study populations
Data from 2 indigenous communities in Chile were collected in this study, the Pehuenche and the Atacameños. According to the last population census (2017), the Atacameños—also known as Likan-Antai—numbered 25,262 individuals in the Antofagasta region (30) where this research took place. The Pehuenche are not recognized as a separate ethnic group by statute, thus census data specific for the Pehuenche do not exist, but it should be noted that many Pehuenche consider themselves to be both Pehuenche and Mapuche.
The Pehuenche are a Mapudungun-speaking and Mapuche subgroup that inhabit the temperate rainforest of both the eastern and western slopes of the Andes Mountains between latitudes ∼37°S and 41°S (Figure 1). Information on the lifestyle and economic activities of this group prior to contact with Spanish colonizers comes mainly from documents of the early colonial period because archaeological studies are scarce in this area. These sources indicate that until 200–300 y ago, the Pehuenche based their subsistence on hunting wild camelids (Lama guanicoe), Andean deer (Hippocamelus bisulcus), rheas (Rhea pennata), among other smaller animals, but mainly on gathering the seeds of the monkey-puzzle tree (Araucaria araucana), which constituted a staple food for this population (31, 32). The timing for the introduction of European cattle is unclear for the Pehuenche, but information from other Mapuche groups nearby indicates that this occurred gradually soon after contact with Spanish colonizers (33). Agriculture, however, seems to have never been practiced by this population, although there is evidence that other Mapuche groups in the valleys nearby adopted wheat and a few other crops during the 18th century (34, 35).
FIGURE 1.
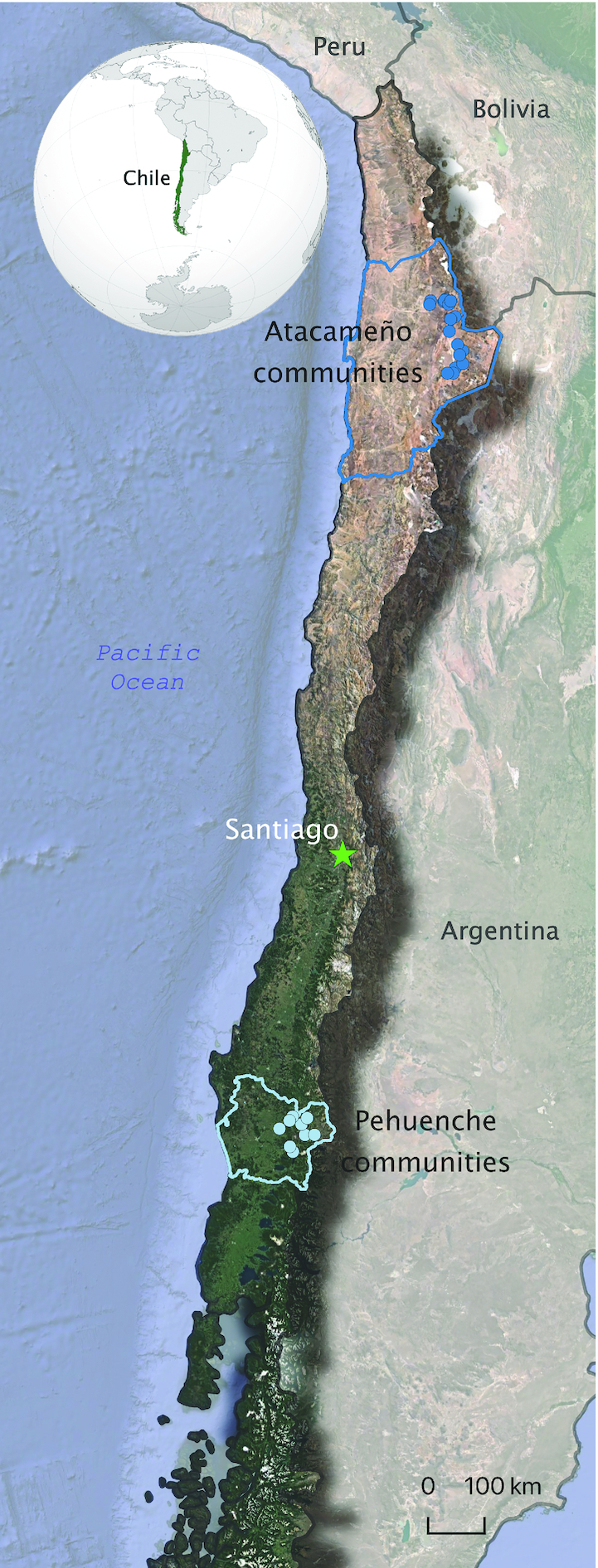
Location of the communities of the study sites. (Figure was produced using the software QGIS, version 3.4.15-Madeira).
Pehuenche communities today are located on indigenous reservations, and most of them have access to market goods—including packaged foods, primary education, public transportation, electricity, and drinking water. However, the majority lack sewerage systems, and access to health care is limited to monthly visits from health professionals. Most Pehuenche individuals speak fluent Spanish, and many have been converted to evangelical Christianity in the last 2–3 decades. Major changes in the foodways, lifestyle, and environment where this population lives are partially the result of the arrival of logging companies in the area, which, among other consequences, has decreased water availability in the communities and provided wage labor opportunities, especially for men. Despite these transformations, some aspects of the Mapuche culture, such as the practice of Nguillatun and We-tripantu religious ceremonies, and their traditional subsistence system the veranada-invernada, are still practiced by the vast majority of the population. This system requires seasonal migration from the lower lands to the mountains where the Araucaria araucana trees grow (i.e., above ∼1000 m) from December to April (veranada). During this period, entire families move to the highlands to gather firewood for cooking and heating, and specially to collect piñones, the fruit of the Araucaria trees, which then can be stored for ≤1 y under the right conditions. Until a few decades ago, these constituted a food staple, especially during the winter months when other foods were very scarce, but today piñones are gathered mostly for informal food markets.
The Atacameños inhabit the high-altitude Atacama Desert oases and ravines of the Loa River between latitudes ∼22°S and 24°S (Figure 1). Evidence of camelid domestication in this area dates as early as 4.5 kya (36) and agriculture was most likely introduced ∼3.5–3 kya (37). They engaged almost exclusively in subsistence agropastoralism until a few decades ago, and some elders continue to do so (38). Communities at the higher altitudes specialized in transhumance pastoralism of llamas (Lama glama) and, among a few other crops, cultivated quinoa (Chenopodium quinoa) and varieties of potatoes (Solanum tuberosum) in agricultural terraces. The populations at the lower spectrum of the high altitude cultivated a much wider range of species and adopted European crops such as wheat and many species of fruits and cattle soon after contact with Spanish colonizers (39, 40). Despite agriculture and pastoralism being the main subsistence strategies for this population, until recently communities along the altitudinal range supplemented their diet by hunting wild camelids and smaller mammals and birds and gathering wild fruits such as algarrobo (Prosopis sp.) and chañar (Geoffroea decorticans) (41, 42). Additionally, trading fish and seafood with coastal populations during pre-Hispanic times has been documented (43–45), but this activity progressively declined with Spanish colonization.
Today, Atacameño communities speak fluent Spanish and most practice Catholicism, although evangelical Christianity has gained support in the last couple of decades. They have access to primary education in their own communities, and secondary education is facilitated through boarding schools in nearby towns or cities. The expansion of tourism and especially the mining industry in this area has incorporated most of the Atacameño population into wage labor and affected the water supply for the human population, cattle, and crops, contributing to a sharp decline in the agriculture and pastoralist activities, and migration to the surrounding cities (46). The dramatic intensification of mining activities over the past few decades has contributed to major dietary and lifestyle changes, such as the decline in the frequency and scale of Atacameño traditional ceremonies, many of which are linked to agricultural cycles (38, 47).
Compared with the Pehuenche communities, the Atacameños tend to have higher incomes due to more stable and well-paid jobs, which have ensured them wider access to market goods, including processed foods and motor vehicles. Despite improvements in their living conditions in the last 2–3 decades, many Atacameño communities do not have sewage treatment systems, drinking water, or regular electricity, particularly the communities along the Loa River ravines. Like the Pehuenche, access to health care is restricted to regular visits from health professionals although most communities have a permanent health office with a paramedic.
