Abstract
As an intermediary between cells and scaffolding biomaterials, the extracellular matrix secreted by the cells offers challenges and opportunities for the design and fabrication of engineered tissues.
Relying on advances in materials science, stem cell biology and bioengineering, engineered tissues are increasingly being considered in a range of fundamental, translational and clinical applications. Engineered tissues can be used for the exploration of fundamental mechanisms in health and disease, as in vitro tissue models for drug screening, and as replacements of tissues damaged by trauma or disease. Building a tissue often requires a material that can mimic the native extracellular matrix (ECM), which provides physical support to the cells and directs a myriad of cellular activities. Hydrogels — a versatile class of biomaterials formed from water-swollen polymer networks — are often used as mimics of the ECM because they can be synthesized to provide precise control over physical properties such as stiffness, and chemically modified to present biologically active peptides. As with the native ECM, the physical and biological properties of hydrogels can be tuned to regulate cellular behaviours. Hydrogels have thus been widely exploited to direct stem cell differentiation and tissue formation1, and as material systems to create tissue and tissue models of disease2,3
However, cells encapsulated within hydrogels can quickly remodel their surroundings by assembling secreted proteins around themselves4–6. Although it has long been known that cells secrete proteins pericellularly, it is now clear that local cell-mediated modifications can quickly override the physical and biological cues presented by the hydrogel. This partly explains why cell responses to physical cues on surfaces can differ from those in three dimensions: not only because cell morphology is likely to differ, but also because of the local assembly of secreted ECM proteins. Moreover, because material properties in three dimensions can impact the retention and distribution of the secreted ECM, these findings may provide insight into why strategies for modulating the properties of hydrogels to create tissues in vitro have not been entirely successful. The secreted matrix drives cellular behaviours (such as stem cell differentiation), and thus should be considered when using biomaterials to form engineered tissues.
In this Comment, we discuss how the fact that the secreted ECM can override cues provided by biomaterial scaffolds impacts the engineering of tissues. The secreted matrix is an intermediary between cells and biomaterials, and so we therefore provide examples of how it can be exploited to direct cell responses. We then present strategies for harnessing the secreted ECM, through the creation of materials that prompt cells to secrete an ‘instructive’ local matrix, and by enriching the cells’ pericellular environment with a desired ECM. We also discuss how to leverage the secreted matrix to create engineered tissues that can be used both therapeutically and as tissue models or disease models.
Cell-mediated matrix remodelling
The ECM is a complex network of proteins and polysaccharides that surrounds cells in tissues. Although once dismissed as a simple passive support for cells, the ECM is now recognized as a transducer of physical signals and as an important regulator of cellular activities, such as proliferation, quiescence, migration and differentiation7. For example, matrix stiffness regulates cancer-cell migration and metastasis8, and adhesive binding sequences within ECM proteins allow cells to directly attach to the ECM and to physically manipulate it. Moreover, large sugar-containing molecules that permeate the ECM act as ‘docking stations’ for cell-signalling molecules such as growth factors and matrix fragments, which can be released back to the cells to regulate their activities.
The intrinsic bioactivity of the ECM has been harnessed by extracting native polymers from animal tissues and cell cultures. However, despite the important biological insights gained by using such naturally derived matrices (which typically consist of either collagen, fibrin or tumour-basement membrane), their utility is often limited, mostly because uncoupling physical properties (such as stiffness) from biological properties (such as ligand density) is often not possible9. Moreover, native matrices often contain undefined growth factors and extracellular vesicles10, which can influence cellular behaviours and preclude translational use. In contrast, synthetic materials can mimic the native ECM and be physicochemically defined, and their properties can be tailored to produce a cell-instructive environment. For example, by incorporating degradability into hydrogels, the stemness of neural progenitors can be maintained11. Similarly, the lineage commitment of mesenchymal stromal cells (MSCs) can be regulated by tuning the hydrogel’s stress relaxation12 and by modulating the stiffness of ionically crosslinked networks13 or the degradability of covalently crosslinked networks14. Also, the contribution of biophysical properties to neural tube13 and epithelial morphogenesis14, as well as conditions that permit the maintenance of mouse15 and human intestinal organoids16, have all been clarified via the use of synthetic hydrogels.
