Abstract
Background and Aims
Tricuspid regurgitation (TR) is a frequent valvular heart disease with relevant adverse impact on patients' prognosis. Adequate TR imaging and evaluation is challenging. In this study, we aimed to compare different imaging modalities (echocardiography and multi‐slice computed tomography) for the assessment of tricuspid valve (TV) function and geometry.
Methods
We retrospectively investigated patients that presented to University Hospital Bonn, Germany, between September 2018 and March 2019, who underwent comprehensive echocardiography and multi‐slice computed tomography (MSCT) to evaluate TR. MSCT was considered the reference approach for dimensional assessment of TV anatomy and echocardiography (transthoracic echocardiography + transesophageal echocardiography) for functional assessment of TV. We used Spearman's Rank order correlation, Bland‐Altman analysis, and intra‐class correlation to compare the different imaging modalities.
Results
Forty patients (Mean Age ± SD: 77.5 ± 7.1 years; 35% female) with high grade TR (effective regurgitant orifice area, EROA: 0.49 ± 0.3 cm2, RegVol: 49.5 ± 13.4 mL) were included. There was a statistically significant but moderate correlation between 2D‐TEE and MSCT for anteroposterior (AP) (r = 0.68, 95% confidence interval [CI]: 0.44‐0.93, P = .05; intraclass correlation [ICC]: 0.77, P = .03) and septolateral (SL) diameters (r = 0.71, 95% CI: 0.33‐0.93, P = .03; ICC = 0.76, P = .05). MSCT and 3D‐TEE showed a strong correlation for determination of TV annulus area (r = 0.94, 95% CI: 0.57‐0.98, P = .002; ICC = 0.95, P = .4), perimeter (r = 0.9, 95% CI: 0.6‐0.98, P = .002; ICC = 0.97, P = .3) and diameters (AP‐Diameter: r = 0.73, 95% CI: 0.06‐0.94, P = .03; ICC = 0.83, P = .09; SL‐Diameter: r = 0.86, 95% CI: 0.47‐0.97, P = .02; ICC = 0.95, P = .1). Only 3D‐TEE allowed for direct measurement of planimetric EROA, which exhibited a significant difference from calculated EROA (0.49 ± 0.4 cm2, 0.67 ± 0.17 cm2, P = .05; r = 0.93, 95% CI: 0.5 to 0.99, P = .006). According to Bland‐Altman analysis, we found a relevant agreement between MSCT and 3D‐TEE only for TV area (bias: −1.95, 95% limits of agreement −3.6 to −0.1).
Conclusion
Only 3D‐TEE allowed for sufficient simultaneous functional and dimensional assessment of TR in our cohort.
Keywords: multislice computed tomography, transesophageal echocardiography, tricuspid valve
Abbreviations
- 3D
three‐dimension
- AP
anteroposterior
- EROA
effective regurgitation orifice area
- ICC
intraclass correlation
- LV
left ventricle
- LV‐EF
left ventricular ejection fraction
- MSCT
multislice computed tomography
- PISA
proximal isovelocity surface area
- SL
septolateral
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
- TV
tricuspid valve
- TR
tricuspid regurgitation
1. INTRODUCTION
Tricuspid regurgitation (TR) is a frequent valvular heart disease, with 1.6 million patients in the United States suffering from severe symptomatic TR. 1 It is associated with increased mortality 2 and morbidity, and TR most frequently occurs as a functional TR caused by tricuspid valve (TV) annular dilation and leaflet tethering secondary to the right atrial (RA) and/or ventricular (RV) dilation. 3 Dedicated treatment options for solitary severe TR are scarce, and the benefit of isolated TV surgery on treated patients' prognosis and morbidity is not clear. 4
Interventional TR annuloplasty with the Cardioband valve reconstruction system (Edwards Lifesciences, Irvine, California) and interventional TR edge‐to‐edge repair with the MitraClip system (Abbott Vascular, Santa Clara, California) have previously been tested in small patients' cohorts and have shown promising functional results.1, 5, 23 For procedural planning, determination of TR severity and accurate imaging of the TV anatomy is the most crucial step. 7 Up to now, current guidelines do not provide unambiguous protocols for preprocedural patient selection and TV geometry assessment.
Several studies have proven multi‐slice computed tomography (MSCT) to be superior for anatomical and pathological assessment of atrioventricular valves compared to 2D imaging modalities in the setting of transcatheter valve therapies. 6 However, MSCT has some essential limitations, such as radiation exposure to the patient and the use of nephrotoxic contrast dye. Importantly, MSCT does not allow for TR grading, real‐time TV imaging, and intraprocedural guiding.
Although 3D transesophageal echocardiography (3D‐TEE) theoretically enables precise determination of the TV geometry, echocardiographic imaging might be limited by poor image quality, and echocardiographic views are not standardized for the assessment of TV annular geometry.7, 8
The adequate global assessment of TV and TR is challenging. There is no imaging modality for both anatomical and functional TV assessment. Therefore, we aimed to compare different imaging modalities, namely 2D‐, 3D‐TEE, and MSCT, for the assessment of TV function, annular dimension, and TR grading, with the goal of attempting to identify a one‐stop‐shop imaging solution for TV and TR assessment.
