Abstract
Purpose:
To develop and test the feasibility of a sub-three-minute imaging strategy for non-contrast evaluation of the extracranial carotid arteries using ungated quiescent interval slice-selective (QISS) MRA, combining single-shot radial imaging with deep neural network-based image processing to optimize image quality.
Methods:
The extracranial carotid arteries of 12 human subjects were imaged at 3T using ungated QISS MRA. In 7 healthy volunteers, the impacts of radial and Cartesian k-space sampling, single-shot and multi-shot image acquisition (1.1–3.3s/slice, 141–423 s/volume), and deep learning-based image processing were evaluated using segmental image quality scoring, arterial temporal signal-to-noise ratio (tSNR), arterial-to-background contrast and apparent contrast-to-noise ratio (aCNR), and structural similarity index (SSIM). Comparison of deep learning-based image processing was made with block-matching and 3D filtering (BM3D) denoising.
Results:
Compared to Cartesian sampling, radial k-space sampling increased arterial tSNR 107% (P<0.001) and improved image quality during 1-shot imaging (P<0.05). The carotid arteries were depicted with similar image quality on the rapid 1-shot and much lengthier 3-shot radial QISS protocols (P=NS), which was corroborated in patient studies. Deep learning-based image processing outperformed BM3D denoising in terms of SSIM (P<0.001). Compared to original QISS source images, deep learning image processing provided 24% and 195% increases in arterial-to-background contrast (P<0.001) and aCNR (P<0.001), and provided source images that were preferred by radiologists (P<0.001).
Conclusion:
Rapid, sub-three minute evaluation of the extracranial carotid arteries is feasible with ungated single-shot radial QISS, and benefits from the use of deep learning-based image processing to enhance source image quality.
Keywords: QISS, MRA, carotid, radial, deep learning
INTRODUCTION
Stroke is the second leading cause of death worldwide, and large vessel disease of the neck is an important cause of stroke (1,2). In patients with suspected stroke and transient ischemic attack, cross-sectional evaluation of the neck arteries is often performed using contrast-enhanced computed tomographic angiography (CTA) or contrast-enhanced MR angiography (CEMRA) to identify potential arterial disorders such as stenoses, aneurysms, and dissections (3–5). However, CTA and CEMRA require the injection of contrast media. In patients with renal impairment, contrast-enhanced CTA carries the potential risk of contrast-induced nephropathy (6), while CEMRA exposes patients to long-term gadolinium deposition in the body as well as to the risk of nephrogenic systemic fibrosis (7–9).
Nonenhanced MRA provides a risk-free alternative to CTA and CEMRA. 2D time-of-flight (TOF) provides rapid evaluation of the extracranial carotid and vertebral arteries and is sensitive to slow flow, while 3D TOF provides higher spatial resolution and less flow dephasing artifact than 2D TOF, but more limited anatomical coverage and greater flow saturation artifact (10–12). The image quality and anatomical coverage provided by TOF MRA is inferior to that of CEMRA. Moreover, image quality is limited near the vessel origins due to respiratory motion and in horizontally directed vessel segments due to in-plane flow saturation.
While several alternative nonenhanced MRA techniques have been proposed (13–18), ungated quiescent interval slice-selective (QISS) using a fast low-angle shot (FLASH) readout has been found to provide better image quality than 2D TOF and whole-neck coverage (19,20). However, a cardiac-gated implementation of QISS lengthens and complicates patient setup (as cardiac leads are not applied during routine neuro imaging), and renders the approach sensitive to heart rate, arrhythmias, and mistriggering from poor lead contact and magnetohydrodynamic effects. To address these problems, ungated QISS FLASH MRA of the neck has been developed and applied with good results in two studies involving over 90 patients (21,22), but so far has carried scan times of ≈7 minutes.
Ungated QISS protocols providing shorter scan times would be desirable to improve patient compliance, comfort, and streamline neurovascular assessment, which would be particularly beneficial in the workup of patients with suspected stroke and to minimize artifact from patient motion. The goal of this study was to develop and test the feasibility of a sub-three-minute imaging strategy for non-contrast evaluation of the extracranial carotid arteries using ungated QISS MRA, combining single-shot radial imaging with deep neural network-based image processing (23,24) to optimize image quality.
