Abstract
An individual’s ability to respond to and align with the behavior of others is a fundamental component of social behavior. Zebra finches form life-long monogamous pair bonds; however, zebra finches are also gregarious and can form strong social bonds with same-sex conspecifics. Here, we quantified behavior during brief 10-minute reunions for males and females in five types of social conditions: monogamously bonded opposite-sex partners, familiar same-sex, familiar opposite-sex, novel same-sex, and novel opposite-sex dyads. We analyzed these interactions in three ways. First, we quantified overall activity levels (call and movement rates) for each individual. Second, we measured how coordinated calls and movements were by calculating (1) the percent difference in activity rates as an estimate of how similar calling and movement activity were between individuals within a dyad, and (2) the sliding correlation coefficients for time-stamped calls and movements for each dyad. Finally, we described multi-modal behavioral profiles of coordination using principal components analyses. Overall, females were more active than males. For both females and males, activity levels as well as the coordination of calls and movements were significantly affected by social condition. In general, the pattern was that monogamous partners, female familiar same-sex dyads and familiar opposite-sex dyads were more coordinated. This effect of familiarity shows that moment-to-moment behavioral coordination can be influenced by prior social experiences. Quantifying patterns of coordination or social synchrony may prove valuable for understanding the effects of social experience on brain and behavior.
Keywords: Behavioral synchrony, interactional synchrony, sex differences
Introduction
Social behavior can be broadly defined as the behaviors of an individual that are affected by others (Whishaw & Kolb, 2006). An individual’s ability to respond to and align with the behaviors of others is a fundamental component of social behavior (Insel & Fernald, 2004). Across species, the temporal alignment of vocal and physical behaviors is a critical component of courtship and pair-bonding. Duetting, courtship displays and biparental care are highly specialized examples of behavioral coordination, which are by definition associated with breeding and monogamous partnerships. Behavioral coordination is also fundamental to other affiliative relationships and social behaviors in various contexts. Most social species engage in highly coordinated activities with conspecifics other than their pair-bonded partners, such as is seen with schooling/flocking (Boinski & Garber, 2000; Greenberg, 2001) and hunting (Bailey et al., 2013; Handegard et al., 2012). More broadly, the coordination of activities is associated with group living (Conradt & Roper, 2005; Focardi & Pecchioli, 2005), an increase in social cohesion (King & Cowlishaw, 2009; Pays et al., 2007), and an increase in affiliative behaviors (Sakai et al., 2010). Coordination between two individuals also promotes prosocial behavior (Ashton–James et al., 2007; Gueguen et al., 2009; Van Baaren et al., 2004) (reviewed in (Duranton & Gaunet, 2016)). Whereas it is clear that behavioral coordination is an essential component of social behavior across a wide range of species, it is unclear how behavioral coordination varies with social and environmental context.
In monogamous bird species, the importance of coordination is seen with temporal alignment and turn-taking in vocal duetting (Elie et al., 2010; Hall, 2009; Odom & Omland, 2017), courtship (Ota et al., 2015), and territorial displays (Hall & Magrath, 2007; Ręk & Wong, 2017). Additionally, behavioral coordination, including turn-taking and division of labor, is necessary for the sharing of parental duties in biparental species. Not only is behavioral coordination important for biparental care, but such division of labor can require active negotiation by both partners. In several avian species, intra-pair calling during nesting is tightly coordinated and can directly reflect the division of labor (Boucaud et al., 2016; Boucaud et al., 2017; Elie et al., 2010; Villain et al., 2016; Villain et al., 2017). Furthermore, this coordination has been positively related to the reproductive fitness of a pair in biparental bird species (Bebbington & Hatchwell, 2015; Fortune et al., 2011; Koenig & Walters, 2016; Mariette & Griffith, 2012, 2015; van Rooij & Griffith, 2013; Wojczulanis-Jakubas et al., 2018)
It is estimated that 90% of bird species form some type of monogamous bond and engage in biparental care (Black & Hulme, 1996; Silver et al., 1985). Furthermore, many bird species are also highly gregarious and socially tolerant, living in large flocks and maintaining numerous social relationships. Individuals can form multiple strong relationships, which can even be behaviorally indistinguishable from monogamous partnerships (Alger et al., 2011; Elie et al., 2011). Such relationships emphasize the importance of social experiences outside of pair-bonded partnerships. However, further research is needed to determine the role of behavioral coordination in social relationships outside of monogamous partnerships.
In our current experiment, we studied zebra finches (Taeniopygia guttata). Amongst small songbirds, zebra finches are unusual in the apparent strength of their monogamous bonds: they form selective life-long pair bonds (Zann, 1996), maintain these bonds during both breeding and non-breeding periods (Prior & Soma, 2015), and have low levels of extra-pair paternity (Birkhead et al., 1990; Griffith et al., 2010). However, zebra finches are also extremely gregarious and exhibit a high degree of social tolerance (Zann, 1996). In the absence of an opposite-sex partner they will form equally strong social bonds with same-sex conspecifics, and they can also maintain multiple social bonds (Alger et al., 2011; Elie et al., 2011; Tomaszycki & Zatirka, 2014).
In order to investigate the role of behavioral coordination across relationships, it is important use a social context that is comparable across different social dyads. Thus, we quantified moment-to-moment behavioral coordination during brief 10-minute reunions (similar to a greeting). As a gregarious species, reunion/greeting behavior is likely to be ethologically relevant between monogamously partnered individuals as well as non-pair-bonded individuals (Zann, 1996). Importantly, while greeting behavior is considered to be prosocial, the significance of behavioral variation during greetings is unclear. Here, we recorded calls and movements during greeting interactions from males and females in five different social conditions: monogamous partners, familiar opposite sex conspecifics, familiar same sex conspecifics, novel opposite sex conspecifics, and novel same sex conspecifics. We analyzed these interactions in three different ways. First, we described the amount of activity (calling and movement) for each individual. Second, we measured the coordination of calls and movements in two ways: (1) calculating the percent difference in activity rates as an estimate of how similar calls and movements are between individuals within a dyad, and (2) calculating the sliding correlation coefficients for time-stamped calls and movements for each dyad. Finally, in order to describe multimodal behavioral profiles for males and females within different social conditions, we conducted separate principal components analyses (PCA) for females and males using four dependent variables (call rate, movement rate, sliding correlation coefficient of time-stamped calls and sliding correlation coefficient of time-stamped movements).
Materials and Methods
Subjects
Zebra finches, 10 males and 10 females (7 to 8 months old), were used as focal birds for this study. Additionally, 2 males and 2 females were used as novel stimulus birds. All birds were paired for the first time during this study. All zebra finches were housed with ad libitum seed, water and grit on a 12L:12D light cycle.
Monogamous partners
Zebra finches were initially housed in same-sex flocks, then transitioned to mixed-sex flocks for 72 hours and given the opportunity to freely form monogamous pairs. Mixed-sex flocks were composed of 2–3 males and 2–3 females and had either a male or female-biased sex ratio, which we find facilitates pair bonding. Pair bonding was assessed visually twice a day (am and pm) by quantifying selective affiliation (clumping, allopreening, and coordinated preening) between individuals during 10 min behavioral observations. Four males and 4 females freely formed clear pair bonds, and the remaining 6 pairs were formed by randomly assigning males to females. Thus, we established 10 zebra finch pairs.
