Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
Prophylactic brincidofovir reduced the incidence and magnitude of plasma HHV-6B detection after allogeneic HCT.
Prophylactic brincidofovir may reduce the morbidity associated with HHV-6B in HCT recipients.
Abstract
Human herpesvirus 6B (HHV-6B) frequently reactivates after allogeneic hematopoietic cell transplantation (HCT). There are no randomized studies of antiviral treatments to prevent HHV-6B reactivation. Brincidofovir has high in vitro activity against HHV-6B and other DNA viruses, but its in vivo activity for HHV-6B has not been demonstrated. We performed a post hoc analysis of a randomized controlled trial of twice-weekly oral brincidofovir for cytomegalovirus prophylaxis after allogeneic HCT to study the effect of brincidofovir on HHV-6B reactivation. We included patients randomized within 2 weeks of HCT and who received at least 6 consecutive doses of study drug after randomization. We tested plasma for HHV-6B through week 6 post-HCT. The cohort consisted of 92 patients receiving brincidofovir and 61 receiving placebo. The cumulative incidence of HHV-6B plasma detection through day 42 post-HCT was significantly lower among patients receiving brincidofovir (14.2%) compared with placebo (32.4%; log-rank, 0.019). In an adjusted Cox model, brincidofovir exposure remained associated with a lower hazard for HHV-6B plasma detection (hazard ratio, 0.40; 95% confidence interval, 0.20-0.80). In conclusion, brincidofovir prophylaxis reduced HHV-6B reactivation after allogeneic HCT in a post hoc analysis of a randomized controlled trial. These data support the study of intravenous brincidofovir for HHV-6B prophylaxis.
Visual Abstract
Introduction
Human herpesvirus 6B (HHV-6B) reactivation after allogeneic hematopoietic cell transplantation (HCT) is frequent and is the most common infectious cause of post-HCT encephalitis.1-4 HHV-6B is also implicated in acute graft-versus-host disease (GVHD), pneumonia, and other complications.5-11 Nonrandomized, noncontrolled studies of ganciclovir or foscarnet for preemptive or prophylactic treatment of HHV-6B demonstrated reductions in the incidence and magnitude of HHV-6B DNA detection in blood, but have not reduced the risk for HHV-6B encephalitis.12-16 Possible reasons include underdosing antivirals because of concern for toxicities. Brincidofovir is a lipid conjugate of cidofovir with a different safety profile (eg, lack of nephrotoxicity) that has high in vitro activity against HHV-6B and demonstrable central nervous system penetration in animal models.17-19 We report the effect of prophylactic oral brincidofovir on HHV-6B reactivation after allogeneic HCT from a post hoc analysis of a randomized, double-blind, placebo-controlled trial.19
Study design
We obtained samples from the SUPPRESS trial, which enrolled adult cytomegalovirus-seropositive allogeneic HCT recipients who were randomly assigned 2:1 to receive oral brincidofovir or placebo twice weekly for cytomegalovirus prophylaxis until week 14 post-HCT (ClinicalTrials.gov: NCT01769170; EudraCT: NCT01769170).19 On the basis of the timing of HHV-6B reactivation and disease post-HCT,4 we selected (a priori) patients who were randomized within 2 weeks of HCT and who received at least 6 doses of brincidofovir or placebo within the first 3 weeks after randomization. We tested weekly plasma samples through 6 weeks post-HCT for HHV-6B with quantitative polymerase chain reaction (Viracor Eurofins, Lee's Summit, MO; polymerase chain reaction for species identification was performed at the University of Washington, Seattle, WA; supplemental Data, available on the Blood Web site). Patients with HHV-6B detection at the baseline randomization visit were excluded. We compared HHV-6B detection between patients who received brincidofovir or placebo, using the Kaplan-Meier method and Cox proportional hazards model. Analyses were stratified by high and low risk, using the cytomegalovirus risk criteria in the SUPPRESS trial, given that randomization was stratified by risk group19 and the overlap in risk factors for HHV-6B reactivation.20,21 Patients were considered high risk if they received cord blood or ex vivo T-cell-depleted grafts or grafts from unrelated, mismatched, or haploidentical donors; received anti-thymocyte globulin or alemtuzumab; or were being treated with prednisone, at least 1 mg/kg/day (or equivalent). Variables in a Cox model with P < .3 were included in an adjusted model. We also compared the incidence of select clinical events through week 7 post-HCT.
