Abstract
Recent demonstrations of human brain organoid transplantation in rodents have accentuated ethical concerns associated with these entities, especially as they relate to potential “humanization” of host animals. Consideration of established scientific principles can help define the realistic range of expected outcomes in such transplantation studies. This practical approach suggests that augmentation of discrete brain functions in transplant hosts is a more relevant ethical question in the near term than the possibility of “conscious” chimeric animals. We hope that this framework contributes to a balanced approach for proceeding with studies involving brain organoid transplantation and other forms of human-animal brain chimeras.
The advent of brain organoids derived from human pluripotent stem cells has generated an avalanche of enthusiasm and interest in the neurobiological and biomedical communities. These entities emulate normal neurodevelopment, producing brain-specific architecture such as neural progenitor zones and rudimentary cortical layers through the principles of self-organization (Lancaster et al., 2013; Paşca et al., 2015; Qian et al., 2016). Because of their recapitulation of certain brain structures, brain organoids could enable the study of human neurodevelopment and cerebral disorders in novel, previously unimaginable ways (Di Lullo and Kriegstein, 2017; Kelava and Lancaster, 2016; Kretzschmar and Clevers, 2016; Lancaster and Knoblich, 2014). One example is the role played by brain organoids in defining the pathogenic mechanisms of Zika virus during the recent global health emergency (Garcez et al., 2016; Ming et al., 2016; Qian et al., 2016) Moreover, there are several potential clinical applications of brain organoids, including personalized models of pathogenesis, therapeutic screening, and repair of damaged cerebral circuitry (Chen et al., 2019).
Although their scientific and translational promise is great, brain organoids also have sparked intense debate among academics (Farahany et al., 2018) and in the press (Begley, 2017; Moody, 2017) regarding the potential ethical challenges they pose. These concerns stem from the ethical and moral implications of generating and using neural tissues that are increasingly similar to the human brain, the source of the higher-order cognitive capacities that are most often equated with being human. From a purely scientific perspective, it may be tempting to dismiss the ethical considerations of brain organoids as not currently relevant. After all, as we will discuss below, the likelihood that current iterations of organoids or animals transplanted with these organoids can develop more complex cognitive abilities is minute. However, engagement of neurobiologists and neuroscientists in brain organoid ethics is important for several reasons. Scientists should help develop the appropriate frameworks for these ethical discussions to prevent faulty conclusions from being drawn, especially in the realm of public policy. There is also the need for scientists to clearly articulate the scientific and translational benefits of brain organoids to society so that any ethical or moral risks can be properly weighed. Finally, there is wisdom in understanding the relevant ethical considerations to avoid potential pitfalls that may arise as organoid technology advances.
The emerging ethical debate regarding brain organoids centers on issues pertaining to organoids themselves, animals subjected to transplantation procedures, and socio-legal governance of organoid generation and storage. These three areas were broadly discussed in a recent summary of two workshops supported by the Duke Initiative for Science and Society and the NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative (Farahany et al., 2018). Other commentaries on brain organoid ethics have also begun to appear in the literature (Lavazza and Massimini, 2018a, 2018b; Munsie et al., 2017; Shepherd, 2018). In this article, we focus on transplantation of brain organoids into animal hosts, which has been the subject of a number of recent publications (Daviaud et al., 2018; Mansour et al., 2018) and scientific abstracts (D. Jgamadze, 2017, Soc. Neurosci., abstract; O. Revah, 2018, Soc. Neurosci., abstract). We first summarize recent progress in the field of brain organoids and provide our perspective on areas of advancement on the horizon. Within this context as well as that of prior literature on the ethics of human-animal brain chimeras, we then evaluate the scientific possibility of enhancing host animal brain function using brain organoid transplantation. Separately, we discuss some of the ethical ramifications of enhanced animals if their creation should become feasible. We hope that this discussion of pertinent ethical issues and associated scientific frameworks facilitates participation of the scientific community in the public discourse on brain organoid development and transplantation.
Current State of Brain Organoid Technology
The modern era of human brain organoids began with the development of whole-brain organoids (Lancaster et al., 2013) and the demonstration that stratified cortical epithelium could arise through self-organization of human pluripotent stem cells (Kadoshima et al., 2013). In the latter case, some of the temporal and spatial features of neocorticogenesis were recapitulated. Subsequent studies refined these aspects of normal neurodevelopment in region-specific organoids, resulting in rudimentary segregation of superficial and deep cortical layers (Paşca et al., 2015; Qian et al., 2016), generation of a distinct layer of outer radial glial cells (Qian et al., 2016), and expansion of cortical folds (Li et al., 2017). Glial populations were observed at later time points, including astrocytes (Dezonne et al., 2017; Paşca et al., 2015; Qian et al., 2016; Sloan et al., 2017) and oligodendrocytes (Matsui et al., 2018). Integration of interneurons into these cortical organoids has been studied through fusion of dorsalized glutamatergic organoids with ventralized organoids containing GABAergic neurons (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017). Other brain region-specific organoids that model the midbrain (Jo et al., 2016; Monzel et al., 2017; Qian et al., 2016), hippocampus (Sakaguchi et al., 2015), pituitary gland (Ozone et al., 2016), hypothalamus (Qian et al., 2016), and cerebellum (Muguruma et al., 2015) have also been reported.
Several approaches have been used to assess the similarity of brain organoids to the human brain. Genetic (Camp et al., 2015; Paşca et al., 2015; Qian et al., 2016), epigenetic (Luo et al., 2016), and epitranscriptomic (Yoon et al., 2017) analyses indicate a high degree of concordance between brain organoids and the human fetal cortex through the second trimester. However, brain organoids are distinctly different from the human brain in several ways. Their maximal size is on the order of millimeters because of the limits of nutrient, gas, and waste exchange via diffusion, and organoids lack endothelial cells, microglia, and other cell types that contribute to the microenvironment of the brain. Furthermore, even within whole-brain organoids, organized structural nodes and the white matter connections among them are absent.
Data are emerging regarding the electrical activity of brain organoids, but our understanding of their neurophysiology is still underdeveloped. Slow neuronal calcium waves, post-synaptic potentials, and induced action potentials have been reported in organoids (Lancaster et al., 2013; Paşca et al., 2015; Qian et al., 2016). Spontaneous action potentials require extended periods of time to appear (Quadrato et al., 2017), and addition of GABAergic neurons to glutamatergic populations promotes synaptic inputs and more robust induced action potentials (Birey et al., 2017). There is some evidence to suggest that brain organoids form local neural networks. Light stimulation of photosensitive cells in brain organoids attenuates the activity of a sub-population of neurons, and statistical analyses of late-organoid activity indicate the interdependency of neural activity (Quadrato et al., 2017). So far, there has been no direct evidence of communication across multiple network nodes in these entities or computational processing required to generate more complex information.