Data collection
Data were collected in June 2016, and December 2017 through May 2018. Community leaders and potential participants were contacted soon after arrival in the communities to explain the purpose of the study and to answer questions. Potential participants received an information sheet about the study and the investigator, and recruitment took place during community meetings and door-to-door. The inclusion criteria for this study were: 1) age ≥18 y; 2) self-identification as Pehuenche or Atacameño/Likan-Antai; and 3) having had ≥2 generations of relatives in a Pehuenche or Atacameño/Likan-Antai community. Excluded were pregnant women and individuals closely related by kinship with previous study participants (i.e., parents-children, grandparents-grandchildren, siblings, aunts/uncles-niece/nephews, and cousins were not included). Participants did not receive any compensation for taking part in this research, and individuals who reported any medical condition were not excluded from the study or analyses. All participants recruited for this study consented verbally and by written informed consent. This study was approved by the Institutional Review Board at Indiana University (Study #1,703,735,479).
Data collection took place largely in the homes of participants, or occasionally at other locations according to the participant's preference and availability. Fifty-seven semistructured interviews were conducted during the pilot study phase and in the first 2–3 wk at each field site to gain insight into subsistence strategies in the past, dietary changes experienced by the individuals during their lifetime, and their drivers.
Individuals were asked to complete a semiquantitative FFQ based on the FFQ used in the Chilean nutritional assessment (ENCA 2010–2011) (48) and adapted to these populations for this study. This FFQ encompassed the last 12 mo and included 112 foods and beverages grouped in 8 categories, 7 of which contain the same products for both populations because they were common foods available in both regions. The “traditional foods” category contains foods identified by the individuals of each population as “traditional,” which are available and customary in one population and not the other, and that represent the most salient “traditional” food items identified on the interviews.
Physical activity was assessed using the Global Physical Activity Questionnaire (GPAQ) developed by the WHO (49) incorporating examples relevant to the local context. Minutes of moderate-to-vigorous physical activity (MVPA) were estimated and included as a variable in the analyses. Additionally, individuals completed a short questionnaire on demographics, and their nutritional, anthropometric, and health history, which included questions about experiences with food scarcity and food insecurity during childhood (up to ∼12 y old) and during their parents’ childhood.
During the same visit, the NCD risk biomarkers—BMI (height and weight), body fat percentage (BFP), waist circumference, blood pressure, and blood biomarkers (total cholesterol, HDL cholesterol, triglycerides, and glucose)—were collected. In most cases, and in both populations, these blood measurements were done in a nonfasting state because it was not feasible in these settings to ask people to fast overnight. For this reason, time since last meal or sweetened beverage was documented and included in the analyses to account for potential variation in blood measurements introduced by food consumption. Height was measured using a portable stadiometer (Seca 213), and weight and body fat percentage were measured using a portable scale and bioelectrical impedance body composition monitor (Tanita Inner Scan BC-543). Waist circumference was measured using fiberglass tape (ADE MZ10021), following the 2013 guidelines used by NHANES (50). Blood pressure was measured 3 times, with a 2-min interval between each measurement, using a portable blood pressure monitor (Omron BP742N), and the average of the 3 readings was used in the following analyses.
Finger puncture blood samples were obtained using a sterile single-use lancet (AccuChek Safe-T-Pro Plus). Cardiocheck Plus Analyzer (PTS Inc.) and the glucose and lipid panel strips were used to measure blood glucose, cholesterol, HDL cholesterol, and triglycerides according to manufacturer's instructions.
Data analyses
FFQ responses were converted to daily portions, and grams or milliliters for food and beverages, respectively, were calculated by converting the portion in the questionnaire to grams and then multiplying to get a daily frequency estimate. Nutrient and energy composition of diets were estimated by multiplying the energy and nutrient content per gram of each food by the grams of each food consumed per individual. The USDA Food Composition Database (51) was the primary source used to estimate nutrient content in foods; alternatively, when foods in the FFQ were not available in the USDA database, information from the FAO/INFOODS databases (52), the Chilean Food Composition Table (53), and the Peruvian Food Composition Tables (54) were used.
Foods were categorized into 4 groups according to degree of processing (unprocessed or minimally processed; processed culinary ingredients; processed foods; and ultraprocessed food and drink products) following the NOVA guidelines provided by Monteiro and colleagues (55). Additionally, a category that contains a sample of 8 of the 112 food items in the FFQ that are high in refined carbohydrates was created as a means of assessing any association of these foods and other variables in the study and NCD risk. The foods included in this category were: 1) tortilla de rescoldo, which is an unleavened bread made with lard and white flour and cooked in the coals of a fire; 2) store-bought flatbread-like bread (hallulla); 3) store-bought French-like bread (marraqueta); 4) noodles; 5) granulated white sugar—usually added to hot beverages; 6) soft drinks prepared with powder mix; 7) carbonated soft drinks; and 8) hard candies. These are referred to as the sample of 8 refined carbohydrate foods in the following analyses.
To investigate whether the 2 populations differed in their prevalence of NCD risk biomarkers, t tests were used to compare the means of each biomarker between the 2 populations. Additionally, individuals were categorized as “at-risk” and “not-at-risk” for each biomarker, according to age, sex, and time since last meal or sweetened beverage for glucose, following the cutoffs suggested by the scale manufacturer (Tanita) (56) or NIH cutoffs (57) for increased NCD risk (Table 1). Frequencies of individuals “at-risk” and “not-at-risk” between populations were compared using chi-square tests.
TABLE 1.
Risk classification according to age and sex for each biomarker1
| Biomarkers | At-risk |
|---|---|
| BFP female | 18 y: ≥31%; 19 y: ≥32%; 20–39 y: ≥33%; 40–59 y: ≥34%; ≥60 y: ≥36% |
| BFP male | 18–39 y: ≥20%; 40–59 y: ≥22%; ≥60 y: ≥25% |
| WC female | ≥88 cm |
| WC male | ≥102 cm |
| BMI | ≥25 kg/m2 |
| CHOL | 18–19 y: ≥170 mg/dL; ≥20 y: ≥200 mg/dL |
| HDL CHOL | 18–19 y: ≤44 mg/dL; ≥20 y: ≤49 mg/dL |
| TRG | ≥150 mg/dL |
| SBP | Average of 3 reads: ≥130 mmHg |
| DBP | Average of 3 reads: ≥80 mmHg |
| GLU | >45 min after meal or beverage: ≥240 mg/dL >120 min after meal or beverage: ≥140 mg/dL >8 h after meal or beverage: ≥100 mg/dL |
1BFP, body fat percentage; CHOL, cholesterol; DBP, diastolic blood pressure; GLU, glucose; HDL CHOL, HDL cholesterol; SBP, systolic blood pressure; TRG, triglycerides; WC, waist circumference.
To assess whether ethnicity (Pehuenche or Atacameño) was associated with chronic disease risk based on each biomarker, several regression models were run that included ethnicity as the variable of interest, and age, sex, food insecurity during childhood, ultraprocessed foods consumption, minutes of MVPA per week, and minutes since last meal or sweetened beverage as confounders. The variable minutes since last meal or sweetened beverage was only included in the models for blood biomarkers that might vary on a fed state. Regression models in this study did not account for potential cluster-correlated data because the number of individuals sampled per community within an ethnic group was often too small to implement this statistical approach.
Data from all the biomarkers except blood glucose were grouped into quintiles to create a score that contains the sum of the number of biomarkers for which an individual was placed into the top quintile. Observations in the highest quintile for all biomarkers (except HDL cholesterol, where the bottom quintile is considered “at-risk”) were assigned a value of 1, and a 0 was assigned for the remaining quintiles resulting in a score from 0 to 8 for each individual. Body fat percentage values were categorized into quintiles according to sex and age range, waist circumference was categorized according to sex, and the biomarkers blood triglycerides, cholesterol, HDL cholesterol, BMI, and systolic and diastolic blood pressure were sorted into quintiles regardless of sex or age. Ten-year cardiovascular Framingham risk scores (FRSs) were calculated for each individual considering age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, smoking and diabetes status, and hypertension medication, following D'Agostino et al. (58).
Categories “at-risk” and “not-at-risk” for each biomarker were considered as the outcome variable in 9 binary logistic regression models that included ethnicity as the variable of interest, and the confounders listed above. FRSs were not normally distributed and therefore log-transformed to be used as the outcome variable in a multiple linear regression model that included sex, age, ethnicity, ultraprocessed food consumption, food insecurity, physical activity, and time since last meal as predictors. All the analyses were performed in RStudio (v. 1.1.456). Statistical significance was set at P < 0.05.