Prior to the start of the experiment, pairs were housed together for over 2 months, and during this time all pairs were observed engaging in pair-directed affiliative behaviors (e.g. clumping and allopreening). After this experiment, all pairs attempted to breed (nest building and/or egg laying) and 8 out of 10 pairs successfully fledged chicks. Therefore, we considered all 10 of these pairs to be strongly bonded (Zann, 1996).
Familiar dyads
The same two monogamous pairs were housed adjacent to one another, separated by an opaque partition, for the entire period post-pairing (2 months). During this period, neighbors were in acoustic but not visual or physical contact. Two weeks prior to behavioral testing the opaque partition was replaced with a wire partition leaving the pairs in both visual and acoustic contact (but not physical contact). These neighboring pairs were used to form same- and opposite-sex familiar dyads. Note that these birds also had varying degrees of prior acoustic visual and physical contact based on the initial 2 months of habituation in same-sex flocks and 3 days of mixed-sex housing during courtship.
Novel stimulus birds
Novel dyads were set up by pairing a focal zebra finch with a bird that had been housed in a separate colony room. These novel stimulus birds had no prior contact of any kind with the focal birds.
Brief reunion behavioral paradigm
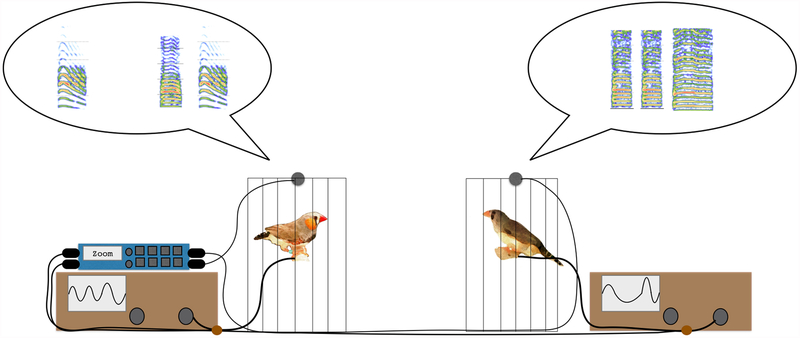
In order to define behavioral coordination in a manner that could be compared across social relationships, we chose to focus on interactions during brief reunions, the 10 minutes following a brief separation or disruption (similar to greeting behavior). Our behavioral set up is illustrated in Figure 1. This behavioral assay is based on partner separation and reunion paradigms used previously (Prior et al., 2018; Prior et al., 2014). However, in this paradigm we focus on the finer, moment-to-moment details of behavior. More specifically, in addition to video and audio recordings, movement data were recorded from a piezo sensor attached to the perch of a smaller cage along with audio recordings using a single multi-channel Zoom recorder (F8). Thus, the audio and movement recordings were temporally synchronized.
Figure 1:
Schematic illustration of the reunion (greet) paradigm. Behavior is recorded using four channels of a Zoom recorder (F8): movement is recorded from a piezo sensor attached to the perch of a smaller cage (indicated via oscilloscope), and acoustic behavior is recorded using tie clip microphones (indicated via spectrogram). Using MATLAB, we quantified (1) the number of calls (2) the number of movements (3) the temporal correlation of calling (sliding correlation of calling between the two birds), and (4) the temporal correlation of movement (sliding correlation of movement between the two birds).
Habituation to behavioral paradigm
The brief reunion test was conducted in a sound-attenuated room, separate from the colony room. Birds were caught in their home cage and individually transported in a small covered cage to the testing room, which took 2–3 min for each bird (4–6 minutes for both birds). In the testing room, birds were not handled and were allowed to enter the testing cage/exit the transport cage on their own. This procedure minimized the stress of handling during and prior to behavioral testing. To further minimize stress of handling, all focal birds were habituated to the testing and transport procedure. Habituation began two months prior to the start of the experiment, before initial pairing. Habituation included 10 consecutive days of transport to the testing room (transport only = 4 days; alone = 3 days; and same-sex flock mate = 3 days). Additionally, over the 2 months of pairing, birds were regularly transported and tested with their monogamous partner. Thus, each focal bird had experienced this testing and transport procedure at least 20 times over 2 months. Novel stimulus birds also received 10 consecutive days of habituation prior to the experiment.
On the one hand, our goal was to minimize stress on birds, in order to get full behavioral responses from each individual. However, in order to elicit a behavioral interaction, we also needed to provide a disruption. Thus, the goal was to elicit a significant behavioral response, while keeping the period of disruption (i.e. separation and transport) as short as possible. Based on previous experiments using other behavioral assays and our pilot experiments using the current reunion paradigm, we decided the 4–6 min separation caused by the time to transport birds was sufficient to elicit greeting behavior. Previous research has shown that brief disruptions of even 30 seconds are sufficient to alter the behavior of paired zebra finches (Prior et al., 2016). Additionally, we tested different separation periods (between 5 min and 24 hours) using our current paradigm and longer separations did not necessarily lead to more robust behavioral responses for paired birds (data not shown).
Testing was conducted on 5 consecutive days. Each bird was recorded once in each type of dyad (social condition). Across pairs, the order of social conditions was counterbalanced and distributed throughout each day. Additional precautions were taken to minimize confounding factors: (1) The two partners from a pair were not tested back-to-back, (2) neighbors were not tested back-to-back, (3) novel stimuli were used for only 2 tests per day, and (4) novel stimulus partners were not tested back-to-back.
Quantifying behavioral coordination
For each individual, we recorded movement data with a piezo sensor attached to the perch of a smaller cage. We verified piezo sensor detection of bird movements in two ways: (1) manually tapping the perch 10 times and confirming that our Matlab classification and analysis program identified 10 discrete movements, and (2) attaching the piezo sensor to an oscilloscope while a live bird was in the cage to identify which bird movements were detectable. From these tests we confirmed that the piezo sensor was sensitive to enough to record both small movements such as head tilts and wing fluffs, as well as larger movements such as perch hops. For the purposes of this experiment we did not distinguish between types of movements. We also video recorded each test using a GoPRO camera. Every video was watched in order to verify that birds were spending a significant portion of the test on the perch. During the first 5 minutes females spent on average 87±9 % and males spent 78±13 % of their time on the perch or hopping between the perch and cage walls (mean ± SEM).
Using a MATLAB program (written by E.S.) we automatically identified time-stamped movements for each bird within a dyad. Calls were semi-automatically classified. After an initial automatic classification of events (noise and vocalizations) on each audio channel, one researcher (N.H.P.) manually classified these auditory events as calls or non-vocalizations. Additionally, each call was assigned to either the right or left channel by visual assessment of the time waveform. In this way, time-stamped calls were identified for each bird within a dyad. Previous experiments using similar partner separation-reunion paradigms have found that this primarily elicits stack-like short calls and some distance calls, and very few tet-like short calls or male songs (Gill et al., 2015; Prior et al., 2018). Although we did not score call types in the current study, the vast majority of vocalisations we heard on the recordings were stack-like calls. From these calls and movements, we quantified activity (number of calls and movements per minute) as well as the sliding correlation coefficient for coordination the time-stamped calls and movements within a dyad.