Generating More Mature and Complex Brain Organoids
Brain organoids have facilitated the study of human neurodevelopment, modeling of congenital brain conditions and neuropsychiatric disorders, and exploration of differences in brain formation among species (Chen et al., 2019; Qian et al., 2019). However, current iterations of these organoids are still imperfect facsimiles of the human brain. Although they faithfully recapitulate certain aspects of cerebral architecture, others, such as the layers of the cerebral cortex, remain rudimentary. Related to this incomplete structure is the relative transcriptomic immaturity of the organoids, which approximate, at best, the late second trimester of human fetal development (Camp et al., 2015; Paşca et al., 2015; Qian et al., 2016; Quadrato et al., 2017; Sloan et al., 2017; Velasco et al., 2019). It is also the case that current brain organoids are comprised of multiple redundant units rather than being a unified organ and that higher-order features, including gyrification and white matter tracts, are missing. These deficiencies have motivated a major push to engineer next-generation organoids with a greater degree of maturity and complexity. Modeling later stages of brain development is especially relevant for cerebral disorders that manifest later in life, such as schizophrenia (young adulthood) and neuro-degenerative diseases (late adulthood). The following sections will discuss areas in need of progress to achieve the objective of “better” brain organoids that expand our capacity to study human cerebral development and disease. Importantly, organoids that more accurately recapitulate the brain provide scientific context for the ethical discussion below.
Overcoming the Limits of Diffusion
One of the fundamental challenges in generating more mature brain organoids that reflect later stages of development is the constraint imposed by diffusion. Organoids can grow up to 4 mm in size using orbital shakers and high-oxygen incubators (Kadoshima et al., 2013; Lancaster et al., 2017; Qian et al., 2016), but a necrotic core inevitably develops because of inadequate nutrient, gas, and waste exchange. Neural progenitors, which preferentially populate the interior of the organoid and have high metabolic demands, are therefore lost, resulting in arrest of further organoid development.
Several strategies for overcoming this obstacle have been investigated. Decreasing the thickness of organoid tissue to improve mass transport can be achieved using classic techniques for maintaining organotypic slice cultures, such as vibratome sectioning and growth at the air-liquid interface (Giandomenico et al., 2019) or physically constraining growth in the z dimension using an on-chip method (Karzbrun et al., 2018). These approaches have resulted in improved neuronal survival, axon growth and alignment, and surface wrinkling reminiscent of cortical folds. However, it should be noted that data showing that these organoids are more mature than previous versions are so far lacking. Introducing a perfusion system into brain organoids is an alternative strategy that bypasses the diffusion problem altogether. Incorporation of endothelial cells into brain organoids leads to primitive vascular networks that are likely not functional (Pham et al., 2018). More advanced vascular networks can be derived from biomaterial casting techniques (Miller et al., 2012), 3D printing (Mirabella et al., 2017), and human blood vessel organoids (Wimmer et al., 2019). Microfluidic devices will likely be needed in vitro to provide the pressure gradients necessary to drive adequate nutrient and oxygen delivery. Efforts are also underway to utilize the in vivo environment to support brain organoid perfusion after transplantation (see below). In this case, host animals are essentially used as bioreactors to generate new vasculature for organoids as a means of maintaining their growth and maturation.
Modeling Interactions among Different Parts of the Brain with Organoids
Most recent work with brain organoids has been directed toward region-specific organoids as opposed to whole-brain organoids. This shift in emphasis has occurred in large part because of the considerable variability accompanying the unguided protocols that produce whole-brain organoids (Qian et al., 2019; Quadrato et al., 2017). The stochastic nature of stem cell differentiation in these protocols results in unpredictable heterogeneity in both the cellular populations derived and their organization, limiting their use in quantitative studies. Although tighter control of the differentiation process with external factors reduces heterogeneity in region-specific organoids, their ability to model interactions among different parts of the brain is inherently restricted. Reintroducing this complexity to brain organoids is crucial if they are to serve as viable alternatives to in vivo systems for answering questions at the level of systems neuroscience, which are often central to understanding neurocognitive and neuropsychiatric disorders.
Effectively modeling multiple regions of the brain with brain organoids could be accomplished in a number of ways. The concept of “assembloids,” the fusion of two organoids, was introduced initially as a means of studying interneuron migration from the ganglionic eminences to the cerebral cortex (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017). More recently, this approach has been applied to other aspects of the brain, including cortico-thalamic connections (Xiang et al., 2019). In this assembloid model, reciprocal connections formed between cortical and thalamic organoids with an apparent increase in the firing rate of thalamic neurons in fused compared with unfused organoids. One downside of assembloids is the lack of long-range axon tracts that connect the different organoids, the equivalent of white matter pathways in the brain. This deficiency likely precludes assembloids from recreating the small-world topology of the brain, a graph theory concept defined by highly intra-connected modules with few connections between modules that helps characterize the network functionality of the brain (Bassett and Bullmore, 2017).
Modularity could potentially be incorporated into brain organoid systems via tissue engineering techniques. Hydrogel micro-columns promote the directional outgrowth of robust axonal processes from cortical organoids, effectively resulting in a “connectome unit,” two neuronal clusters spanned by an intervening axon tract (Cullen et al., 2018). This in vitro platform could expedite the investigation of information encoding, transmission, and decoding across axon pathways, processes that are disrupted or aberrant in many brain diseases and disorders. Ultimately, assembloids and organoid connectome units are placeholders for more advanced whole-brain organoids, although these minimal circuit models may have advantages over whole-brain organoids when limited processing power is an ethical goal. Overcoming the heterogeneity produced by unguided differentiation protocols may require integration of morphogen gradients and other innovations (Jabaudon and Lancaster, 2018; Qian et al., 2019).
Understanding the Cellular and Network Activity of Brain Organoids
As described previously, the neural activity of brain organoids is beginning to be elucidated, but much is still unknown. Spontaneous action potentials are observed in brain organoids, but the timeline for their appearance and evolution remains undefined. Another unknown is the degree of complexity in neural activity that can be achieved in brain organoids. This question may be tied to the development of more mature organoids because most human neurons in the late second trimester are immature, and only specific subsets of neurons are capable of spontaneous firing (Zhong et al., 2018).
An article published in this issue of Cell Stem Cell begins to explore some of the network dynamics that may be present in cortical organoids (Trujillo et al., 2019). This study identified synchronized bursting of neural activity in organoids adhered to and dispersed over a planar multielectrode array surface and reported the purported presence of oscillatory activity. These interesting results represent a preliminary step toward comprehending how ensembles of neurons in organoids function, but they should be interpreted with caution. It is not clear how the activity recorded from these organoids correlates to normal oscillations found in the brain, especially “non-oscillatory gamma activity.” Furthermore, the machine learning-based classifier that was used to compare activity from the organoids and brains of pre-term neonates was trained in a highly selective manner, which may have predisposed it to identify similarities. It must also be noted that oscillatory activity alone is unlikely to be sufficient to produce complex brain function, as evidenced by the observation that a computational model composed of just hundreds of neurons can generate spontaneous gamma oscillations (Wang and Buzsáki, 1996).
As these topics are unraveled, opportunities will arise to examine how neural activity and circuitry in brain organoids can be modulated and modified for specific ends. Identifying the factors relevant to these manipulations will likely provide more control over brain organoids for both basic and translational pursuits, allowing them to be utilized to answer more complex questions. Ultimately, a better understanding of brain organoid activity will be essential to optimize their use as models of neurodevelopment and cerebral diseases and substrates for brain repair.
Ethical Considerations for Brain Organoid Transplantation
As brain organoids capture more of the complexities of the mature human brain, they will become ever more valuable research and clinical tools. However, the very reasons that make advanced brain organoids attractive to scientists and clinicians will provoke escalating ethical concerns. Brain organoids are already conjuring the image of the so-called “brain in a vat,” a disembodied organ capable of perception and thought that is imprisoned in a dehumanizing existence (Lavazza and Massimini, 2018b; Shepherd, 2018). Such a scenario is unlikely to materialize in the near future for a variety of reasons, one being the lack of sophisticated sensory inputs into developing brain organoids that may be necessary for the iterative learning and conditioning that cultivate cognitive processes. One currently plausible situation in which brain organoids could be linked to fully formed sensory (and motor) systems is transplantation into an animal’s brain. This intriguing scenario will be the subject of the remainder of this article.