The basic premise underlying the use of hydrogels as mimics of the ECM centres around the tuning of their biological and physical properties, with the expectation that encapsulated cells will then respond in a predictable manner. However, cells are not merely passive sensors of physical and biological signals. Instead, many cell types actively modify their local environment in processes that are central to tissue development, homeostasis and repair17. Such cell-mediated ECM remodelling is driven by two complementary mechanisms: matrix production, and matrix degradation. Together, they dynamically modulate the cells’ milieu. This interplay between cells and their ECM, which was first described by Mina Bissell (who coined the term ‘dynamic reciprocity’18 to describe how cells continually modulate their surroundings biochemically and mechanically) in turn regulates signalling cascades, gene expression, and thus cell behaviour. Bi-directional interactions between cells and ECM are ubiquitous across many tissues (such as the skin, where the ECM of the epidermal stem cell niche is continually regulated by its constituent cells19; and the breast, where mammary epithelial cells modify their ECM, which in turn regulates tissue-specific functions such as milk production20). However, the dynamic interplay between matrix secretion and degradation, and how it impacts interactions between cells and their surroundings, has often been neglected. That is, although degradation (and degradation-like behaviour) is often designed into cell-instructive materials by making them sensitive to proteolytic enzymes, by rendering them responsive to physical stimuli such as light, or by making them reliant on supramolecular interactions that cells can manipulate21–23, matrix secretion has remained relatively unexplored. Indeed, although the pericellular space can impact fate specification24, the regulatory influence of the secreted matrix itself has largely been overlooked.
However, this is now changing. It has been recently shown that, when encapsulated within hydrogels, human bone-marrow-derived MSCs (hMSCs) quickly modify their surroundings by secreting and assembling proteins around themselves4–6 (Fig. 1). The cell-derived matrix alters both the biochemical composition and local mechanical environment of the cells. As cells secrete more ECM, the cell-derived matrix modulates cellular mechanotransduction and impacts the specification of cellular fate. In other words, the secreted matrix does not simply provide additional instructions to the cell; rather, it masks and overrides the biological and physical cues provided by the hydrogel. Hence, both the composition of a tissue scaffold and the instructions that cells receive from it changes over time.
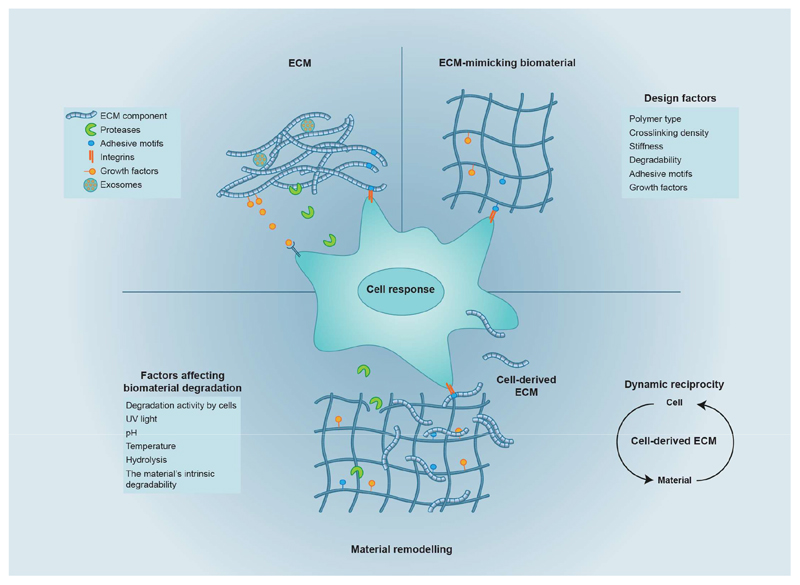
Fig. 1. Biomaterials inspired by the extracellular matrix, and cell-mediated matrix remodelling.
The extracellular matrix (ECM) — the physiological material that surrounds cells in tissues — provides tissues with a three-dimensional physical structure and with bioactive ligands (such as adhesive motifs and growth factors). ECM-mimicking biomaterials can be rendered cell-instructive by incorporating protein-based or peptide-based motifs that recapitulate key features of the native ECM. However, cells encapsulated within hydrogels remodel their surroundings through a combination of matrix secretion and degradation. Over time, the cell-secreted matrix can override cues provided by the hydrogel. This behaviour parallels the phenomenon of dynamic reciprocity observed in tissues, by which cells modulate their surroundings biochemically and mechanically, regulating intracellular signalling, gene expression, and ultimately cell behaviour.
