Abstract
Over the past few decades, a range of vesicle-based drug delivery systems have entered clinical practice and several others are in various stages of clinical translation. While most of these vesicle constructs are lipid-based (liposomes), or polymer-based (polymersomes), recently new classes of vesicles have emerged that defy easy classification. Examples include assemblies with small molecule amphiphiles, biologically derived membranes, hybrid vesicles with two or more classes of amphiphiles, or more complex hierarchical structures such as vesicles incorporating gas bubbles or nanoparticulates in the lumen or membrane. In this review, we explore these recent advances and emerging trends at the edge and just beyond the research fields of conventional liposomes and polymersomes. A focus of this review is the distinct behaviors observed for these classes of vesicles when exposed to physical stimuli - such as ultrasound, heat, light and mechanical triggers - and we discuss the resulting potential for new types of drug delivery, with a special emphasis on current challenges and opportunities.
Keywords: Physical stimulus, vesicle, ultrasound, thermo-responsive, light-responsive, mechano-responsive, amphiphile, self-assembly
1. Introduction
The aim of drug delivery is to increase therapeutic efficacy. Drug delivery vesicles can aid in this pursuit by enhancing on-target effects while reducing off-target toxicity [1, 2]. For example, by increasing local drug concentration in diseased tissues while reducing accumulation in other tissues and organs (e.g. the liver or kidneys), or by releasing a drug in response to a biological stimulus (e.g. the release of insulin in response to a change in blood glucose levels in a diabetic patient). The central challenge is thus endowing drug delivery vesicles with the means to influence the spatiotemporal distribution of therapeutics.
A range of bio-responsive nanomaterials and vesicles have been developed towards addressing this challenge. This includes materials that respond to physiological changes in pH, redox environment, and the presence of enzymes (e.g. upon endocytosis by cells) [3, 4]. A complementary route towards enhanced spatiotemporal control of therapeutics is using drug delivery vesicles that can be triggered by physical stimuli (e.g. ultrasound or light) instead—or in addition to—physiological stimuli [5]. The design and development of drug delivery vesicles integrating responsive and triggering functionalities has therefore received increasing attention.
Liposomes are often considered the archetype of many drug delivery vesicles, originally described half-a-century ago [6] and widely considered the first “nanoscale drug” approved for clinical use [7, 8]. Polymersomes are closely related vesicles, assembled from polymers instead of lipids [9] and first reported in the mid-1990s [10, 11]. Liposomes and polymersomes have received intense research interest since their introduction—especially in biomedical applications—as recently reviewed by Torchilin and co-workers [12] and Gu and co-workers [13].
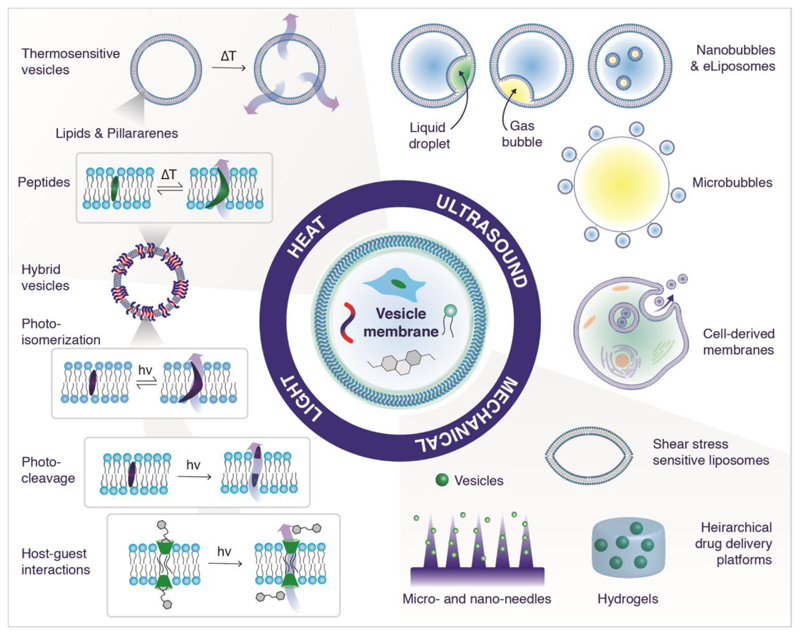
Excitingly, in the last few years a range of new vesicle-based drug delivery systems have emerged that defy easy classification as they go beyond many of the conventional strategies and materials typically associated with liposomes and polymersomes (Figure 1). These developments are fueled by recent advances in materials science and engineering, and the resulting vesicles typically fall at the edge or between main themes of liposome and polymersome research. The potential of these emerging vesicle systems to help drive a step-change in drug delivery responsive to physical stimuli is the subject of intense research, and the focus of this review.
Figure 1.
Examples of vesicle drug delivery systems including non-conventional amphiphiles or hierarchical assemblies, which deliver a payload in response to heat, light, ultrasound and mechanical forces.
In this review, we explore emerging approaches incorporating non-conventional strategies and materials (e.g. hybrid materials, gases and emulsions) of various origins (e.g. synthetic or biological) for the assembly of diverse classes of stimuli-responsive vesicles (Figure 1). Following a short primer on triggers, amphiphiles and hierarchical structures, the discussion is structured around the targeted physical stimuli: (i) ultrasound, (ii) heat, (iii) light, and (iv) mechanical stimuli. We conclude by providing an outlook on current challenges and opportunities, and explore potential research directions towards accelerating the development and translation of the next generation of vesicle-based drug delivery systems.
2. Triggered drug delivery using vesicles
2.1. Endogenous and exogenous triggers
Stimuli-responsive systems can roughly be divided into two groups according to whether they react to endogenous or exogenous stimuli [14]. Nanomaterials that react upon endogenous triggers take advantage of the physiological differences between diseased and healthy tissue, such as chemical differences including pH [15], hypoxia [16, 17], redox [18] or enzymes [19, 20], and physical differences including mechanical triggers [21] such as shear stress-mediated release [22, 23] and temperature changes [24]. Altered environmental changes can be found in tumors, infections, ischemia and inflammatory diseases. Exogenously triggered nanomaterials can react to external physical stimuli like light [25, 26], ultrasound [27, 28] and magnetic fields [29], as well as mechanical triggers [21] such as shear thinning upon injection [30]. Within this review we focus on physically triggered vesicular systems, specifically intrinsic mechanical and temperature changes and extrinsic light, ultrasound and mechanical stimuli. As the nomenclature around responsiveness, triggering, and targeting can sometimes be confusing, we here largely follow the definitions and terminology described by Wang and Kohane [5].
2.2. Conventional vesicle-based nanocarrier systems
Vesicles are an extensively studied and varied class of nanocarriers that consist of an amphiphilic membrane bilayer enclosing an aqueous core. These supramolecular structures can be formulated from a wide range of amphiphilic molecules including lipids, polymers, and organic and inorganic small molecules. Their formulation is driven primarily by thermodynamically favorable clustering of hydrophobic moieties (e.g. lipid tails) while hydrophilic moieties (e.g. phospholipid head groups) face the aqueous solution. [31] The geometry of the constituent amphiphilic building blocks dictates the self-assembled morphology and is often described by the packing parameter, p, defined as:
where, v is the volume of the hydrophobic chains, ao is the optimal head-group area and lc is the hydrophobic tail length. [32] In general, vesicles form when 0.5 ≤ p ≤ 1, spherical micelles form when p ≤ 0.33, cylindrical micelles when 0.33 ≤ p ≤ 0.5, and inverted micelle structures such as the micellar cubic phases at p > 1 [33]. Similar approximations can be applied to dendrimer self-assemblies [34] and block co-polymers [35]. Thanks to impressive progress in material science there are plenty of examples of vesicle-based delivery vectors, predominantly comprising lipid [7, 8, 36–38] and polymer-based [13, 39–42] vesicles typically prepared through self-assembly approaches such as thin film hydration or template-based assembly [43, 44]. Some liposome formulations are already FDA approved and in clinical use, primarily Doxil [45], AmBiosome and their derivatives [46]. A range of others are in translational status towards clinical use [46].
2.3. Vesicles comprising alternative amphiphiles and/or hierarchical structures
There is a plethora of examples of well-established, conventional liposomal and polymersome-based drug delivery systems [12, 13]. Emerging at the edge of these research fields are vesicle-based constructs formulated using alternative amphiphiles and/or higher hierarchical structures for physically triggered drug delivery—although some examples have already reached more advanced stages (notable examples include vesicle-bubble [27] and vesicle-emulsion constructs which have been successful in the field of ultrasound-mediated drug delivery and imaging [47]). These more exotic vesicle constructs have been used to transport a range of active cargos including small molecule pharmaceutics, enzymes, nucleic acids [48, 49] and imaging agents [50], using optimization techniques for specific therapeutic activities commonly used in the design of more conventional liposome and polymersome systems. These emerging approaches can offer new avenues in the preparation of vesicles, with tailored surface chemistry, size, shape and architecture, which may improve their drug release behavior, targeting properties, and biodistribution profiles [51, 52]. Vesicle surfaces can further be functionalized with “stealth” or targeting moieties (e.g. polyethylene glycol and/or antibodies) [53]. The addition of polyethylene glycol (PEG) chains to the outer surface increases steric repulsion and results in longer blood circulation times [54]. In this review, we present an up-to-date summary of vesicle delivery systems responsive to diverse physical triggers. The focus is on vesicle-based constructs comprising “alternative” amphiphiles and/or higher hierarchical structures, with an emphasis on contributions over the last few years.
3. Ultrasound
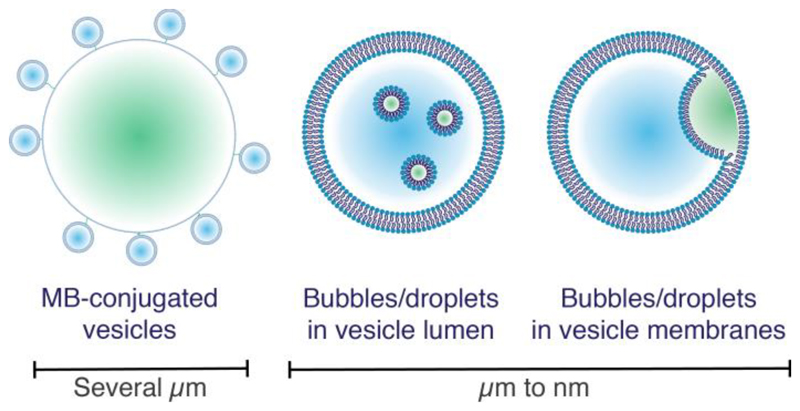
Clinical ultrasound (US) is one of the largest applications of exogenously triggered physical release from vesicles, and is a non-invasive, inexpensive, readily available, and well-established tool for both clinical imaging and therapeutics. Microcarriers for US contrast, typically containing low molecular weight perfluorinated gases, or mixtures of these gases with air, have been used in the clinic since the 1990s [55]. In recent years, US-sensitive constructs comprising gas-filled bubbles and vesicles have been extensively studied for imaging and drug delivery [56]. The gas bubble may be micrometer-sized and have vesicles tethered to its surface, or may be nanometer-sized and incorporated into the vesicle membrane or in the internal aqueous compartment. The possibility of using perfluorocarbons in liquid state at physiological temperature has also been investigated, and the term eLiposomes has been coined to describe liposomes containing such emulsion droplets [57–59]. Here, we discuss the developments over the last few years in the field of vesicle–bubble and vesicle–droplet constructs for US-mediated drug delivery, along with recent applications using biologically-derived membranes (Table 1).
Table 1. Ultrasound-responsive vesicles listed by triggered species, including architectural details on example delivery systems and amphiphiles used.
| Triggered Species | Example Delivery System | Vesicle Amphiphiles | Ref |
|---|---|---|---|
| Perfluorinated gases | Vesicles tethered to MBs | Lipids | [60–62] |
| MBs that form vesicles upon trigger | Lipids | [63] | |
| MBs encapsulated in vesicles | PLA | [64] | |
| Monocytes encapsulating MBs in presence of DOX loaded polymersomes | [65] | ||
| DNA-conjugation cationic liposomes with encapsulated nanobubbles | DOTAP and PEGylated lipids | [66] | |
| Emulsion droplets in vesicle lumen | Vesicles encapsulating droplets = eLiposomes | Lipids | [57–59, 67, 68] |
| Giant cluster sized vesicles with Lipiodol | Polyglycerine polyricinoleate (PGPR) | [69] | |
| Vesicles encapsulating hydrogen peroxide | PLGA | [70] | |
| Emulsion droplets in vesicle membrane | Vesicles encapsulating PFOB in membranes | PEG polymer | [71] |
| Vesicles encapsulating HMME in membranes | Polymer/Lipid | [72] | |
| Direct US on biological membranes | EV shedding, sonoporation, cavitation of MBs | Derived from cell membrane | [73] |
3.1. Microbubbles
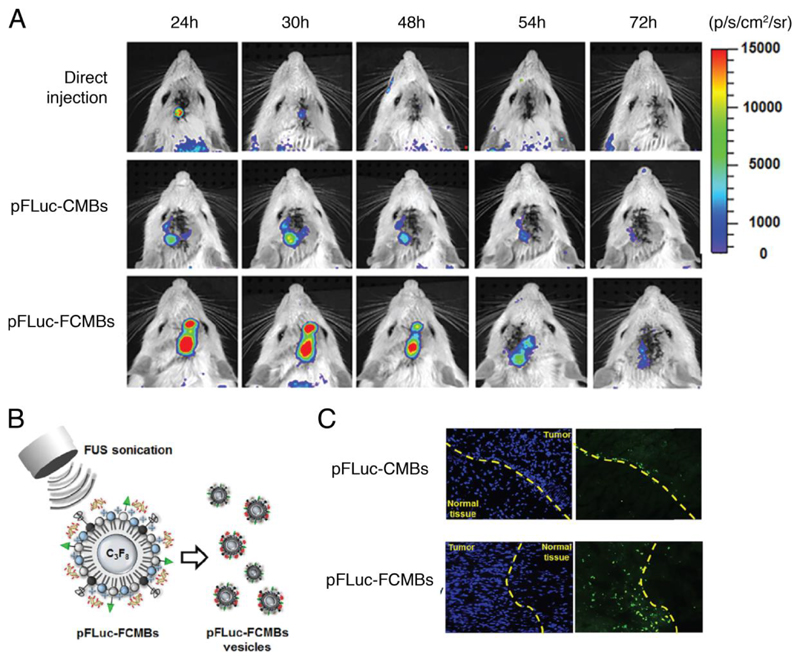
Constructs combining micrometer-sized gas-filled bubbles (microbubbles, MB) and drug-loaded vesicles are promising US-mediated delivery vectors. The pressure wave induced during US can cause these bubbles to oscillate and collapse during a process called inertial cavitation [74]. Depending on the US frequency applied, this process can induce transient permeation or irreversible damage to the membranes of neighboring cells and artificial vesicle constructs, leading to vesicle rupture and release of encapsulated contents. Typically, gas bubbles comprising mixtures of air and perfluorinated gas are stabilized using a monolayer of phospholipids or other amphiphiles including polymers and surfactants [60, 61, 75], and vesicles containing therapeutics can be tethered to their surface. The release of small molecules and enzymes from liposomes conjugated to lipid-stabilized MBs is well reported in the literature, for example in the release of thrombin to accelerate localized blood clotting [62]. In vivo studies have also shown that low frequency ultrasound can be used to increase the permeability of the blood–tumor barrier in a rat model [76]. This effect has been exploited by Fan and co-workers who formulated folate conjugated, DNA-loaded cationic MBs which form vesicles in situ under focused US (FUS) [63]. These vesicles contain the same DNA payload as the parent bubbles and in vitro studies showed they could be used to target tumors (see Figure 2) [63].
