Abstract
The aim of this research was to differentiate dapagliflozin, empagliflozin, and canagliflozin based on their capacity to inhibit sodium‐glucose cotransporter (SGLT) 1 and 2 in patients with type 2 diabetes using a previously developed quantitative systems pharmacology model of renal glucose filtration, reabsorption, and excretion. The analysis was based on pooled, mean study‐level data on 24‐hour urinary glucose excretion, average daily plasma glucose, and estimated glomerular filtration rate collected from phase I and II clinical trials of SGLT2 inhibitors. Variations in filtered glucose across clinical studies were shown to drive the apparent differences in the glucosuria dose–response relationships among the gliflozins. A normalized dose–response analysis demonstrated similarity of dapagliflozin and empagliflozin, but not canagliflozin. At approved doses, SGLT1 inhibition by canagliflozin but not dapagliflozin or empagliflozin contributed to ~ 10% of daily urinary glucose excretion.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The concentration of sodium‐glucose cotransporter (SGLT) 2 inhibitors in kidney cannot be directly measured in humans. This makes it challenging to quantify gliflozin contributions toward SGLT2 vs. SGLT1 inhibition without using in silico methods. Several studies were conducted to investigate gliflozin‐mediated renal inhibition of SGLT1/2; however, no consensus has been reached.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What is the difference in renal SGLT1/2 inhibition between gliflozins (dapagliflozin, empagliflozin, canagliflozin), considering the increased contribution of SGLT1 to renal glucose reabsorption under upstream SGLT2 inhibition conditions and an overall increase in SGLT expression in type 2 diabetes mellitus patients?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ We quantitatively described the similarities between dapagliflozin and empagliflozin vs. canagliflozin glucosuria dose response by identifying mean plasma glucose and estimated glomerular filtration rate as important covariates; subsequently, we characterized the relative roles of renal SGLT1 and SGLT2 in glucose reabsorption and drug response.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ At approved doses, canagliflozin, but not dapagliflozin or empagliflozin, inhibits renal SGLT1, which may reconcile the differences in clinical efficacy and safety among gliflozins.
Type 2 diabetes mellitus (T2DM) is the most common form of diabetic metabolic disorder (> 90% of all cases) and is characterized by abnormally high plasma glucose concentration resulting from resistance to insulin, defects in insulin secretion, or both. Sodium‐glucose cotransporter inhibitors (SGLT inhibitors; gliflozins) are a class of oral glucose lowering compounds approved for the treatment of T2DM.1, 2 Gliflozins (e.g., dapagliflozin, empagliflozin, canagliflozin) competitively block SGLT2 in the proximal tubules, preventing renal glucose reabsorption, causing glucosuria, and leading to lowered plasma glucose and subsequent reduction of glycosylated hemoglobin.3 In addition to glycemic control, each of these SGLT inhibitors has shown cardiovascular benefits in T2DM patients with increased cardiovascular risk factors through several pivotal outcomes trials: dapagliflozin DECLARE‐TIMI584 (dapagliflozin), EMPA‐REG OUTCOME2 (empagliflozin), and CANVAS5 (canagliflozin). CANVAS, unlike EMPA‐REG and DECLARE, reported a greater risk of amputation (primarily toe or metatarsal) and higher rates of bone fractures.5
Although believed to be unimportant to the clinical efficacy of gliflozins,6 human SGLT1 expression has been identified in the apical brush border of the small intestine, in the heart and skeletal muscles, and in the S3 (segment 3 of the proximal tubules, a location of SGLT1) segment of the proximal tubule of the kidney.7 Among the most commonly used SGLT inhibitors, canagliflozin is the least selective for SGLT2 and may inhibit, at approved therapeutic doses, SGLT1 in the small intestine from the luminal side,6 warranting further investigation into the differential pharmacology of the class. In a related model‐based analysis, we demonstrated that SGLT1‐mediated glucose reabsorption capacity was greater in T2DM patients than in healthy individuals.8 Furthermore, the inhibition of SGLT2 in the S1/S2 (segment 1 of the proximal tubule, a location of SGLT2/segment 2 of the proximal tubule, a location of SGLT2) proximal tubule segments raised the glucose availability for SGLT1 located downstream in the S3 segment9, 10 and resulted in a shift to much greater SGLT1‐mediated urinary glucose reabsorption. Taken together, these findings highlight the importance of SGLT1 and SGLT2 selectivity to understand the comparative actions of gliflozins in treating T2DM. The objective of this analysis was to compare the glucosuria dose response of dapagliflozin, canagliflozin, and empagliflozin in T2DM patients, focusing on the relative roles of SGLT1 and SGLT2 in the action of each compound using a quantitative systems pharmacology (QSP) model.