Mansour et al. (2018) recently reported successful transplantation of whole-brain organoids into the adult mouse brain with evidence of anatomic and functional integration. Organoid grafts sent robust axonal projections into the host brain with putative synapse formation, and optogenetic stimulation of the graft evoked field potential responses in the adjacent brain. Other groups have confirmed the feasibility of whole-brain and cortical organoid transplantation (Daviaud et al., 2018; D. Jgamadze, 2017, Soc. Neurosci., abstract; O. Revah, 2018, Soc. Neurosci., abstract). This line of research could yield substantial benefits from both a basic science and translational perspective. Vascularization of the graft by the in vivo environment enables organoid growth that is not currently feasible in vitro, which could lead to more representative models of human neurodevelopment and neurological disorders (Chen et al., 2019). Moreover, a graft that emulates brain architecture is intuitively appealing for repairing the brain after injury (Chen et al., 2016). The strategy of transplanting structured neural tissues builds on the premise of current stem-cell-based treatments, some of which have already reached human clinical trials for stroke (Kalladka et al., 2016; Steinberg et al., 2016), traumatic brain injury (Sanbio’s Study of Modified Stem Cells in Traumatic Brain Injury [STEMTRA] trial, NCT02416492), and Parkinson’s disease (Barker et al., 2017).
Despite these potential advantages, the idea of brain organoid transplantation has evoked a measure of unease (Begley, 2017; Moody, 2017). One of the primary concerns centers on the possibility that animals transplanted with human brain organoids would become more “human.” In unpacking this issue, we first discuss where brain organoid transplantation fits in the broader concept of human-animal brain chimeras and summarize current guidelines for this work. We then revisit the ethical principles that explain what the above assertion could mean and argue that assessing the implications of enhancing specific brain functions is a more practically productive position. Subsequently, we use available scientific frameworks to explore the theoretical limits of animal brain enhancement with human neurons and how thresholds for concern could be established. The special case of host animal “consciousness” or “self-awareness” will be explored via thought experiments as a part of this discussion. Last, we touch briefly on the related but separate topic of the potential socio-legal ramifications of animals with enhanced brain function should they become possible in the future.
Brain Organoid Transplantation on the Spectrum of Human-Animal Brain Chimeras
Although the concept of brain organoid transplantation is new, it fits within the context of prior dialog on human stem cells (National Research Council and Institute of Medicine, 2010) and human-animal chimeras (Greely et al., 2007; Greene et al., 2005; Hyun, 2015; Hyun et al., 2007; Karpowicz et al., 2005; Robert and Baylis, 2003). The accepted definition of chimeras in these discussions is the introduction of human cells into other animal species. Host animals grafted with human brain organoids are a sub-category of brain chimeras based on this definition, and these terms are used interchangeably in the subsequent text. Thus, the ethics of brain organoid transplantation are in many ways equivalent to the ethics of brain chimeras. In revisiting the latter, the relevant consideration is whether brain organoid transplantation moves the field of brain chimeras into more ethically problematic territory. To start, let us consider where brain organoid transplantation fits on the spectrum of brain chimeras.
Potential brain chimeras range from an animal with a single human cell in its brain to an animal in which every brain cell, both neuronal and non-neuronal, is of human origin (Figure 1). Between these extremes are myriad possibilities that arise from the permutation of several variables that potentially affect the ethical import of the resultant chimera. A factor that is particularly relevant to brain organoid transplantation is whether human cells are arranged and connected in a manner reflective of normal brain architecture or in a random, haphazard way. An organized graft, such as a brain organoid, could be reasonably expected to generate more meaningful brain function than a disorganized one. This possibility is likely a primary driver behind the additional scrutiny of brain organoid transplantation. In cases other than the bookends of the chimera spectrum, another important variable is whether human cells are disseminated throughout the brain or confined to a particular location. Disseminated grafts are more likely to influence multiple or distributed brain functions, whereas the effect of focal grafts, which include brain organoids (Daviaud et al., 2018; Mansour et al., 2018), may be limited to a single discrete function. Other significant variables include the percentage of the animal brain that is of human origin, the specific site of brain integration, and host factors such as host species and age.
Figure 1. Spectrum of Human-Animal Brain Chimeras.

Human-animal chimeras are animals into which human cells have been introduced. Brain chimeras can range from introduction of a single human cell into the host animal brain (A, green cell) to a situation where the entire host animal’s brain is composed of human cells (E, green color). Between these extremes is a potentially infinite number of permutations. Noteworthy variables to consider are whether the graft is focal (B) or disseminated (D) and whether a focal graft possesses architecture reflective of normal brain anatomy (C). The differently colored cells in (B)-(D) represent three different neuronal phenotypes that normally have an organized distribution (i.e., layers of the cerebral cortex). The type of host animal (i.e., species, embryonic or early post-natal versus adult) will also affect the degree of brain function enhancement that is theoretically possible.
It is worthwhile to note that the variables discussed above help form the basis of the limited recommendations available for the creation of brain chimeras. Guidelines from the NIH do not directly reference brain chimeras but state that human embryonic stem cells should not be introduced into non-human primate (NHP) blastocysts (National Institutes of Health, 2009). The National Academy of Sciences recommends review by research oversight committees for any work involving introduction of human pluripotent stem cells or their derivatives into NHPs or embryonic or perinatal animals with the potential to develop into adult chimeras (National Research Council and Institute of Medicine, 2010). Experiments in which human cells “could contribute in a major organized way to the brain of the recipient animal” are highlighted as needing review. The International Society of Stem Cell Research endorses review of brain chimera studies, especially “when the degree of functional integration is considerable enough to raise concerns that the nature of the animal host may be substantially altered” (International Society for Stem Cell Research, 2016). Moreover, it is recommended that chimeric animals be monitored for changes in behavior and cognition (Hyun et al., 2007). A separate working group suggested that review of experiments involving human neural transplantation in NHPs specifically consider the factors of “(i) proportion of engrafted human cells, (ii) neural development, (iii) NHP species, (iv) brain size, (v) site of integration, and (vi) brain pathology” (Greene et al., 2005).
Framing the Discussion: “Humanization” versus Brain Enhancement
What does it mean for an animal transplanted with a brain organoid to become more “human”? A common reply is that the chimera has assumed more “human-like” characteristics, such as self-awareness, advanced cognitive capacities, and complex emotions. However, these traits may not be unique to human beings, a notion that has been discussed with respect to a range of non-human species (see below) and artificial intelligence (Chella et al., 2008; Clayton, 2004; Franklin et al., 1997).
An alternative approach often taken in the ethical literature on chimeras is to consider whether these entities have attained moral equivalence to human beings (Hübner, 2018). Several theories of moral status have been debated. One argument is that individuals should be accorded respect simply because of their membership in the human race. Extending this reasoning to human tissue, cells, and genes leads to the conclusion that a chimera harboring any human components would automatically have its moral status elevated. Most scholars have rejected this line of reasoning because it does not hold up to biological scrutiny and is based on species-centric bias with no other logical basis (Greene et al., 2005; Hübner, 2018; Hyun et al., 2007; Karpowicz et al., 2005). A more logically consistent argument for moral status is based on the premise that entities that are capable of making rational, conscious choices possess intrinsic moral value (Kant, 1785). Achieving this ability in a chimeric animal is a very high bar (Hyun, 2015). Making rational, conscious choices may require the use of language to enable meta-cognition (i.e., thinking about thinking) and awareness of one’s own mental states (Allen and Bekoff, 1997). Moreover, these abilities require years of social and educational nurturing to develop, even in humans (Hyun, 2013). Therefore, discussions of the moral equivalency of “extreme chimeras” (Hyun, 2015), self-aware animals with rational thought, may be less germane to the immediate issue of brain organoid transplantation.