Results
One hundred-ninety participants, 90 from 18 Pehuenche communities and 100 from 15 Atacameño communities, were recruited. The number of people sampled in each community ranged from 1 to 22, depending mostly on the size of the community because sampling of related individuals was avoided. The majority of both samples comprised women (71% of the Atacameños and 70% of the Pehuenche) because recruitment and sampling took place at participants’ homes and most men were away working in wage labor. For the same reason, the samples are biased toward older adults, especially among the Atacameños, where the mean age is statistically significantly higher than in the Pehuenche sample (54.2 ± 18.1 y and 48.5 ± 15.5 y, respectively; P < 0.05) (Table 2).
TABLE 2.
Descriptive statistics of sample characteristics and confounders by ethnic group1
| Characteristic | Total (n = 190) | Pehuenche (n = 90) | Atacameño (n = 100) | P |
|---|---|---|---|---|
| Sex female | 134 (71%) | 64 (71%) | 70 (70%) | 0.993 |
| Age, y | 51.52 ± 17.1 | 48.5 ± 15.5 | 54.2 ± 18.1 | 0.020* |
| Smoking (at least 1 cigarette on 1 or more occasions per week) | 17 (8.9%) | 3 (3.3%) | 14 (14%) | —2 |
| MVPA, min/wk | 387.1 ± 631.6 | 488.5 ± 670.5 | 295.8 ± 582.7 | 0.037* |
| Any physical activity, min/wk | 616.9 ± 776.3 | 685.2 ± 750.0 | 555.5 ± 798.0 | 0.250 |
| Physical inactivity, min/wk | 358.7 ± 174.9 | 250.6 ± 119.8 | 456.0 ± 159.2 | <0.001* |
| % kcal carbohydrates | 55.2 ± 6.3 | 54.6 ± 5.9 | 55.7 ± 6.7 | 0.225 |
| % kcal protein | 12.9 ± 1.9 | 12.6 ± 2.0 | 13.1 ± 1.8 | 0.075 |
| % kcal lipids | 31.9 ± 5.8 | 31.5 ± 5.7 | 32.3 ± 5.8 | 0.375 |
| % kcal ultraprocessed foods | 13.7 ± 6.6 | 15.1 ± 6.9 | 12.4 ± 6.1 | 0.005* |
| % kcal refined carbohydrates3 | 26.0 ± 9.4 | 25.2 ± 9.7 | 26.8 ± 9.1 | 0.264 |
| % kcal traditional foods | 1.7 ± 2.7 | 1.3 ± 2.5 | 2.2 ± 2.7 | 0.012* |
| Food insecurity in childhood | 120 (63%) | 70 (78%) | 50 (50%) | <0.001* |
| Food insecurity mother | 147 (82%) | 81 (92%) | 66 (72%) | <0.001* |
| Food insecurity father | 130 (82%) | 77 (91%) | 53 (73%) | 0.006* |
| Body fat, % | 34.5 ± 8.2 | 35.7 ± 7.6 | 35.1 ± 7.9 | 0.291 |
| BMI, kg/m2 | 29.0 ± 4.5 | 29.4 ± 4.4 | 28.7 ± 4.5 | 0.243 |
| Diastolic blood pressure, mmHg | 75.6 ± 9.2 | 76.5 ± 9.4 | 74.8 ± 9.0 | 0.217 |
| Systolic blood pressure, mmHg | 118.8 ± 17.1 | 118.9 ± 16.8 | 118.6 ± 17.4 | 0.910 |
| Blood cholesterol, mg/dL | 163.0 ± 36.8 | 158.7 ± 37.7 | 166.8 ± 35.7 | 0.133 |
| Blood HDL cholesterol, mg/dL | 44.1 ± 13.2 | 43.9 ± 11.1 | 44.3 ± 14.9 | 0.834 |
| Blood triglycerides, mg/dL | 222.1 ± 111.9 | 216.0 ± 116.6 | 227.7 ± 107.7 | 0.475 |
| Waist circumference, cm | 100.7 ± 11.5 | 102.0 ± 11.3 | 99.5 ± 11.6 | 0.156 |
| Blood glucose, mg/dL4 | 138.5 ± 59.3 | 134.9 ± 45.5 | 141.8 ± 69.6 | 0.412 |
| Diagnosed type 2 diabetes | 17 (8.9%) | 9 (10%) | 8 (8%) | 0.820 |
| Time since last meal, min | 174.2 ± 129.2 | 192.1 ± 159.5 | 158.8 ± 94.3 | 0.096 |
1Mean ± SD for continuous variables and frequency (%) for categorical variables. *Statistically significant at P < 0.05. MVPA, moderate-to-vigorous physical activity.
2Less than 5 observations per cell.
3Sample of 8 refined carbohydrate foods.
4Nonfasting blood glucose.
Univariate analyses were performed to identify meaningful predictor variables for multivariate analyses. For each biomarker, t tests between binary categories (ethnicity, sex, and food insecurity) showed that only sex differences were statistically significant for body fat percentage and systolic and diastolic blood pressure, and no other biomarkers significantly differed by sex, ethnicity, or food insecurity categories (Supplemental Table 1). Spearman correlations between biomarker values and age, ultraprocessed food consumption, and MVPA indicated that age was positively associated with total cholesterol, HDL cholesterol, and systolic blood pressure. Ultraprocessed food consumption was positively associated with diastolic blood pressure and negatively associated with HDL cholesterol, whereas minutes of MVPA were negatively associated with body fat percentage and BMI (Supplemental Table 2).
Physical activity patterns
The Atacameño sample reported statistically significantly less MVPA and more physical inactivity per week than the Pehuenche. The mean of total minutes of any physical activity did not differ between the two populations (Table 2).
Food patterns
As Table 2 indicates, energy (kilocalories) intake for both populations came mainly from carbohydrates (55%), followed by lipids (32%), and then by proteins (13%). Over 25% of calories consumed in both populations came from only 8 of the 112 foods in the FFQ, which correspond to “refined carbohydrate foods” such as white bread, noodles, sugar, candies, and sugary beverages. Mean total energy intake did not differ between the 2 populations nor did the proportion of energy from any macronutrients or refined carbohydrate foods (Table 2). Individuals in both populations reported having gradually incorporated these foods, cooking oil, and some ultraprocessed foods into their regular diet over the last ∼30 y. Ultraprocessed foods comprised 23 of the 112 foods in the FFQ and made up ∼14% of average energy intake; this value was higher in the Pehuenche (15.1%) than in the Atacameños (12.4%) (P < 0.05). Age was inversely associated with ultraprocessed foods consumption (r = −0.43; P < 0.001).
In both populations, “traditional foods” are mostly consumed during ceremonies or festivities. On average, only 36% of the “traditional foods” were consumed monthly or more frequently, and the proportion of these foods consumed at least once a month was lower among the Atacameños than the Pehuenche (Wilcoxon rank sum test: P < 0.001). This difference was mainly driven by 1 food: the fruit of the monkey-puzzle tree, piñones (pehuen), which is still consumed frequently by the Pehuenche, with 83% of this population reporting having consumed it at least once per month during the last year. In contrast, the most frequent “traditional” food among the Atacameños was toasted dry corn (tostado), which was consumed regularly by 58% of the sample (Supplemental Tables 3 and 4).
Food insecurity
Food insecurity in childhood was common in both samples, with >50% of the individuals reporting they had faced hunger during their infancy and youth. Although relatively frequent in both populations, the experience of food scarcity and unpredictability during childhood was reported more often in the Pehuenche than in the Atacameños (78% compared with 50%; P < 0.001). Likewise, parents’ food insecurity (i.e., both mother and father, when known by the participant) was reported more frequently in the Pehuenche than in the Atacameños (P < 0.05); mother's and father's food insecurity reached 92% and 91%, respectively, in the Pehuenche, whereas the frequency in the Atacameños was 72% and 73%, respectively (Table 2). In both populations, parents’ food insecurity was more commonly reported than participants’ food insecurity during childhood. Over 70% of Atacameños and 90% of Pehuenche with known parents reported being told about seasonal hunger (i.e., winter and spring months), or constant food scarcity, limited to low quantity and poor-quality foods during most of the year during their parents’ childhood.