2. METHODS
We retrospectively examined consecutive patients presenting at the University Hospital Bonn, Heart Center, Germany, between September 2018 and March 2019, with symptomatic, significant TR (≥severe TR), in whom MSCT and comprehensive echocardiography (transthoracic echocardiography + transesophageal echocardiography) have been performed to evaluate TV function and dimensions for planning interventional TR therapy.
The inclusion criteria of the present study were sufficient image quality for postprocessing analysis (ie, easily identifiable images with all basic requirements from all needed views without missing any anatomical areas or acquisition requirements due to any limitations) and required 3D acquisitions in patients who were admitted for the evaluation of high‐grade symptomatic TR in our center. Patients with incomplete examinations, without required 3D acquisitions, and with images of severely reduced quality were excluded.
All imaging modalities were performed during first hospitalization for TR evaluation on compensated state without any sign for cardiac decompensation under intensive intravenous diuretic therapy. In echocardiography, the acquisitions were done during at least 3 cardiac cycles—5 cardiac cycles in case of atrial fibrillation—to avoid discrepancies due to dysrhythmia. Here, we considered MSCT as the reference modality for dimensional assessment of complex and challenging TV anatomy and echocardiography for functional assessment of TV.
The observer of MSCT images was blinded to the results of 2D‐/3D‐TEE studies and vice versa. All echocardiographic examinations were performed with GE Vivid E9 (GE Health Medical, Horten, Norway) with 3D probes M5Sc‐D and 6VT‐D.
According to current standards for morphological TV assessment, 9 we aimed to determine end‐diastolic septolateral (SL), anteroposterior (AP) diameters, TV annular perimeter, and area for the evaluation of TV annular geometry. In 2D‐TEE, diameters were measured using mid‐esophageal views at 40‐60° (AP‐diameter) and 120‐150° (SL‐diameter). The TV perimeter and area were estimated from a transgastric “en‐face” view of the TV at 20‐40° (Figure 1 ).
Figure 1.
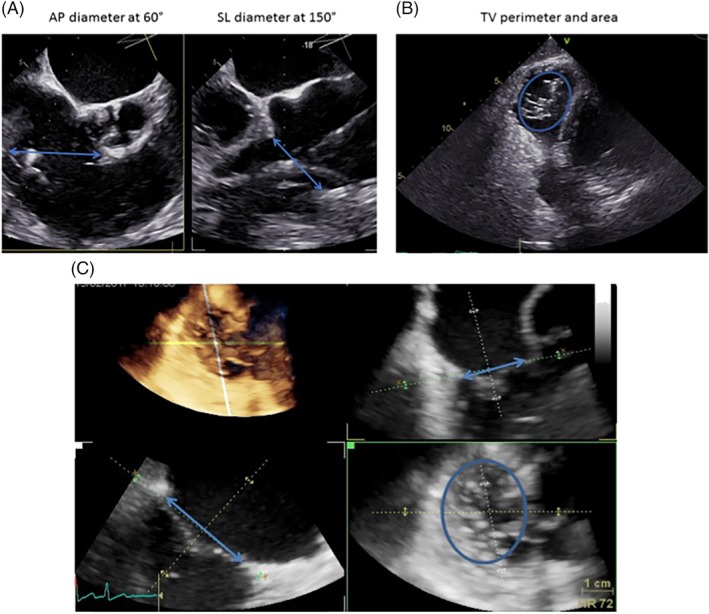
Assessment of tricuspid valve (TV) geometry in 2D‐TEE and 3D‐TEE. A, Biplane grasping view from mid‐esophageal transesophageal echocardiography (TEE) to visualize anteroposterior (AP)‐ and septolateral (SL)‐diameter. B, Transgastric view at 40° to visualize TV annulus. C) Multiplane reconstruction of complete volume dataset from transgastric “en‐face” view at 20‐40° (TV view from the right atrium side): Dimensional assessment of TV
For 3D‐TEE, complete volume data sets with an average frame rate from 15 to 25 Hz were acquired with and without color‐Doppler coming from a mid‐esophageal “inflow‐outflow” view of the TV at 40‐60° and transgastric view at 20‐40° during breath‐holding over four heart‐cycles. Color‐Doppler was performed according to our internal standardized setting for TR evaluation with adapted Color Gain and Nyquist limit between 20 and 30 m/s. Then, 3D datasets were analyzed using commercially available software (EchoPAC, vBT13; GE Healthcare Vingmed Ultrasound, Horten, Norway).