The secreted matrix can be detected around cells as soon as 4 hours after encapsulation5,6. Although cells quickly engage with the secreted matrix (blocking integrin-mediated interactions with soluble peptides results in reduced cell viability4), the secreted matrix does not make integrin-mediated interactions with the hydrogel entirely dispensable, as hMSCs within inert hydrogels lacking adhesive motifs show reduced viability25. Instead, cells appear to simultaneously interact with their own secreted matrix and with the hydrogel. This is supported by observations that hMSCs within hydrogels containing adhesive motifs yet depleted of fibronectin show reduced cell spreading6. Therefore, as the quantity of secreted matrix increases over time, there seems to be a transition during which the secreted ECM increasingly displaces the cells’ initial interactions with the hydrogel. It is however unclear how strongly the initial interactions with the hydrogel regulate cell responses, and how long they last. In particular, it is unknown to what extent initial hydrogel interactions govern the composition, distribution and quantity of the secreted matrix, and how long cell–hydrogel interactions dominate before interactions with the secreted matrix take over. Understanding this interplay will have important implications for the design of regenerative scaffolds, because they may reveal a time window during which materials can most effectively be employed to direct a desired cellular behaviour. Mechanistic experiments in which adhesive motifs can be presented or removed from hydrogels with precise temporal control are needed to reveal the minimum time frame required for ensuring cell survival and to identify the time window in which a biomaterial can direct cellular responses.
Harnessing the cell-secreted ECM
Although the regulatory roles of the secreted ECM remain to be thoroughly understood, promising strategies to harness them are within reach. It has long been known that soluble factors and tissue-derived ECM impact the composition and quantity of the secreted ECM (ref. 26). For example, ascorbic acid as an essential co-factor for a number of hydroxylases can stimulate fibroblasts to form of a collagen-rich ECM (ref. 27). However, relying on matrix secretion alone to engineer a tissue is often insufficient. Instead, the local microenvironment can be tailored to prompt the cells to produce an instructive ECM or to preclude matrix secretion that prompts a deleterious or pathophysiological response. Alternatively, instead of instructing cells to secrete a specific matrix, desired secreted proteins can be enriched pericellularly, or harnessed to modulate cellular mechanotransduction. In what follows and in Fig. 2, we outline a few of these strategies.
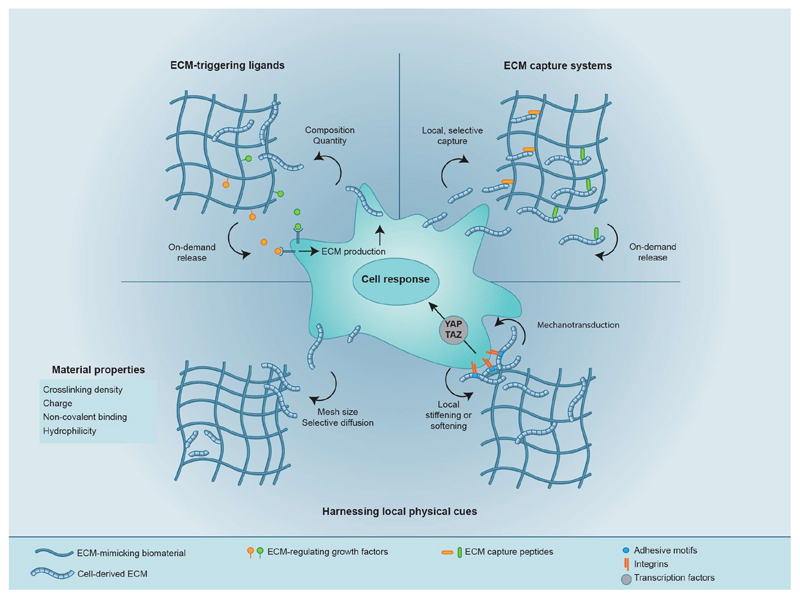
Fig. 2. Biomaterial-based strategies for harnessing the secreted ECM.
By tethering biological molecules or by incorporating physical strategies, biomaterials can be designed to regulate the secreted matrix and, in turn, cellular responses. For example, ECM-triggering ligands, as well as peptides, aptamers or nanobodies that locally and specifically capture secreted proteins, can be incorporated into biomaterials to sequester signalling proteins from the secreted matrix that can then signal back to cells. The physical properties of scaffold biomaterials can also be modulated to regulate cell responses by using the scaffold’s mesh size as well as non-covalent interactions to selectively enrich for particular components of the secreted matrix pericellulary. Moreover, by capturing the secreted matrix pericellularly, the local microenvironment can be stiffened or softened to potentially regulate cellular mechanotransduction and thus cellular behaviour.