Figure 2.
In vivo gene transfection using classical (CMB) and folate-conjugated (FCMB) MBs. (A) In vivo gene transfection on rat brain tumor model with 700 kPa. (B) Outline of technique in which vesicles are formed in situ during US. (C) The distribution of pFLuc-CMBs vesicles or pFLuc-FCMBs vesicles within brain tissue after treatment. The vesicles of pFLuc-FCMBs only largely accumulated in the tumor location, suggesting that the conjugation of folate onto pFLuc-FCMBs shells might promote MB-derived vesicles to enter the tumor tissue from cerebral vessels. Green spots: MB-derived vesicles. Adapted from [63] with permission from Elsevier.
3.2. Vesicles containing microbubbles and nanobubbles
MBs have also been encapsulated into vesicles and used to deliver, for example, chemotherapeutic agents [77]. Wrenn and co-workers found that vesicles formulated from various amphiphiles can be tailored for specific applications [64]. Specifically, microcapsules formed from polylactic acid (PLA) are preferential for imaging because they prevent gas escape into the bulk (increasing bubble longevity), inhibit inertial cavitation and absorb the energy of inertial cavitation where it does not occur (improving safety). They also give contrast-to-tissue ratios that are just as bright as commercial agents and persist at least ten times longer. Liposomes on the other hand, specifically large unilamellar vesicles, showed improved release profiles versus polymersomes in US-mediated drug delivery, since by varying the lipid composition it is possible to tune bilayer phase behavior. While there are several microbubble formulations on the market [78], the production of nanometer-scale bubbles encapsulated into vesicles is also a highly active area of research. These gas bubbles can be incorporated into the lumen or membrane of vesicles which are normally pre-formulated before the gas is introduced [79]. Bubbles as small as around 100 nm have been shown to survive up to 100-fold longer than their micron-sized counterparts under US [80]. Their smaller size can also make them more permeable to capillaries in the tumor vasculature and they could therefore penetrate tissues more easily compared to their larger counterparts [79]. While the junctions in epithelial cells are usually smaller than 8 nm, tumor tissues can on the other hand have a “leaky” vasculature allowing nanoparticles on the order of 100 nm to enter and be retained because of the defective lymphatic drainage system in what is often referred to as the enhanced permeability and retention (EPR) effect [81]. It is important however to remember that the EPR effect is highly tumor specific, and is not present in all tumors [82].
Nanometer-scale bubbles can be formulated with narrower size distributions than MBs, and because of their smaller size can be included in the aqueous core of vesicles [83]. A nanobubble–vesicle construct, where lipid-stabilized nanobubbles are incorporated into the aqueous compartment of liposomes, has been investigated as a treatment for stroke. The authors could deliver plasmin in vitro to human whole blood clots, leading to clot lysis [84]. Negishi and co-workers have also reported DNA-coated cationic liposomes of around 650 nm diameter containing nanometer-sized, phospholipid-stabilized perfluoropropane bubbles. They could deliver bFGF-expressing pDNA locally using focused US, which led to the induction of angiogenic factors and improved blood flow [66].
3.3. Vesicles containing emulsion droplets
In addition to perfluorinated gases, there has been increasing interest in using amphiphile-stabilized droplets of US-sensitive perfluorinated liquids, and the term eLiposomes has been coined to describe liposomes containing such emulsion droplets [58]. Lattin and co-workers have shown that droplets formed from perfluorocarbons (e.g. pentafluoropentane or perfluorohexane) can be stabilized with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and extruded to uniform size [57]. Liposomes, also formed from DPPC, are then assembled around the droplets using the interdigitation method and extrusion, leading to droplets encapsulated in the aqueous core. US-mediated release of encapsulated calcein dye was increased when the droplet size [67] and overall vesicle construct [68] was bigger. However, although 200 nm eLiposome constructs released less encapsulated dye than their larger 800 nm counterparts, this smaller size has the advantage of more easily being endocytozed (by non-phagocytic cells) for cytosolic delivery applications. For example, folate-conjugated eLiposomes with diameters from 200 to 600 nm have been successfully used to deliver a model drug to HeLa cells in vitro [59]. It has further been shown that both free emulsion droplets and eLiposomes loaded with a model drug and labeled with folate can be internalized into the endosome of folate receptor-expressing HeLa cells. Vaporization of the endosome by US led to release of the contents of the fractured endosome into the cytosol, including drugs delivered to the extracellular matrix [85]. This process provides a route to delivering extracellular drugs and drugs encapsulated in eLiposomes to the cytosol. The authors also observed that the perfluorocarbon used has an effect, with perfluoropentane proving more effective than perfluorohexane in delivering the model drug to the cytosol. Efforts have also been made to improve efficacy of commercially available contrast agents. For example, giant cluster-like vesicles (GCVs) have been loaded with the commercially available X-ray contrast agent Lipiodol and fluorescent dye. They are destroyed by an US activated device, commonly used for cutting and sealing tissue in laparoscopic surgeries [69]. This technique could also be applied to delivering therapeutics at the site of US-mediated tissue incision.
In a move away from perfluorinated hydrocarbons, Li and co-workers have reported a hydrogen peroxide filled PLGA polymersome including iron oxide nanoparticles in the membrane [70]. Here, the hydrogen peroxide in the encapsulated solution provides oxygen which acts as the echogenic source. On US-mediated disruption of the membrane the hydrogen peroxide encounters the membrane-bound iron oxide nanoparticles and by the Fenton reaction forms hydroxyl free radicals which act as the therapeutic moiety. It was observed that this non-thermal process eradicated malignant tumors in a nude mouse model.
In the above examples, the emulsion is incorporated into the vesicle lumen, however there are examples of emulsion droplets in the membrane bilayer as well (Figure 3). Polymer-based vesicles allow transport of perfluorinated contrast agents in the liquid state, such as perfluorooctyl bromide (PFOB), which has a boiling point of 144°C. While lipid-based vesicles have been investigated for this use, polymer-based vesicles have been found to be a more promising avenue since the interaction between the phospholipids and the PFOB is not strong enough to easily form vesicles that are stable on the nanoscale. Hao and co-workers have reported a vesicle formulated from poly(ethylene glycol) methyl ether-block-poly(D,L lactide) block copolymers that encapsulates PFOB in the membrane bilayer, which provides good US contrast in blood pool imaging in in vivo studies [71]. Sonodynamic therapy is a technique that uses US and a sonosensitizer encapsulated in vesicles that, upon US treatment, is released and forms reactive oxygen species. Hematoporphyrin monomethyl ether (HMME) is a hydrophobic liquid and a known sonosensitizer and can be easily incorporated into polymer membranes [72] and liposome bilayers. Chen and co-workers have shown in in vitro studies that liposomes labelled with the mitochondria-targeting triphenylphosphonium bromide, with HMME loaded into the lipid membrane, can lead to around 60% cell death upon 3 minutes of US, versus 20% in the liposome-only control [86]. Localized heating by high-intensity focused ultrasound can be used as an external trigger to release content from temperature-sensitive liposomes (TSLs, see section 4.2.1) and polymersomes, in the absence of perfluorinated bubbles or emulsions. This is used to induce thermal ablation of tumors at around 57°C [87], and more commonly, to induce non-destructive hyperthermia in the region of 39-42°C [88, 89]. Extrapolating from this success with conventional techniques, non-lipid or polymer-based vesicles with US sensitivity are another avenue for future investigation. However, so far there is only one report, by Song and Zhang, of non-liposome or non-polymer-based constructs with similar properties [90]. They described tetraphenylethylene macrocyclic compounds that can self-assemble into hollow spheres. When triggered with US, these constructs rearrange to form “bird nest” structures composed of nanorods with potential applications in controlled release of anti-cancer drugs.
Figure 3.
Schematic representation of different kinds of echogenic liposomes. Left, microbubbles with vesicles tethered to their surface. Middle, bubbles or emulsions encapsulated within vesicles. Right, gas or emulsion-in-bilayer vesicles. Blue denotes aqueous encapsulated contents and green denotes perfluorocarbon and/or air.
3.4. Ultrasound and biological membranes
Recently there have been examples of US-triggered constructs including biologically derived membranes. Specifically, loading MB-vesicle mixtures into cells for intracellular drug transport makes for a Trojan horse type delivery. For example, Huang et al. found in mouse studies that monocytes could be loaded with polymeric MBs and subjected to FUS in the presence of doxorubicin-loaded polymer vesicles (DLPV). This enabled the vesicles to target tumors more specifically than if they were directly injected intravenously. [91] Specifically, in the monocyte delivery system 60% was delivered to the tumor vs. 80% delivery to the liver in the case of MBs and DLPVs alone.
Sonoporation uses low intensity US to induce cavitation of MBs. This leads to enhanced endocytosis and formation of transient membrane pores in neighboring cells [92], and has been shown to trigger the release of extracellular vesicles (EVs) [65]. Paproski and co-workers first reported that the release of EVs from cancer cells could be stimulated using US and lipid-coated MBs [73]. This led to a 100-fold increase in the circulating tumor DNA (ctDNA). Analysis of ctDNA is a powerful tool for liquid biopsies and profiling tumor genetics and the authors could characterize the tumor phenotype, including its aggressiveness, based on the protein and nucleic acids presented in these EVs produced under US triggered MB cavitation. The identification of aggressive tumors before they metastasize is critical to improve patient outcomes, as the vast majority of cancer deaths are due to metastatic cancer.
In an example using droplets, red blood cell ghosts formulated into microdroplets with perfluoropentane have been proposed for anti-cancer theranostics. When loaded with a chemotherapeutic agent and triggered with US, they led to around 70% cell death in in vitro experiments with a HeLa cell model [93].
At smaller length scales, gas vesicles (GVs) are nanometer-sized anisotropic vesicles with a 2 nm thick protein shell. Expressed intracellularly in some bacteria and archaea, these gas-permeable vesicles regulate cell buoyancy in aqueous environments. Lakshmanan and co-workers have recently shown that these vesicles can be genetically engineered to modify their harmonic response, surface charge, collapse pressure, targeting specificity, and fluorescent properties. In vivo imaging studies in mice showed that the harmonic contrast was greatly improved in genetically modified GVs vs. the wild-type, and in vitro studies of GVs modified to include several fluorescent dyes showed that cellular targeting was possible [94]. Further discussion of such vesicles and their applications can be found in a review by Aw and Paniwnyk [95].
4. Heat
Temperature regulated processes play a vital role in biology, and many biomedical applications of vesicles have taken inspiration from biology to pursue heat triggerable mechanisms (Table 2). Localized hyperthermia has by itself become an effective tool for the treatment of solid tumors [96, 97]. In this method, tumors are heated to temperatures exceeding 50°C which has a cytotoxic effect and leads to coagulated necrosis. However, this treatment method has the drawback of inhomogeneous heating causing incomplete destruction of tumors as well ineffective targeting of deeply seated tumors. The combination of hyperthermia with thermoresponsive vesicles can improve its efficacy due to accumulation of nanocarriers within the tumor site via passive or active targeting. The success of a thermoresponsive system depends on the availability of a sensitive carrier that readily reacts to small temperature changes.