Association between chronic disease risk prevalence and sample characteristics
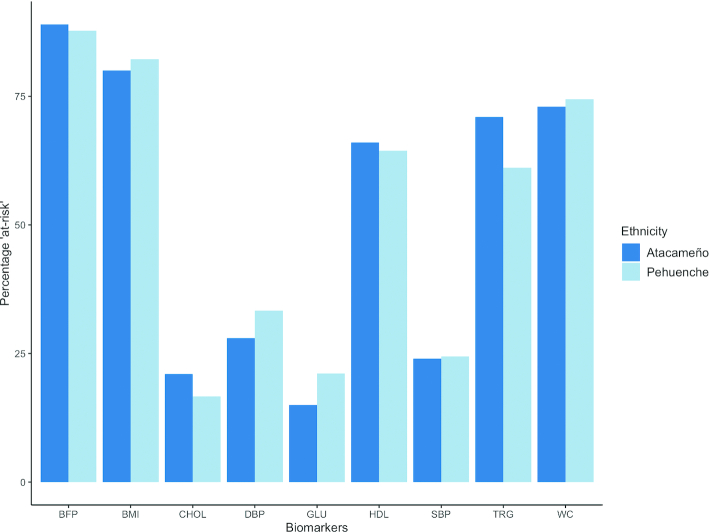
It was shown by t tests that means of each biomarker did not differ between the 2 populations. Furthermore, when individuals were categorized into “at-risk” or “not-at-risk” categories for each biomarker, the proportion of individuals “at-risk” and “not-at-risk” was not different in the 2 populations (Figure 2).
FIGURE 2.
Percentage of population “at-risk” for each biomarker by population. There were no statistically significant differences between the 2 groups at P < 0.05 for any biomarker. BFP, body fat percentage; CHOL, cholesterol; DBP, diastolic blood pressure; GLU, glucose; HDL, HDL cholesterol; SBP, systolic blood pressure; TRG, triglycerides; WC, waist circumference.
Logistic regressions considering each biomarker showed that ethnicity (i.e., belonging to either an Atacameño or Pehuenche community) was not a statistically significant predictor of being “at-risk” for any of the biomarkers when controlling for confounders. Sex, followed by age, were the most common predictors of the risk categories in these samples, reaching significance for 5 and 3 of the 9 biomarkers, respectively. Males were more likely than females to be in the “at-risk” category for systolic and diastolic blood pressure, and less likely than females to be in the “at-risk” category for blood HDL cholesterol, BMI, and waist circumference. Each 1-y increase in age was associated with higher odds of being in the “at-risk” category for blood cholesterol and systolic blood pressure, and lower odds of being in the “at-risk” category for blood HDL cholesterol. The proportion of energy from ultraprocessed foods in the diet increased the odds of being “at-risk” of high systolic blood pressure, and food insecurity almost reached statistical significance (P = 0.067) as a predictor for the risk of elevated glucose. MVPA slightly decreased the odds of elevated triglycerides (P = 0.048) and glucose at-risk was only associated with time since last meal or sweetened beverage. None of the independent variables included statistically significantly predicted the likelihood of being in the “at-risk” category for body fat percentage (Table 3).
TABLE 3.
Logistic regression for each biomarker risk outcome using multiple predictors1
| Predictors | |||||||
|---|---|---|---|---|---|---|---|
| Biomarkers | Age, y | Sex: male | Ethnicity: Pehuenche | Food insecurity | UP foods | MVPA | Time meal |
| BFP “at-risk” | 0.99 (0.96, 1.02) | 0.69 (0.26, 1.90) | 0.82 (0.29, 2.27) | 1.99 (0.70, 5.74) | 0.96 (0.88, 1.03) | 1.00 (1.00, 1.00) | — |
| BMI “at-risk” | 0.99 (0.96, 1.01) | 0.42 (0.19, 0.93)* | 1.02 (0.44, 2.37) | 1.51 (0.64, 3.60) | 1.00 (0.99, 1.00) | 1.00 (1.00, −1.00) | — |
| WC “at-risk” | 0.98 (0.95, 1.00) | 0.089 (0.04, 0.19)* | 1.01 (0.44, 2.34) | 0.96 (0.39, 2.34) | 0.98 (0.92, 1.05) | 1.00 (1.00, 1.00) | — |
| SBP “at-risk” | 1.05 (1.02 −1.08)* | 2.36 (1.09, 5.13)* | 1.28 (0.57, 2.94) | 0.74 (0.31, 1.73) | 1.12 (1.05 −1.20)* | 1.00 (1.00, 1.00) | — |
| DBP “at-risk” | 0.99 (0.97, 1.01) | 3.2 (1.58, 6.64)* | 1.29 (0.59, 2.46) | 1.23 (0.58, 2.46) | 1.02 (0.97, 1.08) | 1.00 (1.00, 1.00) | — |
| CHOL “at-risk” | 1.03 (1.01, 1.06)* | 0.64 (0.23, 1.61) | 1.01 (0.43– 2.41) | 1.11 (0.45, 2.86) | 0.98 (0.92, 1.05) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| TRG “at-risk” | 1.02 (1.00, 1.04) | 0.97 (0.47, 2.07) | 0.79 (0.39, 1.58) | 0.87 (0.41, 1.84) | 1.03 (0.98, 1.09) | 1.00 (1.00, 1.00)* | 1.00 (1.00, 1.00) |
| HDL CHOL “at-risk” | 0.96 (0.94, 0.99)* | 0.38 (0.18, 0.79)* | 0.68 (0.33, 1.39) | 1.43 (0.66, 3.12) | 1.00 (0.95, 1.06) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| GLU “at-risk”2 | 1.01 (0.98, 1.04) | 0.79 (0.27, 2.09) | 1.38 (0.56, 3.49) | 2.81 (0.98, 9.36) | 0.95 (0.88, 1.02) | 1.00 (1.00, 1.00) | 1.01 (1.00, 1.01)* |
| Quintile risk score3 | 1.01 (0.99, 1.02) | 1.55 (0.83, 2.89) | 0.88 (0.48, 1.61) | 1.41 (0.75, 2.67) | 1.02 (0.97, 1.07) | 1.00 (1.00, 1.00)* | 1.00 (1.00, 1.00) |
1Results displayed as ORs (95% CIs). Risk categories were defined according to the cutoffs indicated in Table 1. *Statistically significant at P < 0.05. BFP, body fat percentage; CHOL, cholesterol; DBP, diastolic blood pressure; GLU, glucose; HDL CHOL, HDL cholesterol; MVPA, moderate-to-vigorous activity; SBP, systolic blood pressure; Time meal, minutes since last meal; TRG, triglycerides; UP, ultraprocessed; WC, waist circumference.
2Nonfasting glucose “at-risk” category was adjusted by time of last meal according to the cutoffs indicated in Table 1.
3Ordinal logistic regression.
Using quintile risk scores as the outcome variable in an ordinal logistic regression that considered the same confounders listed above, only minutes of MVPA was a statistically significant predictor (P = 0.030), where fewer minutes were associated with increased odds of higher scores. When FRSs were considered as the outcome variable, a linear regression model showed that increased age and being male were the only statistically significant predictors and that ethnicity was not associated with FRSs in this study (Table 4).
TABLE 4.
Multivariate linear regression for log-transformed Framingham risk scores1
| Predictor | Coefficient (95% CI) | P |
|---|---|---|
| Age | 0.05 (0.05, 0.06) | <0.001* |
| Sex: male | 1.14 (0.94, 1.35) | <0.001* |
| Ethnicity: Pehuenche | −0.06 (−0.25, 0.14) | 0.5735 |
| Food insecurity | 0.10 (−0.11, 0.30) | 0.3595 |
| UP foods | 0.01 (−0.01, 0.02) | 0.3399 |
| MVPA | −0.00 (−0.00, 0.00) | 0.0722 |
1Results displayed as ORs (95% CIs). *Statistically significant at P < 0.05. MVPA, moderate-to-vigorous physical activity; UP, ultraprocessed.
Discussion
Since the 1990s studies on how market integration has affected indigenous peoples’ lifestyle and health in South America have proliferated concomitantly with the nutrition transition model posited by Popkin (11). Across this region, a high degree of variability in the extent of market integration, lifestyle and dietary shifts, NCD risk prevalence, as well as the interplay between these factors has been documented, suggesting complex and heterogeneous interactions between these factors and health outcomes (59–64). With the aim of contributing evidence to this debate, this study assessed the prevalence of NCD risk–associated biomarkers in 2 indigenous populations currently experiencing the nutrition transition, and which until a few decades ago had contrasting lifestyles and diets in highly distinctive environmental settings.