Tuning biological ligands
Synthetic biomaterials can be functionalized with peptides or proteins to render the materials suitable for growing cells. However, if cells secrete matrix around themselves, is the incorporation of biological ligands at all required? In completely inert hydrogels such as those formed from poly(ethylene glycol) (PEG), many cell types may undergo anoikis in the absence of adhesive motifs. However, for highly secretory cells, adhesive ligands may not be necessary. For example, although intestinal organoids formed from human induced pluripotent stem cells (hiPSC) were first derived in Matrigel28 and can be maintained within synthetic hydrogels containing adhesive peptides16, unmodified alginate can similarly support the organoids’ maintenance29. This observation has been attributed to the fact that iPSC-derived organoids contain vimentin-positive mesenchymal cells28 that surround the epithelium and that are likely to secrete a supportive ECM (ref. 30). Hence, in this case the simplest approach of merely allowing the cells to create their own instructive matrix seems sufficient to support viability.
However, when biological ligands are necessary, they can be exploited to direct the composition or quantity of the secreted ECM. For example, by functionalizing materials with signalling proteins such as transforming growth factor-β (TGFβ), it may be possible to direct MSC specification towards a differentiating and secretory phenotype (or, by using fibroblast growth factor-2, towards a quiescent and non-secretory phenotype). Moreover, biological ligands such as Jagged1, which prompts MSCs to create a basement-membrane-like matrix6, could be immobilized within biomaterials. Such ligands could also be tethered in precise spatial arrangements by combining photopatterning with sortase-based protein-modification strategies31, and released on-demand in response to matrix metalloproteinases32 or by using light as a stimulus21. However, because tethered ligands may only impact cells for a short period of time, exploiting such strategies will likely require an understanding of how the pericellular matrix evolves and interacts with the cells.
The ECM impacts cellular behaviours directly via interactions with cellular receptors; however, it also acts as a storage reservoir for secreted signalling proteins that can be released on-demand. Therefore, biomaterials can also be used to prompt cells to secrete ECM that sequesters specific secreted signalling proteins. For example, culturing MSCs on a self-secreted matrix helps to maintain their multipotency, because the matrix binds bone morphogenetic protein-2, a growth factor that they themselves secrete and which would otherwise promote differentiation33. Similarly, many cells secrete TGFβ1, which can drive fibrosis under pathological conditions. However, the proteoglycan decorin binds TGFβ1, rendering it inactive34. Therefore, creating a tissue scaffold that prompts cells to secrete particular ECM proteins that sequester signalling proteins may be a powerful strategy to either direct a desired response, such as stem cell maintenance, or to preclude a deleterious response such as fibrosis.
Local-capture-and-enrichment systems
An alternative to directing the production of specific ECM is to design materials that capture and maintain an instructive ECM pericellularly, where it can signal back to the cells. Peptides that bind fibronectin, laminin and collagen type I have been incorporated into PEG-based hydrogels35. When MSCs are encapsulated within them, these hydrogels capture these ECM proteins specifically in their pericellular space. Such peptide-based protein-capture approaches could be applied to harness the signalling specificity of the secreted ECM with chemistries that allow for on-demand and spatially resolved control. For example, peptide-modified hydrogels have been designed to contain a photolabile caging group on an amino acid that can be removed on exposure to light36, rendering the peptide active. Such chemistries could be used to capture specific secreted proteins, and to do so on-demand and with precise spatial patterns within 3D tissue constructs. Alternative strategies that can similarly capture secreted proteins specifically and locally include aptamer-based traps created from single-stranded oligonucleotides37 and single-domain antibodies38,39 that capture almost any target protein. The chemical versatility of these approaches would similarly allow them to be incorporated into materials with user-controlled chemistries, essentially providing an array of strategies to locally capture and spatiotemporally control any secreted protein.
Harnessing local physical cues
Although biological ligands can be harnessed to direct the assembly of specific proteins pericellularly, physical properties can also be used to modulate the local secreted ECM. Analyses of encapsulated hMSCs (by using stable isotope labelling by amino acids in cell culture) show that their secreted proteome is relatively similar, regardless of hydrogel crosslinking density4. This suggests that physical properties of the biomaterial, such as mesh size, may regulate the pericellular distribution of the secreted ECM by regulating the rate and distance that secreted matrix diffuses away from a cell, and thus its ability to signal back to it. Simple physical parameters such as crosslinking density and degradability have long been known to regulate ECM production and distribution, particularly by chondrocytes40,41. However, such parameters could also be modulated to selectively regulate the local distribution of the secreted proteome, allowing ECM proteins maintained pericellularly on the basis of physical effects such as size to stimulate biological activities. For example, some large proteoglycans can have molecular weights of more than 1,000 kDa and would be captured pericellularly in hydrogels with small mesh sizes that might still allow relatively large glycoproteins such as fibronectin (440 kDa) and growth factors such as TGFβ1 (25 kDa) to diffuse farther away. Charge and other non-covalent interactions could be similarly harnessed to regulate the distribution and local retention of secreted proteins. For example, by tuning the functional groups of hydrogels bearing tethered small molecules with varying charge and hydrophilicity42, it may be possible to locally enrich for particular types of matrix proteins (for example, highly charged proteoglycans).