Table 2. Thermoresponsive vesicles listed by type of triggered vesicle, including details on amphiphile type and triggered mechanism.
| Type of vesicle | Type of amphiphile | Triggered mechanism | Ref |
|---|---|---|---|
| TSL | Lipids | Rearrangement of fatty acid chains above Tm of lipids, leaky membranes | [98–101] |
| Liposomes encapsulating NaCO2 | Lipids | NaCO2 decomposition loaded into liposomes, dissociation of vesicles | [102, 103] |
| Polymersomes | Polymers | Change of amphiphile hydrophilicity upon LCST or UCST, dissociation of vesicles | [13, 42, 104–108] |
| Hybrid vesicle | Mixture of amphiphiles (lipids, polymers, peptides, etc.) | Rearrangement of component mostly peptides, leaky membranes | [109–111] |
| Macrocycle vesicles | Functionalized pillararene macrocycles | Dissociation of vesicles | [112, 113] |
4.1. Conventional thermoresponsive vesicles
4.1.1. Liposomes
Almost all thermoresponsive vesicles described in literature are based on liposomes and polymersomes. The thermoresponsiveness of liposomes can be modulated by tuning the lipid composition to alter the phase transition temperature (Tm), around which conformational changes within the lipid bilayer are triggered. Around the melting temperature of the lipid, fatty acid chains rearrange from a gel state to a liquid crystal state. This can either happen instantaneously at the melting temperature or it can be a more progressive process that begins at the melting temperature and completes at a much higher temperature. In both cases, this process leads to a more fluid and leaky membrane and a drug release as a result [98–100] with a significant increase in the membrane permeability around the transition melting temperature. These conventional thermosensitive liposomes (TSLs) were first introduced in the 1970s by Yatvin et al. [101], and within the last 30 years a large body of research has been generated in their development all the way towards clinical testing [99]. Other thermoresponsive mechanisms have also been investigated. For example, sodium bicarbonate can be loaded into liposomes to facilitate a pH gradient for doxorubicin, but also to trigger release upon heating [102, 103]. Hyperthermia causes the sodium bicarbonate to decompose into ammonia and carbon dioxide bubbles, resulting in liposomal membrane defects and therefore release of the loaded drug. The authors demonstrated high biostability of their nanocarrier after intravenous injection into mice and showed effective tumor-selective chemotherapy upon heat trigger. Combinations of various triggers can often lead to even greater efficacy and targeting ability. For example, Kheirolomoom and co-workers used US-mediated hyperthermia to release a pH-sensitive doxorubicin–copper complex from long-circulating TSLs that gathered in tumors due to the EPR effect. Intravascular release of the drug was achieved by applying US to the tumor for a total of 25 minutes, including 5 minutes prior to injection of the therapeutic. In in vivo experiments with DL-tumor bearing mice, after twice weekly treatments over 28 days, the tumors had disappeared. The tumors were still undetectable eight months after treatment [98].
4.1.2. Polymersomes
Polymers are a very common thermoresponsive material, where the most commonly used examples are poloxamers, poly(N-substituted acrylamide)s, poly(N-vinylcaprolactam)s and chitosans [13, 104–106]. These react reversibly to temperature changes in a very narrow temperature window. The lower critical solution temperature (LCST) describes the temperature at which, upon heating, a hydrophilic polymeric structure changes to a hydrophobic state and becomes insoluble in water. On the other hand, when a polymer becomes soluble upon heating they are described by the upper critical solution temperature (UCST) [42]. For example, the most investigated example of polymeric amphiphiles, poly(N-isopropylacrylamide) (PNIPAM), has a LCST of 32°C which is close to normal physiological temperatures. For both liposomes and polymersomes, plenty of examples can be found in the literature and both have been thoroughly reviewed [2, 13, 14, 107, 108]. Here we instead focus on emerging candidates including hybrid vesicles and supramolecular amphiphiles.
4.2. Thermal rearrangement of peptides embedded in vesicle membranes
Hybrid vesicles are formed from two or more different types of amphiphiles, for example phospholipids, polymers or small molecules. Al-Amahdy et al. were the first to report temperature-responsive hydrid vesicles [109, 110] with their study of vesicles comprising lipids and a leucine-zipper peptide that imparts thermoresponsiveness. This peptide increases the thermostability of the overall lipid vesicles at 42°C by decreasing its membrane fluidity. It is known that the peptide forms a super helix coiled-coil structure at lower temperatures and that this self-assembled structure is dissociated at increased temperatures, which is the proposed mechanism for the thermoresponsive drug release behavior. The authors showed loading of the chemotherapeutic doxorubicin and demonstrated successful delivery into tumor tissue after intravenous injection into mice. The payload was then released upon heat trigger causing local cytotoxicity and tumor regression.
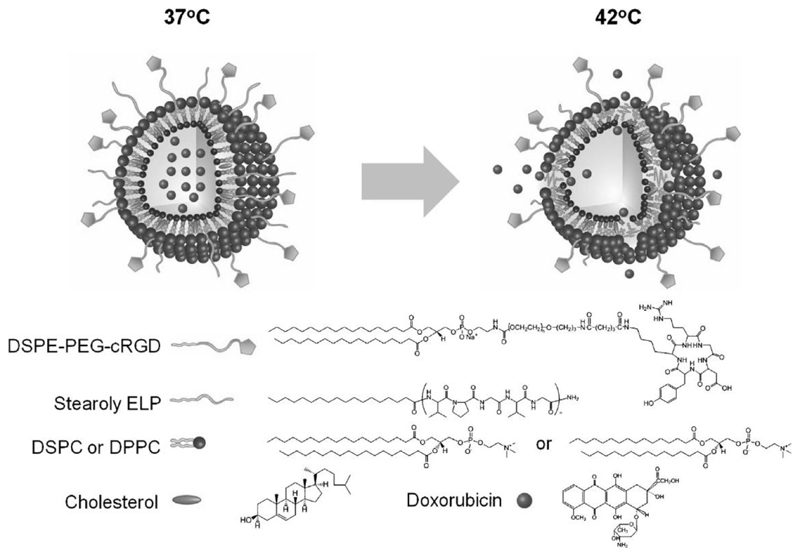
In another hybrid example, vesicles formulated from lipids and an elastin-like polypeptide that show thermally responsive phase transition behavior (Figure 4). Kim and co-workers showed that functionalizing these nanocarriers with cyclic arginine-glycine-aspartic acid (cRGD) ligands resulted in 8-fold and 10-fold higher uptake into αvβ3integrin overexpressing U87MG and HUVEC cells respectively, and efficient cytotoxicity was demonstrated upon heat application [111]. The vesicles showed good loading and release abilities for doxorubicin and thermoresponsive properties were maintained for up to 12 h, with payload release triggered under very mild hyperthermia. Moreover, the nanocarrier showed an approximately 5-fold higher accumulation in tumor tissue compared to non-targeted vesicles after intravenous injection into mice.
Figure 4.
Hybrid vesicles consisting of lipids, a targeting unit and elastin-like peptide for mild hyperthermic release of doxorubicin. Adapted from [111] with permission from Elsevier.
4.3. Pillararenes
Zhou et al. reported vesicles with thermoresponsive properties constructed by glycol chain functionalized amphiphilic Pillar[5]arenes [112]. These macrocycle amphiphiles can self-assemble into 120 nm sized bilayer vesicles that collapse upon heating. Interestingly, this process is reversible as the vesicles reform upon cooling. Moreover, they established magnetic responsiveness by incorporating magnetic nanoparticles within the bilayer. Co-encapsulated fluorescent calcein was then released upon application of a magnetic field due to deformation of the vesicles into irregular structures.
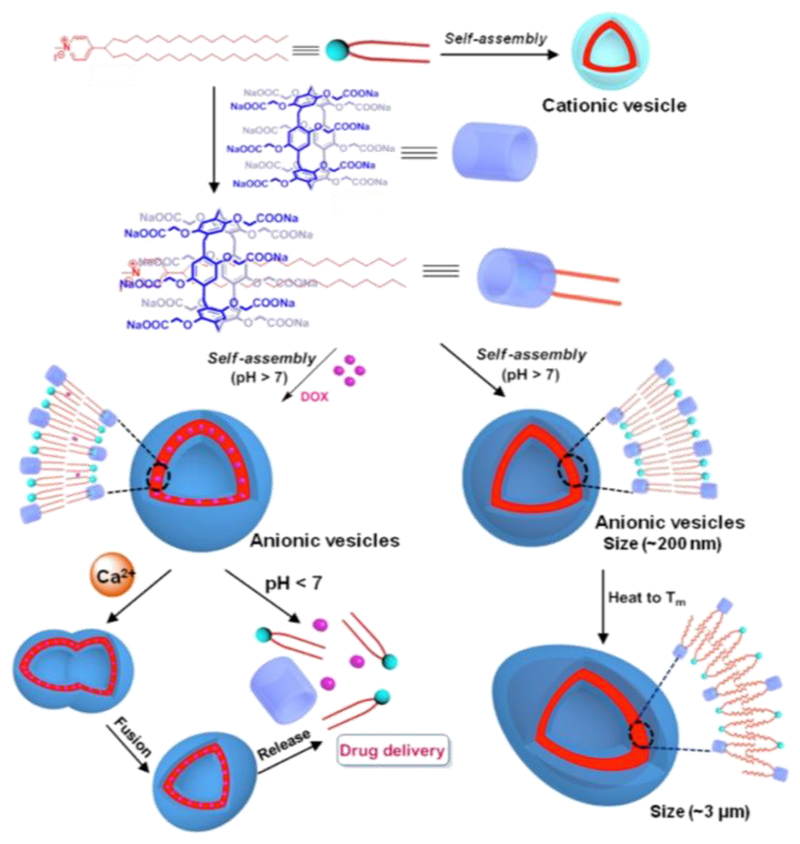
A doxorubicin-loaded supramolecular Pillar[6]arene based vesicle was also reported by Wang and co-workers (Figure 5) [113]. As well as being thermoresponsive, these supramolecular amphiphile vesicles also show responsive behavior to a change in pH and addition of Ca2+. The authors showed via in vitro experiments with MCF-7 cancer cells and healthy NIH3T3 cells that their system does not reduce the toxicity of doxorubicin towards cancer cells. Moreover, they demonstrated that heat could trigger cytotoxicity, and that cytotoxicity without heat trigger towards healthy cells was reduced compared to treatment with free DOX.
Figure 5.
Multi-responsive Pillar[6]arenes. Adapted with permission from [113]. Copyright (2018) American Chemical Society.
5. Light
Photoresponsive vesicles allow for spatial as well as temporal control with non-invasive tissue penetration (Table 3), and most examples have a specific wavelength for illumination in the UV range [114]. UV light is often used because of its high energy, which can induce conformational changes or even the breaking of covalent bonds. However, a major drawback in using UV light is the low penetration depth because of scattering in biological tissue. Recent examples have therefore focused on the near infrared region (NIR), which shows low absorbance by skin and tissue enabling a penetration from hundreds of micrometers to centimeters. Two photon technologies can increase the efficacy of NIR nanocarriers even further where pulsed lasers provide a high density of photons in short time pulses (nanosecond to femtosecond) which are absorbed by photolabile groups with two photon cross sections, for example coumarin derivatives.
Table 3. Photoresponsive vesicles listed by type of light triggered mechanism including details on mechanism and architectural composition of example delivery systems.
| Category | Mechanism (triggered molecule) | Example Delivery System | Ref |
|---|---|---|---|
| Light responsive drug release | Cleavable linker (coumarin and nitrobenzyl) | Dendrimersomes with nitrobenzyl units | [115] |
| Photoisomerization (spiropyran and azobenzene) | Cationic azobenzene amphiphiles forming vesicles | [116] | |
| Amphiphilic cyclodextrin vesicles with surface immobilized azobenzene-conjugated cargo | [48, 49, 117–121] | ||
| Azobenzene functionalized amphiphilic cyclodextrin | [122] | ||
| Supra-amphiphiles via host guest interaction of pillararene and azobenzene | [123] | ||
| Supra-amphiphiles via host guest interaction of cucurbituril, methylviologen and azobenzene | [124] | ||
| Light Upconversion Nanoparticles (UCNPs) | UCNps embedded in hybrid vesicle (lipids and amphiphilic azobenzene units) | [125] | |
| Photothermal therapeutic | Heating (gold nanorods, DIR) | Exosomes with membrane embedded gold nanorods | [126] |
| Cell derived nanovesicles with membrane embedded DIR | [127] | ||
| Photodynamic therapeutic | Generation of reactive species (porphyrin, phthalocyanine, squaraine) | Vesicles with membrane embedded photosensitizer | [50] |
| Amphiphilic cyclodextrin vesicles with surface-immobilized squaraine | [128] | ||
| Amphiphilic cyclodextrin vesicles with surface immobilized phthalocyanine | [129] | ||
5.1. Vesicles for light-responsive drug release
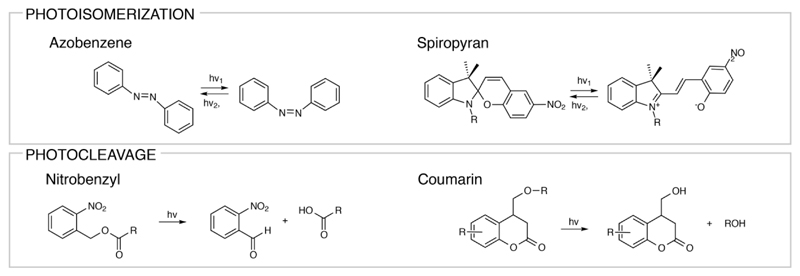
Phototriggerable nanomedicines can usually be categorized into three groups: (i) light-responsive drug releasing therapeutics, (ii) photothermal therapeutics, and (iii) photodynamic therapeutics. Light-sensitive drug release is usually facilitated by implementing light-cleavable linkers, light-induced crosslinking, light-induced degradation, or light-responsive conformational changes. Well-known examples of light-triggerable components that can directly react with light are azobenzenes or spiropyrans that react via photoisomerization [130, 131] and nitrobenzyl or coumarin groups that react via cleavage [132, 133] (Figure 6).
Figure 6.
Chemical structures of photosensitive small molecules that can undergo photoisomerization and photocleavage.