Findings from this study indicate that despite slight differences in physical activity intensity and the consumption of ultraprocessed and “traditional” foods observed between the 2 groups, broader socioeconomic characteristics and dietary (i.e., macronutrient composition) and activity patterns are nowadays fairly similar. These results suggest a convergence in lifestyle and dietary patterns between the Pehuenche and Atacameño communities in the context of ongoing nutrition transition and integration into the wage labor and market economy. Furthermore, this study reported no significant differences in NCD risk biomarkers between the 2 populations, which agrees with the minimal differences observed in NCD risk factors.
The results of multivariate analyses showed that ethnicity was not a predictor for any of the NCD risk biomarkers, nor were the composite quintile scores and FRSs. These analyses instead indicate that while controlling for other confounders, age and sex are the most consistent predictors of NCD risk–associated outcomes, as they are in most populations (65, 66). Compared with populations with low-NCD-risk subsistence lifestyles such as the Tsimane from lowland Bolivia (67), the Atacameño and Pehuenche individuals in this study showed remarkably higher mean BMI, body fat percentage, and blood triglycerides, with slightly higher total blood cholesterol, HDL cholesterol, and diastolic and systolic blood pressures. These results are consistent with other transitioning indigenous populations in South America that have radically changed both their lifestyle (i.e., settlement patterns and occupation) and food habits (60–62, 68, 69)—particularly with the displacement of nutrient- and fiber-rich foods by energy-dense and low-nutrient foods.
In this study, sex seemed to predict most of the differences observed in NCD risk–associated outcomes. Compared with women, men in both populations were more likely to have elevated systolic and diastolic blood pressure. This trend has been observed not only in industrialized populations but also in subsistence populations like the Tsimane (67), and appears to be associated with the role of endogenous estrogens in vasodilatation (70). However, females in both populations were more likely to be associated with the “at-risk” category for BMI, waist circumference, and HDL cholesterol. This is consistent with the global trend of higher prevalence of overweight and obesity in adult females than males (1), and this is becoming more extreme in rural populations (8). A recent nationally representative health assessment in Chile (ENS 2016–2017) showed that obesity prevalence in women was markedly greater than in men (38.4% compared with 30.3%, respectively) (71), and was higher in women from rural areas than from urban areas (8), such as those from the 2 indigenous communities included in this study.
In addition to sex, age was associated with an increased risk for hypercholesterolemia and hypertension consistent with the pattern seen in industrialized populations (72–74). Because this association is commonly observed, it has been assumed that elevated blood pressure and cholesterol are part of the normal process of ageing. However, recent studies conducted on subsistence lifestyle populations have challenged this view, showing that blood pressure, LDL cholesterol, and total cholesterol do not necessarily rise with age, thus suggesting that this is likely a phenomenon associated with the nutrition transition (67, 75, 76).
Time spent in MVPA was a significant predictor only for elevated blood pressure, and some differences in physical activity were found between the 2 populations, which might relate to current lifestyle changes and wage-labor integration. Individuals from the Atacameño communities engaged in less MVPA and more hours of sedentary behavior (e.g., watching TV, knitting, driving, etc.) than the Pehuenche—although total time spent on any physical activity per week did not differ between the 2 populations. This observation was consistent with the ethnographic and interview data, which indicated that most Atacameños, particularly men, but also women, worked for mining companies operating machines while seated for many hours per day (∼10 h) and stayed at home resting when not working. Atacameño women who did not have a wage-earning job tended to dedicate time almost exclusively to domestic labor, which included little MVPA. In contrast to the Atacameños, the vast majority of Pehuenche households did not have a motor vehicle and individuals tended to do strenuous activities such as chopping wood, commuting by bike, or carrying heavy objects (e.g., water buckets) over long distances more often and for longer periods of time. Compared with industrialized populations, like the United States, where MVPA is slightly >1 h/wk on average (77), both samples in this study engaged in considerably higher minutes of MVPA per week but remarkably lower than subsistence populations, like the Hadza, who engaged in >900 min MVPA per week (76).
Food insecurity during childhood almost reached statistical significance as a predictor of high blood triglycerides, and no significant associations with any NCD risk biomarkers were found. These results diverged from the thrifty phenotype hypothesis, which predicts that poor nutritional conditions during fetal and infant development would result in increased susceptibility to impaired glucose-insulin metabolism and thus higher risk of developing type 2 diabetes and metabolic syndrome (78, 79). Although nonsignificant as a predictor of NCD risk, food insecurity was strikingly different between the 2 groups. Individuals from the Pehuenche communities reported significantly higher frequencies of both parents’ and childhood food insecurity compared with the Atacameños, despite no differences in risk for any biomarker being observed between the 2 populations. In both populations, parents’ food insecurity was statistically significantly higher than childhood food insecurity, suggesting that food availability and access have improved over the course of 1 generation. In the interviews, most older individuals (i.e., >60 y) described the contrast between their situation today and the precarious conditions in which they lived during their childhood, emphasizing their feelings of hunger and unpredictability in relation to food.
In both populations, the proportion of energy that comes from the 3 macronutrients—carbohydrates, lipids, and proteins—was within the acceptable macronutrient distribution ranges proposed by the Institute of Medicine (80) for adequate nutrition. The proportion of the total energy intake that comes from each macronutrient was fairly similar for the 2 groups, and closely resembled the average proportion of macronutrients in the current Chilean diet (81). Compared with the US diet (51), the proportion of energy from carbohydrates was slightly higher, and that from protein was lower in both indigenous populations in this study. However, the percentage of energy from ultraprocessed foods was remarkably lower in both of the study samples (∼14%) compared with the United States (∼60%) (82). Because consumption of ultraprocessed foods has been strongly associated with NCD worldwide, the relatively low consumption of this food group should exert a protective effect against NCD. In the multivariate analyses conducted in this study, ultraprocessed food consumption only showed an association with being in the “at-risk” category for systolic blood pressure and did not appear to be a predictor of NCD risk for any of the other health outcomes evaluated. However, this could have been caused by the low consumption of these foods in general, particularly in the elderly, who constituted the majority of the sample and who consumed statistically significantly less ultraprocessed foods than younger individuals in both populations in this study. Ethnographic observations and interview data supported this finding and indicated that the main barriers toward consumption of ultraprocessed foods among the elders were income and/or lack of access to food markets due to relative isolation, but food preferences also played a role. A similar trend has been previously described among the Xavante (Brazil), and appears to be related to higher adherence to traditional subsistence patterns by the elders due to linguistic, cultural, and geographic barriers (62).
A very high intake of refined carbohydrates (e.g., white bread, noodles, sugar, sugary soft drinks, etc.) is noticeable in both populations. Large quantities of white bread are usually served with every meal and soft drinks with added sugar have replaced water consumption in many households at both study sites. According to the participants, ultraprocessed foods and refined carbohydrate foods were gradually introduced in the last 3 decades, and they are rapidly becoming staple foods as employment in the booming mining and logging industries in the respective areas provides a stable income to every household. Access to market foods and the abandonment of agriculture appears to be one of the most frequent drivers of the nutrition transition among the Atacameños. Because, compared with older members of the community, younger individuals consume more refined carbohydrates and ultraprocessed foods and have the economic means to access/provide these foods, it is likely that their consumption will increase in the following decades. Such a pattern has been observed in other populations currently undergoing the nutrition transition, where the incorporation of food additives, such as vegetable oil, salt, and refined sugar, has paralleled the increase in BMI and body fat percentage of the individuals (63, 83).
Consumption of “traditional” foods, usually nutrient-rich, high in fiber, and low in sugar and saturated fats, has been associated with positive health outcomes (84–88). Both populations included in this study exhibited sporadic consumption of most of the foods in this group, although the average intake was higher in the Pehuenche than in the Atacameños due to the persistence of some aspects of their traditional subsistence system—the veranada/invernada—that provides Pehuenche families with the fruit of the Araucaria trees (piñones). Although piñones were still part of the regular diet of many Pehuenche, frequency of consumption seems to have declined in the last decade due to price increases in the market. Thus, many families reported preferring to sell most of the gathered piñones to buy other desirable foods such as white flour, rice, oil, and sugar. This phenomenon of commoditization of food systems and its negative effects on a population's nutrition has been extensively described for rural households Latin America (68, 89). The remaining “traditional” foods identified in this population were only consumed anecdotally or during traditional ceremonies that take place a few times a year.