In framing further discussions of brain organoid transplantation and brain chimeras in general, we argue that determining the degree to which a chimeric animal is similar to a human is less constructive than considering the possibility of specific brain enhancements in chimeras and how these enhancements would influence their moral status. This contention builds on prior calls to focus on the welfare of chimeric animals (Hyun, 2015) and the previously mentioned assertion that the “human” traits that may be conferred to brain chimeras may not, in fact, be uniquely human. Reframing the conversation in this manner has certain advantages. The timeline for attaining lesser degrees of brain enhancement in chimeric animals is likely shorter; thus, these considerations are more germane to the immediate future of brain organoid transplantation. A focus on specific brain enhancements also promotes a more nuanced approach to individual cases rather than applying blanket conclusions to a wide range of potential brain chimera outcomes.
Prospects of Enhancing Brain Function in Brain Organoid Chimeras
Evaluating the scientific possibility of enhancing cerebral function after brain organoid transplantation brings much needed practicality to the ethical discussion of this subject. Such an approach provides real-world context for these conversations by identifying scenarios that are on the horizon and approximating how far in the future the more challenging cases may arise. In this section, we discuss some of the pertinent scientific principles that can help determine what types of brain enhancement are possible after brain organoid transplantation and what limitations may exist.
An important initial point is that current studies involving brain organoid transplantation are more likely to worsen brain function than improve it. Transplantation of organoids, unlike cellular injections or genetic techniques, involves the creation of a surgical cavity, an injury that likely leads to loss of function. Empiric evidence of this negative effect comes from the observation that mice grafted with brain organoids perform worse than un-grafted controls in a spatial memory task (Mansour et al., 2018). For an organoid graft to enhance brain function, it would first need to cross the threshold of restoring normal brain function, which would require appropriate connectivity and functional integration with the brain. Adoption of visual network function can be achieved in neurons transplanted into a lesion made in one cortical layer (Falkner et al., 2016). Human pluripotent stem cell-derived neurons can also respond to peripheral sensory stimulation after transplantation adjacent to a stroke cavity (Tornero et al., 2017). However, complete repair of a large brain lesion has not yet been achieved. Until this objective is obtained, which in and of itself would be quite notable from a translational and clinical perspective, cerebral enhancement via brain organoid transplantation remains firmly planted in the theoretical realm.
If we assume that enhancing brain function via organoid transplantation can eventually be achieved, some definitional issues regarding the quantity and quality of this enhancement require thought. What degree of enhancement is relevant? One could argue that a 1% change is immaterial, whereas a 20% improvement is consequential. The scale of change that matters likely depends on the specific brain function in question. An animal with faster reaction times or finer visual perception would certainly be a novelty and perhaps provoke ethical concerns about the mere fact of enhancement. However, there likely would be more concerns about animals that could learn faster (Han et al., 2013), make decisions more quickly, or remember a maze with greater accuracy. Enhancement resulting in self-awareness and meta-cognitive decision-making would be in an altogether different category. Thus, it may be instructive to think about enhanced brain function in tiers of significance and ethical import (Figure 2). This organizational structure could help direct a systematic series of thought experiments to better delineate the implications of different enhanced brain functions (Greely et al., 2007).
Figure 2. Tiers of Brain Function Enhancement in Chimeric Animals.
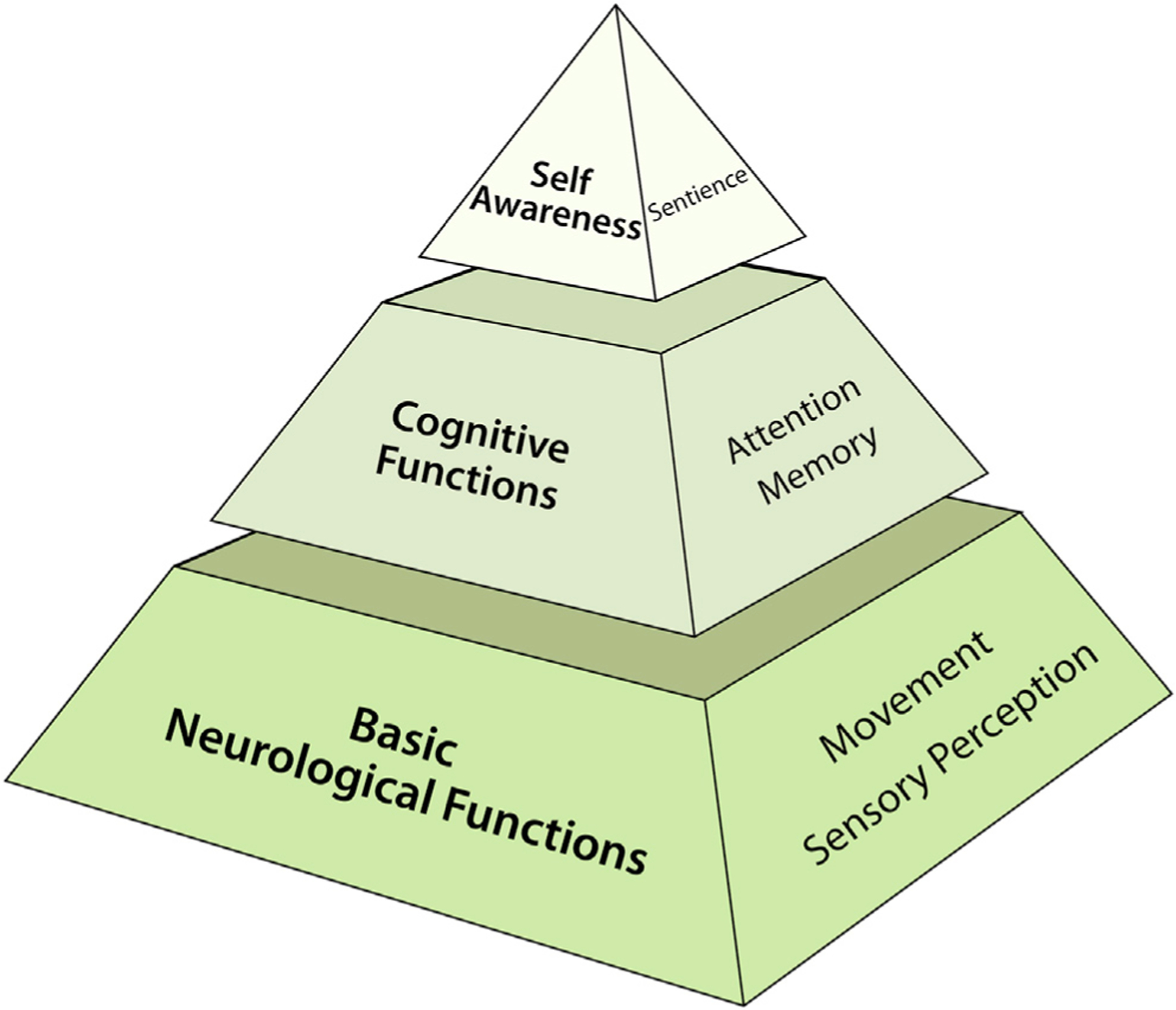
We contend that not all types of brain function have the same import when considering their potential enhancement. Cognitive functions such as memory require greater scrutiny than basic sensorimotor activity. At the top of the pyramid is self-awareness or sentience. The basis for this hierarchy is the degree of moral status that may need to be conferred to brain chimeras that achieve enhancement of these functions.