Beyond modulating the composition and distribution of the pericellular matrix, secreted proteins may regulate stem cell differentiation by mechanisms that exceed the complexity of their canonical signalling functions. As with degradation, which softens the cells’ surroundings and has long been known to regulate cellular behaviours in 3D, the secreted matrix can also play a role in stiffening the cells’ pericellular space4, which may similarly influence fate specification. The secreted matrix can impact the intracellular shuttling of Yap and Taz (ref. 5), which are key co-transcriptional regulators of mechanotransduction in MSCs (ref. 43). Further studies are required to potentially link pericellular stiffening in hydrogels to Yap and Taz or to other mechanosensitive pathways. Identifying such a mechanism would open the possibility of harnessing the secreted matrix to modulate local mechanics around cells as an additional strategy to elicit a desired cellular response. An elegant option for the exploitation of this phenomenon would be the design of biomaterials with different local and bulk properties by encapsulating single cells in a thin layer of hydrogel, thus creating single-cell microgels. As microgels allow for the tuning of the local mechanical properties around individual cells44, they could be exploited to prompt the production of a desired ECM locally. Microgels have recently been combined with 3D printing to uncouple microscopic and macroscopic properties within tissue-engineered scaffolds45.
Applications of the cell-secreted ECM
Native tissue morphogenesis requires cellular self-organization, which is highly dependent on bidirectional cell–matrix interactions46. To facilitate cellular interactions within hydrogels, recombinant ECM proteins or ECM-derived adhesive peptide sequences can be incorporated into the biomaterial. However, adhesive peptides such as RGD often do not elicit cellular responses that are identical to those of the full native protein47, and even tethering full recombinant proteins to hydrogels does not always produce the desired cell responses. Although recombinant proteins provide more native-like binding than minimal peptides, the interactions can still lack the complexity afforded by native proteins, which include post-translational modifications and supramolecular organization. Therefore, the cell-secreted matrix may be crucial for the engineering of tissues, particularly if pericellular composition and distribution can be modulated. In what follows, we outline examples of how the secreted matrix can be leveraged to engineer tissues for applications in regenerative medicine and for modelling tissues and diseases (Fig. 3).
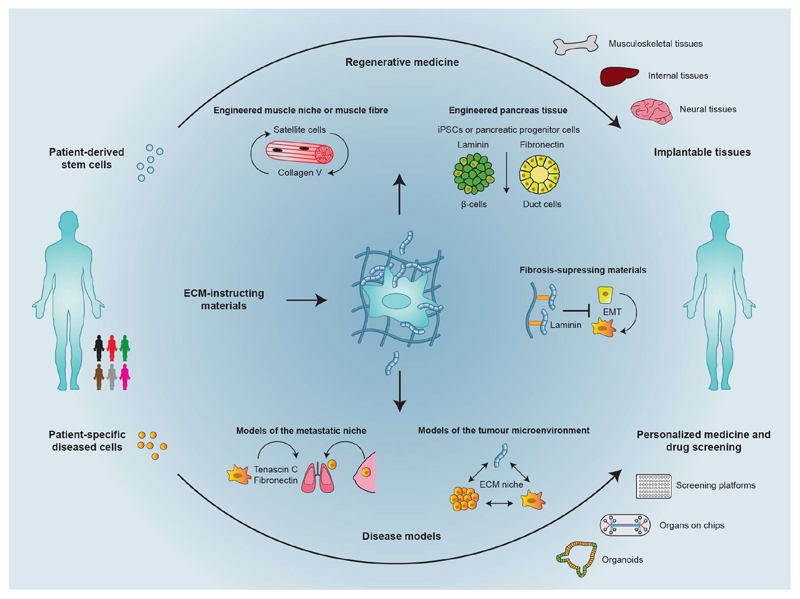
Fig. 3. Applications of the cell-secreted matrix.