Methods
A QSP model of renal glucose filtration, SGLT‐mediated reabsorption, and urinary excretion was developed and reported.8 The structural model explicitly represented flux and concentrations in the S1/S2 and S3 segments of the proximal tubules. Pharmacokinetic (PK) parameters were estimated separately for dapagliflozin, canagliflozin, and empagliflozin using a physiologically based approach guided by the following knowledge:
Gliflozins are absorbed through the intestine following oral administration
Only the unbound fraction in plasma is filtered through the kidney and then excreted in the urine with the same fluid flux as that for glucose
Differences in glomerular filtration rates across clinical trials influence compound exposure in the kidney
The pooled data set included drug plasma concentration‐time profiles and 24‐hour drug excretion data in urine in healthy and T2DM subjects treated with different doses and regimens. These data were used for parameter estimation. To account for differences in glomerular filtration rates, the median estimated glomerular filtration rate (eGFR) value summarizing each SGLT inhibitor’s pool of clinical studies was used for PK parameter estimation. The unbound fraction of each compound was assumed to be filtered through the kidney and considered available to inhibit glucose reabsorption. SGLT‐mediated renal glucose reabsorption in proximal tubules was described by Michaelis‐Menten kinetics, with the inhibition being modeled as simple competition characterized by drug‐specific and SGLT‐specific inhibitory constant (K i) values. SGLT2‐specific K i values were estimated based on cumulative 24‐hour urinary glucose excretion (UGE) in healthy and T2DM subjects treated with gliflozins. SGLT1‐specific K i values were calculated using in vitro SGLT1/SGLT2 ratio values determined through an AstraZeneca SGLT‐mediated glucose uptake assay.8
Most parameters (27/44) specified in the model were defined or calculated using values from the literature, whereas the remainder (17/44) were estimated using a gradient‐driven maximum likelihood method.11 Relative contributions of SGLT1 and SGLT2 to renal glucose reabsorption were estimated from 24‐hour (UGE) data in clinical studies of healthy and T2DM subjects treated with SGLT inhibitors. Detailed information on model development, verification, and validation procedures is available in the original publication.8
A summary of the clinical data used in this analysis is presented in Supplementary Table S1 . Glucose filtration fluxes in the T2DM subjects were profiled using a pooled data set of (i) 24‐hour UGE during SGLT2 inhibitors treatment and (ii) mean daily plasma glucose (MPG) concentrations and eGFR for each treatment arm. When a trial reported only creatinine clearance (CrCl) rather than eGFR, a simple correction (eGFR = 0.9 × CrCl) was used, acknowledging that CrCl overestimates actual GFR by 10% to 20% as a result of the tubular secretion of creatinine.12, 13
The pooled data set used in this analysis included mean aggregated pharmacodynamic data from each cohort in dose‐ranging clinical studies in patients with T2DM for dapagliflozin (n = 7 treatment arms; 2.5–100 mg), canagliflozin (n = 11; 25–400 mg), and empagliflozin (n = 14; 1–100 mg).
Software
The model was developed in R, version 3.5.014 using the IQRtools package for systems pharmacology and pharmacometrics, version 0.9.2 (IntiQuan, Basel, Switzerland). Visualizations of model simulations were created with the ggplot2 package version 2.2.1.15
Model limitations