With the above ideas in mind, let us consider what types of cerebral function could conceivably be enhanced by transplantation of brain organoids. So far, transplantation studies have only grafted organoids into the cerebral cortex of host animals (Daviaud et al., 2018; Mansour et al., 2018). In these transplantation paradigms, organoid grafts remain discrete within the host brain. The focal nature of these transplanted organoids and the small size of the grafts relative to the host brain suggest that their effect on host brain function would be constrained. Brain functions reliant on relatively local circuitry (e.g., motor movement and vision) could be affected in significant ways, but the effect on highly distributed brain functions would be more limited. For example, a brain organoid could potentially participate in pre-existing circuitry for memory storage or higher-order cognition, but it would be far less likely for it to instantiate such functions. This supposition would especially be true if the transplanted organoids were region-specific (e.g., cortical organoids into the cerebral cortex). In the case of whole-brain organoids, significant growth of these grafts in vivo could theoretically lead to emergence of highly distributed brain functions, but this outcome would require proper development of intra-organoid cerebral structures and connectivity and formation of appropriate connections with the host brain. These considerations are equally applicable to all host species of the chimeric animal, including NHPs.
Is Self-Awareness Possible in Brain Organoid Chimeras?
Many would consider self-awareness to be the ultimate form of cerebral enhancement that could arise in a brain chimera. Although brain organoid chimeras are unlikely to develop this trait anytime soon, a fascinating scientific question is what parameters would need to be in place for self-aware chimeras to emerge. Empiric exploration of this topic is not possible, but thought experiments may shed some light on this question. One strategy is to compare a theoretical brain organoid chimera with a known animal species with documented features of self-awareness in terms of their cerebral computational capacity. There are certainly limitations to this approach, including the inherent difficulties of comparing cognitive abilities across species and our fundamental lack of understanding of the neurobiological substrates of self-awareness. Despite these caveats, such a comparison offers a starting point for thinking about how self-awareness could arise in a chimeric animal and defines relevant questions for subsequent investigations.
Before diving into the thought experiments, it is important to define what we mean by “self-awareness.” This term is often used interchangeably with “consciousness” and “sentience.” From a clinical perspective, the term consciousness is divided into the related but distinct ideas of an individual’s level of arousal and the content of consciousness. The concept of arousal is common across animal species and is localized to subcortical areas such as the reticular activating system. This aspect of consciousness is often described as how “awake” a patient or animal is and is the subject of research examining the mechanisms behind anesthesia, sleep, and wakefulness after brain injury (Laureys et al., 2004). In contrast, the content of consciousness refers to an entity’s ability to perceive their internal and external environments and is thought to rely on distributed processing across association cortices as well as thalamocortical and corticothalamic relays. Assessing the content of consciousness in the clinical setting is typically performed by asking patients to follow commands or imagine scenarios. The content of consciousness is predicated on the presence of wakefulness, and it is a continuum, as evidenced by the variety of disorders of consciousness after brain injury (Schiff, 2010). The usage of the term “self-awareness” in this article is explicitly linked to the concept of content of consciousness.
Completely replacing an animal’s brain with human cells would result in the greatest chance for creating a self-aware chimera. A version of this theoretical animal, the human neuron mouse, was proposed in the early 2000s (Greely et al., 2007). Although far-fetched at this time, some experimental routes eventually could make this animal a reality. An organoid graft could grow in vivo to the point of overtaking the native brain. Alternatively, a fully formed “mini-brain” organoid grown in vitro could be transplanted into a host animal. Some form of neural blastocyst complementation (Chang et al., 2018) could also result in a chimera with a human brain. Two questions to address when evaluating the cognitive potential of these hypothetical animals is (1) what would be the computational capacity of their brains and (2) how much computational capacity would be needed to achieve self-awareness.
Let us use the common laboratory rat as a test case. When determining the computational capacity of a human neuron rat, variables that are likely to be important include the number of neurons, the computational capacity of individual neurons, and the structural organization of and connectivity among these neurons. A standard rat brain weighs, on average, 2 g and contains 200 million total and 31 million cortical neurons (Herculano-Houzel and Lent, 2005). Replacing rat neurons with human neurons could affect cerebral cell numbers in two ways. First, rodents and primates have fundamentally different scaling rules that relate neuron number to brain weight (Herculano-Houzel et al., 2006, 2007). Primate brains follow a linear scaling rule; thus, a hypothetical rat brain of 2 g composed of human neurons would be expected to have 143 million total and 26.7 million cortical neurons compared with a human brain of 1,200 g with 86 billion total and 16 billion cortical neurons (Azevedo et al., 2009; Figure 3). Second, if the human neurons were introduced at an early embryonic stage, then cortical expansion driven by the presence of human outer radial glial progenitors could remodel the rat skull to produce a larger brain. Similar skull remodeling is seen in children with untreated hydrocephalus. The extent to which the brain would enlarge is hard to predict, but it would be hard to image a rat’s head expanding beyond 2–3 times its normal size. Thus, it would be improbable that a human neuron rat could have more than around 430 million neurons.
Figure 3. Comparison of Cerebral and Cortical Neuron Numbers across Species.