The sliding correlation coefficient was calculated between the vocalizations and movements separately using MATLAB “corrcoef” function which is based on Pearson correlations. The step size for the sliding correlations was chosen based on the temporal dynamics of the movement and vocal signals. A 1 ms sliding correlation timestep was used for vocal analyses whereas 40 ms was used for movement analyses. This was based on our preliminary observations of the impulse response of the piezo sensor (on the order of 40 ms). Inputs to the sliding correlation computations were two vectors of ones and zeros. The vectors were constructed using the chosen step sizes of (1 or 40 ms). The sensor signal power in each time step was computed. Ones were used in the vectors to represent above-threshold values (a movement or vocalization), and zeros were used to represent below-threshold values. For statistical analyses, here we report the maximum Pearson’s correlation coefficient value (“corrcoef”) based on all possible temporal offsets.
Statistical analysis
All statistical analyses were carried out in R (v.3.2.3, R Foundation for Statistical Computing). We used linear-mixed models (function lmer from the lme4 Package). For each model, prior to interpretation, we transformed data as necessary based on the distribution of the residuals. All graphically presented data are non-transformed. Follow up tests were conducted using pairwise t-tests with Benjamini & Hochberg (1995) corrections for multiple comparisons or function “difflsmeans” (library ‘lmerTest’) or Tukey’s HSD.
To determine how long the natural greeting behavior lasted, our first statistical models assessed the effect of Time (minute 1 through minute 10) as well as Social Condition (monogamous partners, familiar opposite-sex, female familiar same-sex, male familiar same-sex, female novel same-sex, male novel same-sex, female novel opposite-sex, male novel opposite-sex) and Sex of focal individuals. Note that novel opposite sex dyads are divided into female and male social conditions because our focal subjects were in dyads with novel stimulus birds in these conditions. Behavior was not directly assessed for the novel stimulus birds. Behavior was scored at either the individual or pair level, and thus either individuals or pair identity was included as a random factor in each of the models as appropriate.
The first 5 minutes of the behavioral test included the majority of the greeting behavior. Thus, we assessed the effect of Sex and Social Condition (separately) on level of activity, coordination of activity and profiles of behavioral coordination. In order to describe the patterns of behavioral coordination across social conditions, we conducted two PCAs, one for the females and one for males (function ‘prcomp’) (Table 1). For both analyses, we included four dependent variables: call rate, movement rate, sliding correlation of time-stamped calls, sliding correlation of time-stamped movements). Note that only raw behavioral measures (non-transformed) were loaded into the PCA.
Table 1:
PC loadings from the PCA analyses for females and males separately. We considered parameters that loaded on their respective components > 0.30 to be descriptor parameters (bolded), and > 0.50 to be strong descriptors (*).
| Female | Male | |||
|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | |
| Cumulative Variance | 44% | 72% | 56% | 77% |
| Call Rate | 0.58* | −0.28 | 0.58* | −0.26 |
| Movement Rate | 0.32 | −0.78 | 0.51 | −0.61 |
| Calling Correlation Coefficient | 0.56* | 0.39 | 0.44 | 0.62* |
| Movement Correlation Coefficient | 0.48 | 0.40 | 0.46 | 0.41 |
Note: Here the loadings are multiplied by −1, making more positive values more coordinated for both PC 1 and PC 2 (for females and males). Likewise, all the PCs in subsequent figures are multiplied by −1 to facilitate interpretation of the results.
Results
Activity is highest at the beginning of the reunion
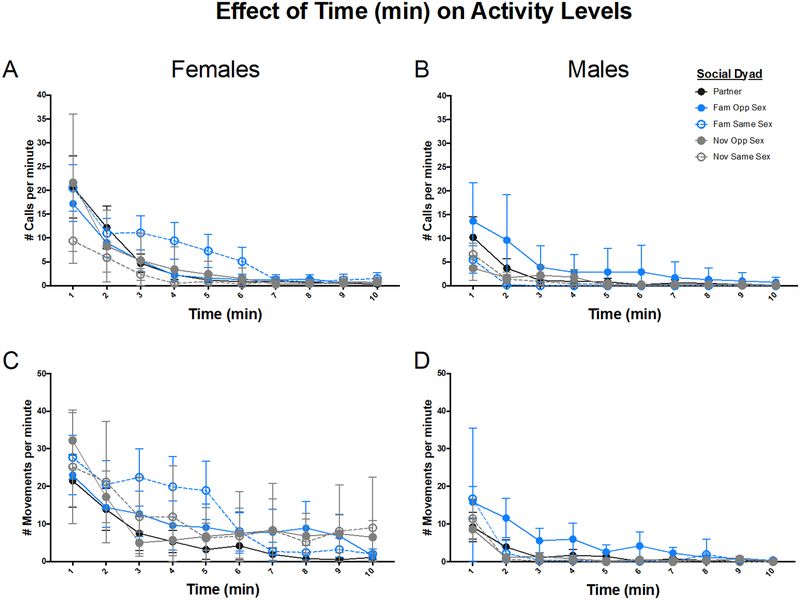
Activity levels changed over the course of the 10-minute reunion. Therefore, we first considered Time as a factor in our statistical models. Both call rate and movement rate were highest at the beginning and decreased over the course of the 10 min reunion (Time (min) for Call Rate χ(1) = 299.82, P < 0.001 (Figure 2A–B); Time (min) for Movement Rate χ(1) = 301.43, P < 0.001 (Figure 2C–D)). The rate of this decrease was affected by both Sex and Social Condition (Sex × Time × Social Condition for Call Rate χ(5) = 15.55, P = 0.001; Sex × Time × Social Condition for Movement Rate χ(4) = 12.73, P = 0.005). Despite recording behavior for 10 minutes, there was very little activity in the second 5 minutes. Therefore, the rest of the results are calculated based on behavior during the first 5 min of the reunion.
Figure 2:
Activity over time: call rate (A and B) and movement rate (C and D) for females (A and C) and males (B and D). Mean ± 95% confidence interval. Open shapes = same-sex dyads, closed shapes = opposite-sex dyads, blue = familiar dyads, gray = novel dyads, and purple = monogamous partners.
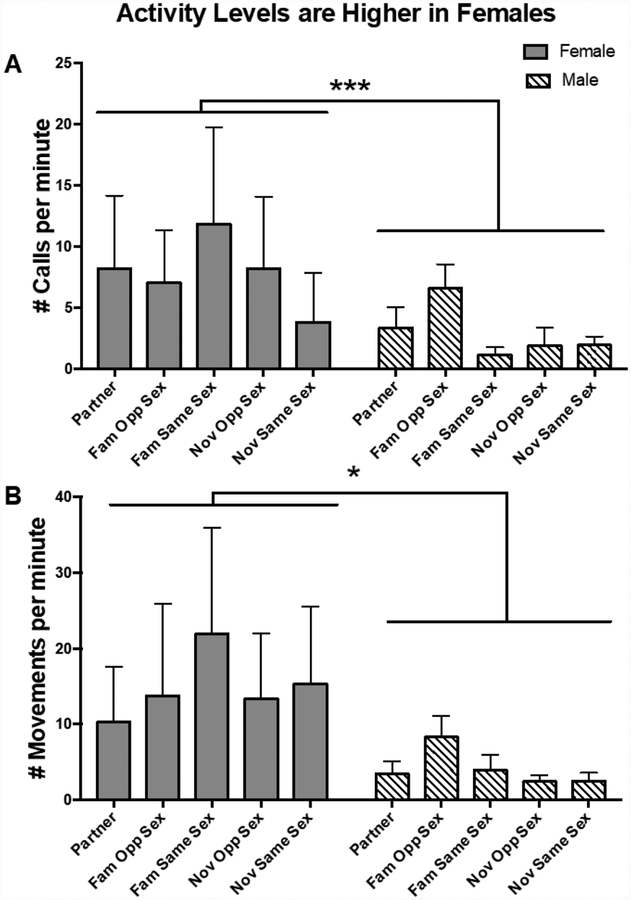
Females were more active than males
Overall, females called more than males (Sex χ(1) = 13.84, P < 0.001) (Figure 3A). For females, Social Condition did not affect Call Rate (χ(4) = 4.28, P = 0.370); however, for males Social Condition did significantly affect Call Rate (χ(4) = 11.45, P = 0.022). More specifically, there was a trend for males to call more in familiar opposite sex dyads (Pairwise comparison: Familiar Opposite Sex – Male Familiar Same Sex, P = 0.054; Familiar Opposite Sex – Novel Opposite Sex P = 0.054).