This analysis was based on publicly available, study‐level, mean aggregated data. We only considered plasma glucose averaged over 24 hours and cumulative UGE (g/day); therefore, the resulting model may not replicate plasma glucose concentrations and detailed glucosuria dynamics throughout the day. Glucose homeostasis is a highly complex physiological process; instantaneous measurements depend on multiple long‐term and short‐term factors (e.g., prandial state, wakefulness, hormonal regulation, exercise/lifestyle). Previous studies demonstrated that the oral administration of canagliflozin delays postprandial glucose absorption from the intestine and enhances glucose‐induced glucagon‐like peptide‐1 secretion,16, 17, 18 likely as a result of the SGLT1 inhibition in the small intestine. Because the model was focused on glucose distribution and gliflozin action in the kidney, other tissue sites of SGLT1 inhibition were not considered. By using daily MPG concentrations, we intended, in the model, to capture the total glucose reabsorbed or excreted during the day while minimizing detailed mechanisms required to replicate fluctuations caused by meals, activity, or other acute behaviors. Also, adaptive changes in kidney filtration rates—caused by SGLT2 treatment—were not considered in this modeling analysis because the model was built with a focus on short‐term UGE data. We refer the reader to a discussion of overall model limitations and qualifications, as presented in a companion publication.8
Results
Dose–response analysis for dapagliflozin, canagliflozin, and empagliflozin
To best study comparative potencies of gliflozins with respect to glucose reabsorption inhibition and corresponding glucosuria dose–response characteristics, data from similar clinical studies were analyzed together in a common framework. When data originate from studies in differing populations, a quantitative model can be used to explicitly account for such differences. UGE arises from the difference between glucose filtered by the kidney and the glucose reabsorbed through SGLT in the proximal tubules over a given time interval (e.g., 24 hours). Glucose filtered by the kidney, i.e., the glucose filtration flux, was estimated by the product of MPG and eGFR, each of which varies across clinical study cohorts and between development programs for the gliflozins studied (Table S1 ). Variation in the amount of glucose filtered makes the comparisons of glucosuria (the net amount of glucose loss) across studies challenging to accurately interpret for dose–response analyses. To correct for this variation and enable the fairest comparison of glucosuria dose response among gliflozins, the MPG, eGFR, and resulting glucose flux characteristics were profiled and summarized for each SGLT inhibitor in the pool of clinical studies for patients with T2DM (Figure 1). Trials of empagliflozin and canagliflozin in T2DM patients exhibited significantly greater MPG and glucose filtration flux than T2DM patients in dapagliflozin trials. Specifically, median MPG values for dapagliflozin, empagliflozin, and canagliflozin were, respectively, 7.8, 9.24, and 10.43 mM (Figure 1 a). Renal status (eGFR) was similar for all three gliflozins: the median eGFR value for dapagliflozin was 98.1 mL/minutes/1.73 m2 and 100 mL/minutes/1.73 m2 for canagliflozin and empagliflozin (Figure 1 b). Taken together, MPG and eGFR variations resulted in glucose fluxes of 45.89, 55.48, and 62.61 mmol/hour (Figure 1 c) for dapagliflozin, empagliflozin, and canagliflozin, respectively. The cohort‐specific values of eGFR and MPG were used in the estimation of the model.8
Figure 1.
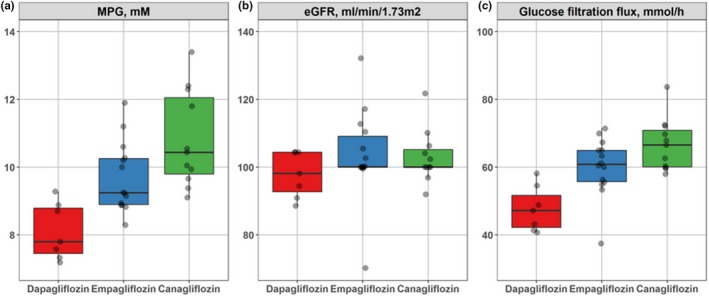
Summary of type 2 diabetes mellitus subject characteristics: (a) daily MPG, (b) eGFR, and (c) glucose kidney filtration flux in the pooled data set for dapagliflozin (red, n = 7), empagliflozin (blue, n = 14), and canagliflozin (green, n = 11). eGFR, estimated glomerular filtration rate; MPG, mean daily plasma glucose.
Dose–response relationships for dapagliflozin, empagliflozin, and canagliflozin were simulated using a mechanistic QSP model based on the following two scenarios: (i) an in situ scenario, with drug‐specific median MPG and eGFR characteristics corresponding to the actual values observed in each study (Figure 2 a), and (ii) a normalized scenario, with common median MPG (9.33 mM) and eGFR (100 mL/minutes/1.73 m2) values applied to all three drugs (Figure 2 b). For the in situ case, dapagliflozin appears to have less capacity for glucosuria than empagliflozin (at comparable doses) and canagliflozin (at higher doses); this finding, however, may be driven by differences in filtered glucose between study patient populations.
Figure 2.