Cardiac MSCT was performed in spiral mode during 5 to 10 cardiac cycles. The heart is captured with low pitch of 0.2‐0‐5. ECG editing was used to overcome dysrhythmia‐related insufficient image quality such as “misregistration” or “blurred hazy.” Moreover, 5 mg Bisoprolol or Metoprolol was given if required, to maintain low and regular hearth rate. The triphasic contrast agent injection protocol was performed to assess right heart side, tricuspid valve, and right and left ventricular global function. The contrast agent protocol was performed via antecubital vein in three phases: 30‐40 mL contrast agent was first applied at 5 mL/s, followed by a 1:1 contrast agent and saline (15‐25 mL at 3.5 mL/s), and finally, 20 mL saline injection at 3.5‐4 mL/s. MSCT images were evaluated offline using a dedicated widely used DICOM viewer (OsiriX MD, Pixmeo SARL, Bernex, Switzerland) offering post‐processing techniques in each dimension as well as 3D navigation. We performed offline a multiplanar reconstruction of MSCT images to assess AP‐Diameter from the two‐chamber long‐axis, SL‐Diameter from the RV inflow‐outflow view, and Perimeter and Area from the short‐axis. All measurements were performed at end‐diastole (Figure 2). 24
Figure 2.
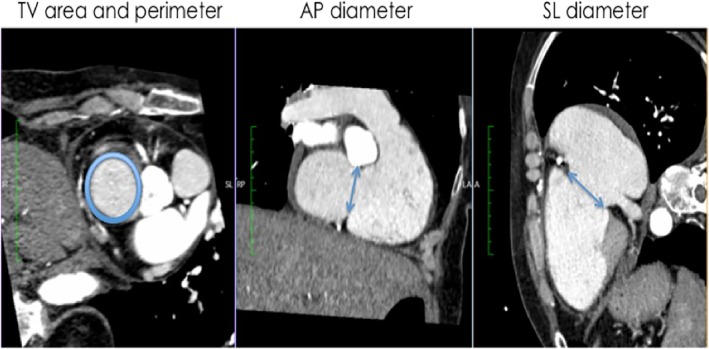
Assessment of TV geometry in multislice computed tomography (MSCT). In short axis view (image on the left): tricuspid valve (TV) perimeter und cross sectional area; in two‐chamber long‐axis view (image in the middle): anteroseptal diameter; in right ventricle (RV) inflow‐outflow view (image on the right): TV septolateral diameter
TR grading was performed according to available recommendations from international guidelines and more recent suggestions for a more precise determination of TR severity 11 ; in accordance with the latter imaging protocol, 3D color volume data sets from the transgastric view at 20‐40° with standardized color gain and baseline shift in mid‐ to end‐systole were used for the direct assessment of regurgitation volumes by determination of effective regurgitant orifice area (EROA),10, 11 (Figure S1 ). We compared semi‐quantitative measures on TR severity with direct planimetric measurements from 3D‐TEE for correlation and reproducibility. We used postprocessing analysis of 3D volume data sets of transgastric TV “en‐face” view” to assess TV dimension employing multiplanar reconstruction (Supplemental Figure S2).
3. STATISTICAL ANALYSIS
Continuous data is expressed as mean values (± SD), if normally distributed. Categorical data is shown in percentage values. Shapiro Wilk test was performed to assess normal distribution. Spearman's Rank correlation analysis was performed to evaluate strength and direction of monotonic relationship between parameters of TV geometry. Student's two‐tailed t test was performed to compare differences in continuous variables.
Bland‐Altman analysis was performed to compare two measurement methods, TEE vs MSCT. The results of the Bland‐Altman analysis are presented as mean difference (bias) and 95% limits of agreement. Mean differences were compared by using the paired t test (two‐tailed). Inter‐method reliability was evaluated by intra‐class correlation (ICC) for total agreement, with good agreement defined as >0.80. Mean values and SDs between the measurements were obtained, and total agreement among the observation was calculated using intra‐class correlation analysis. Two‐tailed P‐values were considered to be significant if <.05.
Statistical analyses were performed using SPSS for Windows (PASW statistic, Version 20.0.0.0, SPSS Inc., Chicago, Illinois).
4. ETHICAL CONSIDERATIONS
The study was approved by the Ethics committee of the Faculty of Medicine at University Hospital Bonn and was done in accordance with the Declaration of Helsinki. All patients signed written informed consent before study inclusion.
5. RESULTS
We evaluated 55 patients who were admitted for TR evaluation in our heart center between September 2018 and March 2019. A total of 40 consecutive patients (Mean Age ± SD: 77.5 ± 7.1 years; 14 [35%] female) at high surgical risk (Mean EuroSCORE II ± SD: 8.8 ± 2.1%) were ultimately included in the present study and underwent complete 2D‐/3D‐TEE and MSCT for evaluation of the severity of TR and TV anatomy. Clinical and demographic characteristics are presented in Table 1. Echocardiographic characteristics are shown in Table 2. All patients showed high‐grade TR.
Table 1.