5.1.1. Vesicles with Photoisomerisable or Photocleavable units
Azobenzene derivatives are among the most investigated examples in the UV-range due to their trans-to-cis isomerization at 300–400 nm [134]. Transition back to the more thermodynamically stable trans form can be obtained by either irradiation in the visible range or heating. The isomerization is accompanied by physical changes (like polarity) which is often used to control the self-assembly of its nanocarrier. Despite their extensive use, azobenzenes have two major drawbacks. As already mentioned, UV-light has a high scattering rate in biological tissue that can be damaging to healthy tissue, and the cis-isomer has low thermodynamic stability [135]. As a result, current research efforts are more directed towards the functionalization of azobenzenes (e.g. fluorinated azobenzenes) to shift the wavelength for isomerization into the visible or even NIR region and to obtain more stable cis-isomers [136–138]. However, examples for visible or NIR region trans to cis switching of azobenzene derivatives have not yet been reported in vesicles. The following examples for azobenzenes focus therefore on recent and innovative examples of UV light responsive switches.
The synthesis of a new cationic azobenzene (CAB) derivative that self-assembles into vesicles when mixed with anionic SDS in aqueous solution was recently reported by Geng et al. [116]. The authors show that these CAB/SDS vesicles undergo photoresponsive drug release via a cis-trans isomerization upon irradiation of the azobenzene derivative, and that irradiated doxorubicin-loaded CAB/SDS vesicles exhibit a higher cytotoxicity than non-irradiated ones.
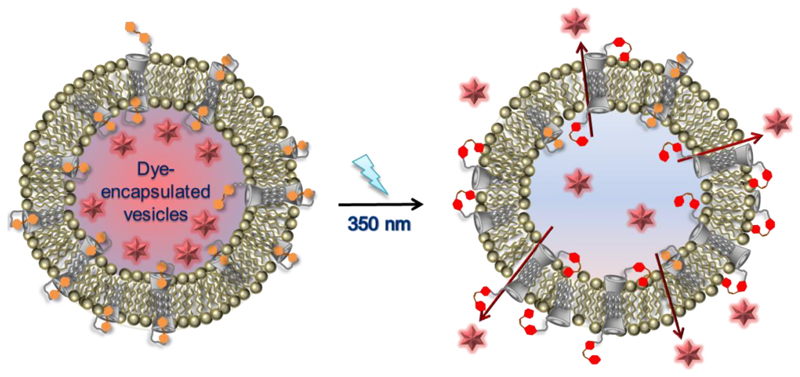
In another example, Ravoo and collaborators have also incorporated photoresponsive azobenzene units into vesicles, in this case via host–guest inclusion into vesicles formulated from amphiphilic cyclodextrin molecules [139]. These cyclodextrin vesicles are biocompatible, non-toxic, versatile and easy to functionalize, with guest molecules bound via reversible host–guest recognition to the surface of their surface [139, 140]. Their work has shown reversible recognition processes on vesicles emulating biological systems [118–120, 141, 142] as well as drug release and targeting with a variety of different biomolecules including carbohydrates [143–146], peptides [147–149], proteins [48, 121] and nucleotides [48, 49, 117]. Building on this, Kauscher et al. have synthesized an amphiphilic β-cyclodextrin that is covalently labelled with azobenzene [122] and embedded into a liposomal membrane consisting of DOPE, DOPC and cholesterol. Here, azobenzene binds into the cyclodextrin cavity in its trans form, but once it is isomerized to its cis form the inclusion complex dissociates. The authors showed that this light-responsive switching can be used to control the vesicle membrane permeability, where UV light irradiation was followed by content release (Figure 7).
Figure 7.
Amphiphilic cyclodextrin with covalent azobenzene side functionalization for phototriggered drug release from hybrid vesicles [122]. Reproduced with permission from The Royal Society of Chemistry.
Stricker and collegues enhanced the photoswitching ability of their cyclodextrin vesicles by introducing a new arylazopyrazole photoswitch, including water soluble carboxylated derivaties.[136] While in azobenzene molecules the absorance spectra of the cis and trans isomers overlap, arylazopyrazole derivatives can be isomerized from cis to trans with green light, and the absorbances of the cis and trans isomer do not overlap. As a result the authors show nearly quatitative isomerization with UV (trans to cis) and green light (cis to trans). This is clearly advantageous to the conventionally used azobenzenes.
Photoresponsive host–guest recognition-based vesicles comprising Pillar[6|arenes and azobenzenes were first formulated into vesicles by Huang et al. in 2012 [123], and are a good alternative to the more established cyclodextrin–azobenzene systems. This group has since presented azobenzene-conjugated polymers that form amphiphiles with Pillar[6]arenes [124]. Under UV irradiation they dissociate and the azobenzene-functionalized polymers self-assemble into micelles. Visible light irradiation reverses the formation of micelles back to vesicles. Giant supramolecular vesicles have also been observed when the host molecule cucurbit[8]uril is mixed with maleimide-modified methylviologen and hydrophobic 3,4,5-tris(n-dodecyloxy)benzoylamide with an azobenzene moiety [150]. Again, azobenzene isomerization causes the supra-amphiphile to dissociate leading to release of an encapsulated drug. The maleimide moiety allows for easy functionalization with target molecules like RGD or BSA and authors demonstrated cellular uptake by three different human cancer cell lines as well as stability and drug delivery abilities of the nanocarrier.
Spiropyran units have been incorporated into liposomes as early as 1998 by Yuichi Ohya et al. [151]. They synthesized a single chain lipid containing the spiropyran unit at its hydrophobic terminus, which could release a dye on UV irradiation. Recently, Kwangmettatam et al reported the synthesis of a small amphiphile bearing two spiropyran moieties and an ethylenglycol backbone [152]. These amphiphiles assemble into vesicles in water, and expand to double their size upon irradiation and resulting photoisomerization. Interestingly this behavior is reversible and as soon as irradiation is stopped the vesicles shrink back to their initial size. They envision incorporation of their synthesized amphiphile into a range of self-assembled structures which can undergo complex shape transformations, due to regions of bilayer phase separation.
Finally, dendrimer-based vesicles, termed dendrimersomes, have been designed to contain photodegradable hydrophobic blocks with o-nitrobenzyl units throughout their backbones. Upon exposure to UV light, the dendrimers undergo complete photolytic backbone cleavage, causing dissociation of the dendrimersome self-assemblies and release of both hydrophilic and hydrophobic payloads [115].
5.1.2. Upconversion of NIR light
Light can also be used for indirect photoactivation via a so-called light upconversion [153]. Light upconversion describes the absorption of NIR light by the carrier into more energetic visible or UV light. The upconverted light then causes the cleavage or isomerization of the phototriggerable functionality [154, 155]. In one example, Yao et al. loaded upconversion nanoparticles (UCNPs) into hybrid vesicles consisting of lipids and an amphiphilic azobenzene-molecule [125]. NIR irradiation at 980 nm is upconverted to UV light by the encapsulated UCNPs, which is in turn absorbed by the azobenzene moieties within the vesicle membrane. The azobenzene units then undergo a trans-to-cis isomerization, allowing the release of encapsulated drugs. Simultaneous irradiation with visible light causes a continuous rotation-inversion mechanism that results in leaky membranes. The authors showed how vesicles loaded with the cancer drug doxorubicin caused tumor growth inhibition after intravenous injection into nude mice bearing MCF-7/ADR tumors and NIR irradiation of the tumor tissue.
5.2. Photothermal therapy
Photothermal therapy describes the transformation of NIR into heat and the subsequent treatment and ablation of diseased tissue, mostly cancer tissue [156–158]. Given the high spatial and temporal selectivity, and minimal toxicity to healthy tissue, this technique exhibits some unique advantages compared to conventional therapeutic modalities [25, 159]. Since heat can be used to change the fluidity of bilayer membranes, combination of photothermal agents with vesicles is often used to create responsive drug delivery systems.
Vesicles derived from natural membranes have been investigated for this purpose. In one example, Liu and coworkers engineered exosomes to be nanoplatforms for chemo- and photothermal tumor therapy [126]. Exosomes are naturally derived vesicles shed from many cell types [160, 161]. In their work [126], the authors cultured donor cells with RGD and sulphydryl groups in their membranes, and exosomes collected from these cells showed the same surface modification. Gold nanorods immobilized via Au–S bonds on the surface of these exosomes and further covalent modification with tumor targeting moiety folic acid was carried out. The authors demonstrated accumulation of the exosomes in tumor tissue after intravenous injection into mice, due the dual targeting of folic acid and RGD, and enhanced drug release in response to the heat generated by gold nanorods in response to NIR irradiation.
The near infrared dye DIR has also been used to transform NIR into heat by Wu et al. in a naturally derived vesicle system [138]. They used cell-based nanovesicles obtained via extrusion of DC2.4 cells. Several extrusion steps with diminishing extrusion pore sizes resulted in vesicles with a diameter of about 100 nm. By co-extruding the cells with doxorubicin in solution, DOX-loaded vesicles were obtained, and DIR was subsequently inserted into the vesicle membrane by adding an ethanol solution of the dye to the vesicle suspension. The authors report that NIR irradiation causes heat that eradicates tumor cells but also destroys the nanovesicle membrane resulting in DOX release. In vitro tumor cytotoxicity and in vivo tumor growth inhibition in a murine model was demonstrated.
5.3. Photodynamic therapy
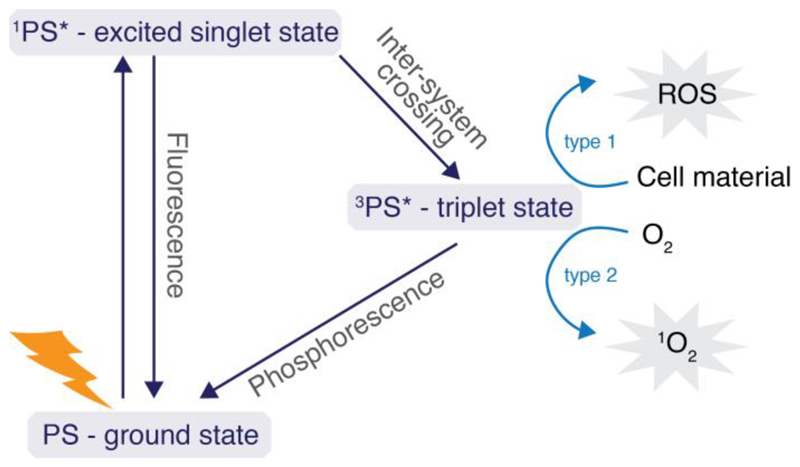
Photodynamic therapy (PDT) is the non-invasive treatment of cancerous or diseased tissue, where toxic species that can cause cell death are produced via the irradiation of a photosensitizer [162, 163]. Upon irradiation, the photosensitizer is excited to its singlet state and reaches a triplet state via intersystem crossing (Figure 8). Upon relaxation, electrons are transferred either to the surrounding cell material causing the production of radicals, or singlet oxygen is generated via the interaction of the photosensitizer with oxygen. Most PDT nanosystems are based on the photosensitizer porphyrin with Photofrin being the first sensitizer approved for treating cancer in the 1990s.[164] However, porphyrin absorbs mostly in the visible region, which is a disadvantage because of the low penetration depths of visible light for biological tissue [165, 166]. Research has therefore been directed to photosensitizers that absorb in the visible or near infrared region and has included examples of squarain [167], porphyrin, cyanin, bodipy and phthalocyanine [168] derivatives. [169] Most of these photosensitizers are macrocylic compounds with poor water solubility. [170] Due to their extended conjugated π-system they tend to form H-aggregates, which reduce their ability to produce singlet oxygen. Combination of these photosenisitizers with a delivery platform has been shown to reduce aggregation and to increase their photosensitizing ability. [171, 172]
Figure 8.
Jablonski diagram illustrating photosensitizer excitation and relaxation in photodynamic therapy. Upon relaxation of the photosensitizer from the triplet to ground state, reactive oxygen species (ROS) or singlet oxygen (1O2) is formed.
Vesicles have been used to transport photosensitizers or have been directly constructed out of photosensitizers. Porphysomes, vesicles consisting of porphyrin-functionalized lipids, have been reported by Lovell et al. [50]. These photo-therapeutic vesicles can also act as imaging agents, as quenched porphysomes allow for visualization of the lymphatic system by photoacoustic tomography. NIR fluorescence was triggered by degradation of enzymatic linkers allowing for low-background fluorescence imaging. The photo-therapeutic application was demonstrated by systemic administration, upon which the porphysomes accumulated within tumor tissue. The tumor was then successfully ablated via laser irradiation.
Kauscher et al. synthesized a squaraine molecule covalently equipped with two adamantane molecules [128]. This symmetric molecule could be bound via host–guest interaction to cyclodextrin vesicles, where it generated radicals on their surface upon irradiation with NIR light. This approach has potential for medical application due to the use of NIR and since target molecules can be attached to the vesicle surface in a host–guest driven manner. The same group reported cyclodextrin-based vesicles decorated with phthalocyanine-derivatives (Figure 9) [129, 173]. Phthalocyanines are macrocycles with outstanding photosensitizing properties due to their high absorption values within the near infrared region, their high intersystem crossing yields, long triplet state lifetimes, and high singlet oxygen quantum yields [174–176]. Within a study by Ravoo and Kauscher, phthalocyanines were functionalized with adamantane groups, which bind via host guest inclusion into cyclodextrin cavities [129]. The authors show that the immobilization of the photosensitizer on the surface of cyclodextrin-based vesicles prohibits inactivation via aggregation and enhances the phototherapeutic action of the phthalocyanines. The system was then improved by changing the zinc(II) phthalocyanine core to a Si(IV) core for higher phototherapeutic performance, and changing their symmetric functionalization with adamantane units to an asymmetric one for axial immobilization on cyclodextrin vesicles, which further prohibits undesired inactivation via H-aggregation in water. In vitro phototoxicity testing against methicillin resistant staphylococcus aureus bacteria (MRSA) showed almost entire inactivation.
Figure 9.