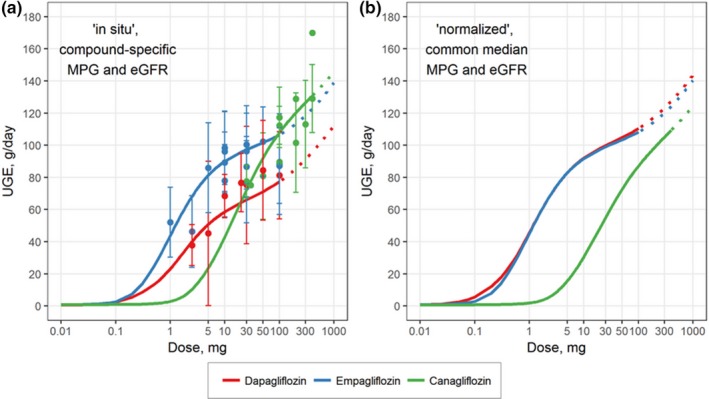
Dose response of UGE (g/day) for type 2 diabetes mellitus patients treated with dapagliflozin (red), canagliflozin (blue), or empagliflozin (green). Model‐predicted UGE dose response for (a) the in situ scenario using the median MPG and eGFR calculated from all cohorts for each drug and (b) the normalized scenario in which the dose responses are based on the median MPG and eGFR calculated based on the pooled data set for all three drugs. Symbols, clinical data; curves, model‐predicted dose response. eGFR, estimated glomerular filtration rate; MPG, mean daily plasma glucose; UGE, urinary glucose excretion.
To assess the influence of filtered glucose in clinical study results, the glucosuria dose response of each gliflozin was simulated instead using a common normalized median glucose filtration flux. The corrected relationships show near identical dose responses for dapagliflozin and empagliflozin, indicating that the apparent differences between the two are largely a function of studied patient characteristics and not intrinsic properties of the two molecules. Canagliflozin displays a similar dose–response shape although at considerably higher doses (300 mg vs. 10–25 mg), for a comparable effect.
In the normalized scenario, our quantitative model‐based analysis shows that glucosuria of the gliflozins differed in the following way: UGE values for 5 mg and 10 mg dapagliflozin were 82.3 and 91.4 g/day; for 10 mg and 25 mg empagliflozin, 91.1 and 98.4 g/day; and for 100 mg and 300 mg canagliflozin, 86.4 and 104.3 g, respectively.
Simulated SGLT1‐dependent and SGLT2‐dependent glucose reabsorption inhibition in T2DM subjects
The QSP model used in this analysis describes mechanistic differences in SGLT2‐mediated and SGLT1‐mediated renal glucose reabsorption and glucosuria across healthy volunteers and T2DM patients. The results from our previously published analysis indicate higher renal glucose reabsorption in T2DM patients vs. healthy subjects, associated with 54% and 28% greater maximum reabsorption rate (Vmax) for SGLT1 and SGLT2, respectively.8 Importantly, SGLT2 inhibition in the S1/S2 segments results in considerably more glucose available for SGLT1‐mediated reabsorption, downstream in the S3 tubule segment, and thus makes any corresponding SGLT1 inhibition increasingly important.8 Model‐predicted drug distributions in plasma and kidney (S1/S2 and S3 segments) and the resultant glucose reabsorption fluxes (through SGLT2 and SGLT1, respectively) were computed for approved doses of 10 mg dapagliflozin, 25 mg empagliflozin and 300 mg canagliflozin (Figure 3). Key PK characteristics for each treatment are summarized, along with the ratio of free drug concentrations (a model‐predicted peak concentration of the compound (Cmax) or a model‐predicted average concentration of the compound (Cavg) to K i important for that segment: K i,SGLT2 S1/S2 and K i,SGLT1 for S3 (Table 1).
Figure 3.
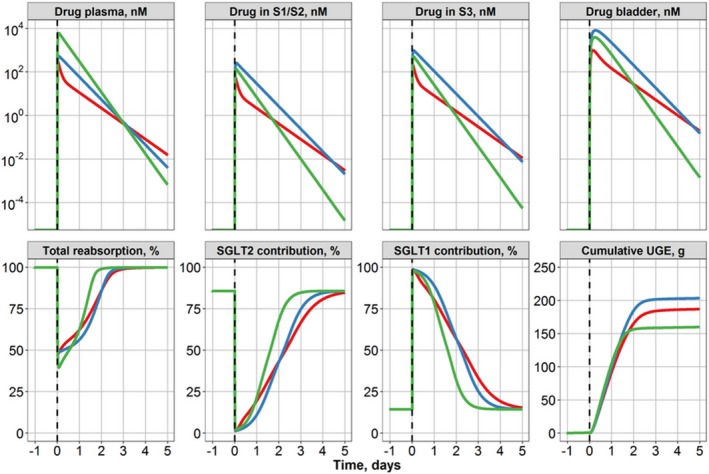
Model‐predicted drug concentration profiles in plasma, proximal convoluted and straight tubules, and bladder in a typical type 2 diabetes mellitus patient (estimated glomerular filtration rate = 100 mL/minutes/1.73 m2, mean daily plasma glucose = 9.33 mM; top row) and model‐predicted total reabsorption rate and individual contributions from SGLT1 and SGLT2 transporters to renal reabsorption and placebo‐normalized cumulative urinary glucose after a single dose of 10 mg dapagliflozin (red), 25 mg empagliflozin (blue), or 300 mg canagliflozin (green) (bottom row). S1, segment 1 of the proximal tubule, a location of SGLT2; S2, segment 2 of the proximal tubule, a location of SGLT2; S3, segment 3 of the proximal tubules, a location of SGLT1; SGLT1, sodium‐glucose cotransporter 1; SGLT2, sodium‐glucose cotransporter 2; UGE, urinary glucose excretion.