METHODS
Imaging System and Human Subjects
Imaging was performed on a 3 Tesla system (MAGNETOM SkyraFit, Siemens Healthineers, Erlangen) under an institutional review board-approved research protocol. All participants provided written informed consent prior before entering the study. A total of 12 human subjects (9 men, 3 women, mean age 51±16 years) participated in the study. Eight subjects were healthy volunteers; 7 of 8 healthy subjects were imaged with protocols of varying scan times (imaging volunteer group), whereas 6 of 8 healthy subjects provided k-space data for training a deep neural network (training volunteer group). The four remaining subjects were patients with a recent history (<3 months) of stroke.
QISS MR Imaging Protocols
Imaging was performed using an ungated QISS FLASH protocol, which was adapted from prior reports (20,21). In summary, the QISS protocol applies tracking and in-plane inversion RF pulses for suppression of venous and background signals, and uses a FLASH readout in the neck. Unless noted otherwise, imaging parameters for ungated QISS FLASH were as follows: 416 mm×416 mm field of view, 128 2.0-mm-thick slices acquired in a descending order with 0.6 mm slice overlap and 45° axial-to-coronal slice tilt, acquisition of 68 k-space phase-encoding lines (in the case of Cartesian sampling) or radial views (in the case of radial sampling) in each QISS shot (a QISS shot was defined as the time period between successive in-plane inversion RF pulses), QISS shot TR of 1100 ms, FLASH readout TR/TE of 15 ms/2.1 ms, flip angle of 30°, 530 ms delay time from the application of in-plane inversion RF pulse to the center of the FLASH readout.
Impacts of Scan Time and K-Space Sampling: Methods
To examine the potential for shortening scan time, 1-shot, 2-shot, and 3-shot QISS protocols were collected in the imaging volunteer group. These three protocols had scan times of 2 min 21 s, 4 min 42 s, and 7 min 3 s, respectively, and acquired a total of 68, 136, and 204 total k-space lines (in the case of Cartesian sampling) or views (in the case of radial sampling). The acquisition of one slice during the 1-shot, 2-shot, and 3-shot QISS protocols was done in 1, 2 and 3 QISS shot TRs. To counter the expected reduced signal-to-noise ratio of the shorter 1-shot and 2-shot protocols in comparison to the 3-shot protocol, the acquired in-plane spatial resolution was modestly reduced, with 1-shot, 2-shot, and 3-shot protocols acquiring in-plane spatial resolutions of 1.44 mm×1.44 mm (288×288 imaging matrix), 1.18 mm×1.18 mm (352×352 matrix), and 1.08 mm×1.08 mm (384×384 matrix), respectively. All images were thereafter two-fold interpolated to provide reconstructed resolutions of 0.72 mm×0.72 mm, 0.59 mm×0.59 mm, and 0.54 mm×0.54 mm, respectively.
The impact of k-space trajectory was examined by acquiring QISS using both Cartesian and radial sampling. To match the scan times, field-of-views, and number of acquired k-space lines of the radial acquisitions, Cartesian 3-shot, 2-shot, and 1-shot QISS protocols were acquired using GRAPPA undersampling factors of 2, 3, and 6, respectively. Radial scans were acquired in a sequential manner with a golden view angle increment of ≈111.25° (i.e., view angle schedules of ≈111.25°, ≈222.49°, ≈333.74° and so forth), even in the case of multi-shot imaging. Receiver bandwidths were 915 Hz/pixel, 945 Hz/pixel, and 930 Hz/pixel for 1-shot, 2-shot and 3-shot QISS imaging, respectively.
The temporal signal-to-noise ratio (tSNR) of arterial signal was computed using the multiple acquisition method (25). In computing tSNR, one QISS imaging slice was acquired ten times through the common carotid artery ≈1 cm below the carotid bifurcation. tSNR was first computed on a pixel-wise manner; the final arterial tSNR reported equaled the mean of pixel-wise tSNR values contained within a region of interest placed over the cross-section of the right common carotid artery.