ECM-instructing materials can be used to engineer tissues for regenerative medicine, tissue modelling and disease modelling. To create tissues that can be used therapeutically, secreted collagen type V could be captured locally to regulate the quiescence of satellite cells, and the differentiation of pancreatic progenitor cells controlled by regulating the local retention of fibronectin versus laminin. The secreted ECM can also be harnessed to create tissue models and disease models. Such models may be particularly useful in designing and testing therapies that disrupt metastasis in situations in which cancer cells secrete ECM to create a niche that allows them to form tumours in other tissues. Tissue models and disease models that use patient-derived cells could be used for personalized medicine (for example, to pre-screen chemotherapies prior to use in patients). iPSCs, induced pluripotent stem cells.
Regenerative medicine
A variety of culture systems can be used maintain, expand and differentiate the cells required to create tissues. For example, there are artificial stem cell niches that can maintain muscle stem cells (satellite cells) ex vivo in their highly potent and quiescent state48. However, even optimized engineered muscle fibres cannot fully recapitulate the properties of the native tissue. Collagen type V, which is secreted by muscle satellite cells in their niche, regulates their own quiescence49. Therefore, engineering a muscle fibre using materials that locally and specifically capture collagen type V could form the basis of an in vitro system that would allow for the further study of niche regulation in normal human cells, and also enable the testing of potential therapies in diseased cells, such as those derived from patients with Duchenne muscular dystrophy. Moreover, such a construct could be implanted therapeutically to maintain satellite cells in their quiescent state. One could even use chemistries controlled by light to release the sequestered collagen, thereby stimulating the cells to differentiate and create new muscle tissue on-demand.
The secreted matrix may similarly be harnessed to create cell-based therapies to treat diabetes. hiPSCs could be used for diabetes therapy because in principle hiPSCs can be expanded to provide the billions of cells necessary to regulate blood-glucose levels50; however, the efficiency of their differentiation is one factor that currently limits their therapeutic use. The differentiation of pancreatic progenitor cells into duct cells is known to be regulated by fibronectin (and by laminin in their differentiation into insulin-producing β-cells)51. A tissue scaffold that selectively binds laminin over fibronectin by using peptide-based capture or aptamer-based capture could thus promote the formation of insulin-producing pancreatic islets. This could be combined with anti-fibrotic hydrogels52 for the development of iPSC-based therapies that could alleviate the dependence of diabetic patients on insulin injections.
Humanized tissue models and disease models
There is a pressing need to create in vitro disease models that can outperform small-animal models, which cannot faithfully recapitulate many human diseases53. In vitro disease models should allow for the study of the influence of molecular factors, cell types and microphysiological stimuli on functional tissue responses, and permit the screening of potential treatments on patient-derived cells. Also, because ECM remodelling is central to many human pathologies, tissue models that incorporate the secreted ECM could enable a better understanding of the role of the matrix in disease.
Cancer cells respond to the physical properties of their environment, and hydrogels can be used to probe the impact of the environment’s stiffness on the susceptibility of cancer cells to chemotherapies54. However, the tumour microenvironment is also regulated by the matrix, which is secreted by both cancer cells and cancer-associated fibroblasts. Synthetic materials could be exploited as diagnostic devices for the investigation of how cell-derived ECM impacts cancer-cell growth and metastasis-like behaviour in the presence of different chemotherapies. In combination with emerging imaging55 and proteomics-based26,56 techniques for the visualization and determination of the composition of the secreted ECM, they could be used for therapy development. For example, exosomes from pancreatic cancer cells create a fibronectin-rich metastatic niche in the liver57, and breast-cancer cells express the glycoprotein Tenascin C to create an appropriate microenvironment for colonization in the lung58. Therefore, by creating tissue models that can either present exogenous Tenascin C or capture secreted Tenascin C, it may be possible to dissect the individual components of the tumour environment that drive metastasis, and to build a diagnostic device for testing the efficacy of inhibitory drugs. This strategy has been exploited by creating implantable tumour microenvironments containing stromal cells that assembled tumour-niche-like matrix and provided insight into how the local environment impacts the ability of disseminated prostate cancer cells to form metastases59.
Similarly, tissue models engineered from materials that modulate the secreted matrix could be used to study fibrosis. For example, in liver fibrosis and lung fibrosis pathological matrix secretion stiffens the local environment and signals back to the cells in a pernicious positive feedback loop60,61. Fibrosis could be inhibited by implantable materials that enrich the pericellular space for proteins that can preclude the local accumulation of proteins involved in fibrosis. For example, matrix metalloproteinase-2 (MMP-2) drives fibrosis by compromising the basement membrane and thus prompting cells to undergo an epithelial-to-mesenchymal transition. However, the laminin β1-chain can attenuate the expression of MMP-2. Tethering laminin β1 to an electrospun biomaterial can prevent peritoneal fibrosis in mice62. However, as laminin β1 is ubiquitous in the body and constantly being turned over, a material that could specifically capture the laminin β1-fragment might suppress the formation of fibrotic tissue around an implant. It could also be incorporated into injectable materials to discourage fibrosis, particularly in wound-healing applications.