Table 1.
Estimated Ki for SGLT2 and SGLT1 transporters,8 the ratio between the proximal tubule segment concentration of a gliflozin and respective SGLT Ki, and model‐predicted peak and average compound concentration in the plasma, kidney (S1/S2 and S3), and bladder
| Means of estimation | Dapagliflozin (10 mg) | Empagliflozin (25 mg) | Canagliflozin (300 mg) | |
|---|---|---|---|---|
| Ki,SGLT2 (nM) | Fitted | 0.031 | 0.195 | 0.111 |
| Ki,SGLT1 (nM) | Calculated | 36.35 | 243.03 | 17.55 |
| SGLT2:SGLT1 | Calculated | 1,200‐fold | 1,300‐fold | 160‐fold |
| Cmax (nM) | ||||
| Plasma | Predicted | 275 | 533 | 6,156 |
| S1–S2 | 52.5 | 260 | 137 | |
| S3 | 196 | 976 | 513 | |
| Bladder | 987 | 8,172 | 3,922 | |
| Cmax,S1S2:Ki,SGLT2 | Calculated | 1,700‐fold | 1,300‐fold | 1,200‐fold |
| Cmax,S3:Ki,SGLT1 | 5.4‐fold | 4.0‐fold | 29‐fold | |
| Cavg,0–24 (nM) | ||||
| Plasma | Predicted | 51.7 | 229 | 2109 |
| S1/S2 | 9.87 | 112 | 46.9 | |
| S3 | 37.0 | 420 | 176 | |
| Bladder | 462 | 5164 | 2205 | |
| Cavg,0–24,S1S2:Ki,SGLT2 | Calculated | 320‐fold | 570‐fold | 420‐fold |
| Cavg,0–24,S3:Ki,SGLT1 | 1.0‐fold | 1.7‐fold | 10‐fold | |
Fold calculations were rounded to two decimals.
Cavg, 0‐24, a model‐predicted average concentration of the compound during 24‐hours period after dose administration; Cmax, a model‐predicted peak concentration of the compound; K i, inhibitory constant; S1, segment 1 of the proximal tubule, a location of SGLT2; S2, segment 2 of the proximal tubule, a location of SGLT2; S3, segment 3 of the proximal tubules, a location of SGLT1; SGLT1, sodium‐glucose cotransporter 1; SGLT2, sodium‐glucose cotransporter 2.
Simulations clearly indicate that a single dose for each of the gliflozins (at the highest approved dose) results in both a Cmax and a model‐predicted average concentration of the compound during 24‐hours period after dose administration (Cavg,0–24) within the S1/S2 segment that almost completely inhibits SGLT2‐driven reabsorption (Table 1; Cmax,S1S2:K i,SGLT2> 1,000, Cavg,0‐24,S1S2:K i,SGLT2> 300). However, total glucose reabsorption via both SGLT1 and SGLT2 transporters was only decreased by 50% for dapagliflozin and empagliflozin, whereas canagliflozin exhibited a 60% reduction. We propose that such a difference arises from a shift to SGLT1‐mediated reabsorption and a greater inhibition of SGLT1 by canagliflozin, which has a considerably higher exposure (10‐fold relative to K i,SGLT1) in the S3 segment vs. the other two selective SGLT2 inhibitors (Table 1).
UGE was computed for the first 24 hours (24‐hour UGE) and over several days (cumulative UGE) after a single dose of the gliflozins for a typical T2DM subject from the data set (eGFR = 100 mL/minutes/1.73 m2, MPG = 9.33 mM). The 24‐hour UGE was greater after canagliflozin administration (104 g) vs. dapagliflozin (92 g) or empagliflozin (98 g). Conversely, cumulative UGE (0–5 days; Figure 3) arising from a single dose of each gliflozin was lowest for canagliflozin (157 g) vs. dapagliflozin (184 g) and empagliflozin (200 g); it is unclear, however, whether such differences would be meaningful in a clinical setting of a repeated daily dosing regimen.