The protocol was approved by each center's ethics committees and conducted in accordance to the International Conference on Harmonization guideline for Good Clinical Practice and the Declaration of Helsinki.
Results and discussion
Of the 452 randomized patients in the SUPPRESS Trial, 98 patients who received brincidofovir and 64 who received placebo met this analysis’s inclusion criteria and had a baseline sample available from the randomization visit. Four patients in the brincidofovir group and 3 in the placebo group had plasma HHV-6B detected at baseline and were excluded; an additional 2 patients in the brincidofovir group had no subsequent samples available (supplemental Figure 1). Patient baseline characteristics remained balanced in the study groups (Table 1). Sixty-nine (75%) patients in the brincidofovir group and 39 (64%) in the placebo arm were categorized as high risk for HHV-6B reactivation. During the first 6 weeks post-HCT, GVHD grades 2 to 4 was reported in 37 (40%) patients receiving brincidofovir (median onset, 28 days) and 6 (10%) patients receiving placebo (median onset, 35 days). Details of GVHD grading in this cohort are provided in supplemental Table 1; additional details for the entire cohort were previously described.19 The median duration of study drug administration through transplant week 6 was 32 days in the brincidofovir group and 30 days in the placebo group. Most patients remained on study drug by week 6 (89% for brincidofovir; 70% for placebo).
Table 1.
Clinical and demographic characteristics
| Characteristics | Overall | HHV-6B cohort* | ||
|---|---|---|---|---|
| Brincidofovir (n = 303) | Placebo (n = 149) | Brincidofovir (n = 92) | Placebo (n = 61) | |
| Age in years, median (range) | 56 (18-77) | 54 (20-75) | 57 (18-76) | 59 (20-73) |
| Sex, n (%) | ||||
| Male | 163 (53.8) | 98 (65.8) | 57 (62.0) | 41 (67.2) |
| Female | 140 (46.2) | 51 (34.2) | 35 (38.0) | 20 (32.8) |
| Race, n (%) | ||||
| White | 255 (84.2) | 123 (82.6) | 76 (82.6) | 52 (85.2) |
| African American | 24 (7.9) | 14 (9.4) | 8 (8.7) | 4 (6.6) |
| Asian | 17 (5.6) | 10 (6.7) | 7 (7.6) | 4 (6.6) |
| Other | 7 (2.3) | 2 (1.3) | 1 (1.1) | 1 (1.6) |
| Underlying disease, n (%) | ||||
| Acute myelogenous leukemia | 129 (42.6) | 64 (43.0) | 37 (40.2) | 26 (42.6) |
| Myelodysplasia | 52 (17.2) | 24 (16.1) | 17 (18.5) | 13 (21.3) |
| Non-Hodgkin’s lymphoma | 28 (9.2) | 18 (12.1) | 9 (9.8) | 6 (9.8) |
| Acute lymphocytic leukemia | 29 (9.6) | 13 (8.7) | 6 (6.5) | 6 (9.8) |
| Other | 65 (21.5) | 30 (20.1) | 23 (25.0) | 10 (16.4) |
| Conditioning regimen, n (%) | ||||
| Myeloablative | 162 (53.5) | 86 (57.7) | 40 (43.5) | 32 (52.5) |
| Nonmyeloablative | 141 (46.5) | 63 (42.3) | 52 (56.5) | 29 (47.5) |
| Alemtuzumab use, n (%) | 26 (8.6) | 12 (8.1) | 10 (10.9) | 4 (6.6) |
| Antithymocyte globulin use, n (%) | 85 (28.1) | 47 (31.5) | 30 (32.6) | 18 (29.5) |
| Ex vivo T-cell depletion, n (%) | 36 (11.9) | 20 (13.4) | 13 (14.1) | 6 (9.8) |
| Graft source, n (%) | ||||
| Bone marrow | 41 (13.5) | 24 (16.1) | 13 (14.1) | 10 (16.4) |
| Peripheral blood | 241 (79.5) | 113 (75.8) | 73 (79.3) | 48 (78.7) |
| Cord blood | 19 (6.3) | 11 (7.4) | 6 (6.5) | 3 (4.9) |
| Other† | 2 (0.7) | 1 (0.7) | 0 | 0 |
| Donor type, n (%) | ||||
| Haploidentical | 14 (4.6) | 8 (5.4) | 6 (6.5) | 2 (3.3) |
| Matched related | 97 (32.0) | 52 (34.9) | 29 (31.5) | 26 (42.6) |
| Matched unrelated | 148 (48.8) | 62 (41.6) | 45 (48.9) | 23 (37.7) |
| Mismatched | 34 (11.2) | 27 (18.1) | 12 (13.0) | 10 (16.4) |
| Days from transplant to first dose, n (%) | ||||
| ≤1 weeks | 63 (20.8) | 32 (21.5) | 37 (40.2) | 26 (42.6) |
| >1 to ≤2 weeks | 85 (28.1) | 43 (28.9) | 55 (59.8) | 35 (57.4) |
| >2 to ≤3 weeks | 84 (27.7) | 37 (24.8) | 0 | 0 |
| >3 to ≤4 weeks | 69 (22.8) | 36 (24.2) | 0 | 0 |
| >4 weeks | 2 (0.7) | 1 (0.7) | 0 | 0 |
| Risk category | ||||
| High-risk | 223 (73.6) | 109 (73.2) | 69 (75) | 39 (63.9) |
| Low-risk | 80 (26.4) | 40 (26.8) | 23 (25) | 22 (36.1) |
Data are presented as number (%) unless otherwise indicated.