Clinical and demographic characteristics of patients included in the study (N = 40)
| Characteristic | Number (%) or Mean ± SD |
|---|---|
| Age (years) | 77.5 ± 7.1 |
| EuroSCORE II (%) | 8.8 ± 2.1 |
| Female, n (%) | 14 (35) |
| Heart rate (BPM) | 68 ± 13 |
| Systolic blood pressure (mmHg) | 110 ± 19 |
| Diastolic blood pressure (mmHg) | 67 ± 8 |
| Creatinine levels (mg/dL) | 1.6 ± 0.5 |
| NT‐proBNP levels (ng/L) | 5816.1 ± 991.3 |
| Walking distance (6‐MWT) (meters) | 91 ± 55 |
| Functional NYHA class, n (%) | |
| a. NYHA III | 28 (70) |
| b. NYHA IV | 12 (30) |
| Previous myocardial infarction, n (%) | 7 (17.5) |
| Previous cardiac surgery, n (%) | 15 (37.5) |
| Hypertension, n (%) | 35 (87.5) |
| Smoking, n (%) | 10 (25) |
| Diabetes mellitus, n (%) | 7 (17.5) |
| COPD, n (%) | 17 (42.5) |
| Pacemaker, n (%) | 10 (25) |
| Symptoms, n (%) | |
| a. Fatigue | 37 (92.5) |
| b. Palpitations | 5 (12.5) |
| c. Abdominal bloating | 27 (67.5) |
| d. Cachexia | 7 (17.5) |
| e. Edema | 40 (100) |
| f. Ascites | 25 (62.5) |
Abbreviations: BPM, beats per minute; COPD, chronic obstructive pulmonary disease; 6‐MWT, six minutes walking test; NYHA, New York Heart Association; NT‐proBNP, N terminal pro brain natriuretic peptide.
Table 2.
Baseline echocardiographic characteristics of included patients (N = 40)
| Characteristic | Number (%) or Mean ± SD |
|---|---|
| LV‐EF, (%) | 57 ± 11 |
| Severity of TR, n (%) | |
| TR grade III | 32 (80) |
| TR grade IV | 8 (20) |
| Etiology of TR, n (%) | |
| Functional TR | 37(92.5) |
| Degenerative TR | 3(7.5) |
| TR PISA (mm) | 7.8 ± 1.7 |
| TR VC (mm) | 8.3 ± 2.3 |
| TR EROA (cm2) | 0.49 ± 0.13 |
| TR RegVol (ml) | 49.5 ± 13.4 |
| Right atrial area (cm2) | 57.2 ± 22.7 |
| TAPSE (mm) | 1.7 ± 0.4 |
| sPAP (mmHg) | 31.1 ± 13.2 |
| S′ velocity(cm/s) | 7.4 ± 0.9 |
| RV‐FAC, (%) | 34.1 ± 11.1 |
Abbreviations: EROA, effective regurgitant orifice area; FAC, fractional area change; LV‐EF, left ventricle ejection fraction; sPAP, systolic pulmonary artery pressure; PISA; proximal isovelocity surface area; RegVol, regurgitant volume; RV, right ventricle; S′ velocity; systolic myocardial velocity; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VC, vena contracta widths.
5.1. Quantification of TV geometry
In general, 2D‐TEE showed lower diameters than MSCT, with a significant but moderate correlation between both imaging modalities for AP‐diameters (2D‐TEE: 41.4 ± 7.8 mm, MSCT: 47.2 ± 8.9 mm, r = 0.68, 95% CI: 0.44‐0.93, P = .05; ICC: 0.77, P = .03) and for SL‐diameters (2D‐TEE: 41.6 ± 5.3 mm, MSCT: 46.6 ± 4.6 mm, r = 0.71, 95% CI: 0.33‐0.93, P = .03; ICC = 0.76, P = .05). We observed neither a statistically significant correlation nor inter‐method agreement between 2D‐TEE and MSCT concerning annulus perimeter (2D‐TEE: 117.6 ± 18.9 mm, MSCT: 130.3 ± 21.5 mm, r = 0.3, 95% CI: 0.29‐0.92, P = .4, ICC = 0.64, P = .09) and annulus area (2D‐TEE: 10.1 ± 3.3 cm2, MSCT: 13.4 ± 4.1 cm2, r = 0.5, 95% CI: 0.27‐0.9, P = .4, ICC = 0.63, P = .07) (Tables 3 and 5).
Table 3.
Correlations between 2D‐TEE and MSCT concerning TV geometry
| Variable | 2D‐TEE | MSCT | r (95% CI) | P value |
|---|---|---|---|---|
| AP‐diameter, mm | 41.4 ± 7.8 | 47.2 ± 8.9 | 0.68 (0.44 to 0.93) | .05 |
| SL‐diameter, mm | 41.6 ± 5.3 | 46.6 ± 4.6 | 0.71 (0.33 to 0.93) | .03 |
| TV‐area, cm2 | 10.1 ± 3.3 | 13.4 ± 4.1 | 0.5 (0.27 to 0.90) | .4 |
| TV‐perimeter, mm | 117.6 ± 18.9 | 130.3 ± 21.5 | 0.3 (0.29 to 0.92) | .4 |
Abbreviations: AP, anteroposterior; CI, confidence interval; MSCT, multislice computed tomography; r, correlation coefficient; SL, septolateral; TEE, transesophageal echocardiography; TV, tricuspid valve.