Gliflozin differentiation based on SGLT2:SGLT1 selectivity
To evaluate the relative importance of SGLT1 transporter inhibition in the kidneys by the three gliflozins, model‐predicted concentrations of dapagliflozin, empagliflozin, and canagliflozin in the S3 proximal tubule segments were computed and compared with the corresponding K i,SGLT1 (Figure 4 a). At 24 hours following dosing, average dapagliflozin concentrations in the S3 segment remained below (0.5‐fold, 5 mg) or equal to (1.0‐fold, 10 mg) the K i,SGLT1 values. In contrast, 25 mg empagliflozin and 300 mg canagliflozin led to drug concentrations in the S3 segment exceeding their respective K i,SGLT1 values, 1.7‐fold and 10‐fold, respectively. The total reabsorption flux through each transporter type was calculated to determine the fractional contribution of SGLT1 inhibition toward total glucosuria (Figure 4 b). Owing to its greater Cavg,S3:K i,SGLT1 ratio, SGLT1 inhibition by canagliflozin contributed to approximately 10% of daily urinary glucose excretion, whereas for dapagliflozin and empagliflozin, SGLT1 contributed much less (only 1.59% and 2.41%, respectively).
Figure 4.
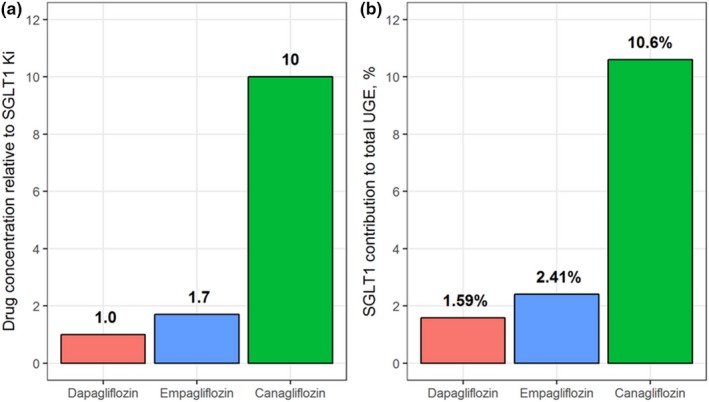
Analysis of SGLT1 inhibition by gliflozins. (a) comparison of dapagliflozin (red), empagliflozin (blue), and canagliflozin (green) drug concentrations in the S3 segment of the proximal tubules and fold‐change relative to model‐estimated K i for SGLT1 inhibition. (b) Contribution of SGLT1 inhibition to cumulative 24‐hour UGE for each drug. K i, inhibitory constant; S3, segment 3 of the proximal tubules, a location of SGLT1; SGLT1, sodium‐glucose cotransporter 1; SGLT2, sodium‐glucose cotransporter 2; UGE, urinary glucose excretion.
Discussion
Effect of T2DM patient baseline characteristics on UGE
Populations of T2DM patients studied in clinical trials exhibit significant differences in baseline characteristics (daily MPG and eGFR), which influences how much glucose is filtered by the kidneys. Not surprisingly, the amount of glucose presented for reabsorption in the proximal tubules and, therefore, the amount escaping to the urine (UGE) are also dependent on patients’ MPG and eGFR. An analysis of the pooled data set showed greater filtered glucose in the empagliflozin and canagliflozin study populations than those receiving dapagliflozin. Correcting for filtered glucose across clinical studies resolved apparent differences in the glucosuria dose–response relationships among dapagliflozin and empagliflozin, demonstrating similar efficacies in terms of highly selective SGLT2 inhibitors. Canagliflozin remained distinct, likely because of its bioavailability, PK, and lesser selectivity for SGLT2 vs. SGLT1. This finding confirms the importance of MPG and eGFR alignment in the interpretation of existing and new UGE clinical findings and the dose–response relationships reported for the gliflozin class.