Figure 3:
Activity rates calculated during the 5 minute of the reunion (A) Calls and (B) Movements. Mean ± 95% confidence interval.
Movement rate was significantly affected by both sex and social condition (Sex χ(1) = 8.43, P = 0.004) (Figure 3B). Again, the effect of social condition was stronger in males than females (Females, χ(4) = 8.07, P = 0.089; Males, χ(4) = 10.45, P = 0.033). Although the effect of social condition on the pattern of activity rate was similar for movement and call rate, follow-up pairwise comparisons suggested that there were no significant differences between groups.
Familiarity influences moment-to-moment behavioral coordination of calling and movement
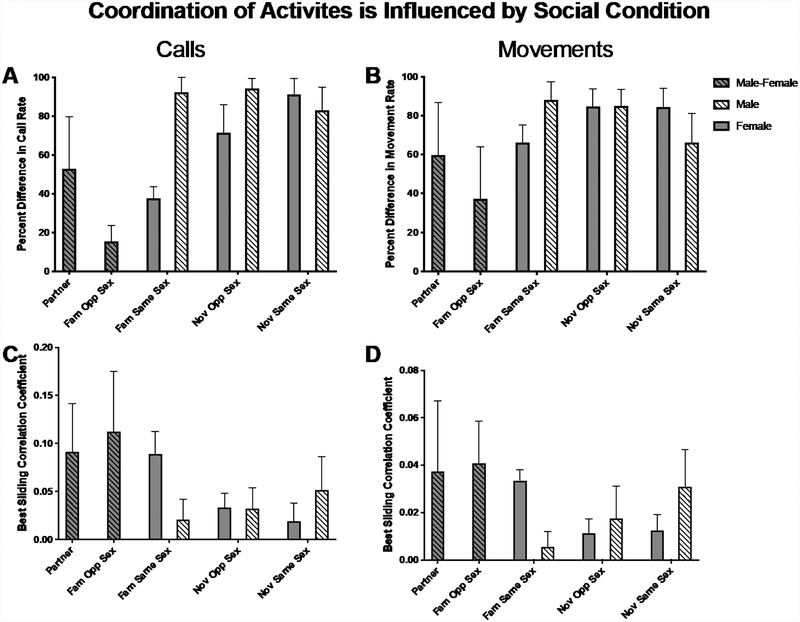
We assessed moment-to-moment coordination of calls and movements in two ways: first by quantifying the sliding correlation coefficient of time-stamped calls and movements (Figure 4; Figure 5A–B) and second by quantifying how similar activity levels were between the two individuals within the dyad (percent difference) (Figure 5C–D). Social condition significantly affected behavioral coordination both of calls (seen in both dependent measures) and movements (seen in the percent difference in movement rate) (Sliding Correlation of Calls χ(7) = 16.63, P = 0.020; Percent Difference in Call Rate χ(7) = 45.57 P < 0.001; Sliding Correlation of Movements χ(7) = 11.79, P = 0.110; Percent Difference in Movement Rate χ(7) = 17.67, P = 0.014) (Figure 5).
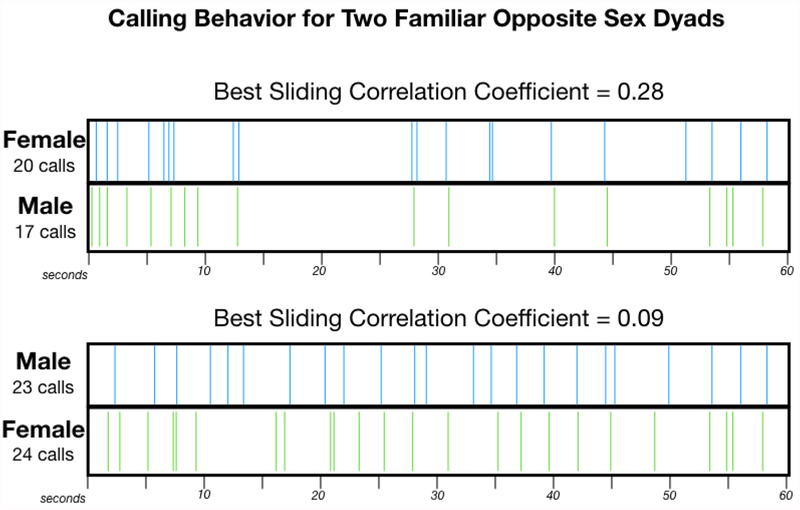
Figure 4:
Calling exchanges over the course of 1 minute are illustrated for two different Familiar Opposite-Sex Dyads. The corresponding sliding correlation coefficient is given. The top panel displays calling activity for a dyad with a relatively high sliding correlation coefficient, and bottom panel shows calling activity for a dyad with a lower sliding correlation coefficient. For each panel, the blue tick marks represent the onset of calls on channel 1 and the green tick marks represent the onset of calls on channel 2. These two example recordings were chosen because both individuals from each dyad called a lot. Thus, it is easier to visualize how the sliding correlation coefficient relates to variation in the pattern of calling behavior. Of particular note, the dyad represented in the top panel had organized calling bursts together and had longer periods of silence.
Figure 5:
Effect of social condition type on behavioral coordination. A and B the similarity in call rate within a dyad calculated as percent difference in the number of calls individuals. C and D sliding cross correlation coefficient of time-stamped calls. Coordination of calls is presented in A and C, and coordination of movements B and D. Mean ± 95% confidence interval.
Qualitatively, call rate was more similar in familiar dyads (including partners, familiar opposite sex and familiar same sex dyads) and likewise calling behavior was more coordinated in these dyads. Post Hoc analyses (Tukey’s HSD) point to Familiar Opposite Sex dyads and Female familiar same sex dyads as particularly coordinated (Sliding Correlation of Calling Familiar Opposite Sex – Female Novel Same Sex P = 0.026; Percent Difference in Call Rate: Familiar Opposite Sex – Female Novel Opposite Sex P = 0.002; Familiar Opposite Sex – Female Novel Same Sex P < 0.001; Familiar Opposite Sex – Male Familiar Same Sex P < 0.001; Familiar Opposite Sex – Male Novel Opposite Sex P < 0.001; Familiar Opposite Sex – Male Novel Same Sex P < 0.001). Social Condition affected the coordination of movements similarly to coordination of calling (Percent Difference in Movement Opposite Sex – Female Novel Opposite Sex P = 0.027; Familiar Opposite Sex – Female Novel Same Sex P = 0.029, Familiar Opposite Sex – Male Novel Opposite Sex P = 0.032).