Among the Atacameños, the average consumption of “traditional” foods was even lower than in the Pehuenche and mostly associated with ceremonies or festivities. Many are no longer readily available because the population has largely abandoned the agricultural and pastoralist practices that provided them with these products. Several individuals reported that because ceremonies still practiced in these communities require the preparation of these foods, they have to acquire them from Bolivia. In both populations, participants noted that consumption of local and “traditional” foods has steadily declined in the last 20–30 y, but due to the lack of longitudinal data documenting changes in the food system and the population's health, this study could not ascertain the direct impact of diet delocalization on health outcomes.
The Pehuenche and Atacameños are both transitioning to a Western diet high in processed and nonlocal foods and progressively incorporating noticeable amounts and variety of ultraprocessed foods. Despite the changes in dietary and physical activity patterns observed, the fourth stage of the nutrition transition described by Popkin (11) has not been completed yet because “traditional” foods obtained through gathering and small-scale agriculture still complement their diets. This is particularly distinctive in older individuals, who also tended to be at lower NCD risk than middle-aged individuals. Because both populations are being progressively incorporated to the market economy and often emigrating from their communities of origin, it is likely that “traditional” foodways will be abandoned and consumption of ultraprocessed foods will continue to rise concomitantly with the noticeable increases in obesity (8) and other NCD risk factors in rural areas of the country (71).
Study limitations
This study has several limitations that prevent generalization of these findings to the broader Atacameño and Pehuenche populations. First, the cross-sectional nature of these data limits the ability of this study to establish causal relations between environmental and behavioral patterns and chronic disease risk. Second, the small sample size could contribute to the failure to detect subtle differences in NCD risk between the 2 populations. Third, due to the voluntary and nonrandomized sampling strategy, some bias toward women and older individuals—who were available and more willing to participate—is apparent in this study. This selection bias implies that findings might not be extrapolated to the entire populations. Fourth, food consumption and physical activity collected through an FFQ and GPAQ, respectively, rely on participant memory, and individuals had difficulty determining food quantities, frequencies, seasonality, and time spent on a given activity. These limitations inherent to these methods have been extensively described elsewhere (90–92) and several precautions to avoid further biases were taken. These included the administration of the questionnaires through interview by a trained researcher instead of giving a paper-pencil version to the participants for completion, the incorporation of local cognitive categories for foods, and context-appropriate examples of physical activities. Fifth, NCD blood biomarkers were not assessed in a fasting state and could have reflected recent diet, although this was controlled for in the statistical analyses by incorporating the time since last meal in the regressions and categorizations for blood glucose risk. Sixth, due to the small number of individuals per community within an ethnic group and lack of data on household belonging, data on this study were not suitable to implement a mixed-effects model or other approach that could account for potential cluster correlation at the household or community level. Data clustering at the household level is, however, unlikely in this dataset because sampling avoided individuals related by kinship. Lastly, the prompts in the questionnaire about food insecurity during childhood might not have captured the true extent of maternal and early-life nutritional stress, thus this study cannot certainly rule out the role of developmental mismatches related to predictive adaptive responses (93).
Conclusions
To my knowledge, this is the first study documenting food patterns and NCD risk–associated outcomes in indigenous populations in Chile. Compared with other indigenous populations in the region who live a more traditional subsistence lifestyle (67, 94), the Atacameños and Pehuenche exhibited a remarkably higher NCD risk. These results agree with similar studies on transitioning hunting-horticulturalist populations where changes in food resources and market integration have paralleled an increase in body size and other NCD risk biomarkers (60, 62, 63, 69). Milton (95) has argued that populations that follow traditional diets and lifestyles, regardless of proportions of dietary macronutrients or if they include agricultural products, have very low NCD prevalence, indicating that energy-dense and low-fiber foods characteristic of contemporary Western diets along with sedentary behavior are driving the high NCD burden.
Despite contrasting past subsistence strategies and environments, the highly similar NCD risk pattern in the 2 studied populations suggests that if genetic variation related to dietary adaptations plays a role in NCD risk, this is either not related to glucose-insulin metabolism and thus reduced NCD risk, or that current dietary and physical activity patterns are overruling the expected phenotypic outcomes. Analyses of selection signatures in these populations’ genomes will provide further information to evaluate the role of dietary adaptations in NCD risk and support or rule out evolutionary mismatch hypotheses as an explanation for the rise in chronic diseases in contemporary societies.
Considering the abrupt and profound transformations of these populations’ landscapes, economies, and lifestyles in the last few decades, and the relatively high NCD risk in young and middle-aged individuals, the results of this study suggest that these changes in the environment might be related to the current high burden of NCD risk in both groups. In particular, the matched increased consumption of processed and ultraprocessed foods and low levels of physical activity observed among the Atacameño and Pehuenche populations could be playing a critical role in the lack of differences in NCD risk between them. Future research along these lines should consider longitudinal assessments and incorporate standardized measures of market integration, income, and food insecurity to provide a more complete understanding of the effects of the nutrition transition on population health in diverse settings. Identifying drivers and effects of this transition and their particular interplay with health outcomes at a local level could potentially contribute to developing context-specific public policies addressing food, nutrition, and health.
Supplementary Material
ACKNOWLEDGEMENTS
I express my gratitude to the community leaders and study participants for their permissions and contributions, to Francisca Adasme, Rayen Aguilar, Paula Candia, Daniela Castillo, Daniela Cordero, Josefa Guerra, Camila Muñoz, and Marcela Pfaff for their invaluable help on fieldwork data collection, and to Jennifer Cullin, Andrea Wiley, and 2 anonymous reviewers for their very helpful comments, which greatly improved the quality of this manuscript.
The sole author was responsible for all aspects of this manuscript.
Notes
This material is based upon work supported by the National Science Foundation under the Doctoral Dissertation Research Improvement Grant No. 1752114, Wenner-Gren Foundation Fieldwork Research Grant #9530, and the National Commission for Scientific and Technological Research (CONICYT) Becas Chile Fellowship. Funders had no role in the design, implementation, analysis, and interpretation of the data included in this study.
Author disclosures: The author reports no conflicts of interests.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviation used: FRS, Framingham risk score; GPAQ, Global Physical Activity Questionnaire; MVPA, moderate-to-vigorous physical activity; NCD, noncommunicable diseases.
References
- 1. WHO. Noncommunicable diseases country profiles 2018. Geneva (Switzerland):World Health Organization; 2018. [Google Scholar]
- 2. Hotez PJ, Peiperl L. Noncommunicable diseases: a globalization of disparity?. PLoS Med. 2015;12(7):3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Islam SMS, Purnat TD, Phuong NTA, Mwingira U, Schacht K, Fröschl G. Non communicable diseases (NCDs) in developing countries: a symposium report. Global Health. 2014;10:(81). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albala C, Vio F, Kain J, Uauy R. Nutrition transition in Chile: determinants and consequences. Public Health Nutr. 2002;5(1a):123–8. [DOI] [PubMed] [Google Scholar]
- 5. Uauy R, Albala C, Kain J. Symposium: obesity in developing countries: biological and ecological factors. J Nutr. 2001;131(3):893–9. [DOI] [PubMed] [Google Scholar]
- 6. Popkin BM, Reardon T. Obesity and the food system transformation in Latin America. Obes Rev. 2018;19(8):1028–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Cesare M, Khang YH, Asaria P, Blakely T, Cowan MJ, Farzadfar F, Guerrero R, Ikeda N, Kyobutungi C, Msyamboza KP et al.. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381(9866):585–97. [DOI] [PubMed] [Google Scholar]
- 8. NCD-RisC. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petermann F, Durán E, Labraña AM, Martínez MA, Leiva AM, Garrido-Méndez A, Poblete-Valderrama F, Díaz-Martínez X, Salas C, Celis-Morales C. Factores asociados al desarrollo de obesidad en Chile: resultados de la Encuesta Nacional de Salud 2009–2010. Rev Med Chil. 2017;145(6):716–22. [DOI] [PubMed] [Google Scholar]
- 10. Wagner KH, Brath H. A global view on the development of non communicable diseases. Prev Med (Baltim). 2012;54(Suppl):S38–41. [DOI] [PubMed] [Google Scholar]
- 11. Popkin B. Nutritional patterns and transitions. Popul Dev Rev. 1993;19(1):138–57. [Google Scholar]
- 12. Tagle MA. Cambios en los patrones de consumo alimentario en América Latina. Arch Latinoam Nutr. 1988;XXXVIII(3):750–65. [PubMed] [Google Scholar]
- 13. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY et al.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popkin B. An overview on the nutrition transition and its health implications: the Bellagio meeting. Public Health Nutr. 2002;5(1a):93–103. [DOI] [PubMed] [Google Scholar]
- 15. Koopman JJE, Van Bodegom D, Ziem JB, Westendorp RGJ. An emerging epidemic of noncommunicable diseases in developing populations due to a triple evolutionary mismatch. Am J Trop Med Hyg. 2016;94(6):1189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19(1):1–19. [DOI] [PubMed] [Google Scholar]
- 17. Uauy R, Kain J, Corvalan C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries?. Am J Clin Nutr. 2011;94(6):1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med. 1988;84(4):739–49. [DOI] [PubMed] [Google Scholar]
- 19. Lieberman LS. Dietary, evolutionary, and modernizing influences on the prevalence of type 2 diabetes. Annu Rev Nutr. 2003;23(1):345–77. [DOI] [PubMed] [Google Scholar]
- 20. O'Keefe JH, Cordain L. Cardiovascular disease resulting from a diet and lifestyle at odds with our paleolithic genome: how to become a 21st-century hunter-gatherer. Mayo Clin Proc. 2004;79(1):101–8. [DOI] [PubMed] [Google Scholar]
- 21. Lindeberg S. Paleolithic diets as a model for prevention and treatment of Western disease. Am J Hum Biol. 2012;24(2):110–5. [DOI] [PubMed] [Google Scholar]
- 22. Pampel FC, Denney JT, Krueger PM. Obesity, SES, and economic development: a test of the reversal hypothesis. Soc Sci Med. 2012;74(7):1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urlacher SS, Ellison PT, Sugiyama LS, Pontzer H, Eick G, Liebert MA, Cepon-Robins TJ, Gildner TE, Snodgrass JJ. Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists. Proc Natl Acad Sci U S A. 2018;115(17):E3914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bulka CM, Daviglus ML, Persky VW, Durazo-Arvizu RA, Lash JP, Elfassy T, Lee DJ, Ramos AR, Tarraf W, Argos M. Association of occupational exposures with cardiovascular disease among US Hispanics/Latinos. Heart. 2019;105(6):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. SBU. Occupational chemical exposures and cardiovascular disease: a systematic review and assessment of the social, medical and ethical aspects[Internet]. [Accessed 2020 Feb 20]. Stockholm (Sweden): SBU—Swedish Agency for Health Technology Assessment and Assessment of Social Services; 2017. Available from:https://www.sbu.se/en/publications/sbu-assesses/occupational-chemical-exposures-and-cardiovascular-disease/.