162 patients were randomly assigned within 2 weeks of HCT, received at least 6 doses of brincidofovir or placebo within the first 3 weeks after randomization, and had a baseline sample available (n = 98, brincidofovir; n = 64, placebo); 7 patients had HHV-6B detected in plasma at baseline and were excluded (n = 4, brincidofovir; n = 3, placebo); 2 patients in the brincidofovir group were negative at baseline but had no subsequent samples (supplemental Figure 1).
Combination of adult haploidentical peripheral blood and cord blood grafts.
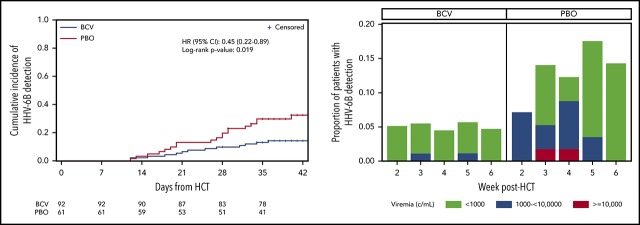
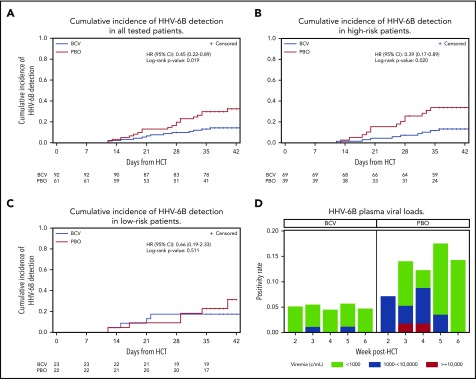
The cumulative incidence of HHV-6B plasma detection by 6 weeks post-HCT was significantly lower among patients randomly assigned to brincidofovir (14.2%) compared with placebo (32.4%; log-rank, 0.019; Figure 1A). When stratified by risk for HHV-6B reactivation, brincidofovir appeared to have a greater effect on preventing HHV-6B reactivation among high-risk patients (Figure 1B-C). In an adjusted Cox model, randomization to brincidofovir remained associated with a lower hazard for HHV-6B plasma detection (adjusted hazard ratio, 0.40; 95% confidence interval, 0.20-0.80; supplemental Table 2). Patients receiving brincidofovir also had a lower magnitude of HHV-6B detection (Figure 1D). Two patients (2%) in the brincidofovir group had HHV-6B viremia above 1000 copies/mL compared with 7 patients (11%) in the placebo group. No patients had findings consistent with donor-derived inherited chromosomally integrated HHV-6.22
Figure 1.
Cumulative incidence and magnitude of HHV-6B reactivation stratified by brincidofovir vs placebo for post-HCT prophylaxis. (A-C) Cumulative incidence of any plasma detection of HHV-6B by quantitative polymerase chain reaction in the overall cohort (A) and stratified by high risk (B) or low risk (C) for HHV-6B reactivation. The number of patients at risk in the brincidofovir (BCV) and placebo (PBO) groups are indicated underneath the figures. (D) Proportion of patients with maximum plasma detection of HHV-6B at different viral load thresholds in the BCV and PBO study groups.