Multimodal profiles of behavioral coordination
To capture the relation between activity levels and the coordination of activity (call rate, movement rate, sliding correlations of time-stamped calls and movements between the two individuals in a dyad), and to assess the effect of social condition on the pattern of these behavioral profiles, we conducted PCAs for female and male behavior separately.
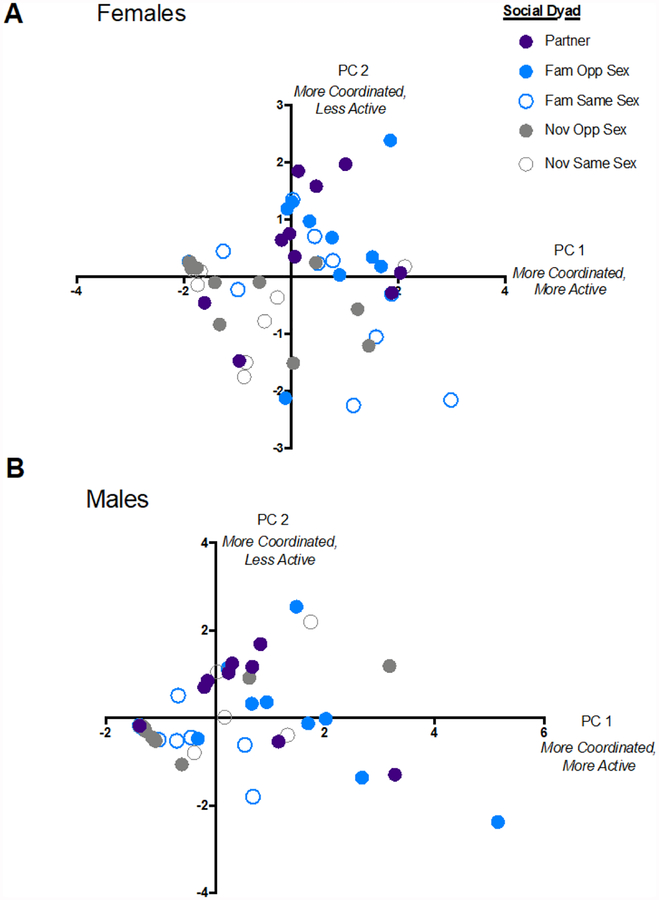
Overall, the behavioral profiles (PCs) describing variation in female and male behavior were similar. For both males and females, the predominant behavioral profile (PC 1) described elevated coordinated activity: all four dependent variables were positively correlated, with call rate being a particularly strong explanatory variable in both males and females (loadings > 0.50) (Table 1). This first component explained 44% of the variation for females and 56% of the variation for males.
For both females and males, PC 2 disentangled activity levels (call rate and movement rate) from coordination of activities (sliding correlations of calling and movement) (Table 1; Figure 6). Thus, PC 2 described low, but coordinated levels of activity. For both females and males, this effect is driven by the strong negative relationship between movement rate and the correlation of calling activity (loadings > 0.50). PC 2 explains 28% of variation for females and 21% for males. Together, we consider PC 1 and PC 2 as multi-modal profiles of behavioral coordination.
Figure 6:
Multi-modal behavior coordination profiles for females (A) and males (B) across relationship types. Open shapes = same-sex dyads, closed shapes = opposite-sex dyads, blue = familiar dyads, gray = novel dyads, and purple = monogamous partners. PC 1 describes elevated coordinated activity whereas PC 2 describes coordination, but low activity.
Familiarity influences profiles of behavioral coordination
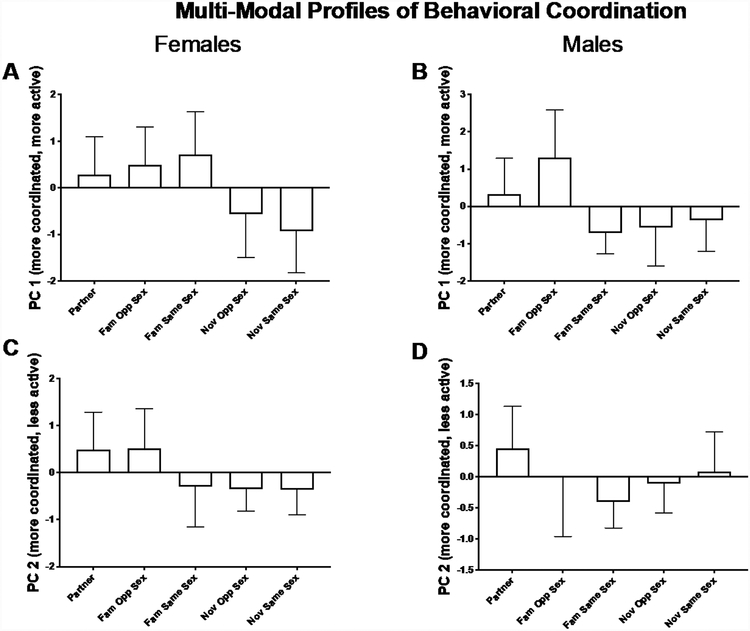
Social condition significantly affected profiles of behavioral coordination for both female and male focal subjects (Female PC 1 χ(4) = 18.05, P = 0.001; Female PC 2 χ(4) = 17.50, P = 0.002; Male PC 1 χ(4) = 16.99, P = 0.002; Male PC 2 χ(4) = 3.32, P = 0.505) (Figure 7). Again, the pattern was for familiar dyads to be more coordinated (Female PC 1 Pairwise comparisons Familiar Opposite Sex – Novel Same Sex P = 0.013; PC 2 Partner – Novel Same Sex P=0.018) (Male PC 1 pairwise comparison Familiar Opposite Sex – Familiar Same Sex P = 0.38, Familiar Opposite Sex – Novel Opposite Sex P = 0.011) (Figure 7).
Figure 7:
Effect of social condition on female (A and C) and male (B and D) multi-modal behavior coordination profiles. PC 1 (A) describes elevated coordinated activity whereas PC 2 (B) describes coordination, but low activity. Mean ± 95% confidence interval
Discussion
Initially, we did not have a specific prediction for how greeting behavior and behavioral coordination would be modulated by social condition. At the one extreme, zebra finches are highly gregarious; therefore, we could have expected that greeting behavior would have been non-discriminatory and similar across social groups. On the other hand, zebra finches are strongly monogamous; therefore, we could have expected that monogamous partners would have been more coordinated. We found that greeting behavior differed across dyads and that familiarity, particularly with females, resulted in more robust and more coordinated greeting behavior. More specifically, monogamous partners, familiar opposite sex dyads, and female familiar same sex dyads were more coordinated in both calling and movement (as evidenced by the raw coordination measures and the PCA analysis). This effect of familiarity is evidence that prior social experiences modulate moment-to-moment coordination. The fact that male familiar same sex dyads did not have robust behavioral responses suggests that either male-male relationships are less important or, that in this context, female behavior is driving these social interactions. Consistent with the latter is the fact that we report several sex differences in behaviors, including that females call and move almost twice as much as males. Importantly, while the PCAs show that multi-modal behavioral profiles of coordination were similar for females and males (all four dependent variables were positively correlated), the coordination in this paradigm is linked to activity. It is inherently challenging to disentangle the contribution of each individual within a dyad to pair-level metrics such as the sliding correlation coefficient. Thus, our results raise many questions about the significance of this sex difference for behavioral coordination as well as the ultimate significance of behavioral coordination across social contexts.