- 26. Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, Bergman RN. Inverse association between diabetes and altitude: a cross-sectional study in the adult population of the United States. Obesity. 2014;22(9):2080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okumiya K, Sakamoto R, Ishimoto Y, Kimura Y, Fukutomi E, Ishikawa M, Suwa K, Imai H, Chen W, Kato E et al.. Glucose intolerance associated with hypoxia in people living at high altitudes in the Tibetan highland. BMJ Open. 2016;6:e009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kralova Lesna I, Rychlikova J, Vavrova L, Vybiral S. Could human cold adaptation decrease the risk of cardiovascular disease?. J Therm Biol. 2015;52:192–8. [DOI] [PubMed] [Google Scholar]
- 29. Hertz-Picciotto I, Arrighi HM, Hu SW. Does arsenic exposure increase the risk for circulatory disease?. Am J Epidemiol. 2000;151(2):174–81. [DOI] [PubMed] [Google Scholar]
- 30. Instituto Nacional de Estadisticas (INE). Radiografía de género: pueblos originarios en Chile 2017, [Internet] 2018; Santiago (Chile): Instituto Nacional de Estadisticas (INE), Last accesed: April 13, 2020 Available from: https://historico-amu.ine.cl/genero/files/estadisticas/pdf/documentos/radiografia-de-genero-pueblos-originarios-chile2017.pdf. [Google Scholar]
- 31. Torrejón F. Variables geohistóricas en la evolución del sistema económico Pehuenche durante el período colonial. Universum. 2001;16, 219–36. [Google Scholar]
- 32. de Quiroga J. Memoria de los sucesos de la guerra de Chile. Santiago (Chile): Editorial Andrés Bello; 1979.. pp. 20–9. [Google Scholar]
- 33. Guevara T. Historia de la civilización de Araucania. Santiago (Chile): Imprenta Cervantes; 1908. [Google Scholar]
- 34. Aldunate C, Villagrán C. Recolectores de los bosques templados del cono sur Americano. In: Aldunate C, Villagrán C, editors. Botánica indígena de Chile. Santiago (Chile): Editorial Andrés Bello; 1992.. pp. 20–38. [Google Scholar]
- 35. Montalba R, Stephens N. Ecological change and the “Ecological Mapuche”: a historical sketch of the human ecology of Chile's Araucania region. Hum Ecol. 2014;42(4):637–43. [Google Scholar]
- 36. Mengoni G, Yacobacio H. The domestication of South American camelids: a view from the South-Central Andes. In: Zeder M, Bradley D, Emshwiller E, Smith B, editors. Documenting domestication: new genetic and archaeological paradigms. University of California Press, Berkeley and Los Angeles (California); 2006.. pp. 228–44. [Google Scholar]
- 37. Núñez L, Grosjean M, Cartajena I. Análisis secuencial de los Patrones de ocupación humana y explotación de recursos en el desierto de atacama. Chungara Rev Antropol Chil. 2010;42(October):363–91. [Google Scholar]
- 38. Fernández C, Pfaff M, Candia P, Aguilar R. Tradición y transformación de las huertas en los oasis del Desierto de Atacama. In: Ibarra T, Barreau A, Caviedes J, Pessa N, editors. Huertas familiares y comunitarias: cultivando soberanía alimentaria. Santiago (Chile): Ediciones Universidad Católica de Chile; 2019;85–93. [Google Scholar]
- 39. Villagrán C, Castro V, Sánchez G, Romo M, Latorre C, Hinojosa LF. La tradición surandina del desierto: etnobotánica del área del Salar de Atacama (Provincia de El Loa, Región de Antofagasta, Chile). Estud Atacameños Arqueol y Antropol surandinas. 1998;16:7–105. [Google Scholar]
- 40. Villagrán C, Castro V. Ciencia indígena de los Andes del Norte de Chile. Santiago (Chile): Editorial Universitaria; 2003. [Google Scholar]
- 41. Núñez L. Cultura y conflicto en los Oasis de San Pedro de Atacama. Santiago (Chile): Editorial Universitaria; 1992. [Google Scholar]
- 42. Martínez JL. Pueblos del Chañar y el Algarrobo. Los Atacamas en el siglo XVII. Santiago (Chile): DIBAM; 1998. [Google Scholar]
- 43. Santoro C, Osorio D, Ugalde P, Sepúlveda M, Cartajena I, Standen V, Gayó E, Maldonado A, Rivadeneira MM, Latorre C et al.. Cazadores recolectores y pescadores arcaicos del desierto de Atacama. Entre el Pacífico y los Andes, norte de Chile (ca. 10.000 a 3.700 años a.p). In: Falabella F, Uribe M, Sanhueza L, Aldunate C, Hidalgo J, editors. Prehistoria en Chile: desde sus primeros habitantes hasta los incas. 1st ed Santiago (Chile): Editorial Universitaria; 2016. pp. 117–80. [Google Scholar]
- 44. Santana-Sagredo F, Lee-Thorp JA, Schulting R, Uribe M. Isotopic evidence for divergent diets and mobility patterns in the Atacama Desert, Northern Chile, during the Late Intermediate Period (AD 900–1450). Am J Phys Anthropol. 2015;156(3):374–87. [DOI] [PubMed] [Google Scholar]
- 45. Pestle WJ, Torres-Rouff C, Gallardo F, Ballester B, Clarot A. Mobility and exchange among marine hunter-gatherer and agropastoralist communities in the formative period Atacama Desert. Curr Anthropol. 2015;56(1):121–33. [Google Scholar]
- 46. Gundermann H. Pastoralismo andino y transformaciones sociales en el norte de Chile. Estud Atacameños Arqueol y Antropol Surandinas. 1998;(16):293–319. [Google Scholar]
- 47. Yañez N, Molina R. Los pueblos indígenas y el agua. In: Yañez N, Molina R, editors. Las aguas indígenas en Chile. Santiago (Chile): LOM Ediciones; 2011, pp. 13–64. [Google Scholar]
- 48. Ministerio de Salud. Encuesta nacional de consumo alimentario (ENCA 2010–2011), Ministerio de Salud; Santiago (Chile); 2012. [Google Scholar]
- 49. Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health (Bangkok). 2006;14(2):66–70. [Google Scholar]
- 50. NHANES. National Health and Nutrition Examination Survey (NHANES).Anthropometry procedures manual. NHANES and Centers for Disease Control; 2013.