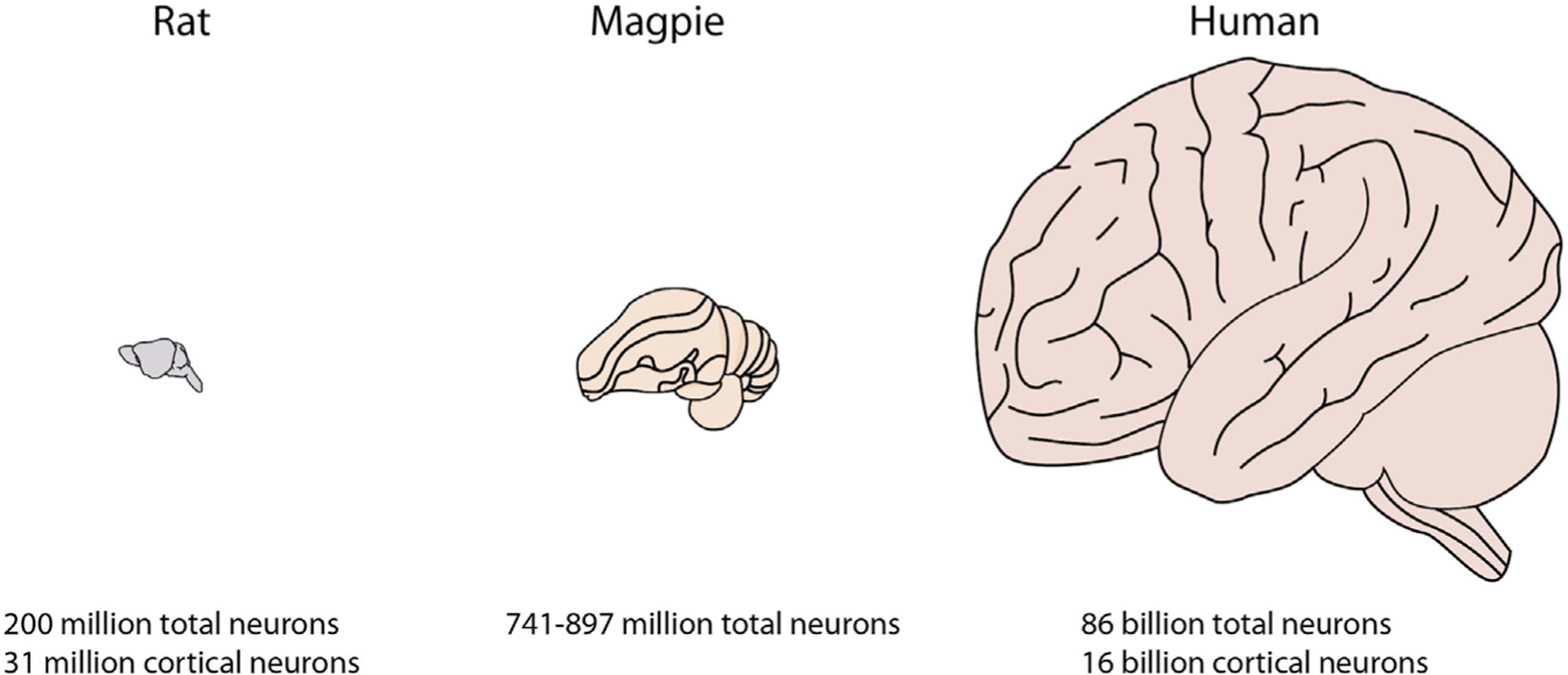
The number of cerebral and cortical neurons in the common laboratory rat is orders of magnitude smaller than the respective number of neurons in humans. A more interesting comparison for the laboratory rat is the magpie, a bird that passes the mirror test for self-recognition. These neuronal numbers can be used as the basis for thought experiments on the degree of brain expansion or computational augmentation that might be necessary to make a chimeric rat self-aware.
Human neurons would theoretically also provide a computational boost compared with rat neurons. Although the cortical synaptic density is, if anything, higher in rodents than in humans (DeFelipe et al., 2002), the dendritic arbors of human layer V pyramidal neurons are significantly longer than their rat counterparts, which results in electrical compartmentalization and input-output properties that potentially augment cortical computation (Beaulieu-Laroche et al., 2018). Certain neural cell types, such as dopamine interneurons, also only exist in the human brain (Sousa et al., 2017). Further gains in computational capacity could be realized if the cerebral structure in this hypothetical chimera resembled that of the human brain. However, it is questionable whether human brain architecture could truly be replicated with only 0.5% of the cells. Ultimately, determining the magnitude of the computational enhancement multiplier provided by human neurons may be best done using in silico models such as the Blue Brain Project, which has so far been able to model a network of 31,000 rat neurons with 36 million synapses (Gal et al., 2017; Markram et al., 2015).
Would the collective changes enumerated above enable a human neuron rat to achieve self-awareness? One way to tackle this question would be to compare the cerebral computational capacity of this chimera with that of the magpie, a highly intelligent bird and one of the few animals that passes the mirror test for recognition of self (Prior et al., 2008). The magpie brain weighs 5 g and is composed of 741–897 million neurons (Olkowicz et al., 2016). Although the avian brain lacks a laminar cortex and cortical folds, its forebrain is capable of supporting sophisticated cognitive abilities and has a higher neuronal density than the primate brain (Jarvis et al., 2005; Olkowicz et al., 2016). A human neuron rat brain with between 200–400 million neurons would require a computational enhancement multiplier of ~2–4 to match the larger number of neurons in the magpie brain. Assuming that human neurons provided a multiplier on this scale, some degree of self-awareness could perhaps arise in this chimeric animal. This comparison is limited by many assumptions and unknowns. Nevertheless, the thought experiment of comparing a human neuron rat with a magpie provides a sketch of the parameter space within which a brain organoid chimera could potentially achieve limited self-awareness.
What are the implications of larger host animals, such as pigs and NHPs, for development of self-awareness in brain organoid chimeras? As mentioned above, focal transplantation of brain organoids, including in NHPs, could affect discrete brain functions but would be unlikely to generate higher-order cognitive abilities, especially distributed functions such as self-awareness. However, the “dose” of human neurons in a more sophisticated host animal brain becomes increasingly important to consider (Greene et al., 2005). Larger brains can accommodate more neurons and greater computational power, and higher-order mammals possess brain architectures that more closely approximate the human brain, especially with regards to the cortex. These features suggest that a smaller leap would be needed to produce self-awareness in these chimeric animals versus rats. Further discourse on human neuron pigs or NHPs is beyond the scope of this article, but it would be sensible to evaluate brain organoid transplantation and other chimera technologies in lower-order species before proceeding to large mammals.
Defining Thresholds for Concern
It will take significant progress in organoid and chimera science before any scenario involving cerebral enhancement of a brain organoid chimera will surface. This being said, continuing discussions of what types of brain enhancements are concerning for society and how these augmented functions can be recognized would be prudent. If an animal improves its visual discrimination as a result of brain organoid transplantation into its visual system, what are the reasons for this outcome being objectionable, and do these reasons stand up to logical scrutiny? For brain enhancements that are deemed to be objectionable, corresponding behavioral tasks would be needed to determine whether enhancement had occurred and to quantify the degree of enhancement (Hyun et al., 2007).
Self-awareness is clearly a special case of brain enhancement. Because we do not yet comprehend the biological substrate of self-awareness, determining its presence or, perhaps more importantly, its absence is not straightforward. Lacking the means to measure self-awareness now may be less of a concern, but it will become a more urgent matter as brain organoid technology, transplantation strategies, and other laboratory methodologies advance. The prior discussion of the hypothetical human neuron rat suggests that extrapolation of known data and computational modeling are currently inadequate for these purposes. One strategy may be to borrow electrophysiological metrics from the field of coma research to measure self-awareness in brain organoid chimeras (Lavazza and Massimini, 2018b). These theoretical measures, such as the perturbational complexity index, are based on electroencephalographic recordings and have been used to stratify patients with disorders of consciousness (Casali et al., 2013; Casarotto et al., 2016). However, they have yet to be validated in animals or in in vitro cultures. Assigning specific threshold values for self-awareness is also problematic because this construct is not a binary phenomenon. Determining what parts of the continuum of self-awareness are of concern for a brain organoid chimera is itself an interesting question. Would a chimera with a level of self-awareness equivalent to a brain-injured patient in a minimally conscious state be concerning, or are greater degrees of self-awareness required to trigger concern?
The alternative is to use behavioral testing to assess self-awareness. Asking chimeric animals to follow commands is not a reliable method because some animals follow commands but are not thought to be sentient (e.g., dogs) whereas other animals may lack the ability to convey their understanding of commands. One of the only tests for self-awareness that currently exists is the aforementioned mirror test. The ability to recognize one’s reflected image in a mirror as oneself as opposed to another individual is evidence of self-awareness, although it is by no means a perfect or comprehensive measure. Humans beyond the age of 2 years of age generally pass this test (Amsterdam, 1972), as do chimpanzees (Gallop, 1970), bottlenose dolphins (Reiss and Marino, 2001), Asian elephants (Plotnik et al., 2006), and magpies (Prior et al., 2008). If an animal lacking self-awareness began routinely passing the mirror test after brain organoid transplantation or another cerebral manipulation, a pause in experimentation, more thorough cognitive and behavioral testing, and discussion with research oversight committees would be obligatory.