Table 5.
Assessment of inter‐method reliability from the different imaging modalities using intraclass correlation
| Variable | Intraclass correlation | (95% CI) | P value | |
|---|---|---|---|---|
| Inter‐method agreement | ||||
| 2D‐TEE vs MSCT | AP‐diameter | 0.77 | 0.54 to 0.89 | .03 |
| SL‐ diameter | 0.76 | 0.28 to 0.91 | .05 | |
| TV‐perimeter | 0.64 | 0.34 to 0.88 | .09 | |
| TV‐area | 0.63 | 0.29 to 0.92 | .07 | |
| Inter‐method agreement | ||||
| 3D‐TEE vs MSCT | AP‐diameter | 0.83 | 0.5 to 0.94 | .09 |
| SL‐ diameter | 0.95 | 0.87 to 0.98 | .1 | |
| TV‐perimeter | 0.97 | 0.93 to 0.99 | .3 | |
| TV‐area | 0.95 | 0.86 to 0.98 | .4 | |
Abbreviations: AP, anteroposterior; CI, confidence interval; MSCT, multislice computed tomography; SL, septolateral; TEE, transesophageal echocardiography; TV, tricuspid valve.
Between 3D‐TEE and MSCT, we found significant agreement and correlation in diameters (AP‐diameter; 3D‐TEE: 43.8 ± 3.2 mm, MSCT: 47.2 ± 8.9 mm, r = 0.73, 95% CI: 0.06‐0.94, P = .03, ICC = 0.83, P = .09 and SL.‐diameters; 3D‐TEE: 44.5 ± 3.6 mm, MSCT: 46.6 ± 4.6 mm, r = 0.86, 95% CI: 0.47‐0.97, P = .02, ICC = 0.95, P = .1). With regards to TV annulus areas, we observed a strong correlation between the values obtained with these two techniques (3D‐TEE: 12.9 ± 2.6 cm2, MSCT: 13.4 ± 4.1 cm2, r = 0.94, 95% CI: 0.57‐0.98, P = .002, ICC = 0.95, P = .4). Similarly, we observed strong correlation for perimeter (3D‐TEE: 130.1 ± 12.4 mm, MSCT: 130.3 ± 21.5 mm, r = 0.9, 95% CI: 0.6‐0.98, P = .002, ICC = 0.97, P = .3) (Tables 4 and 5).
Table 4.
Correlations between 3D‐TEE and MSCT concerning TV geometry
| Variable | 3D‐TEE | MSCT | r (95% CI) | P value |
|---|---|---|---|---|
| AP‐diameter, mm | 43.8 ± 3.2 | 47.2 ± 8.9 | 0.73 (0.06 to 0.94) | .03 |
| SL‐diameter, mm | 44.5 ± 3.6 | 46.6 ± 4.6 | 0.86 (0.47 to 0.97) | .02 |
| TV‐area, cm2 | 12.9 ± 2.6 | 13.4 ± 4.1 | 0.94 (0.57 to 0.98) | .002 |
| TV‐perimeter, mm | 130.1 ± 12.4 | 130.3 ± 21.5 | 0.90 (0.60 to 0.98) | .002 |
Abbreviations: AP, anteroposterior; CI, confidence interval; MSCT, multislice computed tomography; r, correlation coefficient; SL, septolateral; TEE, transesophageal echocardiography; TV, tricuspid valve.
The Bland‐Altman analysis revealed an absolute mean difference (bias) of −5.3 mm (95% limits of agreement −12.6 to 2.4) for AP‐Diameter, −0.8 mm (95% limits of agreement −4.8 to 3.05) for SL‐Diameter, −1.95 mm2 (95% limits of agreement: −3.6 to −0.1) for TV area, and −8.3 mm (95% limits of agreement −19.8 to 1.8) for TV perimeter (Figure 3).
Figure 3.