- 51. USDA Agricultural Research Service. What we eat in America. Usual nutrient intake from food and beverages, by gender and age (NHANES 2013–2016) [Internet] [Accessed 2019 Nov 1]. 2019. Available from: www.ars.usda.gov/nea/bhnrc/fsrg.
- 52. FAO. FAO/INFOODS food composition database for biodiversity version 4.0 – BioFoodComp4.0. User guide and datasheets[Internet]. [Accessed 2019 Jul 14]. Rome (Italy): FAO; 2017. Available from:http://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases/en/. [Google Scholar]
- 53. Schmith-Hebbel H, Pennacchiotti M, Masson L, Mella MA. Tabla de composición quimica de alimentos Chilenos, Santiago (Chile): Departamento de Ciencia de los Alimentos y Tecnologia Quimica, Universidad de Chile; 1990. p. 62. [Google Scholar]
- 54. Ministerio de Salud del Peru. Tablas peruanas de composición de alimentos. Perú. Lima, Perú.2009.; 64p.
- 55. Monteiro C, Cannon G, Levy RB, Moubarac J-C, Jaime P, Martins AP, Canella D, Louzada M, Parra D. NOVA. The star shines bright. World Nutr. 2016;7(1–3):28–38. [Google Scholar]
- 56. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. [DOI] [PubMed] [Google Scholar]
- 57. NIH, National Heart, Lung, and Blood Institute (NHLBI). Health topics | NHLBI. [Internet]; 2019. [cited 2019 Nov 22]. Available from: https://www.nhlbi.nih.gov/health-topics.
- 58. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 59. Tavares EF, Vieira-Filho JPB, Andriolo A, Sañudo A, Gimeno SGA, Franco LJ. Metabolic profile and cardiovascular risk patterns of an Indian tribe living in the Amazon region of Brazil. Hum Biol. 2003;75(1):31–46. [DOI] [PubMed] [Google Scholar]
- 60. Lagranja ES, Phojanakong P, Navarro A, Valeggia CR. Indigenous populations in transition: an evaluation of metabolic syndrome and its associated factors among the Toba of northern Argentina. Ann Hum Biol. 2015;42(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lourenço AEP, Santos RV, Orellana JDY, Coimbra CEA. Nutrition transition in Amazonia: obesity and socioeconomic change in the Suruí Indians from Brazil. Am J Hum Biol. 2008;20(5):564–71. [DOI] [PubMed] [Google Scholar]
- 62. Soares LP, Fabbro ALD, Silva AS, Sartorelli DS, Franco LF, Kuhn PC, Moises RS, Vieira-Filho JPB, Franco LJ. Prevalence of metabolic syndrome in the Brazilian Xavante indigenous population. Diabetol Metab Syndr. 2015;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kraft TS, Stieglitz J, Trumble BC, Martin M, Kaplan H, Gurven M. Nutrition transition in 2 lowland Bolivian subsistence populations. Am J Clin Nutr. 2018;108(6):1183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Valeggia CR, Snodgrass JJ. Health of indigenous peoples. Annu Rev Anthropol. 2015;44:117–35. [Google Scholar]
- 65. Mosca L, Barrett-Connor E, Kass Wenger N. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Forouzanfar MH, Afshin A, Alexander LT, Biryukov S, Brauer M, Cercy K, Charlson FJ, Cohen AJ, Dandona L, Estep K et al.. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, Frohlich B, Sutherland ML, Sutherland JD, Stieglitz J et al.. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet. 2017;389(10080):1730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cantor AR, Chan I, Baines K. From the Chacra to the Tienda: dietary delocalization in the Peruvian Andes. Food Foodways. 2018;26(3):198–222. [Google Scholar]
- 69. Orden AB, Oyhenart EE. Prevalence of overweight and obesity among Guaraní-Mbyá from Misiones, Argentina. Am J Hum Biol. 2006;18(5):590–9. [DOI] [PubMed] [Google Scholar]
- 70. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–7. [DOI] [PubMed] [Google Scholar]
- 71. Ministerio de Salud. Encuesta nacional de salud 2016–2017. Primeros resultados. [Internet] [Accessed 2019 Jul 15]. Ministerio de Salud, Gobierno de Chile;2018. Available from: www.minsal.cl/wp-content/uploads/2017/11/ENS-2016-17_PRIMEROS-RESULTADOS.pdf.
- 72. Kreisberg RA, Kasim S. Cholesterol metabolism and aging. Am J Med. 1987;82(1 Suppl 2):54–60. [DOI] [PubMed] [Google Scholar]
- 73. Schaefer EJ, Lamon-Fava S, Cohn SD, Schaefer MM, Ordovas JM, Castelli WP, Wilson PWF. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res. 1994;35(5):779–92. [PubMed] [Google Scholar]
- 74. Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodríguez-Artalejo F et al.. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. J Am Med Assoc. 2003;289(18):2363–9. [DOI] [PubMed] [Google Scholar]
- 75. Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raichlen DA, Pontzer H, Harris JA, Mabulla AZP, Marlowe FW, Snodgrass JJ, Eick G, Colette Berbesque J, Sancilio A, Wood BM. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am J Hum Biol. 2016;29(2):1–13. [DOI] [PubMed] [Google Scholar]
- 77. Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S. adults: compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40(4):454–61. [DOI] [PubMed] [Google Scholar]
- 78. Hales N, Barker D. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 79. Hales N, Barker D. The thrifty phenotype hypothesis revisited. Br Med Bull. 2001;60(1):5–20. [DOI] [PubMed] [Google Scholar]
- 80. Institute of Medicine. Dietary Reference Intakes: the essential guide to nutrient requirements (2006), vol. 55 Washington (DC): The National Academies Press; 2006. pp. 319–26. [Google Scholar]
- 81. Amigo H, Bustos P, Pino P, editors. Alimentación y nutrición de los chilenos. Santiago (Chile): Editorial Universitaria; 2018. [Google Scholar]
- 82. Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8:e020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuhnlein HV, Receveur O, Soueida R, Egeland GM.. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J Nutr. 2004;134(6):1447–53. [DOI] [PubMed] [Google Scholar]
- 84. O'Dea K. Traditional diet and food preferences of Australian aboriginal hunter-gatherers. Philos Trans R Soc Lond B Biol Sci. 1991;334(1270):233–41. [DOI] [PubMed] [Google Scholar]
- 85. Shintani TT, Hughes CK, Beckham S, O'Connor HK. Obesity and cardiovascular risk intervention through the ad libitum feeding of traditional Hawaiian diet. Am J Clin Nutr. 1991;53(6 Suppl):1647S–51S. [DOI] [PubMed] [Google Scholar]
- 86. Grivetti LE, Ogle BM. Value of traditional foods in meeting macro- and micronutrient needs: the wild plant connection. Nutr Res Rev. 2000;13(1):31–46. [DOI] [PubMed] [Google Scholar]
- 87. Milburn MP. Indigenous nutrition: using traditional food knowledge to solve contemporary health problems. Am Indian Q. 2004;28(3):411–34. [Google Scholar]
- 88. Trichopoulou A. Mediterranean diet, traditional foods, and health: evidence from the Greek EPIC cohort. Food Nutr Bull. 2007;28(2):236–40. [DOI] [PubMed] [Google Scholar]
- 89. Dewey KG. Nutrition and the commoditation of food systems in Latin America and the Caribbean. Soc Sci Med. 1989;28(5):415–24. [DOI] [PubMed] [Google Scholar]
- 90. Willett WC. Food frequency methods. In: Willett WC, editor Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998, pp. 74–100. [Google Scholar]
- 91. Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 2014;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:1–14. [DOI] [PubMed] [Google Scholar]
- 93. Gluckman PD, Hanson MA, Low FM. Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk. Philos Trans R Soc B Biol Sci. 2019;374(1770):20180109 doi:10.1098/rstb.2018.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liebert MA, Snodgrass JJ, Madimenos FC, Cepon TJ, Blackwell AD, Sugiyama LS. Implications of market integration for cardiovascular and metabolic health among an indigenous Amazonian Ecuadorian population. Ann Hum Biol. 2013;40(3):228–42. [DOI] [PubMed] [Google Scholar]
- 95. Milton K. Hunter-gatherer diets—a different perspective. Am J Clin Nutr. 2000;71:665–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.