Potential inhibitory effect of gliflozins on SGLT1 in proximal tubules
The proximal tubular luminal concentrations of the SGLT2 inhibitors cannot be directly measured in humans, making it challenging to quantify their potential to inhibit renal SGLT1 without the use of in silico methods. Some previous studies suggested that systemic steady‐state exposure of unbound canagliflozin, despite having lesser selectivity for SGLT2 vs. SGLT1 than the other gliflozins, would still not be sufficiently high to impact SGLT1‐mediated reabsorption anywhere other than in the small intestine6, 19 as a result of substantial plasma protein binding (~ 99%). According to Mori et al.,19 the renal SGLT1 inhibition capacity estimated for both canagliflozin and dapagliflozin was less than 1%. In their work, the authors developed a detailed physiologically‐based PK (PBPK) model and combined it with a pharmacodynamic (PD) model of renal glucose reabsorption, as reported by Lu et al.,20 to predict gliflozin concentrations in the small intestine and renal proximal tubules and to subsequently apply the model to predict UGE rates in diabetic patients. However, the modeling approach used by Lu et al. to describe glucose filtration, reabsorption, and excretion is appropriate for scenarios in which renal glucose recovery does not affect plasma glucose levels, such as the following:
Experimental procedures with fixed plasma glucose levels (e.g., stepwise glycemic clamp).
Subjects with normal glucose tolerance who can efficiently dispose of the absorbed mass to maintain plasma glucose constant at the fasting state.20 As such, we believe that considering acute glycemic changes as well as a drop in glucose levels during the treatment period might increase the accuracy of model‐based predictions of the PBPK/PD model and explain the discrepancy between these modeling exercises, given that the incorporation of MPG and eGFR was shown to be important for interpretation of UGE dose–response relationships reported for the SGLT2 inhibitors.
SGLT2 inhibition amplifies the importance of SGLT1‐mediated renal glucose reabsorption
The QSP model used in this analysis demonstrated, here and in a previous publication,8 that SGLT1‐mediated renal glucose reabsorption is increased when SGLT2 was inhibited, particularly in T2DM patients, where both transporters are further expressed relative to healthy subjects. Analyses of drug distributions in kidney proximal tubules showed strong SGLT2 inhibition in the S1/S2 segment by all three gliflozins, whereas canagliflozin alone exhibited sustained exposures, 10‐fold above the SGLT1 K i in the S3 segment. SGLT1 inhibition by canagliflozin promotes additional glucose excretion in T2DM subjects, totaling 10% of the overall glucosuria response. Neither dapagliflozin nor empagliflozin showed significant contributions of SGLT1 inhibition toward cumulative UGE. It is plausible that renal SGLT1 inhibition by canagliflozin at approved doses contributes to its safety profile, differing from other SGLT inhibitors.
The goal of this analysis was to quantify the effects of SGLT2 inhibitors on renal glucose reabsorption, whereas the challenge of characterizing nonspecific SGLT1 inhibition in other tissues such as the intestine, heart, or skeletal muscles, as well as the evaluation of a potential role for SGLT1 inhibition in serious adverse events (e.g., amputation and bone fracture) associated with canagliflozin5 but not dapagliflozin or empagliflozin,21 are yet to be addressed.
Funding
This study was supported by AstraZeneca.
Conflict of Interest
L.C., W.T., P.J.G., H.P.S., S.J., G.H., D.W.B., and R.C.P. are employees of AstraZeneca LP, the manufacturer of dapagliflozin. V.S., T.Y., and K.P. are employees of M&S Decisions LLC, a modeling research consultancy contracting with AstraZeneca.
Author Contributions
V.S., T.Y., L.C., W.T., P.J.G., S.J., K.P., G.H., D.W.B., and R.C.P. wrote the manuscript. V.S., T.Y., L.C., P.J.G., K.P., G.H., D.W.B., and R.C.P. designed the research. V.S., T.Y., and R.C.P. performed the research. V.S., T.Y., L.C., W.T., P.J.G., S.J., K.P., G.H., D.W.B., and R.C.P. analyzed the data.
Supporting information
Figure S1. Pharmacokinetic model diagnostic plots: observed vs. predicted. Plasma drug concentrations (dapagliflozin and canagliflozin concentrations in ng/mL, empagliflozin concentrations in nM; (top row); 24‐hour drug excretion in urine in mg (bottom row).
Figure S2. Dapagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Figure S3. Canagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Figure S4. Empagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Table S1. Pooled type 2 diabetes mellitus data set of experimental data.
Table S2. Drug‐specific and common median mean daily plasma glucose and estimated glomerular filtration rate characteristics.
Table S3. Ordinary differential equations' system and rate laws.
SGLT Model Code. File with model code (SGLT_model_code.txt).
Script_Fitting_Simulation. File with R script for model fitting and simulations (Script_Fitting_Simulation.R).