Moment-to-moment behavioral coordination and affiliative bonds
Given that behavioral coordination is associated with social bonding, it is natural to try to interpret the results of our current experiment within the framework of how strong different social relationships may be. If behavioral coordination during greeting interactions had been elevated in monogamous partners compared to other social conditions, this would provide evidence that monogamous partnerships are indeed the strongest relationship for zebra finches. Thus, the fact that female familiar same sex dyads and familiar opposite sex dyads were similarly coordinated, raises questions about the nature of these other familiar relationships both for the birds in our current study as well as for zebra finches more generally. We discuss these points below; however, it is also important to recognize that there is no clear evidence that behavioral coordination in our paradigm is related to the strength of social bonds.
In our current experiment social relationship type (dyad) is very likely confounded with the strength of affiliative bonds. The only opposite sex conspecific that individuals were housed with was their pair bonded partner, with the exception of the brief period during courtship. Neighboring pairs were kept physically and visually separated by an opaque barrier to minimize social interactions. Thus, based on social exposure alone we would predict that the monogamous partners would be the most strongly bonded of the social dyad. Furthermore, for monogamous partners, it was our goal was to ensure monogamous partnerships were strong. First birds were given the opportunity to freely form pair bonds in mixed sex flocks and 5 of the 10 pairs formed selective bonds (note that no clear same-sex bonds were formed during this time). Second after pairing, all the partners used in our experiment were observed engaging in affiliative behaviors and following the completion of this experiment all pairs went on to breed (8 out of the 10 pairs successfully fledged chicks). Whereas we took precautions to ensure that the social conditions (experience with partners and extent of familiarity) were similar across all birds; we did not assess the relationship between familiar neighbors outside of the greeting paradigm used here. The effect of social condition, previous social experience and the degree of selective affiliation on moment-to-moment behavioral coordination could be disentangled, in future studies, by studying flock-housed zebra finches with known social networks.
The robust behavioral responses and elevated coordination within female familiar same sex and familiar opposite sex dyads, could be interpreted as evidence that affiliative bonds with females are particularly important. Across species, considerably less is known about relationships outside of offspring-parent bonds and monogamous partnerships (Beery et al., 2008; Goodson et al., 2006; Tang-Martínez, 2003). In many species of primates, female affiliative bonds are essential for group function and have even been found to increase social integration and individual fitness (Henzi & Barrett, 1999; Palombit et al., 1997; Silk et al., 2009; Sterck et al., 1997). In zebra finches, however, previous research suggests that male-male bonds are stronger (Alger et al., 2011), and that males may have a greater proclivity to maintain social bonds (Madison et al., 2018; Prior et al., 2014). Furthermore, from research on wild flocks, there is no known function of female-female affiliative bonds, rather group movement seems to be organized around monogamous partnerships (McCowan et al., 2015; Zann, 1996).
Sex differences in behavioral coordination
The differences we report in behavioral coordination are largely driven by variation in activity level. There was a robust sex difference in the overall activity (females > males) as well as a difference in the number of calls that familiar females receive. This is not the first report that females have higher call rates than males in similar behavioral paradigms (Prior et al., 2018; Prior et al., 2014). Interestingly, more comprehensive studies, which examine thousands of calls produced in a naturalistic group context, do not find that females call more than males; however, they do suggest that in certain contexts (e.g. early in the nesting phase) females may produce more of the stack-like short calls than males (the main call type produced during our partner separation and reunion paradigms) (Gill et al., 2015; Ter Maat et al., 2014). Importantly, our current results show this elevated level of female activity is not just in the vocal domain, but also true of overall movement rate, suggesting the sex difference in activity is general across modalities. Although there is a precedent for elevated calling activity in females, previous work has shown that preferential calling is highest between monogamous partners (Fernandez et al., 2017; Gill et al., 2015; Stowell et al., 2016). However, when the monogamous partner is not present, then calling synchrony between non-partners increases (Fernandez et al., 2017). Thus, the question remains why, in the absence of their pair-bonded partners, females elicit and engage in elevated behavioral coordination with familiar conspecifics.
Whereas there is not clear evidence that female-female affiliative bonds are particularly important for zebra finches, there is evidence that females may be the more flexible “social caller”, displaying a greater ability to adjust the timing of their calls and responses (Benichov et al., 2015). Combined with the fact that females call more than males in similar paradigms (Prior et al., 2018), these findings would be consistent with the notion that females are driving behavior and communication in this context. For now, the potential function of this sex difference in social proclivity requires further study.
Moment-to-moment behavioral coordination and behavioral synchrony
Altogether, while it is difficult to interpret our results in terms of the importance of specific types of social relationships, our results clearly suggest that prior social experiences enhance moment-to-moment coordination. More specifically, our results support the hypothesis that patterns of prior experience, not simply the overall amount of experience, influences behavioral coordination. If amount of experience alone was the most important factor, then monogamous partners would have been the most coordinated social dyad. However, it is notable that the only prior experience familiar neighbors had was while visually separated, which is very similar to the behavioral assay. This raises the question of whether behavioral coordination would have been highest amongst monogamous partners if we had quantified behavior while the dyads were fully co-housed (able to interact physically). Another interesting question is whether zebra finches are particularly good at coordinating with conspecifics as a result of being so gregarious. The question remains what the function of such moment-to-moment behavioral coordination is. At a proximate level, the significance of variation in behavioral coordination across timescales and contexts is not well understood. The majority of research that aims to understand moment-to-moment behavioral coordination has focused on synchrony in human interactions.
Behavioral or social synchrony (i.e. broadly encompassing the temporal and/or spatial coordination of behaviors, internal biological states and/or social interactions) has been used to discuss affiliative bonds across a range of social species: including insects, birds, and mammals (Bernieri & Rosenthal, 1991; Feldman, 2012). In humans social synchrony has been defined as the non-verbal coordination of behaviors during interpersonal exchanges (Feldman, 2007); whereas interactional synchrony refers to both vocal and physical behaviors that are temporally aligned (non-random) (Bernieri & Rosenthal, 1991).
In humans, there is evidence that social or interactional synchrony on acute timescales (on the order of minutes) promotes the formation and reinforcement of affiliative bonds (Feldman & Eidelman, 2007; Feldman et al., 2011), reflects the relationship of the social partners (Kinreich et al., 2017), and may even galvanize individuals to respond to group threats (Mu et al., 2017). Human social synchrony is also assumed to communicate interest and positive affect (Bernieri & Rosenthal, 1991). During periods of increased gaze and social affect, neural synchrony is higher in romantic partners compared to strangers (Kinreich et al., 2017). However, such brain-to-brain coupling in humans does not require a strong social bond, and it can be achieved via shared memory or response to narratives (Chen et al., 2017; Hasson, 2016; Liu et al., 2017; Mu et al., 2017).
Duetting in birds is arguably one of the best examples of interactional synchrony studied in non-human animals. Intriguingly, research on duetting in the monogamously bonded plain-tailed wrens suggests there is similar brain-to-brain coupling during behavioral interactional synchrony in birds as described in humans. Neural recordings have demonstrated that the partners’ synchronized vocal duet is associated with tight correlation in the partners’ neural responses (Fortune et al., 2011). It is particularly remarkable that the synchrony of neural firing between mates occurs in response to the entire duet (both female and male components) but not to each individual component alone (Fortune et al., 2011).