Ramifications of Enhanced Chimeras
What may be more important for enhanced chimeras is the welfare protections afforded them (Hyun, 2015). If a chimera were to develop a greater degree of cognitive sophistication, perhaps it would require a more stimulating environment to prevent depressive symptoms. If there is evidence of rudimentary self-awareness, perhaps the chimeras should be removed from the research setting and retired to colonies such as chimpanzee sanctuaries. If there is further development of self-awareness, it may even be necessary to afford chimeras legal protections similar humans, including consent for procedures and the right of self-determination. Even if brain organoid transplantation does nothing to enhance the chimera’s brain function, attention must still be paid to the welfare of these animals to minimize any possible pain or suffering.
It should be highlighted that current laws and regulations do not necessarily take into account scientific evidence on self-awareness in animals, although movement toward restricting research based on these considerations is occurring. Chimpanzees, our closest evolutionary relatives, are no longer used for any scientific experimentation in Europe and are afforded special protections in the United States (Institute of Medicine and National Research Council, 2011). However, most species that pass the mirror test for self-awareness are not treated differently than other animals. An interesting case is the domestic pig. Although pigs do not pass the classic mirror test of self-awareness, they are capable of using mirrors to find hidden food, a level of cognitive ability that is quite sophisticated and has been interpreted as some degree of self-awareness (Broom et al., 2009). These results certainly have not resulted in a moratorium on using pigs as a food source.
Other socio-legal issues should be considered along with animal welfare protections (Farahany et al., 2018). One question is consent for the generation of induced pluripotent stem cells (iPSCs), which are often the starting point for brain organoids. Should the possibility of creating enhanced or self-aware chimeras be disclosed during the consent process for obtaining human cells for generating iPSC lines? Should subjects be given the explicit ability to opt out of their cells being used to generate brain chimeras? Along these lines is the question of who would legally own enhanced or conscious chimeras. These topics deserve further exposition in future discussions.
Conclusion
The ethical implications of transplanting human brain organoids into animals fall within the larger context of the discussion on human-animal brain chimeras, which has been ongoing for nearly two decades. In this article, we have argued that considering enhancement of specific brain functions in these chimeras is a more practical framework than debating their degree of “humanization.” Augmentation of cerebral function in brain organoid chimeras is currently not feasible, and the degree to which an animal’s brain can be enhanced, even if it were to be completely replaced with human neurons, has limits. Further inquiry into the fundamental question of how neuronal networks give rise to cerebral function will help delineate the realistic limits and possibilities of brain organoid transplantation as well as other brain chimera techniques. In the meantime, however, it would be prudent to ponder issues raised by such brain function enhancement, such as determining what qualifies as enhancement and defining rational thresholds for concern. Neither of these tasks is easy or straightforward. Last, additional discussion is needed regarding the socio-legal matters related to brain organoid transplantation, some of which are already very much relevant today, whereas others pertain to the societal place of potential chimeras with enhanced brain function in the future.
ACKNOWLEDGMENTS
H.I.C. is supported by the Department of Veterans Affairs (RR&D Career Development Award IK2-RX002013).
REFERENCES
- Allen C, and Bekoff M (1997). Species of Mind: the Philosophy and Biology of Cognitive Ethology (MIT Press; ). [Google Scholar]
- Amsterdam B (1972). Mirror self-image reactions before age two. Dev. Psychobiol 5, 297–305. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol 513, 532–541. [DOI] [PubMed] [Google Scholar]
- Bagley JA, Reumann D, Bian S, Lévi-Strauss J, and Knoblich JA (2017). Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Parmar M, Studer L, and Takahashi J (2017). Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell 21, 569–573. [DOI] [PubMed] [Google Scholar]
- Bassett DS, and Bullmore ET (2017). Small-World Brain Networks Revisited. Neuroscientist 23, 499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, and Harnett MT (2018). Enhanced Dendritic Compartmentalization in Human Cortical Neurons. Cell 175, 643–651.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley S (2017). Tiny Human Brain Organoids Implanted into Rodents, Triggering Ethical Concerns. STAT, November 6, 2017 https://www.statnews.com/2017/11/06/human-brain-organoids-ethics/. [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DM, Sena H, and Moynihan KL (2009). Pigs learn what a mirror image represents and use it to obtain information. Anim. Behav 78, 1037–1041. [Google Scholar]
- Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräauninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, et al. (2015). Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 112, 15672–15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, Casarotto S, Bruno MA, Laureys S, Tononi G, and Massimini M (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med 5, 198ra105. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, Pigorini A, G Casali A, Trimarchi PD, Boly M, et al. (2016). Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol 80, 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Liang Z, Dai HQ, Chapdelaine-Williams AM, Andrews N, Bronson RT, Schwer B, and Alt FW (2018). Neural blastocyst complementation enables mouse forebrain organogenesis. Nature 563, 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chella A, Frixione M, and Gaglio S (2008). A cognitive architecture for robot self-consciousness. Artif. Intell. Med 44, 147–154. [DOI] [PubMed] [Google Scholar]
- Chen HI, Jgamadze D, Serruya MD, Cullen DK, Wolf JA, and Smith DH (2016). Neural Substrate Expansion for the Restoration of Brain Function. Front. Syst. Neurosci 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Song H, and Ming GL (2019). Applications of human brain organoids to clinical problems. Dev. Dyn 248, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton P (2004). Mind and Emergence: From Quantum to Consciousness (Oxford University Press; ). [Google Scholar]
- Cullen DK, Struzyna LA, Jgamadze D, Gordian-Velez WJ, Lim J, Wofford KL, Brown KD, and Chen HI (2018). Three-dimensional human axon tracts derived from cerebral organoids. bioRxiv. 10.1101/253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N, Friedel RH, and Zou H (2018). Vascularization and Engraftment of Transplanted Human Cerebral Organoids in Mouse Cortex. eNeuro 5, ENEURO.0219–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, and Arellano JI (2002). Microstructure of the neocortex: comparative aspects. J. Neurocytol 31, 299–316. [DOI] [PubMed] [Google Scholar]
- Dezonne RS, Sartore RC, Nascimento JM, Saia-Cereda VM, Romão LF, Alves-Leon SV, de Souza JM, Martins-de-Souza D, Rehen SK, and Gomes FC (2017). Derivation of Functional Human Astrocytes from Cerebral Organoids. Sci. Rep 7, 45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo E, and Kriegstein AR (2017). The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci 18, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner S, Grade S, Dimou L, Conzelmann KK, Bonhoeffer T, Götz M, and Hübener M (2016). Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539, 248–253. [DOI] [PubMed] [Google Scholar]
- Farahany NA, Greely HT, Hyman S, Koch C, Grady C, Pașca SP, Sestan N, Arlotta P, Bernat JL, Ting J, et al. (2018). The ethics of experimenting with human brain tissue. Nature 556, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin S, Wolpert S, McKay SR, and Christian W (1997). Artificial minds. Comput. Phys 11, 258–259. [Google Scholar]
- Gal E, London M, Globerson A, Ramaswamy S, Reimann MW, Muller E, Markram H, and Segev I (2017). Rich cell-type-specific network topology in neocortical microcircuitry. Nat. Neurosci 20, 1004–1013. [DOI] [PubMed] [Google Scholar]
- Gallop GG Jr. (1970). Chimpanzees: self-recognition. Science 167, 86–87. [DOI] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, and Rehen SK (2016). Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818. [DOI] [PubMed] [Google Scholar]
- Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, et al. (2019). Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci 22, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greely HT, Cho MK, Hogle LF, and Satz DM (2007). Thinking about the human neuron mouse. Am. J. Bioeth 7, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Schill K, Takahashi S, Bateman-House A, Beauchamp T, Bok H, Cheney D, Coyle J, Deacon T, Dennett D, et al. (2005). Ethics: Moral issues of human-non-human primate neural grafting. Science 309, 385–386. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. (2013). Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, and Lent R (2005). Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci 25, 2518–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, and Lent R (2006). Cellular scaling rules for rodent brains. Proc. Natl. Acad. Sci. USA 103, 12138–12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, and Kaas JH (2007). Cellular scaling rules for primate brains. Proc. Natl. Acad. Sci. USA 104, 3562–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner D (2018). Human-Animal Chimeras and Hybrids: An Ethical Paradox behind Moral Confusion? J. Med. Philos 43, 187–210. [DOI] [PubMed] [Google Scholar]
- Hyun I (2013). Bioethics and the Future of Stem Cell Research (Cambridge University Press; ). [Google Scholar]
- Hyun I (2015). From naïve pluripotency to chimeras: a new ethical challenge? Development 142, 6–8. [DOI] [PubMed] [Google Scholar]
- Hyun I, Taylor P, Testa G, Dickens B, Jung KW, McNab A, Robertson J, Skene L, and Zoloth L (2007). Ethical standards for human-to-animal chimera experiments in stem cell research. Cell Stem Cell 1, 159–163. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council (2011). Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity (The National Academies Press; ). [PubMed] [Google Scholar]
- International Society for Stem Cell Research (2016). Guidelines for stem cell research and clinical translation. http://www.isscr.org/docs/default-source/all-isscr-guidelines/guidelines-2016/isscr-guidelines-for-stem-cell-research-and-clinical-translationd67119731dff6ddbb37cff0000940c19.pdf?sfvrsn=.