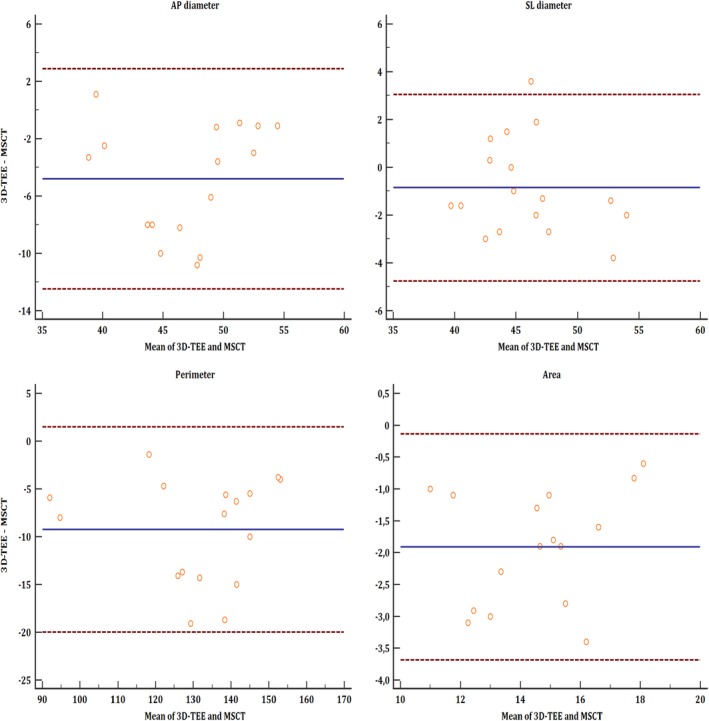
Bland–Altman plot: Comparison of 3D‐transesophageal echocardiography (TEE) and multislice computed tomography (MSCT) concerning tricuspid valve (TV) geometry in patients included in this study (N = 40), for anteroposterior (AP)‐Diameter, septolateral (SL)‐Diameter, perimeter, and area. AP‐Diameter; Mean (bias): −5.3, 95% limits of agreement: −12.1 to 3.05. SL‐Diameter; Mean (bias): −0.8, 95% limits of agreement: −4.8 to 3.05. Perimeter; Mean (bias): −8.3, 95% limits of agreement: −19.8 to 1.8. Area; Mean (bias): −1.95, 95% limits of agreement: −3.6 to −0.1
5.2. TR grading
MSCT is unsuitable for TR grading, since essential parameters for TR grading (PISA, VC, EROA, RegVol) cannot be determined from MSCT. Only echocardiography allows for TR grading in line with guidelines. 10 Importantly, we found significant differences between the 2D‐TEE‐based calculated EROAs according to semi‐quantitative PISA‐method and directly planimetric assessed EROA by use of 3D color volume data sets (0.49 ± 0.4 cm2, 0.67 ± 0.17 cm2, P = .05; r = 0.93, 95% CI: 0.5‐0.99, P = .006). In all cases, 2D‐TEE led to a significant underestimation of TR severity.
5.3. Assessment of TV anatomy
Compared to MSCT, only echocardiography enables real‐time dynamic simultaneous assessment of TV function and anatomy, which allows precise identification of the underlying TR pathology. 2D‐TEE identified functional TR in 36 cases, degenerative valve disease in three cases, and mixed disease in one of our cases. Of note, multiplanar reconstruction of 3D volume data sets with offline post‐processing analysis of TR leaflet mobility and anatomy changed the categorization of the underlying TR pathologies in 10 patients (25%) in comparison with 2D imaging. All of these patients were re‐classified from functional TR assessed by 2D‐TEE to degenerative TR caused by eccentric tricuspid valve prolapse.
6. DISCUSSION
The major findings of our study are that 2D echocardiography significantly underestimates TV annular dimension when compared with 3D imaging modalities (3D‐TEE and MSCT). Compared to MSCT, echocardiography provides adequate functional TV evaluation and is not significantly limited for dimensional TV assessment employing post‐processing multiplanar reconstruction, unless there is poor image. When planning TR treatment, only 3D echocardiography allows for a “one‐stop‐ shop” approach, with (a) quantification of TV dimensions without significant difference when compared to MSCT, (b) TR grading, and (c) identification of the TR underlying pathology.
From a more clinical perspective, TEE has several important advantages, as it can be performed on site, during the intervention, in order to confirm TR severity and procedural planning. In general, echocardiography overcomes the disadvantages of radiation exposure and the need of nephrotoxic contrast dye.
6.1. How to approach the “forgotten valve”
The assessment of TV anatomy is difficult due to its complex 3D shape and variability, and lacks guidance from available evidence.12, 13 Although 2D echocardiography is defined as the gold standard for initial assessment of patients with suspected TV disease, 20 it gives only a “raw estimation” of right heart pathologies and does not allow precise quantification of TV annular dimensions and geometry. 8 In this study, we found that 2D echocardiography led to a systematic underestimation of tricuspid annular diameters, which is in line with the results of other colleagues on this topic8, 22; this is of vital importance and directly impacts treatment planning and decision making for available TR therapies.14, 15 Furthermore, 2D‐TEE failed to identify the TR underlying pathology in a considerable percentage of our patients. In these cases, reassessment of the TV with 3D imaging led to re‐classification of functional to degenerative valve disease.
Consequently, we found that 2D echocardiography alone is inadequate for planning complex cardiac procedures such as TV annuloplasty or edge‐to‐edge repair.
6.2. 3D imaging of the TV: Is it echocardiography or MSCT?