Taken together, these lines of research raise the question of whether moment-to-moment interactional synchrony provides the basis of larger-scale behavioral alignment as evidenced by the impact of behavioral synchrony on brain-to-brain coupling. Indeed, this shared biological foundation of social alignment may provide an entry point to conduct rich comparative studies investigating the function of behavioral coordination across timescales, species and contexts. Conceptualizing social behavior within the framework of interactional synchrony may be useful for describing the nuances of how social interactions impact the brain and behavior.
Conclusion
The vast majority of research has focused on describing the behavioral and physiological mechanisms that support the formation of ‘extreme’ types of social bonds, such as parental and monogamous bonds; however, a deeper understanding of social systems requires the study of many types of social relationships. There are many challenges when it comes to extending our understanding of sociality beyond those more extreme bonds. In many instances social behaviors are not obviously linked to life history timepoints that would guide our understanding of etiology and function of those behaviors. Furthermore, there have been no clear metrics for assessing the quality of a range of social bonds. Here we provide evidence that moment-to-moment behavioral coordination during brief social reunions varies depending on sex, social condition and potentially social experience. Studying mechanisms underlying brief moment-to-moment synchrony could provide insights into the effects of social experiences on brain and behavior.
Acknowledgements
We would like to thank the entire Ball/Dooling lab for help with animal husbandry, data collection, and discussion of results. An earlier version of these results was presented at the Animal Behavior Society meeting in Milwaukee, WI in August 2018. We are grateful for all of the feedback we received there. For feedback on this manuscript, we thank Dr. Matthew D. Taves, Dr. Benjamin A. Sandkam, and three anonymous reviewers.
Funding
A T32 training grant to N.H.P (NIDCD T-32 DC00046).
Footnotes
Ethics Statement
This work was conducted in accordance with Association for the Study of Animal Behaviour (ASAB) guidelines and was approved by the Institutional Animal Care and Use Committee (IACUC) (R-15–09), University of Maryland, College Park.
References
- Alger SJ, Juang C, & Riters LV (2011). Social affiliation relates to tyrosine hydroxylase immunolabeling in male and female zebra finches (Taeniopygia guttata). Journal of chemical neuroanatomy, 42(1), 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton–James C, Van Baaren RB, Chartrand TL, Decety J, & Karremans J (2007). Mimicry and me: The impact of mimicry on self–construal. Social cognition, 25(4), 518–535 [Google Scholar]
- Bailey I, Myatt JP, & Wilson AM (2013). Group hunting within the Carnivora: physiological, cognitive and environmental influences on strategy and cooperation. Behavioral ecology and sociobiology, 67(1), 1–17. [Google Scholar]
- Bebbington K, & Hatchwell BJ (2015). Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird. Behavioral Ecology, 27(2), 652–659. [Google Scholar]
- Beery AK, Loo TJ, & Zucker I (2008). Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Hormones and behavior, 54(1), 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernieri FJ, & Rosenthal R (1991). Interpersonal coordination: Behavior matching and interactional synchrony In Feldman RS & Rimé B (Eds.), Studies in emotion & social interaction. Fundamentals of nonverbal behavior (pp. 401–432). New York, NY, US: Cambridge University Press. [Google Scholar]
- Birkhead TR, Burke T, Zann R, Hunter FM, & Krupa AP (1990). Extra-pair paternity and intraspecific brood parasitism in wild zebra finches Taeniopygia guttata, revealed by DNA fingerprinting. Behavioral ecology and sociobiology, 27(5), 315–324. [Google Scholar]
- Black JM, & Hulme M (1996). Partnerships in birds: The Study of Monogamy: Oxford University Press, UK. [Google Scholar]
- Boinski S, & Garber PA (2000). On the move: how and why animals travel in groups: University of Chicago Press. [Google Scholar]
- Boucaud I, Mariette M, Villain A, & Vignal C (2016). Vocal negotiation over parental care? Partners adjust their time spent incubating based on their acoustic communication at the nest. Biol. J. Linnean Soc, 117, 322–336. [Google Scholar]
- Boucaud IC, Perez EC, Ramos LS, Griffith SC, & Vignal C (2017). Acoustic communication in zebra finches signals when mates will take turns with parental duties. Behavioral Ecology, 28(3), 645–656. [Google Scholar]
- Conradt L, & Roper TJ (2005). Consensus decision making in animals. Trends in Ecology & Evolution, 20(8), 449–456 [DOI] [PubMed] [Google Scholar]
- Duranton C, & Gaunet F (2016). Behavioural synchronization from an ethological perspective: Overview of its adaptive value. Adaptive Behavior, 24(3), 181–19 [Google Scholar]
- Elie JE, Mariette MM, Soula HA, Griffith SC, Mathevon N, & Vignal C (2010). Vocal communication at the nest between mates in wild zebra finches: A private vocal duet? Animal Behaviour, 80(4), 597–605. [Google Scholar]
- Elie JE, Mathevon N, & Vignal C (2011). Same-sex pair-bonds are equivalent to male–female bonds in a life-long socially monogamous songbird. Behavioral ecology and sociobiology, 65(12), 2197–2208 [Google Scholar]
- Feldman R (2007). Parent–infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. Journal of Child psychology and Psychiatry, 48(3–4), 329–354 [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Hormones and behavior, 61(3), 380–391 [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman AI (2007). Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: The role of neonatal vagal tone. Developmental psychobiology, 49(3), 290–302 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: Considering stress and affiliation components of human bonding. Developmental science, 14(4), 752–761 [DOI] [PubMed] [Google Scholar]
- Fernandez MSA, Vignal C, & Soula HA (2017). Impact of group size and social composition on group vocal activity and acoustic network in a social songbird. Animal Behaviour, 127, 163–178. [Google Scholar]
- Focardi S, & Pecchioli E (2005). Social cohesion and foraging decrease with group size in fallow deer (Dama dama). Behavioral ecology and sociobiology, 59(1), 84–91 [Google Scholar]
- Fortune ES, Rodríguez C, Li D, Ball GF, & Coleman MJ (2011). Neural mechanisms for the coordination of duet singing in wrens. Science, 334(6056), 666–670. [DOI] [PubMed] [Google Scholar]
- Gill LF, Goymann W, Ter Maat A, & Gahr ML (2015). Patterns of call communication between group-housed zebra finches change during the breeding cycle. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, & Wang Y (2006). Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Hormones and behavior, 50(2), 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RS (2001). Birds of many feathers: The formation and structure of mixed species flocks of forest birds In Boinski S& Garber PA (Eds.), On the Move: How and Why Animals Travel in Groups (pp. 521–558): University of Chicago Press [Google Scholar]
- Griffith SC, Holleley CE, Mariette MM, Pryke SR, & Svedin N (2010). Low level of extrapair parentage in wild zebra finches. Animal Behaviour, 79(2), 261–264. [Google Scholar]