Pilot Evaluation in Patient Subjects
Four patients with recent stroke (3 men, 1 woman, mean age 72±12 years) and carotid stenosis were imaged to evaluate the feasibility of the ungated 1-shot and multi-shot QISS protocols (radial and Cartesian) described in the volunteer studies. Multi-shot Cartesian QISS was not acquired in one patient due to a scanner technical issue. Correlation of nonenhanced QISS MRA was made with contrast-enhanced MRA or CTA acquired within the prior 3 months.
Deep Learning-Based Image Processing
A deep neural network-based image processing approach was developed and applied to determine if it could potentially provide ungated QISS images that were preferred for diagnostic use. All calculations were done on a computer workstation equipped with a Xeon W3550 central processing unit (Intel Corporation, Santa Clara, CA) and a GTX 750Ti graphics processing unit (Nvidia Corporation, Santa Clara, CA).
Training Data:
In training the network, raw k-space data from 3-shot radial QISS FLASH protocols (acquired with 1.08mm×1.08mm spatial resolution) obtained from 6 healthy subjects (training volunteer group) were exported from the MRI system for offline image reconstruction using in-house routines implemented in commercial software (MATLAB, The MathWorks, Inc., Natick, MA). Using gridding-based reconstruction and sum-of-squares coil combination, two reconstructions were made from the 3-shot radial k-space data: a “1-shot” reconstruction obtained by reconstructing data from the first of three imaging shots (i.e., first 68 radial views); and a “3-shot” reconstruction obtained by reconstructing all three shots of imaging data (204 radial views). So that the first reconstruction roughly matched the spatial resolution and the signal-to-noise ratio of the prospectively-acquired 1-shot protocols described above, low-pass filtering of peripheral k-space for the “1-shot” reconstruction was done to reduce the in-plane spatial resolution from 1.08mm×1.08mm to 1.44 mm×1.44 mm. Both reconstructions were zero-filling interpolated in the Fourier domain to a 0.54 mm×0.54 mm grid prior to further processing.
Network Architecture, Training, and Reconstruction:
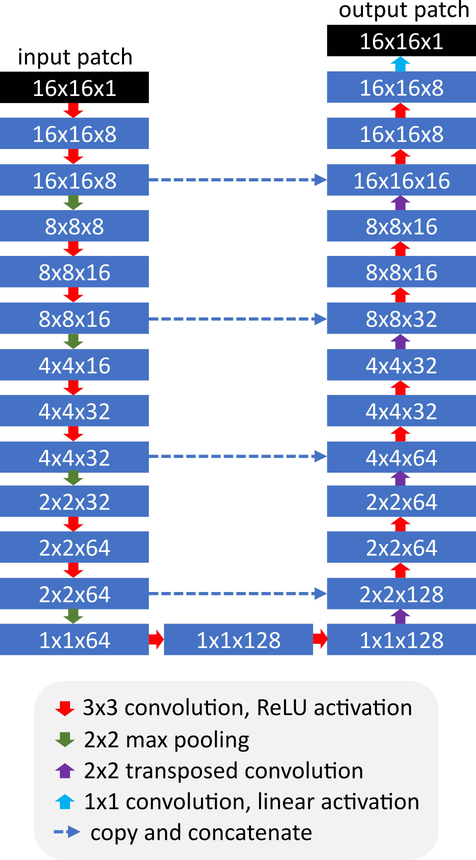
Deep learning-based image processing was done using a U-net deep convolutional neural network (26) which took 16×16 voxel image patches as inputs and produced 16×16 voxel image patches as outputs. The architecture of the network is shown in Figure 1. The U-net was trained using corresponding 16×16 image patches obtained from the “1-shot” and “3-shot” offline reconstructions, which were used as input and output patches, respectively. Network training was done using either a leave-one-out strategy (in the training volunteer group), or its closest analog (in the imaging volunteer group), whereby the deep neural network was trained from all available data obtained in the training volunteer group so long as that data was not obtained from the subject being processed. Image formation for the U-net was done on a patch-by-patch basis. Detailed network training and image reconstruction methodology is provided in Appendix 1.
Figure 1.