In addition to the virologic outcome, we explored differences in incident rashes, pneumonia, or encephalitis through week 7 post-HCT. Rash was reported less frequently among patients receiving brincidofovir (n = 8; 9%) compared with placebo (n = 16; 26%; P = .006). HHV-6B viremia and timing in patients with rash, and all patients with GVHD, are in the supplemental Data. There was no difference in the incidence of pneumonia (1 patient on brincidofovir and 2 patients on placebo) or HHV-6 encephalitis (0 patients receiving brincidofovir and 1 patient receiving placebo), but these comparisons were limited by few events.
This study demonstrates that prophylactic brincidofovir was associated with a significant reduction in both the incidence and magnitude of HHV-6B reactivation in the first 6 weeks after allogeneic HCT. Given the relationship between peak HHV-6B DNA detection in blood and clinical outcomes, wherein viral loads higher than 10 000 copies/mL are sensitive and specific for HHV-6B encephalitis,20,21 prophylactic brincidofovir could mitigate HHV-6B-associated complications. The high in vitro activity of brincidofovir for HHV-6B,17 coupled with evidence for central nervous system penetration in animal models,18,23 make it a promising candidate for prevention and treatment of HHV-6B.
We demonstrated that rash occurred less frequently in patients randomly assigned to brincidofovir compared with placebo. Rash commonly accompanies both primary HHV-6B infection and reactivation after HCT.8,11 HHV-6B-associated rash after HCT may be misdiagnosed as GVHD and lead to unnecessary treatment.24 However, patients receiving oral brincidofovir were more frequently diagnosed with gastrointestinal GVHD because of gastrointestinal toxicity from the drug, which resulted in higher steroid administration.19 Thus, rashes may have been prevented by excess glucocorticoid treatment in the brincidofovir group. Furthermore, the gastrointestinal toxicity of oral brincidofovir precluded analyses of the effect of brincidofovir on reducing HHV-6B-associated GVHD.19 This study was not powered to evaluate an effect on pneumonia or encephalitis. These data support future studies using the intravenous formulation of brincidofovir, which is expected to have less gastrointestinal toxicity while achieving similar blood concentrations,19,25 to prevent HHV-6B reactivation and associated complications (eg, encephalitis, GVHD, and mortality) in HCT patients at high risk for HHV-6B reactivation.
We note that these results are from a post hoc subgroup analysis. Given our goal of studying the in vivo activity of brincidofovir for HHV-6B prevention, this study was restricted to patients randomized within the first 2 weeks after HCT and who received consecutive doses of study drug for at least 3 weeks after randomization. It is possible that these selection criteria affected our findings and may reduce the generalizability of this study, although baseline characteristics of the selected cohort aligned with those of the entire randomized cohort. There may be a rebound of HHV-6B reactivation after discontinuation of brincidofovir, but we did not test samples beyond 6 weeks in the context of this trial, given potential confounding by the increased rate of GVHD diagnoses attributable to the oral brincidofovir formulation.
In conclusion, brincidofovir prophylaxis appeared to reduce the incidence and magnitude of HHV-6B reactivation after allogeneic HCT in a post hoc analysis of a randomized controlled trial. These data support further study of intravenous brincidofovir to prevent HHV-6B reactivation and associated complications after HCT.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This study was supported by Chimerix, Inc. and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (K23 AI119133 to J.A.H.). The authors acknowledge the contributions of the SUPPRESS Trial Clinical Study Group in implementing the SUPPRESS trial. The authors also acknowledge the University of Washington Clinical Virology Laboratory for HHV-6B species testing.
Footnotes
This work was previously presented at the 2019 annual meeting of the American Society for Transplantation and Cellular Therapy, Houston, TX, 23 February 2019.
For original data, please contact jahill3@fredhutch.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.H., W.G.N., and M.J.B. designed the study; J.A.H., F.M.M., G.A.P., R.L., and M.J.B. collected the data; T.M.B. analyzed the data and created the figures; J.A.H., W.G.N., T.M.B., D.M.Z., and M.J.B. interpreted the data; J.A.H. drafted the initial manuscript; and all authors contributed to the writing and revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: J.A.H. has served as a consultant for Gilead Sciences. F.M.M. has received research support from Ansun, Cidara, Chimerix, F2G, Merck, Scynexis, and Shire and has served a consultant for Amplyx, F2G, Janssen, Kyorin, Merck, Regeneron, and ReViral. M.J.B. has served as a consultant and received research support from Chimerix Inc. and Gilead Sciences. G.A.P. has served as a consultant and received research support from Chimerix Inc. W.G.N., T.M.B., and R.L. are employees of Chimerix Inc. D.M.Z. declares no competing financial interests.