Cyclodextrin vesicles with immobilized asymmetric functionalized Si(IV)phthalocyanine via host-guest interaction. Reprinted with permission from [129] Copyright 2018 American Chemical Society.
In an example of multi-stimuli responsive vesicles, Wilhelm and co-workers used magnetically induced hyperthermia combined with photodynamic therapy for tumor ablation [177]. Their hybrid liposomes encapsulate iron oxide nanoparticles inside their hydrophilic core and exhibit the photosensitizer mTHPC payload within their membranes. The authors first showed in vitro that each treatment individually (hyperthermia and PDT) resulted in tumor cell death. Combined methods resulted in complete cell destruction with synergistic apoptotic signaling pathways. In vivo studies showed tumor regression with single treatments and complete eradication with combined treatment.
6. Mechanical triggers
Characteristic mechanical environments within the body can be harnessed as endogenous triggers for drug delivery for a range of self-assembled constructs, as reviewed recently by Gu and co-workers [21]. Mechanical stimuli, despite their interesting applications, are the least commonly studied response triggers, with very few successful examples in the literature. In this area, new mechano-responsive materials and innovative combinations of vesicles within more complex delivery systems have an important role to play (Table 4).
Table 4. Vesicle systems with mechanical triggers listed by type of mechanical trigger including information on treated disease as well as details on example delivery systems and their triggered mechanism.
| Mechanical trigger | Type of delivery system | Triggered mechanism | Ref |
|---|---|---|---|
| Shear stress in diseased heart | 1,3-diamidophospholipid vesicles | Lenticular morphology, destabilization in the membrane along the equator under shear stress, drug release | [23, 178] |
| EggPC/surfactant Brij S10 vesicles | Increased release compared to EggPC liposomes | [179, 180] | |
| Compressive forces across joints in osteoarthritis | Oxidized dextran hydrogel embedded DPPC/DPPE liposomes | Content release due to mechanosensitive synergy of vesicles and hydrogel | [181] |
| Physical perturbation of skin | Hollow microneedles with cationic niosomes | Vesicles delivered into skin due to perturbation of stratum cornium | [182] |
| Shear force | Cyclodextrin vesicles as crosslinker for adamantane functionalized cellulose fibers | Host guest complex disassembles and gel dissociates | [183] |
6.1. Shear stress as an endogenous trigger
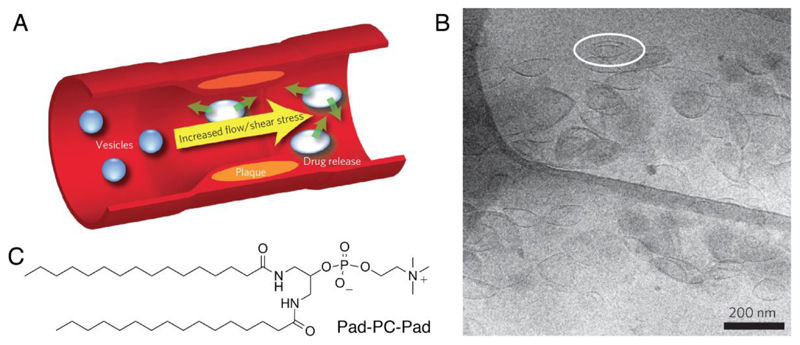
The increased endogenous shear stress observed in arteries of patients with cardiovascular disease has been investigated as a triggering mechanism for localized delivery of e.g. vasodilators [184] or anti-thrombolytic drugs [22]. The shear stresses in healthy vessels range from 0.1–7 Pa depending on the type and location [185]. However, flow simulations estimate that local shear stresses in critically constricted coronary arteries are as high as 20 Pa [178]. Holme and co-workers were the first to report vesicles sensitive to the shear stresses commonly seen in the diseased heart [23]. They formulated liposomes made from an artificial 1,3-diamidophospholipid, Pad-PC-Pad, which are stable in low shear stress but release their payload at elevated shear stress (Figure 10). Using a model cardiovascular system based on X-ray tomography images of diseased human coronary arteries [178], they showed that these circulating vesicles released 25% more of their encapsulated cargo after only one pass through a constricted model artery, versus vesicles passed through a “healthy” model artery control. This unusual property was attributed to the vesicles’ lenticular morphology, which is due to a preferential destabilization in the lipid bilayer along the equator. The vasodilator nitroglycerine was successfully incorporated into these liposomes with 20-50% encapsulation efficiency, and complement activation in human sera and whole pigs showed the constructs to be non-toxic [179, 180]. Holme et al. also reported that long-circulating liposomes formulated from a natural mixture of phospholipids extracted from chicken egg, EggPC, with a proportion of the surfactant Brij S10 showed increased release over time in the high shear stress, diseased artery model versus liposomes formulated from entirely EggPC [23].
Figure 10.
Shear stress delivery from a synthetic, mechanosensitive liposome. A, concept overview of a stenosed artery. B, Cryo-TEM of Pad-PC-Pad vesicles showing lenticular morphology. C, Pad-PC-Pad chemical structure. A and B reproduced with permission from [23].
6.2. Compressive forces in osteoarthritis
In the treatment of osteoarthritis, compressive forces between bones across joints can act as a trigger for release. To this end, mechanically-responsive liposome–hydrogel constructs have been reported by Stalder and Zumbuehl [181]. The authors chemically tethered liposomes comprising a mixture of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) and DPPC to an oxidized dextran hydrogel and observed the release of vesicle contents in response to hydrogel compression. Here, the mechanosensitive release is due to the synergy of the hydrogel and vesicles. The DPPE in the vesicle formulation was found to be crucial for release, with hydrogel-vesicle constructs comprising only DPPC showing no release upon compression.
6.3. Dermal and transdermal drug delivery
Encapsulating hydrophilic therapeutics within vesicles for increased uptake is a cornerstone of dermal and transdermal vesicular delivery systems. Many vesicle constructs have been proposed for this purpose including ethosomes (phospholipid and ethanol-based vesicles), niosomes (non-ionic surfactant based vesicles), pro-niosomes and vesosomes (nested liposomal structures) [186]. These predominantly rely on enhanced passive diffusion through the skin compared to conventional liposomes and polymersomes. Vesicle permeability can be vastly improved by physical perturbation of the skin via e.g. microneedles [187, 188], low frequency ultrasound, electroporation or iontophoresis [189]. These exogenous delivery methods can allow vesicles and other active therapeutics to traverse the otherwise impenetrable outermost layer of dead skin (the stratum corneum) and facilitate, for example, transcutaneous immunization [190]. Pamornpathomkul et al. have recently reported cationic niosomes loaded into hollow microneedles as a vector for transcutaneous immunization, which showed higher immune response in comparison with a subcutaneous injection control and no infection or bleeding in the skin [182]. Microneedle patches have also been integrated with hypoxia-sensitive hyaluronic acid vesicles encapsulating insulin and glucose oxidase, for the fast and glucose-dependent delivery of insulin to treat diabetes. The vesicles in this “smart insulin patch” dissociate under hypoxic conditions found in hypoglyemia, releasing insulin. In a mouse model, the patch was capable of regulating blood glocuse levels to within normal values. [191] On the single cell level, recent work by Chiappini et al. has studied the feasibility of needles loaded with a therapeutic moiety for delivery across the cell membrane. Specifically, they have shown that mesoporous silicon nanoneedles are able to deliver nucleic acids [192] and quantum dots [193] into the cytosol with minimum impact on cell viability. Micro- and nanoneedle platforms for delivering vesicle constructs are an interesting avenue for future research in both transdermal and transmembrane delivery.
6.4. Shear-thinning injectables
Mechanical forces can be used as a tool during administration by means of shear-thinning injectables. Himmelein et al. presented a hydrogel using cyclodextrin vesicles as multivalent junctions between cellulose fibers [183]. The fibers are equipped with adamantane functions that can interact via host–guest inclusion with the cyclodextrin cavities on the surface of the vesicles. Due to the reversibility of the inclusion complexes, the gel shows shear thinning and self-healing properties. This work could be extended to other hydrogels such as hyaluronic acid hydrogels, which are of interest due to their biocompatibility. Recently Leobel et al. have published a protocol for preparing HA hydrogels that are stabilized using the same adamantine–cyclodextrin host–guest interactions, albeit without vesicle moieties [30].
7. Summary and Outlook
Vesicle constructs for drug delivery have seen significant advances over the last few years. Examples include constructs prepared with bubbles or perfluorinated emulsions which can be combined with clinical ultrasound, and constructs assembled from biologically derived membranes. While the possibility of leveraging external actuation such as ultrasound or electromagnetic radiation is of great interest scientifically, additional consideration is needed when considering these emerging types of vesicle constructs for clinical translation.
Consider a vesicle engineered to release an anti-cancer drug upon irradiation with light. Upon the design of such a system one should ask what the additional benefit is compared to directly treating the tumor with ionizing radiation (i.e. the well-established clinical practice of radiotherapy [194]). Potential answers could involve the possibility of leveraging synergistic drug–radiotherapy responses [195], or using vesicles that enhance the “abscopal effect” to improve patient outcomes during immunotherapy [196]. A related challenge when using light-activated vesicles is the depth dependency which often becomes an issue when moving from small animal models to large animal models (and eventually patients), although recent reports show that nanophotosensitizers combined with Cherenkov radiation can potentially address this challenge [197].
When designing new types of vesicles for drug delivery and translational purposes there is always an inherent challenge of balancing new (and often exciting) functionality with simplicity and (often less exciting) tried-and-true approaches. This includes the biocompatibility of the vesicles (which involves different considerations for drug delivery systems compared to other biomaterial-based devices [198]) and performing a careful cost–benefit analysis before adding functionality to any vesicles intended for clinical translation [199]. These are multifaceted challenges which are best addressed through inter- and multidisciplinary approaches, for example through “convergent science” [200, 201].
Exploring strategies for bridging the gap between more fundamental bio–nano science and translational research remains one of the most challenging—but also potentially one of the most rewarding—research directions in the field [82]. For example, there is currently intense debate around the concept and clinical relevance of “targeting” [202, 203], and the strengths and weaknesses associated with many commonly used animal models [82]. Vesicles that respond to physical stimuli may provide a complementary avenue for exploring these and similar controversial topics. Many of the external triggers associated with these vesicles (e.g. ultrasound and electromagnetic radiation) can enable precise, clinically-relevant triggering mechanisms with very high spatial and temporal resolution, compared to vesicles that are triggered in response to endogenous cues (such as changes in pH or enzyme concentrations) which may display substantial heterogeneity between different tissues, patients and patient populations. Using externally actuated vesicles could therefore potentially help decouple some of the compounding effects that arise due to this biological heterogeneity. As a result, these emerging vesicle technologies may prove instrumental for furthering the development of property–performance relationships and predictive design rules, for advancing fundamental understanding in bio–nano science, and for guiding the development of the next-generation of drug delivery vesicles.
Acknowledgements
U.K. acknowledges support from the Deutsche Forschungsgemeinschaft [KA 4370/1-1]. M.N.H. acknowledges support from the FP7 Marie Curie Intra-European Fellowship "SMase LIPOSOME" [626766] and the National Research Programme "Smart Materials" of the Swiss National Science Foundation [P300PA_171540 and 406240_147493]. M.B. acknowledges support from Horizon 2020 (European Union) through a Marie Skłodowska-Curie Individual Fellowship [grant agreement no. 745676]. M.M.S. acknowledges support from a Wellcome Trust Senior Investigator Award [098411/Z/12/Z], the grant from the UK Regenerative Medicine Platform “Acellular / Smart Materials – 3D Architecture” [MR/R015651/1], the ERC Seventh Framework Programme Consolidator grant “Naturale CG” [616417] and the Swedish Foundation of Strategic Research through the Industrial Research Centre “FoRmulaEx” [IRC15-0065].