Architecture of the U-net used for image processing. The U-net was applied with eight initial channels, a channel increase rate of two, skip connections, and a dropout factor of 0.5. ReLU = rectified linear unit.
Comparison with BM3D Denoising in the Training Volunteer Group:
In a simplistic view, the deep learning-based image processing approach developed in this study could be thought as a self-configured and self-trained denoising filter. Using the offline-reconstructed training data, the deep learning image processing strategy was compared to block-matching and 3D filtering (BM3D) (27), which is a prominent denoising approach. For each subject in the training volunteer group, deep learning processing and BM3D denoising was done on 1-shot training images on two slices, ≈3 cm below and above the right carotid bifurcation. The structural similarity index (SSIM) (28) with respect to the 3-shot training result was computed for the resultant images. Arterial-to-background contrast was computed as Sa/Sb−1, where Sa and Sb refer to the mean arterial and background muscle signals obtained in circular regions of interest. Apparent contrast-to-noise ratio (aCNR) was computed as (Sa-Sb)/σb, where σb is the standard deviation of signal in the background region. Scatter plots were constructed to examine the correspondence of voxel signal intensities before and after deep learning image processing and BM3D denoising, with the intraclass correlation coefficient computed with respect to target 3-shot data.
Application to the Imaging Volunteer Group:
Seven U-nets were trained in this portion of the study (in the manner described in the ‘Network Architecture, Training, and Reconstruction’ section above), corresponding to each of the seven subjects in the imaging volunteer group in whom 1-shot, 2-shot, and 3-shot radial and Cartesian QISS protocols were acquired. Unlike in the training volunteer group where offline reconstructed image data was used, the input images to the U-net were obtained using the standard inline image reconstruction of the MRI system, which involved gridding reconstruction and adaptive coil combination. The U-net was applied to all six configurations of prospectively acquired ungated QISS data (1-shot, 2-shot, and 3-shot; radial and Cartesian sampling) to examine whether the trained U-net was generalizable and applicable to multi-shot as well as 1-shot QISS data irrespective of k-space trajectory. BM3D denoising was also performed on QISS data obtained in the imaging volunteer group to evaluate its impact on arterial cross-sectional area and sharpness.
Quantitative Image Analysis
To evaluate potential changes in arterial morphometry and image appearance, quantitative analysis of standard and deep learning processed reconstructions was done to measure arterial cross-sectional area, arterial sharpness, arterial-to-muscle contrast, and apparent arterial-to-muscle contrast-to-noise ratio (aCNR). Contrast and aCNR were computed as defined previously. Arterial cross-sectional area and sharpness was measured ≈3 cm below the right carotid bifurcation on 2 mm-thick axial reformatted images. Arterial cross-sectional area was obtained by full-width-at-half-maximum signal analysis of 60 radial rays emanating from the arterial center after the axial reformatted images were bilinearly interpolated by a factor of 5. Arterial sharpness was computed as the inverse of the distance between the 20th and 80th percentile points of the signal profile of each ray; the median sharpness across all rays was chosen.
Qualitative Image Analysis
Qualitative evaluation of acquired and deep learning processed QISS data from the imaging volunteer group was done through image quality scoring of rotating maximum intensity projection (MIP) images (45 projections, 8° separation) and forced choice selection of preferred source images.
Image quality scoring was carried out in three phases. In a first phase, which examined the impacts of radial and Cartesian sampling, scoring of 42 rotating conventional (i.e. non deep learning processed) rotating MIP images was done independently by the two radiologists. Scoring of rotating MIP images was done on a segmental basis using a 4-point scale: 1, non-diagnostic image quality; 2, fair image quality with moderate artifacts; 3, good image quality with mild artifacts; 4, excellent image quality with minimal to no artifacts. Eight arterial segments were scored bilaterally, including the common, proximal internal, cervical internal, and petrous internal segments of the carotid arteries. Image quality scores from multiple reviewers were averaged on a segmental basis, and a composite image quality score for each subject was obtained by averaging the scores for all eight segments.