In line with Addetia and colleagues, we found a strong correlation between MSCT and 3D‐TEE for the quantification of TV areas and perimeters in our patient cohort. 16 In our study, we used 3D imaging for procedural planning of patients with significant TR, and 3D‐TEE was able to determine annular geometry in the presence of significant tricuspid valve and/or right ventricular heart disease.
When compared with MSCT, only 3D‐TEE allows for dynamic and real‐time assessment of the TV anatomy and function simultaneously, which might be of importance for decision‐making and interventional TR therapy in patients with high‐grade TR.17, 18 More importantly, 3D‐TEE but not MSCT enables precise analysis of TV leaflet mobility and anatomy in real‐time during multiple heart cycle, which remains an apparent limitation of static anatomic assessment with MSCT.
6.3. Method of choice for TR grading
The complexity and difficulties of TR grading have been recently addressed by different expert statements, 19 which have already been in part adopted in current guidelines. 20 MSCT lacks the ability for the determination of TR‐defining parameters such as PISA, VC, EROA, and RegVol. Therefore, MSCT is unsuitable for TR grading. Only echocardiography allows for TR grading in line with guidelines. 10
TR and right heart diseases are complex entities with wide variation in the individuals' underlying patho‐anatomies.1, 2 Therefore, assessment of TR severity by the use of the EROA method has significant limitations, since it is based on the assumption of complex geometrical and stable hemodynamic conditions.16, 20 Thus, quantification of TR by the use of the semi‐quantitative PISA method differs significantly from the direct assessment of EROA with 3D‐TEE. This is in line with findings in patients presenting with functional mitral regurgitation.21, 22 We additionally found that 3D‐TEE offers promising assessment of TV geometry compared to cardiac computed tomography as well, which is essential for planning and evaluation of interventional therapy. The complexity of TR grading has already been addressed by Hahn and Baumgartner et al.11, 20 The authors emphasized the benefit of 3D imaging in this setting. Therefore, we would advise using 2D echocardiography as a fast and straightforward screening method to identify patients with significant TV disease. Procedural planning and decision‐making, on the other hand, should be based on imaging with 3D echocardiography.
7. LIMITATIONS
This single‐center, retrospective study has several limitations. We report data from a small number of patients, and all echocardiographical analyses were not analyzed for inter‐observer reproducibility. Furthermore, 15 patients were excluded, 9 due to incomplete examinations, and 6 due to insufficient image quality, which could have resulted in selection bias. Therefore, our results should be validated by a core‐lab in multicentric studies with a larger number of patients.
8. CONCLUSION
3D‐TEE is highly comparable to MSCT and preferable than 2D imaging for the assessment of tricuspid annulus. 3D‐TEE allows sufficient grading of TR and geometrical assessment of TV without significant differences compared to MSCT. Therefore, 3D‐TEE offers a promising one‐stop‐shop imaging solution for adequate TR and TV evaluation.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Can Öztürk, Christoph Hammerstingl
Formal Analysis: Robert Schueler, Can Öztürk, Christoph Hammerstingl
Investigation: Can Öztürk
Methodology: Can Öztürk, Marcel Weber, Georg Nickenig, Christoph Hammersingl
Supervision: Christoph Hammerstingl, Georg Nickenig
Writing—Original Draft Preparation: Can Öztürk
Writing—Review & Editing: Robert Schueler, Christoph Hammerstingl, Georg Nickenig
All authors have read and approved the final version of the manuscript.
Can Öztürk had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
Can Öztürk affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Figure S1. Planimetric assessment of 3D‐EROA
Transgastric view with color Doppler; planimetry of the effective regurgitant orifice area (EROA) in mid‐ to end‐systole
Figure S2. 3D‐Assessment complex tricuspid regurgitation (TR) pathology using multiplanar reconstruction
Multiplanar reconstruction of complete of transgastric view at 20‐40° to adequately assess the main pathology: coaptation deficiency
Öztürk C, Schueler R, Weber M, Nickenig G, Hammerstingl C. Comparison of different imaging modalities for the quantification of tricuspid valve geometry and regurgitation: a retrospective, single‐center study. Health Sci Rep. 2020;3:e159 10.1002/hsr2.159
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Nickenig G, Kowalski M, Hausleiter J, et al. Transcatheter treatment of severe tricuspid regurgitation with the edge‐to‐edge MitraClip technique. Circulation. 2017;135(19):1802‐1814. [DOI] [PubMed] [Google Scholar]
- 2. Hung J. The pathogenesis of functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg. 2010;22(1):76‐78. [DOI] [PubMed] [Google Scholar]