Abbreviations
- ctDNA
circulating tumor DNA
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- DLPV
doxorubicin-loaded polymer vesicles
- EPR effect
enhanced permeability and retention effect
- EV
extracellular vesicle
- GCVs
giant cluster-like vesicles
- HMME
Hematoporphyrin monomethyl ether
- MB
microbubble
- CMB
classical MB
- FCMB
folate-conjugated MB
- NIR
near infra-red
- PEG
polyethylene glycol
- PDT
photo-dynamic therapy
- PGPR
Polyglycerine polyricinoleate
- PLA
polylactic acid
- PNIPAM
poly(N-isopropylacrylamide)
- ROS
reactive oxygen species
- 1O2
singlet oxygen
- Tm
transition melting temperature (of lipids)
- TSL
thermosensitive liposome
- UCNp
light upconversion nanoparticle
- UCST and LCST
upper critical solution temperature and lower critical solution temperature (of polymersomes)
- US
ultrasound
Footnotes
ORCIDs
U.K.: 0000-0002-2681-5692
M.N.H.: 0000-0002-7314-9493
M.B.: 0000-0002-9876-7079
M.M.S.: 0000-0002-7335-266X
References
- [1].Yao J, Feng J, Chen J. External-stimuli responsive systems for cancer theranostic. AJPS. 2016;11:585–595. [Google Scholar]
- [2].Gu M, Wang X, Toh TB, Chow EK. Applications of stimuli-responsive nanoscale drug delivery systems in translational research. Drug Discov Today. 2017 doi: 10.1016/j.drudis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- [3].Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2017;2 16075. [Google Scholar]
- [4].Cui J, Richardson JJ, Björnmalm M, Faria M, Caruso F. Nanoengineered Templated Polymer Particles: Navigating the Biological Realm. Acc Chem Res. 2016;49:1139–1148. doi: 10.1021/acs.accounts.6b00088. [DOI] [PubMed] [Google Scholar]
- [5].Wang YF, Kohane DS. External triggering and triggered targeting strategies for drug delivery. Nat Rev Mater. 2017;2 17020. [Google Scholar]
- [6].Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- [7].Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- [8].Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23:3319–3329. doi: 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- [9].Christian DA, Cai S, Bowen DM, Kim Y, Pajerowski JD, Discher DE. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur J Pharm Biopharm. 2009;71:463–474. doi: 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang L, Eisenberg A. Multiple Morphologies of "Crew-Cut" Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science. 1995;268:1728–1731. doi: 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]
- [11].van Hest JC, Delnoye DA, Baars MW, van Genderen MH, Meijer EW. Polystyrene-dendrimer amphiphilic block copolymers with a generation-dependent aggregation. Science. 1995;268:1592–1595. doi: 10.1126/science.268.5217.1592. [DOI] [PubMed] [Google Scholar]
- [12].Pattni BS, Chupin VV, Torchilin VP. New Developments in Liposomal Drug Delivery. Chem Rev. 2015;115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- [13].Hu X, Zhang Y, Xie Z, Jing X, Bellotti A, Gu Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules. 2017;18:649–673. doi: 10.1021/acs.biomac.6b01704. [DOI] [PubMed] [Google Scholar]
- [14].Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang X-J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [16].Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- [17].Dachs GU, Dougherty GJ, Stratford IJ, Chaplin DJ. Targeting gene therapy to cancer: a review. Oncol Res. 1997;9:313–325. [PubMed] [Google Scholar]
- [18].Huo M, Yuan J, Tao L, Wei Y. Redox-responsive polymers for drug delivery: from molecular design to applications. Polymer Chemistry. 2014;5:1519–1528. [Google Scholar]
- [19].Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273–12286. doi: 10.1039/c4nr04249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Yu J, Bomba HN, Zhu Y, Gu Z. Mechanical Force-Triggered Drug Delivery. Chem Rev. 2016;116:12536–12563. doi: 10.1021/acs.chemrev.6b00369. [DOI] [PubMed] [Google Scholar]
- [22].Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- [23].Holme MN, Fedotenko IA, Abegg D, Althaus J, Babel L, Favarger F, Reiter R, Tanasescu R, Zaffalon PL, Ziegler A, Muller B, et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat Nanotechnol. 2012;7:536–543. doi: 10.1038/nnano.2012.84. [DOI] [PubMed] [Google Scholar]
- [24].Klouda L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur J Pharm Biopharm. 2015;97:338–349. doi: 10.1016/j.ejpb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- [25].Shanmugam V, Selvakumar S, Yeh C-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43:6254–6287. doi: 10.1039/c4cs00011k. [DOI] [PubMed] [Google Scholar]
- [26].Chen H, Zhao Y. Applications of Light-Responsive Systems for Cancer Theranostics. ACS Appl Mater Interfaces. 2018 doi: 10.1021/acsami.8b01114. [DOI] [PubMed] [Google Scholar]
- [27].Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- [29].Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem Rev. 2015;115:10637–10689. doi: 10.1021/acs.chemrev.5b00112. [DOI] [PubMed] [Google Scholar]
- [30].Loebel C, Rodell CB, Chen MH, Burdick JA. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat Protoc. 2017;12:1521. doi: 10.1038/nprot.2017.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tanford C. The hydrophobic effect: formation of micelles and biological membranes. John Wiley; New York: 1973. [Google Scholar]
- [32].Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics. 1976;72:1525–1568. [Google Scholar]
- [33].Sakya P, Seddon JM, Templer RH, Mirkin RJ, Tiddy GJT. Micellar Cubic Phases and Their Structural Relationships: The Nonionic Surfactant System C12EO12/Water. Langmuir. 1997;13:3706–3714. [Google Scholar]
- [34].Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chemical Reviews. 2009;109:6275–6540. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- [35].Mai YY, Eisenberg A. Self-assembly of block copolymers. Chemical Society Reviews. 2012;41:5969–5985. doi: 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- [36].Bibi S, Lattmann E, Mohammed AR, Perrie Y. Trigger release liposome systems: local and remote controlled delivery? J Microencapsul. 2012;29:262–276. doi: 10.3109/02652048.2011.646330. [DOI] [PubMed] [Google Scholar]
- [37].Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng AC, Yuan JY. Smart Nanocontainers: Progress on Novel Stimuli-Responsive Polymer Vesicles. Macromolecular Rapid Communications. 2014;35:767–779. doi: 10.1002/marc.201300866. [DOI] [PubMed] [Google Scholar]
- [40].Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- [41].Müller LK, Landfester K. Natural liposomes and synthetic polymeric structures for biomedical applications. Biochem Biophys Res Commun. 2015;468:411–418. doi: 10.1016/j.bbrc.2015.08.088. [DOI] [PubMed] [Google Scholar]
- [42].Aghabegi Moghanjoughi A, Khoshnevis D, Zarrabi A. A concise review on smart polymers for controlled drug release. Drug Deliv Transl Res. 2016;6:333–340. doi: 10.1007/s13346-015-0274-7. [DOI] [PubMed] [Google Scholar]
- [43].Björnmalm M, Cui J, Berdeff-Zieschang N, Song D, Faria M, Rahim MA, Caruso F. Nanoengineering Particles through Template Assembly. Chem Mater. 2017;29:289–306. [Google Scholar]
- [44].Richardson JJ, Björnmalm M, Caruso F. Technology-driven layer-by-layer assembly of nanofilms. Science. 2015;348:aaa2491. doi: 10.1126/science.aaa2491. [DOI] [PubMed] [Google Scholar]
- [45].Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [46].Naeem S, Viswanathan G, Misran MB. Liposomes as colloidal nanovehicles: on the road to success in intravenous drug delivery. Rev Chem Eng. 2018;34:365–383. [Google Scholar]
- [47].Huang SL. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [48].Moratz J, Samanta A, Voskuhl J, Mohan Nalluri SK, Ravoo BJ. Light-triggered capture and release of DNA and proteins by host-guest binding and electrostatic interaction. Chemistry. 2015;21:3271–3277. doi: 10.1002/chem.201405936. [DOI] [PubMed] [Google Scholar]
- [49].Nalluri SK, Voskuhl J, Bultema JB, Boekema EJ, Ravoo BJ. Light-responsive capture and release of DNA in a ternary supramolecular complex. Angew Chem Int Ed Engl. 2011;50:9747–9751. doi: 10.1002/anie.201103707. [DOI] [PubMed] [Google Scholar]
- [50].Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- [51].Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- [52].Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- [53].Dai Q, Bertleff-Zieschang N, Braunger JA, Björnmalm M, Cortez-Jugo C, Caruso F. Particle Targeting in Complex Biological Media. Adv Healthc Mater. 2018;7:1700575. doi: 10.1002/adhm.201700575. [DOI] [PubMed] [Google Scholar]
- [54].Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- [55].Ignee A, Atkinson NSS, Schuessler G, Dietrich CF. Ultrasound contrast agents. Endosc Ultrasound. 2016;5:355–362. doi: 10.4103/2303-9027.193594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lattin JR, Belnap DM, Pitt WG. Formation of eLiposomes as a drug delivery vehicle. Colloids Surf B Biointerfaces. 2012;89:93–100. doi: 10.1016/j.colsurfb.2011.08.030. [DOI] [PubMed] [Google Scholar]
- [58].Javadi M, Pitt WG, Tracy CM, Barrow JR, Willardson BM, Hartley JM, Tsosie NH. Ultrasonic gene and drug delivery using eLiposomes. J Control Release. 2013;167:92–100. doi: 10.1016/j.jconrel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [59].Javadi M, Pitt WG, Belnap DM, Tsosie NH, Hartley JM. Encapsulating Nanoemulsions Inside eLiposomes for Ultrasonic Drug Delivery. Langmuir. 2012;28:14720–14729. doi: 10.1021/la303464v. [DOI] [PubMed] [Google Scholar]
- [60].Yang Q, Chen HL, Bai Y, Cao Y, Hu WJ, Zhang LK. Facile Synthesis of Lipid-Perfluorocarbon Nanoemulsion Coated with Silica Shell as an Ultrasound Imaging Agent. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201700816. [DOI] [PubMed] [Google Scholar]
- [61].Niu DC, Wang X, Li YS, Zheng YY, Li FQ, Chen HR, Gu JL, Zhao WR, Shi JL. Facile Synthesis of Magnetite/Perfluorocarbon Co-Loaded Organic/Inorganic Hybrid Vesicles for Dual-Modality Ultrasound/Magnetic Resonance Imaging and Imaging-Guided High-Intensity Focused Ultrasound Ablation. Adv Mater. 2013;25:2686–2692. doi: 10.1002/adma.201204316. [DOI] [PubMed] [Google Scholar]
- [62].Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. Ultrasound-triggered release of materials entrapped in microbubble–liposome constructs: A tool for targeted drug delivery. J Control Release. 2010;148:13–17. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fan CH, Chang EL, Ting CY, Lin YC, Liao EC, Huang CY, Chang YC, Chan HL, Wei KC, Yeh CK. Folate-conjugated gene-carrying microbubbles with focused ultrasound for concurrent blood-brain barrier, opening and local gene delivery. Biomaterials. 2016;106:46–57. doi: 10.1016/j.biomaterials.2016.08.017. [DOI] [PubMed] [Google Scholar]
- [64].Wrenn SP, Dicker SM, Small EF, Dan NR, Mleczko M, Schmitz G, Lewin PA. Bursting Bubbles and Bilayers. Theranostics. 2012;2:1140–1159. doi: 10.7150/thno.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yuana Y, Jiang LL, Lammertink BHA, Vader P, Deckers R, Bos C, Schiffelers RM, Moonen CT. Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Negishi Y, Endo-Takahashi Y, Matsuki Y, Kato Y, Takagi N, Suzuki R, Maruyama K, Aramaki Y. Systemic Delivery Systems of Angiogenic Gene by Novel Bubble Liposomes Containing Cationic Lipid and Ultrasound Exposure. Mol Pharm. 2012;9:1834–1840. doi: 10.1021/mp200554c. [DOI] [PubMed] [Google Scholar]
- [67].Lattin JR, Pitt WG, Belnap DM, Husseiniz GA. Ultrasound-induced calcein release from eLiposomes. Ultrasound Med Biol. 2012;38:2163–2173. doi: 10.1016/j.ultrasmedbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [68].Lattin JR, Pitt WG. Factors Affecting Ultrasonic Release from eLiposomes. J Pharm Sci. 2015;104:1373–1384. doi: 10.1002/jps.24344. [DOI] [PubMed] [Google Scholar]
- [69].Yahagi R, Yoshida K, Zhang YT, Ebata M, Toyota T, Yamaguchi T, Hayashi H. Destruction of giant cluster-like vesicles by an ultrasonically activated device. JJAP. 2016;55 [Google Scholar]
- [70].Li WP, Su CH, Chang YC, Lin YJ, Yeh CS. Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome. ACS Nano. 2016;10:2017–2027. doi: 10.1021/acsnano.5b06175. [DOI] [PubMed] [Google Scholar]
- [71].Li H, Wang P, Wang X, Yin TH, Zhou GF, Shuai XT, Zheng RQ. Perfluorooctyl bromide traces self-assembled with polymeric nanovesicles for blood pool ultrasound imaging. Biomater Sci. 2016;4:979–988. doi: 10.1039/c6bm00080k. [DOI] [PubMed] [Google Scholar]
- [72].Yan S, Lu M, Ding X, Chen F, He X, Xu C, Zhou H, Wang Q, Hao L, Zou J. HematoPorphyrin Monomethyl Ether polymer contrast agent for ultrasound/photoacoustic dual-modality imaging-guided synergistic high intensity focused ultrasound (HIFU) therapy. Sci Rep. 2016;6:31833. doi: 10.1038/srep31833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Paproski RJ, Jovel J, Wong GKS, Lewis JD, Zemp RJ. Enhanced Detection of Cancer Biomarkers in Blood-Borne Extracellular Vesicles Using Nanodroplets and Focused Ultrasound. Cancer Res. 2017;77:3–13. doi: 10.1158/0008-5472.CAN-15-3231. [DOI] [PubMed] [Google Scholar]