Outlook
The understanding of the regulatory impacts of secreted ECM presents exciting opportunities and challenges for the engineering of tissues. The premises that hydrogels provide unidirectional signals to direct cellular behaviours and that tailored material cues alone can provide persistent instructions to cells are understood to be invalid. Instead, the secreted matrix is dynamically instructive24. This is particularly challenging for translational applications, where uncontrolled cellular responses to ill-defined cues might prove deleterious. Moreover, the secreted matrix may not simply override material cues, but also drive aberrant cellular behaviours, limit diffusion to and from the cells, and even preclude cell–cell interactions. To address these challenges, a thorough mechanistic understanding of how the secreted ECM regulates cell responses, and to what degree this can be manipulated in engineered tissues, is needed. At present, it is still unclear to what extent cell responses are driven by adhesive interactions with their own secreted matrix, whether specific signalling molecules maintained locally play a role, and if alterations in mechanotransduction by the secreted matrix regulate cellular behaviours. Protein-labelling4,5 and proteomics4,63 techniques are available to visualize and identify the composition of the secreted matrix in 3D hydrogels. Used in situ, the omics-based identification of the secreted matrix and time-resolved imaging have the potential to foster a step change in understanding of the spatiotemporal formation and impact of the secreted ECM on cells. A clear picture of the dynamic changes at the cell/material interface is also needed to guide materials-based strategies for tissue engineering. All observations to date are limited to hMSCs cultured in a handful of hydrogel materials, so it will be vital and exciting to learn how secreted ECM influences other cell types, particularly hiPSC, which are a promising cell source for regenerative medicine. The roles of the more limited matrix secretion by epithelial cells, which often exist as single cell layers (and thus more akin to 2D culture), are completely unknown. Furthermore, almost all work thus far has focused on the proteinaceous components of the ECM. However, the ECM is also rich in signalling proteins, such as growth factors, and sugars (in particular, proteoglycans), which are also captured pericellularly63 and may be even more potent regulators of cell responses. Answering these questions will widen opportunities for harnessing the secreted ECM to create the next generation of engineered tissues.
Acknowledgements
U.B. acknowledges postdoctoral-funding support from the Balgrist University Hospital Zurich. M.M.S and E.G. acknowledge support from the UK Regenerative Medicine Platform ‘Acellular / Smart Materials – 3D Architecture’ (MR/R015651/1).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Sharma B, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5:167ra166. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Occhetta P, et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat Biomed Eng. 2019;3:545–557. doi: 10.1038/s41551-019-0406-3. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen EH, et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat Biomed Eng. 2017;1 doi: 10.1038/s41551-017-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira SA, et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nat Commun. 2018;9:4049. doi: 10.1038/s41467-018-06183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loebel C, Mauck RL, Burdick JA. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat Mater. 2019;18:883–891. doi: 10.1038/s41563-019-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blache U, et al. Notch-inducing hydrogels reveal a perivascular switch of mesenchymal stem cell fate. EMBO Rep. 2018;19 doi: 10.15252/embr.201845964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans ND, Gentleman E. The role of material structure and mechanical properties in cell-matrix interactions. J Mater Chem B. 2014;2:2345–2356. doi: 10.1039/c3tb21604g. [DOI] [PubMed] [Google Scholar]
- 10.Huleihel L, et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madl CM, et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat Mater. 2017;16:1233–1242. doi: 10.1038/nmat5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhuri O, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranga A, et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci USA. 2016;113:E6831–e6839. doi: 10.1073/pnas.1603529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enemchukwu NO, et al. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J Cell Biol. 2016;212:113–124. doi: 10.1083/jcb.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Acuna R, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissell MJ, Hall HG, Parry G. How Does the Extracellular-Matrix Direct Gene-Expression. J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 19.Morgner J, et al. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat Commun. 2015;6:8198. doi: 10.1038/ncomms9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roskelley CD, Bissell MJ. Dynamic reciprocity revisited: A continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem Cell Biol. 1995;73:391–397. doi: 10.1139/o95-046. [DOI] [PubMed] [Google Scholar]