References
- 1. Zhang, L. , Feng, Y. , List, J. , Kasichayanula, S. & Pfister, M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes. Metab. 12, 510–516 (2010). [DOI] [PubMed] [Google Scholar]
- 2. Zinman, B. et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015). [DOI] [PubMed] [Google Scholar]
- 3. van Bommel, E.J.M. , Muskiet, M.H.A. , Tonneijck, L. , Kramer, M.H.H. , Nieuwdorp, M. & van Raalte, D.H. SGLT2 inhibition in the diabetic kidney—from mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 12, 700–710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiviott, S.D. et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019). [DOI] [PubMed] [Google Scholar]
- 5. Neal, B. et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017). [DOI] [PubMed] [Google Scholar]
- 6. Ohgaki, R. et al Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2. J. Pharmacol. Exp. Ther. 358, 94–102 (2016). [DOI] [PubMed] [Google Scholar]
- 7. Song, P. , Onishi, A. , Koepsell, H. & Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets. 20, 1109–1125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yakovleva, T. et al Comparison of the urinary glucose excretion contributions of SGLT2 and SGLT1: a quantitative systems pharmacology analysis in healthy individuals and patients with type 2 diabetes treated with SGLT2 inhibitors. Diabetes Obes. Metab. 21, 2684–2693 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Hummel, C.S. , Lu, C. , Loo, D.D.F. , Hirayama, B.A. , Voss, A.A. & Wright, E.M. Glucose transport by human renal Na+/D‐glucose cotransporters SGLT1 and SGLT2. AJP Cell Physiol. 300, C14–C21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown, G.K. Glucose transporters: Structure, function and consequences of deficiency. J. Inherit Metab. Dis. 23, 237–246 (2000). [DOI] [PubMed] [Google Scholar]
- 11. Raue, A. et al Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics 25, 1923–1929 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Ricos, C. , Alvarez, V. & Jimenez, C.V. Biological variation in urine samples used for analyte measurements. Clin. Chem. 40, 472–477 (1994). [PubMed] [Google Scholar]
- 13. Payne, R.B. Creatinine clearance: a redundant clinical investigation. Ann. Clin. Biochem. 23, 243–250 (1986). [DOI] [PubMed] [Google Scholar]
- 14. R Core Team . R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). <https://www.R-project.org/>. [Google Scholar]
- 15. Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Springer, New York, 2009). [Google Scholar]
- 16. Kinoshita, S. & Kondo, K. Evaluation of pharmacokinetic and pharmacodynamic interactions of canagliflozin and teneligliptin in Japanese healthy male volunteers. Expert Opin. Drug Metab. Toxicol. 11, 7–14 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Polidori, D. , Sha, S. , Ghosh, A. , Plum‐Mörschel, L. , Heise, T. & Rothenberg, P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin‐treated subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 98, E867–E871 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sha, S. et al Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double‐blind, crossover study. Diabetes Obes. Metab. 17, 188–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori, K. , Saito, R. , Nakamaru, Y. , Shimizu, M. & Yamazaki, H. Physiologically based pharmacokinetic‐pharmacodynamic modeling to predict concentrations and actions of sodium‐dependent glucose transporter 2 inhibitor canagliflozin in human intestines and renal tubules: PBPK/PD modeling of canagliflozin. Biopharm. Drug Dispos. 37, 491–506 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Lu, Y. , Griffen, S.C. , Boulton, D.W. & Leil, T.A. Use of systems pharmacology modeling to elucidate the operating characteristics of SGLT1 and SGLT2 in renal glucose reabsorption in humans. Front. Pharmacol. 5 (2014). 10.3389/fphar.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabbour, S. , Seufert, J. , Scheen, A. , Bailey, C.J. , Karup, C. & Langkilde, A.M. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes. Metab. 20, 620–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pharmacokinetic model diagnostic plots: observed vs. predicted. Plasma drug concentrations (dapagliflozin and canagliflozin concentrations in ng/mL, empagliflozin concentrations in nM; (top row); 24‐hour drug excretion in urine in mg (bottom row).
Figure S2. Dapagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Figure S3. Canagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Figure S4. Empagliflozin plasma concentration vs. time profiles. Number on plot indicates the number of a particular treatment arm in the pooled pharmacokinetic data set. Symbols, clinical data; curves, model‐predicted plasma drug concentrations.
Table S1. Pooled type 2 diabetes mellitus data set of experimental data.
Table S2. Drug‐specific and common median mean daily plasma glucose and estimated glomerular filtration rate characteristics.
Table S3. Ordinary differential equations' system and rate laws.
SGLT Model Code. File with model code (SGLT_model_code.txt).
Script_Fitting_Simulation. File with R script for model fitting and simulations (Script_Fitting_Simulation.R).