- [74].Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- [75].Sirsi S, Borden M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci Eng Technol. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xia CY, Liu YH, Wang P, Xue YX. Low-Frequency Ultrasound Irradiation Increases Blood-Tumor Barrier Permeability by Transcellular Pathway in a Rat Glioma Model. J Mol Neurosci. 2012;48:281–290. doi: 10.1007/s12031-012-9770-0. [DOI] [PubMed] [Google Scholar]
- [77].Ibsen S, Simberg D, Schutt C, Steiner J, Esener S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J Control Release. 2011;155:358–366. doi: 10.1016/j.jconrel.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Appis AW, Tracy MJ, Feinstein SB. Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res Pract. 2015;2:R55–R62. doi: 10.1530/ERP-15-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang Y, Liu GJ, Hu H, Li TY, Johri AM, Li XY, Wang J. Stable Encapsulated Air Nanobubbles in Water. Angew Chem Int Ed Engl. 2015;54:14291–14294. doi: 10.1002/anie.201505817. [DOI] [PubMed] [Google Scholar]
- [80].Perera RH, Wu HP, Peiris P, Hernandez C, Burke A, Zhang H, Exner AA. Improving performance of nanoscale ultrasound contrast agents using N,N-diethylacrylamide stabilization. Nanomedicine: NBM. 2017;13:59–67. doi: 10.1016/j.nano.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5:216rv214. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- [82].Björnmalm M, Thurecht KJ, Michael M, Scott AM, Caruso F. Bridging Bio-Nano Science and Cancer Nanomedicine. ACS Nano. 2017;11:9594–9613. doi: 10.1021/acsnano.7b04855. [DOI] [PubMed] [Google Scholar]
- [83].Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, Macdonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90:1917–1926. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- [84].Kandadai MA, Meunier JM, Hart K, Holland CK, Shaw GJ. Plasmin-Loaded Echogenic Liposomes for Ultrasound-Mediated Thrombolysis. Transl Stroke Res. 2015;6:78–87. doi: 10.1007/s12975-014-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lattin JR, Javadi M, McRae M, Pitt WG. Cytosolic delivery via escape from the endosome using emulsion droplets and ultrasound. J Drug Target. 2015;23:469–479. doi: 10.3109/1061186X.2015.1009074. [DOI] [PubMed] [Google Scholar]
- [86].Chen MJ, Xu AR, He WY, Ma WC, Shen S. Ultrasound triggered drug delivery for mitochondria targeted sonodynamic therapy. J Drug Deliv Sci Technol. 2017;39:501–507. [Google Scholar]
- [87].Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- [88].Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release. 2012;161:317–327. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- [89].Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Song S, Zheng YS. Hollow Spheres Self-Assembled by a Tetraphenylethylene Macrocycle and Their Transformation to Bird Nests under Ultrasound. Org Lett. 2013;15:820–823. doi: 10.1021/ol3035005. [DOI] [PubMed] [Google Scholar]
- [91].Huang WC, Chiang WH, Cheng YH, Lin WC, Yu CF, Yen CY, Yeh CK, Chern CS, Chiang CS, Chiu HC. Tumortropic monocyte-mediated delivery of echogenic polymer bubbles and therapeutic vesicles for chemotherapy of tumor hypoxia. Biomaterials. 2015;71:71–83. doi: 10.1016/j.biomaterials.2015.08.033. [DOI] [PubMed] [Google Scholar]
- [92].Zeghimi A, Escoffre JM, Bouakaz A. Role of endocytosis in sonoporation-mediated membrane permeabilization and uptake of small molecules: a electron microscopy study. Phys Biol. 2015;12 doi: 10.1088/1478-3975/12/6/066007. 066007. [DOI] [PubMed] [Google Scholar]
- [93].Hsieh CC, Kang ST, Lin YH, Ho YJ, Wang CH, Yeh CK, Chang CW. Biomimetic Acoustically-Responsive Vesicles for Theranostic Applications. Theranostics. 2015;5:1264–1274. doi: 10.7150/thno.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lakshmanan A, Farhadi A, Nety SP, Lee-Gosselin A, Bourdeau RW, Maresca D, Shapiro MG. Molecular Engineering of Acoustic Protein Nanostructures. ACS Nano. 2016;10:7314–7322. doi: 10.1021/acsnano.6b03364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Aw MS, Paniwnyk L. Overcoming T. gondii infection and intracellular protein nanocapsules as biomaterials for ultrasonically controlled drug release. Biomater Sci. 2017;5:1944–1961. doi: 10.1039/c7bm00425g. [DOI] [PubMed] [Google Scholar]
- [96].Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21:779–790. doi: 10.1080/02656730500271668. [DOI] [PubMed] [Google Scholar]
- [97].Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, Beck M, Nadobny J, Budach V, Wust P, Ghadjar P. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015;31:609–614. doi: 10.3109/02656736.2015.1040471. [DOI] [PubMed] [Google Scholar]
- [98].Kheirolomoom A, Lai CY, Tam SM, Mahakian LM, Ingham ES, Watson KD, Ferrara KW. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release. 2013;172:266–273. doi: 10.1016/j.jconrel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Al-Ahmady Z, Kostarelos K. Chemical Components for the Design of Temperature-Responsive Vesicles as Cancer Therapeutics. Chem Rev. 2016;116:3883–3918. doi: 10.1021/acs.chemrev.5b00578. [DOI] [PubMed] [Google Scholar]
- [100].Fameau A-L, Arnould A, Saint-Jalmes A. Responsive self-assemblies based on fatty acids. Curr Opin Colloid Interface Sci. 2014;19:471–479. [Google Scholar]
- [101].Yatvin M, Weinstein J, Dennis W, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- [102].Chen KJ, Chaung EY, Wey SP, Lin KJ, Cheng F, Lin CC, Liu HL, Tseng HW, Liu CP, Wei MC, Liu CM, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8:5105–5115. doi: 10.1021/nn501162x. [DOI] [PubMed] [Google Scholar]
- [103].Chen KJ, Liang HF, Chen HL, Wang Y, Cheng PY, Liu HL, Xia Y, Sung HW. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano. 2013;7:438–446. doi: 10.1021/nn304474j. [DOI] [PubMed] [Google Scholar]
- [104].Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [105].Song S, Song A, Hao J. Self-assembled structures of amphiphiles regulated via implanting external stimuli. RSC Adv. 2014;4:41864–41875. [Google Scholar]
- [106].Kozlovskaya V, Liu F, Xue B, Ahmad F, Alford A, Saeed M, Kharlampieva E. Polyphenolic Polymersomes of Temperature-Sensitive Poly(N-vinylcaprolactam)-block-Poly(N-vinylpyrrolidone) for Anticancer Therapy. Biomacromolecules. 2017;18:2552–2563. doi: 10.1021/acs.biomac.7b00687. [DOI] [PubMed] [Google Scholar]
- [107].Zangabad PS, Mirkiani S, Shahsavari S, Masoudi B, Masroor M, Hamed H, Jafari Z, Taghipour YD, Hashemi H, Karimi M, Hamblin MR. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnology Reviews. 2018;7:95–122. doi: 10.1515/ntrev-2017-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Maiti C, Dhara D. Energy-Transfer Phenomena in Thermoresponsive and pH- Switchable Fluorescent Diblock Copolymer Vesicles. Langmuir. 2017;33:12130–12139. doi: 10.1021/acs.langmuir.7b01891. [DOI] [PubMed] [Google Scholar]
- [109].Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, Kostarelos K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano. 2012;6:9335–9346. doi: 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Al-Ahmady ZS, Scudamore CL, Kostarelos K. Triggered doxorubicin release in solid tumors from thermosensitive liposome-peptide hybrids: Critical parameters and therapeutic efficacy. International Journal of Cancer. 2015;137:731–743. doi: 10.1002/ijc.29430. [DOI] [PubMed] [Google Scholar]
- [111].Kim MS, Lee DW, Park K, Park SJ, Choi EJ, Park ES, Kim HR. Temperature-triggered tumor-specific delivery of anticancer agents by cRGD-conjugated thermosensitive liposomes. Colloids Surf B Biointerfaces. 2014;116:17–25. doi: 10.1016/j.colsurfb.2013.12.045. [DOI] [PubMed] [Google Scholar]
- [112].Zhou J, Chen M, Diao G. Magnetic-responsive supramolecular vesicles from self-organization of amphiphilic pillar[5]arene and application in controlled release. ACS Appl Mater Interfaces. 2014;6:18538–18542. doi: 10.1021/am5057147. [DOI] [PubMed] [Google Scholar]
- [113].Cao Y, Hu XY, Li Y, Zou X, Xiong S, Lin C, Shen YZ, Wang L. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J Am Chem Soc. 2014;136:10762–10769. doi: 10.1021/ja505344t. [DOI] [PubMed] [Google Scholar]
- [114].Lino MM, Ferreira L. Light-triggerable formulations for the intracellular controlled release of biomolecules. Drug Discov Today. 2018 doi: 10.1016/j.drudis.2018.01.019. [DOI] [PubMed] [Google Scholar]
- [115].Nazemi A, Gillies ER. Dendrimersomes with photodegradable membranes for triggered release of hydrophilic and hydrophobic cargo. Chem Commun. 2014;50:11122–11125. doi: 10.1039/c4cc05161k. [DOI] [PubMed] [Google Scholar]
- [116].Geng S, Wang Y, Wang L, Kouyama T, Gotoh T, Wada S, Wang JY. A Light-Responsive Self-Assembly Formed by a Cationic Azobenzene Derivative and SDS as a Drug Delivery System. Sci Rep. 2017;7 doi: 10.1038/srep39202. 39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Moratz J, Stricker L, Engel S, Ravoo BJ. Controlling Complex Stability in Photoresponsive Macromolecular Host-Guest Systems: Toward Reversible Capture of DNA by Cyclodextrin Vesicles. Macromol Rapid Commun. 2018;39 doi: 10.1002/marc.201700256. [DOI] [PubMed] [Google Scholar]
- [118].Nalluri SK, Bultema JB, Boekema EJ, Ravoo BJ. Photoresponsive molecular recognition and adhesion of vesicles in a competitive ternary supramolecular system. Chemistry. 2011;17:10297–10303. doi: 10.1002/chem.201100789. [DOI] [PubMed] [Google Scholar]
- [119].Nalluri SK, Ravoo BJ. Light-responsive molecular recognition and adhesion of vesicles. Angew Chem Int Ed Engl. 2010;49:5371–5374. doi: 10.1002/anie.201001442. [DOI] [PubMed] [Google Scholar]
- [120].Samanta A, Ravoo BJ. Metal ion, light, and redox responsive interaction of vesicles by a supramolecular switch. Chemistry. 2014;20:4966–4973. doi: 10.1002/chem.201304658. [DOI] [PubMed] [Google Scholar]
- [121].Samanta A, Stuart MCA, Ravoo BJ. Photoresponsive Capture and Release of Lectins in Multilamellar Complexes. J Am Chem Soc. 2012;134:19909–19914. doi: 10.1021/ja3101837. [DOI] [PubMed] [Google Scholar]
- [122].Kauscher U, Samanta A, Ravoo BJ. Photoresponsive vesicle permeability based on intramolecular host-guest inclusion. Org Biomol Chem. 2014;12:600–606. doi: 10.1039/c3ob41893f. [DOI] [PubMed] [Google Scholar]
- [123].Yu G, Han C, Zhang Z, Chen J, Yan X, Zheng B, Liu S, Huang F. Pillar[6]arene-Based Photoresponsive Host–Guest Complexation. J Am Chem Soc. 2012;134:8711–8717. doi: 10.1021/ja302998q. [DOI] [PubMed] [Google Scholar]
- [124].Xia D, Yu G, Li J, Huang F. Photo-responsive self-assembly based on a water-soluble pillar[6]arene and an azobenzene-containing amphiphile in water. Chem Commun. 2014;50:3606–3608. doi: 10.1039/c3cc49686d. [DOI] [PubMed] [Google Scholar]
- [125].Yao C, Wang P, Li X, Hu X, Hou J, Wang L, Zhang F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv Mater. 2016;28:9341–9348. doi: 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- [126].Wang J, Dong Y, Li Y, Li W, Cheng K, Qian Y, Xu G, Zhang X, Hu L, Chen P, Du W, et al. Designer Exosomes for Active Targeted Chemo-Photothermal Synergistic Tumor Therapy. Adv Funct Mater. 2018;28 1707360. [Google Scholar]
- [127].Wu T, Zhang D, Qiao Q, Qin X, Yang C, Kong M, Deng H, Zhang Z. Biomimetic Nanovesicles for Enhanced Antitumor Activity of Combinational Photothermal and Chemotherapy. Mol Pharm. 2018;15:1341–1352. doi: 10.1021/acs.molpharmaceut.7b01142. [DOI] [PubMed] [Google Scholar]
- [128].Kauscher U, Ravoo BJ. A self-assembled cyclodextrin nanocarrier for photoreactive squaraine. Beilstein J Org Chem. 2016;12:2535–2542. doi: 10.3762/bjoc.12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Galstyan A, Kauscher U, Block D, Ravoo BJ, Strassert CA. Silicon(IV) Phthalocyanine-Decorated Cyclodextrin Vesicles as a Self-Assembled Phototherapeutic Agent against MRSA. ACS Appl Mater Interfaces. 2016;8:12631–12637. doi: 10.1021/acsami.6b02132. [DOI] [PubMed] [Google Scholar]
- [130].Bandara HM, Burdette SC. Photoisomerization in different classes of azobenzene. Chem Soc Rev. 2012;41:1809–1825. doi: 10.1039/c1cs15179g. [DOI] [PubMed] [Google Scholar]
- [131].Huang CQ, Wang Y, Hong CY, Pan CY. Spiropyran-based polymeric vesicles: preparation and photochromic properties. Macromol Rapid Commun. 2011;32:1174–1179. doi: 10.1002/marc.201100197. [DOI] [PubMed] [Google Scholar]
- [132].Schmidt R, Geissler D, Hagen V, Bendig J. Mechanism of photocleavage of (coumarin-4-yl)methyl esters. J Phys Chem A. 2007;111:5768–5774. doi: 10.1021/jp071521c. [DOI] [PubMed] [Google Scholar]
- [133].Nagasaki T, Taniguchi A, Tamagaki S. Photoenhancement of Transfection Efficiency Using Novel Cationic Lipids Having a Photocleavable Spacer. Biocunjug Chem. 2003;14:513–516. doi: 10.1021/bc0256603. [DOI] [PubMed] [Google Scholar]
- [134].Wang D, Wang X. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Progress in Polymer Science. 2013;38:271–301. [Google Scholar]