- 21.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutolf MP, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater. 2016;1 doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott KE, Rychel K, Ranamukhaarachchi S, Rangamani P, Fraley SI. Emerging themes and unifying concepts underlying cell behavior regulation by the pericellular space. Acta Biomater. 2019;96:81–98. doi: 10.1016/j.actbio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 26.Devaud YR, et al. Label-Free Quantification Proteomics for the Identification of Mesenchymal Stromal Cell Matrisome Inside 3D Poly(Ethylene Glycol) Hydrogels. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800534. [DOI] [PubMed] [Google Scholar]
- 27.Murad S, et al. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78:2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence JR, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–U120. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capeling MM, et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019;12:381–394. doi: 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora N, et al. A process engineering approach to increase organoid yield. Development. 2017;144:1128–1136. doi: 10.1242/dev.142919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shadish JA, Benuska GM, DeForest CA. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat Mater. 2019 doi: 10.1038/s41563-019-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzger S, et al. Cell-Mediated Proteolytic Release of Growth Factors from Poly(Ethylene Glycol) Matrices. Macromol Biosci. 2016;16:1703–1713. doi: 10.1002/mabi.201600223. [DOI] [PubMed] [Google Scholar]
- 33.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 35.Hezaveh H, et al. Encoding Stem-Cell-Secreted Extracellular Matrix Protein Capture in Two and Three Dimensions Using Protein Binding Peptides. Biomacromolecules. 2018;19:721–730. doi: 10.1021/acs.biomac.7b01482. [DOI] [PubMed] [Google Scholar]
- 36.Lee TT, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stejskalova A, Oliva N, England FJ, Almquist BD. Biologically Inspired, Cell-Selective Release of Aptamer-Trapped Growth Factors by Traction Forces. Adv Mater. 2019;31 doi: 10.1002/adma.201806380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamperman T, et al. Spatiotemporal material functionalization via competitive supramolecular complexation of avidin and biotin analogs. Nat Commun. 2019;10:4347. doi: 10.1038/s41467-019-12390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridy PC, et al. A robust pipeline for rapid production of versatile nanobody repertoires. Nat Methods. 2014;11:1253–1260. doi: 10.1038/nmeth.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64:70–79. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 41.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 42.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 44.Mao AS, et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat Mater. 2017;16:236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamperman T, et al. Single Cell Microgel Based Modular Bioinks for Uncoupled Cellular Micro- and Macroenvironments. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201600913. [DOI] [PubMed] [Google Scholar]
- 46.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264:1437–1442. [PubMed] [Google Scholar]
- 48.Quarta M, et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol. 2016;34:752–+. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baghdadi MB, et al. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature. 2018;557:714–+. doi: 10.1038/s41586-018-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millman JR, Pagliuca FW. Autologous Pluripotent Stem Cell-Derived beta-Like Cells for Diabetes Cellular Therapy. Diabetes. 2017;66:1111–1120. doi: 10.2337/db16-1406. [DOI] [PubMed] [Google Scholar]
- 51.Mamidi A, et al. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature. 2018;564:114–118. doi: 10.1038/s41586-018-0762-2. [DOI] [PubMed] [Google Scholar]
- 52.Bochenek MA, et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat Biomed Eng. 2018;2:810–821. doi: 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tissue-engineered disease models. Nat Biomed Eng. 2018;2:879–880. doi: 10.1038/s41551-018-0339-2. [DOI] [PubMed] [Google Scholar]
- 54.Shin JW, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc Natl Acad Sci U S A. 2016;113:12126–12131. doi: 10.1073/pnas.1611338113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLeod CM, Mauck RL. High fidelity visualization of cell-to-cell variation and temporal dynamics in nascent extracellular matrix formation. Sci Rep. 2016;6 doi: 10.1038/srep38852. 38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayorca-Guiliani AE, et al. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat Med. 2017;23:890–898. doi: 10.1038/nm.4352. [DOI] [PubMed] [Google Scholar]
- 57.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–U256. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carpenter RA, Kwak JG, Peyton SR, Lee J. Implantable pre-metastatic niches for the study of the microenvironmental regulation of disseminated human tumour cells. Nat Biomed Eng. 2018;2:915–929. doi: 10.1038/s41551-018-0307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker MW, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li CX, et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater. 2017;16:379–389. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 62.Horejs CM, et al. Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information. Nat Commun. 2017;8 doi: 10.1038/ncomms15509. 15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira SA, et al. Neighboring cells override 3D hydrogel matrix cues to drive human MSC quiescence. Biomaterials. 2018;176:13–23. doi: 10.1016/j.biomaterials.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]