Correspondence: Joshua A. Hill, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail Stop E-400, Seattle, WA 98109; e-mail: jahill3@fredhutch.org.
REFERENCES
- 1.Ogata M, Oshima K, Ikebe T, et al. . Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(11):1563-1570. [DOI] [PubMed] [Google Scholar]
- 2.Abidi MZ, Hari P, Chen M, et al. . Virus detection in the cerebrospinal fluid of hematopoietic stem cell transplant recipients is associated with poor patient outcomes: a CIBMTR contemporary longitudinal study. Bone Marrow Transplant. 2019;54(8):1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2013;48(4):574-580. [DOI] [PubMed] [Google Scholar]
- 4.Hill JA, Mayer BT, Xie H, et al. . The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood. 2017;129(16):2316-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan TL, Carlin K, Ljungman P, et al. . Human Herpesvirus-6B reactivation is a risk factor for grades II to IV acute graft-versus-host disease after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2018;24(11):2324-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, O’Dwyer DN, Xia M, et al. . First onset herpesviral infection and lung injury in allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med. 2019;200(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill JA, Vande Vusse LK, Xie H, et al. . Human herpesvirus 6B and lower respiratory tract disease after hematopoietic cell transplantation. J Clin Oncol. 2019;37(29):2670-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux J, Battistella M, Fornecker L, et al. . Human Herpesvirus-6 cytopathic inclusions: an exceptional and recognizable finding on skin biopsy during HHV6 reactivation after autologous stem-cell transplantation. Am J Dermatopathol. 2012;34(6):e73-e76. [DOI] [PubMed] [Google Scholar]
- 9.Admiraal R, de Koning CCH, Lindemans CA, et al. . Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140(6):1643-1650. [DOI] [PubMed] [Google Scholar]
- 10.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2018;2(4):428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muramatsu H, Watanabe N, Matsumoto K, et al. . Primary infection of human herpesvirus-6 in an infant who received cord blood SCT. Bone Marrow Transplant. 2009;43(1):83-84. [DOI] [PubMed] [Google Scholar]
- 12.Ishiyama K, Katagiri T, Ohata K, et al. . Safety of pre-engraftment prophylactic foscarnet administration after allogeneic stem cell transplantation. Transpl Infect Dis. 2012;14(1):33-39. [DOI] [PubMed] [Google Scholar]
- 13.Ogata M, Satou T, Inoue Y, et al. . Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplant. 2013;48(2):257-264. [DOI] [PubMed] [Google Scholar]
- 14.Ogata M, Takano K, Moriuchi Y, et al. . Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transplant. 2018;24(6):1264-1273. [DOI] [PubMed] [Google Scholar]
- 15.Ogata M, Satou T, Kawano R, et al. . Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant. 2008;41(3):279-285. [DOI] [PubMed] [Google Scholar]
- 16.Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant. 2011;46(6):863-869. [DOI] [PubMed] [Google Scholar]
- 17.Chemaly RF, Hill JA, Voigt S, Peggs KS. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antiviral Res. 2019;163:50-58. [DOI] [PubMed] [Google Scholar]
- 18.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202(10):1492-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marty FM, Winston DJ, Chemaly RF, et al. ; SUPPRESS Trial Clinical Study Group . A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill JA, Koo S, Guzman Suarez BB, et al. . Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata M, Satou T, Kadota J, et al. . Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;57(5):671-681. [DOI] [PubMed] [Google Scholar]
- 22.Pellett PE, Ablashi DV, Ambros PF, et al. . Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22(3):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tippin T, Srnka A, Savina P, Van Sickle K, Naderer O. Tissue distribution of radioactivity after intravenous and oral administration of brincidofovir to rats . Poster presented at the American Association of Pharmaceutical Scientists Annual Meeting and Exposition. November 2016. Denver, CO. [Google Scholar]
- 24.Pichereau C, Desseaux K, Janin A, et al. . The complex relationship between human herpesvirus 6 and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1):141-144. [DOI] [PubMed] [Google Scholar]
- 25.Wire MB, Morrison M, Anderson M, et al. . Pharmacokinetics (PK) and safety of intravenous (IV) brincidofovir (BCV) in healthy adult subjects. . Open Forum Infect. Dis. 2017;4(suppl_1):S311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.