- Gueguen N, Jacob C, & Martin A (2009). Mimicry in social interaction: Its effect on human judgment and behavior. European Journal of Social Sciences, 8(2), 253–259. [Google Scholar]
- Hall ML (2009). A review of vocal duetting in birds. Advances in the Study of Behavior, 40, 67–121. [Google Scholar]
- Hall ML, & Magrath RD (2007). Temporal coordination signals coalition quality. Current Biology, 17(11), R406–R407 [DOI] [PubMed] [Google Scholar]
- Handegard NO, Boswell KM, Ioannou CC, Leblanc SP, Tjøstheim DB, & Couzin ID (2012). The dynamics of coordinated group hunting and collective information transfer among schooling prey. Current Biology, 22(13), 1213–1217. [DOI] [PubMed] [Google Scholar]
- Henzi SP, & Barrett L (1999). The value of grooming to female primates. Primates, 40(1), 47–59 [DOI] [PubMed] [Google Scholar]
- Insel TR, & Fernald RD (2004). How the brain processes social information: Searching for the social brain. Annu. Rev. Neurosci, 27, 697–722 [DOI] [PubMed] [Google Scholar]
- King AJ, & Cowlishaw G (2009). All together now: Behavioural synchrony in baboons. Animal Behaviour, 78(6), 1381–1387 [Google Scholar]
- Kinreich S, Djalovski A, Kraus L, Louzoun Y, & Feldman R (2017). Brain-to-brain synchrony during naturalistic social interactions. Scientific Reports, 7(1), 17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig WD, & Walters EL (2016). Provisioning patterns in the cooperatively breeding acorn woodpecker: Does feeding behaviour serve as a signal? Animal Behaviour, 119, 125–134. [Google Scholar]
- Madison FN, Kesner AJ, Alward BA, & Ball GF (2018). Sex differences in hippocampal mineralocorticoid and glucocorticoid receptor mRNA expression in response to acute mate pair separation in zebra finches (Taeniopygia guttata). Hippocampus, 28, 698–706. [DOI] [PubMed] [Google Scholar]
- Mariette MM, & Griffith SC (2012). Nest visit synchrony is high and correlates with reproductive success in the wild zebra finch Taeniopygia guttata. Journal of Avian Biology, 43(2), 131–140. [Google Scholar]
- Mariette MM, & Griffith SC (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. The American Naturalist, 185(2), 270–280. [DOI] [PubMed] [Google Scholar]
- McCowan LSC, Mariette MM, & Griffith SC (2015). The size and composition of social groups in the wild zebra finch. Emu, 115(3), 191–198. [Google Scholar]
- Mu Y, Han S, & Gelfand MJ (2017). The role of gamma interbrain synchrony in social coordination when humans face territorial threats. Social cognitive and affective neuroscience, 12(10), 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom KJ, & Omland KE (2017). Females and males respond more strongly to duets than to female solos: comparing the function of duet and solo singing in a tropical songbird (Icterus icterus). Behaviour, 154, 1377–1395. [Google Scholar]
- Ota N, Gahr M, & Soma M (2015). Tap dancing birds: the multimodal mutual courtship display of males and females in a socially monogamous songbird. Scientific Reports, 5, 16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombit RA, Seyfarth RM, & Cheney DL (1997). The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Animal Behaviour, 54(3), 599–614 [DOI] [PubMed] [Google Scholar]
- Pays O, Jarman PJ, Loisel P, & Gerard J-F (2007). Coordination, independence or synchronization of individual vigilance in the eastern grey kangaroo? Animal Behaviour, 73(4), 595–604 [Google Scholar]
- Prior NH, Fernandez MSA, Soula HA, & Vignal C (2018). Rapid effects of sex steroids on zebra finch (Taeniopygia guttata) pair maintenance. Behavioral neuroscience, 132(6), 536. [DOI] [PubMed] [Google Scholar]
- Prior NH, & Soma KK (2015). Neuroendocrine regulation of long-term pair maintenance in the monogamous zebra finch. Hormones and behavior, 76, 11–22. [DOI] [PubMed] [Google Scholar]
- Prior NH, Yap KN, Liu TQD, Vignal C, & Soma KK (2016). Context-dependent effects of testosterone treatment to males on pair maintenance behaviour in zebra finches. Animal Behaviour, 114, 155–164. [Google Scholar]
- Prior NH, Yap KN, & Soma KK (2014). Acute and chronic effects of an aromatase inhibitor on pair-maintenance behavior of water-restricted zebra finch pairs. General and comparative endocrinology, 196, 62–71. [DOI] [PubMed] [Google Scholar]
- Ręk P, & Wong B (2017). Multimodal coordination enhances the responses to an avian duet. Behavioral Ecology, 29, 411–417. [Google Scholar]
- Sakai M, Morisaka T, Kogi K, Hishii T, & Kohshima S (2010). Fine-scale analysis of synchronous breathing in wild Indo-Pacific bottlenose dolphins (Tursiops aduncus). Behavioural processes, 83(1), 48–53 [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, … Cheney DL (2009). The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society of London B: Biological Sciences, 276(1670), 3099–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Andrews H, & Ball GF (1985). Parental care in an ecological perspective: a quantitative analysis of avian subfamilies. American Zoologist, 25(3), 823–840 [Google Scholar]
- Sterck EHM, Watts DP, & van Schaik CP (1997). The evolution of female social relationships in nonhuman primates. Behavioral ecology and sociobiology, 41(5), 291–309 [Google Scholar]
- Stowell D, Gill LF, & Clayton D (2016). Detailed temporal structure of communication networks in groups of songbirds. Journal of the Royal Society Interface, 13(119), 20160296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Martínez Z (2003). Emerging themes and future challenges: forgotten rodents, neglected questions. Journal of Mammalogy, 84(4), 1212–1227 [Google Scholar]
- Ter Maat A, Trost L, Sagunsky H, Seltmann S, & Gahr M (2014). Zebra finch mates use their forebrain song system in unlearned call communication. PloS one, 9(10), e109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki ML, & Zatirka BP (2014). Same-sex partner preference in zebra finches: Pairing flexibility and choice. Archives of sexual behavior, 43(8), 1469–1475 [DOI] [PubMed] [Google Scholar]
- Van Baaren RB, Holland RW, Kawakami K, & Van Knippenberg A (2004). Mimicry and prosocial behavior. Psychological science, 15(1), 71–74 [DOI] [PubMed] [Google Scholar]
- van Rooij EP, & Griffith SC (2013). Synchronised provisioning at the nest: parental coordination over care in a socially monogamous species. PeerJ, 1, e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain AS, Fernandez MSA, Bouchut C, Soula HA, & Vignal C (2016). Songbird mates change their call structure and intrapair communication at the nest in response to environmental noise. Animal Behaviour, 116, 113–129 [Google Scholar]
- Villain AS, Mahamoud-Issa M, Doligez B, & Vignal C (2017). Vocal behaviour of mates at the nest in the white-throated dipper Cinclus cinclus: Contexts and structure of vocal interactions, pair-specific acoustic signature. Journal of Ornithology, 158(4), 897–910 [Google Scholar]
- Whishaw IQ, & Kolb B (2006). Analysis of behavior in laboratory rats In Suckow MA & Weisbroth SHF, Craig L (Eds.), The Laboratory Rat (2 ed., pp. 191–218). San Diego California: Elsevier. [Google Scholar]
- Wojczulanis-Jakubas K, Araya-Salas M, & Jakubas D (2018). Seabird parents provision their chick in a coordinated manner. PloS one, 13(1), e0189969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA (1996). The zebra finch: a synthesis of field and laboratory studies: Oxford University Press. [Google Scholar]