- [135].Browne WR, Feringa BL. Making molecular machines work. Nat Nanotechnol. 2006;1:25–35. doi: 10.1038/nnano.2006.45. [DOI] [PubMed] [Google Scholar]
- [136].Stricker L, Fritz EC, Peterlechner M, Doltsinis NL, Ravoo BJ. Arylazopyrazoles as Light-Responsive Molecular Switches in Cyclodextrin-Based Supramolecular Systems. J Am Chem Soc. 2016;138:4547–4554. doi: 10.1021/jacs.6b00484. [DOI] [PubMed] [Google Scholar]
- [137].Dong M, Babalhavaeji A, Collins CV, Jarrah K, Sadovski O, Dai Q, Woolley GA. Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions. J Am Chem Soc. 2017;139:13483–13486. doi: 10.1021/jacs.7b06471. [DOI] [PubMed] [Google Scholar]
- [138].Weis P, Wu S. Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared. Macromol Rapid Commun. 2018;39 doi: 10.1002/marc.201700220. [DOI] [PubMed] [Google Scholar]
- [139].Ravoo BJ, Darcy R. Cyclodextrin Bilayer Vesicles. Angew Chem Int Ed Engl. 2000;39:4324–4326. doi: 10.1002/1521-3773(20001201)39:23<4324::AID-ANIE4324>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [140].Falvey P, Lim CW, Darcy R, Revermann T, Karst U, Giesbers M, Marcelis AT, Lazar A, Coleman AW, Reinhoudt DN, Ravoo BJ. Bilayer vesicles of amphiphilic cyclodextrins: host membranes that recognize guest molecules. Chemistry. 2005;11:1171–1180. doi: 10.1002/chem.200400905. [DOI] [PubMed] [Google Scholar]
- [141].Kauscher U, Stuart MCA, Drücker P, Galla H-J, Ravoo BJ. Incorporation of Amphiphilic Cyclodextrins into Liposomes as Artificial Receptor Units. Langmuir. 2013;29:7377–7383. doi: 10.1021/la3045434. [DOI] [PubMed] [Google Scholar]
- [142].Voskuhl J, Fenske T, Stuart MC, Wibbeling B, Schmuck C, Ravoo BJ. Molecular recognition of vesicles: host-guest interactions combined with specific dimerization of zwitterions. Chemistry. 2010;16:8300–8306. doi: 10.1002/chem.201000623. [DOI] [PubMed] [Google Scholar]
- [143].Kauscher U, Ravoo BJ. Mannose-decorated cyclodextrin vesicles: The interplay of multivalency and surface density in lectin–carbohydrate recognition. Beilstein J Org Chem. 2012;8:1543–1551. doi: 10.3762/bjoc.8.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vico RV, Voskuhl J, Ravoo BJ. Multivalent Interaction of Cyclodextrin Vesicles, Carbohydrate Guests, and Lectins: A Kinetic Investigation. Langmuir. 2011;27:1391–1397. doi: 10.1021/la1038975. [DOI] [PubMed] [Google Scholar]
- [145].Voskuhl J, Stuart MC, Ravoo BJ. Sugar-decorated sugar vesicles: lectin-carbohydrate recognition at the surface of cyclodextrin vesicles. Chemistry. 2010;16:2790–2796. doi: 10.1002/chem.200902423. [DOI] [PubMed] [Google Scholar]
- [146].Himmelein S, Ravoo BJ. A Self-Assembled Sensor for Carbohydrates on the Surface of Cyclodextrin Vesicles. Chemistry. 2017;23:6034–6041. doi: 10.1002/chem.201603115. [DOI] [PubMed] [Google Scholar]
- [147].Versluis F, Tomatsu I, Kehr S, Fregonese C, Tepper AW, Stuart MC, Ravoo BJ, Koning RI, Kros A. Shape and release control of a peptide decorated vesicle through pH sensitive orthogonal supramolecular interactions. J Am Chem Soc. 2009;131:13186–13187. doi: 10.1021/ja9026264. [DOI] [PubMed] [Google Scholar]
- [148].Versluis F, Voskuhl J, Stuart MCA, Bultema JB, Kehr S, Ravoo BJ, Kros A. Power struggles between oligopeptides and cyclodextrin vesicles. Soft Matter. 2012;8:8770–8777. [Google Scholar]
- [149].Versluis F, Voskuhl J, Vos J, Friedrich H, Ravoo BJ, Bomans PHH, Stuart MCA, Sommerdijk NAJM, Kros A. Coiled coil driven membrane fusion between cyclodextrin vesicles and liposomes. Soft Matter. 2014;10:9746–9751. doi: 10.1039/c4sm01801j. [DOI] [PubMed] [Google Scholar]
- [150].Hu C, Ma N, Li F, Fang Y, Liu Y, Zhao L, Qiao S, Li X, Jiang X, Li T, Shen F, et al. Cucurbit[8]uril-Based Giant Supramolecular Vesicles: Highly Stable, Versatile Carriers for Photoresponsive and Targeted Drug Delivery. ACS Appl Mater Interfaces. 2018;10:4603–4613. doi: 10.1021/acsami.8b00297. [DOI] [PubMed] [Google Scholar]
- [151].Ohya Y, Okuyama Y, Fukunaga A, Ouchi T. Photo-sensitive lipid membrane perturbation by a single chain lipid having terminal spiropyran group. Supramol Sci. 1998;5:21–29. [Google Scholar]
- [152].Kwangmettatam S, Kudernac T. Light-fuelled reversible expansion of spiropyran-based vesicles in water. Chem Commun. 2018;54:5311–5314. doi: 10.1039/c8cc01780h. [DOI] [PubMed] [Google Scholar]
- [153].Moller N, Hellwig T, Stricker L, Engel S, Fallnich C, Ravoo BJ. Near-infrared photoswitching of cyclodextrin-guest complexes using lanthanide-doped LiYF4 upconversion nanoparticles. Chem Commun. 2017;53:240–243. doi: 10.1039/c6cc08321h. [DOI] [PubMed] [Google Scholar]
- [154].Auzel F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem Rev. 2004;104:139–174. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- [155].Chen G, Ma B, Xie R, Wang Y, Dou K, Gong S. NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier. J Control Release. 2017 doi: 10.1016/j.jconrel.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- [157].Song X, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Research. 2015;8:340–354. [Google Scholar]
- [158].Rengan AK, Bukhari AB, Pradhan A, Malhotra R, Banerjee R, Srivastava R, De A. In Vivo Analysis of Biodegradable Liposome Gold Nanoparticles as Efficient Agents for Photothermal Therapy of Cancer. Nano Letters. 2015;15:842–848. doi: 10.1021/nl5045378. [DOI] [PubMed] [Google Scholar]
- [159].Zou L, Wang H, He B, Zeng L, Tan T, Cao H, He X, Zhang Z, Guo S, Li Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics. 2016;6:762–772. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Armstrong JPK, Holme MN, Stevens MM. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Fuhrmann G, Herrmann IK, Stevens MM. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. Nano Today. 2015;10:397–409. doi: 10.1016/j.nantod.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- [164].Ormond A, Freeman H. Dye Sensitizers for Photodynamic Therapy. Materials. 2013;6:817. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev. 1995;24:19–33. [Google Scholar]
- [166].Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- [167].Avirah RR, Jayaram DT, Adarsh N, Ramaiah D. Squaraine dyes in PDT: from basic design to in vivo demonstration. Organic & biomolecular chemistry. 2012;10:911–920. doi: 10.1039/c1ob06588b. [DOI] [PubMed] [Google Scholar]
- [168].Bian Y, Jiang J. Recent Advances in Phthalocyanine-Based Functional Molecular Materials. In: Mingos DMP, editor. 50 Years of Structure and Bonding – The Anniversary Volume. Springer International Publishing; Cham: 2016. pp. 159–199. [Google Scholar]
- [169].Abrahamse H, Michael Hamblin R. New photosensitizers for photodynamic therapy. Biochemical Journal. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lucky SS, Soo KC, Zhang Y. Nanoparticles in Photodynamic Therapy. Chemical Reviews. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- [171].Debele TA, Peng S, Tsai HC. Drug Carrier for Photodynamic Cancer Therapy. Int J Mol Sci. 2015;16:22094–22136. doi: 10.3390/ijms160922094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Nishiyama N, Morimoto Y, Jang WD, Kataoka K. Design and development of dendrimer photosensitizer-incorporated polymeric micelles for enhanced photodynamic therapy. Advanced drug delivery reviews. 2009;61:327–338. doi: 10.1016/j.addr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [173].Voskuhl J, Kauscher U, Gruener M, Frisch H, Wibbeling B, Strassert CA, Ravoo BJ. A soft supramolecular carrier with enhanced singlet oxygen photosensitizing properties. Soft Matter. 2013;9:2453–2457. [Google Scholar]
- [174].Bottari G, de la Torre G, Guldi DM, Torres T. Covalent and Noncovalent Phthalocyanine−Carbon Nanostructure Systems: Synthesis, Photoinduced Electron Transfer, and Application to Molecular Photovoltaics. Chem Rev. 2010;110:6768–6816. doi: 10.1021/cr900254z. [DOI] [PubMed] [Google Scholar]
- [175].Mack J, Kobayashi N. Low Symmetry Phthalocyanines and Their Analogues. Chem Rev. 2011;111:281–321. doi: 10.1021/cr9003049. [DOI] [PubMed] [Google Scholar]
- [176].Gottfried JM. Surface chemistry of porphyrins and phthalocyanines. Surf Sci Rep. 2015;70:259–379. [Google Scholar]
- [177].Di Corato R, Bealle G, Kolosnjaj-Tabi J, Espinosa A, Clement O, Silva AK, Menager C, Wilhelm C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015;9:2904–2916. doi: 10.1021/nn506949t. [DOI] [PubMed] [Google Scholar]
- [178].Holme MN, Schulz G, Deyhle H, Weitkamp T, Beckmann F, Lobrinus JA, Rikhtegar F, Kurtcuoglu V, Zanette I, Saxer T, Müller B. Complementary X-ray tomography techniques for histology-validated 3D imaging of soft and hard tissues using plaque-containing blood vessels as examples. Nat Protoc. 2014;9:1401. doi: 10.1038/nprot.2014.091. [DOI] [PubMed] [Google Scholar]
- [179].Buscema M, Matviykiv S, Mészáros T, Gerganova G, Weinberger A, Mettal U, Mueller D, Neuhaus F, Stalder E, Ishikawa T, Urbanics R, et al. Immunological response to nitroglycerin-loaded shear-responsive liposomes in vitro and in vivo. J Control Release. 2017;264:14–23. doi: 10.1016/j.jconrel.2017.08.010. [DOI] [PubMed] [Google Scholar]
- [180].Bugna S, Buscema M, Matviykiv S, Urbanics R, Weinberger A, Meszaros T, Szebeni J, Zumbuehl A, Saxer T, Mueller B. Surprising lack of liposome-induced complement activation by artificial 1,3-diamidophospholipids in vitro. Nanomedicine: NBM. 2016;12:845–849. doi: 10.1016/j.nano.2015.12.364. [DOI] [PubMed] [Google Scholar]
- [181].Stalder E, Zumbuehl A. Liposome-Containing Mechanoresponsive Hydrogels. Macromol Mater Eng. 2017;302 [Google Scholar]
- [182].Pamornpathomkul B, Niyomtham N, Yingyongnarongkul BE, Prasitpuriprecha C, Rojanarata T, Ngawhirunpat T, Opanasopit P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles. AAPS PharmSciTech. 2018;19:481–488. doi: 10.1208/s12249-017-0855-5. [DOI] [PubMed] [Google Scholar]
- [183].Himmelein S, Lewe V, Stuart MCA, Ravoo BJ. A carbohydrate-based hydrogel containing vesicles as responsive non-covalent cross-linkers. Chem Sci. 2014;5:1054–1058. [Google Scholar]
- [184].Saxer T, Zumbuehl A, Mueller B. The use of shear stress for targeted drug delivery. Cardiovasc Res. 2013;99:328–333. doi: 10.1093/cvr/cvt102. [DOI] [PubMed] [Google Scholar]
- [185].Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303:607–621. doi: 10.1007/s00403-011-1166-4. [DOI] [PubMed] [Google Scholar]
- [187].S C, C A, J B. Microneedles and other physical methods for overcoming the stratum corneum. Crit Rev Ther Drug Carrier Syst. 2006;23:205–258. doi: 10.1615/critrevtherdrugcarriersyst.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- [188].Ita K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics. 2015;7:90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Pegoraro C, MacNeil S, Battaglia G. Transdermal drug delivery: from micro to nano. Nanoscale. 2012;4:1881–1894. doi: 10.1039/c2nr11606e. [DOI] [PubMed] [Google Scholar]
- [190].Kaurav M, Minz S, Sahu K, Kumar M, Madan J, Pandey RS. Nanoparticulate mediated transcutaneous immunization: Myth or reality. Nanomedicine: NBM. 2016;12:1063–1081. doi: 10.1016/j.nano.2015.12.372. [DOI] [PubMed] [Google Scholar]
- [191].Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proceedings of the National Academy of Sciences. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Chiappini C, De Rosa E, Martinez JO, Liu X, Steele J, Stevens MM, Tasciotti E. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat Mater. 2015;14:532. doi: 10.1038/nmat4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Chiappini C, Martinez JO, De Rosa E, Almeida CS, Tasciotti E, Stevens MM. Biodegradable Nanoneedles for Localized Delivery of Nanoparticles in Vivo: Exploring the Biointerface. ACS Nano. 2015;9:5500–5509. doi: 10.1021/acsnano.5b01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, Clay R, Adams RA, Baird RD, Billingham L, Brown SR, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627–642. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- [196].Min YZ, Roche KC, Tian SM, Eblan MJ, McKinnon KP, Caster JM, Chai SJ, Herring LE, Zhang LZ, Zhang T, DeSimone JM, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Kohane DS, Langer R. Biocompatibility and drug delivery systems. Chem Sci. 2010;1:441–446. [Google Scholar]
- [199].Cheng ZL, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Björnmalm M, Faria M, Caruso F. Increasing the Impact of Materials in and beyond Bio-Nano Science. J Am Chem Soc. 2016;138:13449–13456. doi: 10.1021/jacs.6b08673. [DOI] [PubMed] [Google Scholar]
- [201].Sharp P, Hockfield S. Convergence: The future of health. Science. 2017;355:589–589. doi: 10.1126/science.aam8563. [DOI] [PubMed] [Google Scholar]
- [202].Torrice M. Does Nanomedicine Have a Delivery Problem? Acs Central Sci. 2016;2:434–437. doi: 10.1021/acscentsci.6b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Cancer nanomedicine: is targeting our target? Nat Rev Mater. 2016;1 doi: 10.1038/natrevmats.2016.69. 16069. [DOI] [PMC free article] [PubMed] [Google Scholar]