- Jabaudon D, and Lancaster M (2018). Exploring landscapes of brain morphogenesis with organoids. Development 145, dev172049. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, et al. ; Avian Brain Nomenclature Consortium (2005). Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci 6, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan CP, Lokman H, et al. (2016). Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, and Sasai Y (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, and Muir KW (2016). Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 388, 787–796. [DOI] [PubMed] [Google Scholar]
- Kant I (1785). The Groundwork of the Metaphysics of Morals (Cambridge University Press; ). [Google Scholar]
- Karpowicz P, Cohen CB, and van der Kooy D (2005). Developing human-nonhuman chimeras in human stem cell research: ethical issues and boundaries. Kennedy Inst. Ethics J 15, 107–134. [DOI] [PubMed] [Google Scholar]
- Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, and Reiner O (2018). Human Brain Organoids on a Chip Reveal the Physics of Folding. Nat. Phys 14, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I, and Lancaster MA (2016). Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol 420, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K, and Clevers H (2016). Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev. Cell 38, 590–600. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, and Knoblich JA (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, and Knoblich JA (2017). Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 35, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Owen AM, and Schiff ND (2004). Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 3, 537–546. [DOI] [PubMed] [Google Scholar]
- Lavazza A, and Massimini M (2018a). Cerebral organoids and consciousness: how far are we willing to go? J. Med. Ethics 44, 613–614. [DOI] [PubMed] [Google Scholar]
- Lavazza A, and Massimini M (2018b). Cerebral organoids: ethical issues and consciousness assessment. J. Med. Ethics 44, 606–610. [DOI] [PubMed] [Google Scholar]
- Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, and Jaenisch R (2017). Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell 20, 385–396.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, and Ecker JR (2016). Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 17, 3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, and Gage FH (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol 36, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, et al. (2015). Reconstruction and Simulation of Neocortical Microcircuitry. Cell 163, 456–492. [DOI] [PubMed] [Google Scholar]
- Matsui TK, Matsubayashi M, Sakaguchi YM, Hayashi RK, Zheng C, Sugie K, Hasegawa M, Nakagawa T, and Mori E (2018). Six-month cultured cerebral organoids from human ES cells contain matured neural cells. Neurosci. Lett 670, 75–82. [DOI] [PubMed] [Google Scholar]
- Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, et al. (2012). Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater 11, 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Tang H, and Song H (2016). Advances in Zika Virus Research: Stem Cell Models, Challenges, and Opportunities. Cell Stem Cell 19, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, and Chen CS (2017). 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng 1, 0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, Jarazo J, Walter J, Büggemann, Boussaad I, et al. (2017). Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Reports 8, 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody O (2017). Human brain cells thrive inside the skull of a rat. (The Times), November 9, 2017. https://www.thetimes.co.uk/article/human-brain-cells-thrive-inside-the-skull-of-a-rat-6dk8lsmbx.
- Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, and Sasai Y (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550. [DOI] [PubMed] [Google Scholar]
- Munsie M, Hyun I, and Sugarman J (2017). Ethical issues in human organoid and gastruloid research. Development 144, 942–945. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2009). National Institutes of Health Guidelines for Human Stem Cell Research.. https://stemcells.nih.gov/policy/2009-guidelines.htm.
- National Research Council and Institute of Medicine (2010). Final Report of the National Academies’ Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research. (The National Academies Press; ). [PubMed] [Google Scholar]
- Olkowicz S, Kocourek M, Lučan RK, Porteš M, Fitch WT, Herculano-Houzel S, and Němec P (2016). Birds have primate-like numbers of neurons in the forebrain. Proc. Natl. Acad. Sci. USA 113, 7255–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, and Sasai Y (2016). Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun 7, 10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, and Waldau B (2018). Generation of human vascularized brain organoids. Neuroreport 29, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik JM, de Waal FB, and Reiss D (2006). Self-recognition in an Asian elephant. Proc. Natl. Acad. Sci. USA 103, 17053–17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior H, Schwarz A, and Güntürkün O (2008). Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition. PLoS Biol. 6, e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Song H, and Ming GL (2019). Brain organoids: advances, applications and challenges. Development 146, dev166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, et al. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, and Marino L (2001). Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc. Natl. Acad. Sci. USA 98, 5937–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert JS, and Baylis F (2003). Crossing species boundaries. Am. J. Bioeth 3, 1–13. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, and Sasai Y (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun 6, 8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND (2010). Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 33, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J (2018). Ethical (and epistemological) issues regarding consciousness in cerebral organoids. J. Med. Ethics 44, 611–612. [DOI] [PubMed] [Google Scholar]
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, and Paşca SP (2017). Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 95, 779–790.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, et al. (2017). Molecular and cellular reorganization of neural circuits in the human lineage. Science 358, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, et al. (2016). Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 47, 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero D, Tsupykov O, Granmo M, Rodriguez C, Grønning-Hansen M, Thelin J, Smozhanik E, Laterza C, Wattananit S, Ge R, et al. (2017). Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain 140, 692–706. [DOI] [PubMed] [Google Scholar]
- Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, et al. (2019). Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 25, this issue, 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, and Buzsáki G (1996). Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J. Neurosci 16, 6402–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, et al. (2019). Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, et al. (2017). Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 21, 383–398.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, et al. (2019). hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 24, 487–497.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. (2017). Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 171, 877–889.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhang S, Fan X, Wu Q, Yan L, Dong J, Zhang H, Li L, Sun L, Pan N, et al. (2018). A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555, 524–528. [DOI] [PubMed] [Google Scholar]