- 3. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83(6):897‐902. [DOI] [PubMed] [Google Scholar]
- 4. Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104(10):798‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammerstingl C, Schueler R, Malasa M, Werner N, Nickenig G. Transcatheter treatment of severe tricuspid regurgitation with the MitraClip system. Eur Heart J. 2016;37(10):849‐853. [DOI] [PubMed] [Google Scholar]
- 6.21Willmann JK, Weishaupt D, Lachat M, et al. Electrocardiographically gated multi‐detector row CT for assessment of valvular morphology and calcification in aortic stenosis. Radiology. 2002;225:120‐128. [DOI] [PubMed] [Google Scholar]
- 7. Kim SM, Singh HS, Nati J, Ginns JN. Multi‐modality imaging in the evaluation and treatment of tricuspid regurgitation. Curr Treat Options Cardiovasc Med. 2018;20(9):77. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt HV, Spivack J, Patel PR, et al. Correlation of 2‐dimensional and 3‐dimensional echocardiographic analysis to surgical measurements of the tricuspid valve annular diameter. J Cardiothorac Vasc Anesth. 2018;2:137‐145. [DOI] [PubMed] [Google Scholar]
- 9. Muraru D, Badano LP, Sarais C, Soldà E, Iliceto S. Evaluation of tricuspid valve morphology and function by transthoracic three‐dimensional echocardiography. Curr Cardiol Rep. 2011;13:242‐249. [DOI] [PubMed] [Google Scholar]
- 10. Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). European Journal of Echocardiography: The Journal of the Working Group on Echocardiography of the European Society of Cardiology. 2010;11:307‐332. [DOI] [PubMed] [Google Scholar]
- 11. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18(12):1342‐1343. [DOI] [PubMed] [Google Scholar]
- 12. Buzzatti N, Taramasso M, Latib A, et al. Transcatheter mitral repair and replacement: state of the art and future directions. J Heart Valve Dis. 2014. Jul;23(4):492‐505. [PubMed] [Google Scholar]
- 13. Hammerstingl C. Echocardiographic imaging of the tricuspid valve. Herz Springer Medizin. 2017;42(7):629‐633. [DOI] [PubMed] [Google Scholar]
- 14. Anwar AM, Soliman OII, Nemes A, van Geuns R‐JM, Geleijnse ML, Cate Ten FJ. Value of assessment of tricuspid annulus: real‐time three‐dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging Springer Netherlands. 2007;23(6):701‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ancona F, Stella S, Taramasso M, et al. Multimodality imaging of the tricuspid valve with implication for percutaneous repair approaches. Heart. 2017. Jul;103(14):1073‐1081. [DOI] [PubMed] [Google Scholar]
- 16. Addetia K, Muraru D, Veronesi F, et al. 3‐dimensional echocardiographic analysis of the tricuspid annulus provides new insights into tricuspid valve geometry and dynamics. JACC Cardiovasc Imaging. 2017. Nov;10:401‐412. [DOI] [PubMed] [Google Scholar]
- 17. Boyd AC, Thomas L. Left atrial volumes: two‐dimensional, three‐dimensional, cardiac magnetic resonance and computed tomography measurements. Curr Opin Cardiol. 2014. Sep;29(5):408‐416. [DOI] [PubMed] [Google Scholar]
- 18. Ancona F, Stella S, Capogrosso C, et al. Tricuspid valve imaging. Minerva Cardioangiol. 2018;66(6):680‐690. [DOI] [PubMed] [Google Scholar]
- 19. Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. 2009;119(20):2718‐2725. [DOI] [PubMed] [Google Scholar]
- 20. The 2017 ESC/EACTS guidelines on the management of valvular heart disease: What is new and what has changed compared to the 2012 guidelines? Eur Heart J. 2018;130(5–6):168‐171. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt FP, Gniewosz T, Jabs A, et al. Usefulness of 3D‐PISA as compared to guideline endorsed parameters for mitral regurgitation quantification. Int J Cardiovasc Imaging Springer Netherlands. 2014;30(8):1501‐1508. [DOI] [PubMed] [Google Scholar]
- 22. Chen T‐E, Kwon SH, Enriquez‐Sarano M, Wong BF, Mankad SV. Three‐dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two‐dimensional measures. J Am Soc Echocardiogr. 2013;26(10):1143‐1152. [DOI] [PubMed] [Google Scholar]
- 23. Nickenig G, Weber M, Schueler R, Hausleiter J, Näbauer M, von Bardeleben RS. At al. 6‐month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. 2019;73(15):1905‐1915. [DOI] [PubMed] [Google Scholar]
- 24. Huttin O, Voilliot D, Mandry D, Venner C, Juillière Y, Selton‐Suty C. All you need to know about the tricuspid valve: tricuspid valve imaging and tricuspid regurgitation analysis. Arch Cardiovasc Dis. 2016;109(1):67‐80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Planimetric assessment of 3D‐EROA
Transgastric view with color Doppler; planimetry of the effective regurgitant orifice area (EROA) in mid‐ to end‐systole
Figure S2. 3D‐Assessment complex tricuspid regurgitation (TR) pathology using multiplanar reconstruction
Multiplanar reconstruction of complete of transgastric view at 20‐40° to adequately assess the main pathology: coaptation deficiency
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