A second phase of qualitative evaluation was done to examine the impact of deep learning reconstruction on image quality scoring. In this phase, one radiologist scored 84 rotating MIP series, using the 4-point 8 arterial segment scoring system defined previously. Half of the rotating MIP series were obtained with standard reconstruction, while the other half were obtained with deep learning image processing.
A third phase of qualitative evaluation was done to examine the impact of deep learning processing on source (i.e. non-MIP) image quality. Two radiologists were displayed the two corresponding source image sets (obtained with standard reconstruction and deep learning image processing) on a side-by-side display. The viewing panel (either left or right) used to display the two corresponding source image sets was randomized. For each pair of image sets, radiologists were asked to select the preferred image set based on image quality for use in a diagnostic setting, consistent with the two-alternative forced-choice paradigm.
Statistical Analysis
Differences in continuous quantitative data and composite image quality scores were evaluated using paired t-tests. Two-alternative forced-choice data were analyzed using two-sided binomial tests with a test probability of 0.5. Two-tailed P-values of <0.05 were considered statistically significant.
RESULTS
Impacts of Scan Time and K-Space Sampling
The impact of k-space trajectory on ungated QISS MRA is shown in Figure 2. Compared to Cartesian k-space sampling, the use of radial k-space sampling tended to improve the image quality of the carotid arteries with respect to Cartesian sampling, which was more apparent for shortened acquisitions.
Figure 2.
Coronal maximum intensity projection images obtained with 1-shot (2 min 21 s), 2-shot (4 min 42 s), and 3-shot (7 min 3 s) ungated QISS FLASH MRA obtained with radial and Cartesian k-space sampling with standard reconstruction. Note the reduced arterial signal variability observed with radial as compared with Cartesian k-space sampling, and the overall similar appearance of the angiograms (most evident for radial k-space sampling) despite the large differences in acquisition time. Insets show magnified views of the right carotid bifurcation.
Table 1 summarizes the composite image quality scores obtained with radial and Cartesian QISS using the 1-shot (scan time 2 min 21 s), 2-shot (4 min 42 s), and 3-shot (7 min 3 s) protocols in the imaging volunteer group. Radial k-space sampling provided slightly higher composite image quality scores (range: 0.1–0.2 points) than Cartesian sampling for all comparisons. With respect to significant differences, radial sampling provided significantly better image quality than Cartesian-based QISS for displaying the carotid arteries during rapid 1-shot imaging (P<0.05). There were no significant differences in image quality scores provided by rapid 1-shot imaging (2 min 21 s) and more conventional but slower three-shot imaging (7 min 3 s) for either k-space trajectories. Likewise, there were no significant differences in image quality scores between 2-shot and 3-shot protocols in portraying the carotid arteries.
Table 1.
Image Quality Scores by Scan Duration and K-Space Trajectory
| Radial | Cartesian | |
|---|---|---|
| 1-shot (2 min 21 s) | 2.9±0.4a | 2.7±0.3 |
| 2-shot (4 min 42 s) | 2.9±0.4 | 2.8±0.3 |
| 3-shot (7 min 3 s) | 3.1±0.4 | 2.9±0.5 |
| All Scan Durations | 3.0±0.4 | 2.8±0.4 |
Data are from the imaging volunteer group, are composite/mean image quality scores obtained from two scorers, and are presented as mean ± standard deviation. Scoring scale: 1, non-diagnostic image quality; 2, fair image quality with moderate artifacts; 3, good image quality with mild artifacts; 4, excellent image quality with minimal to no artifacts.
P<0.05, matched pairs t-test, versus 1-shot Cartesian sampling.
Table 2 summarizes arterial tSNR values obtained in the common carotid artery. The use of radial k-space sampling approximately doubled arterial tSNR with respect to Cartesian k-space sampling (P<0.001) for all imaging configurations tested (i.e. 1-shot, 2-shot, 3-shot). Importantly, the faster 2 min 21 s 1-shot and 4 min 42 s 2-shot protocols provided tSNR levels similar to those of the longer 7 min 3 s 3-shot protocols, which was especially true for radial k-space sampling where 89% and 96% of the tSNR obtained with 3-shot imaging was retained by the 1-shot and 2-shot protocols, respectively.
Table 2.
Impact of Scan Duration and Sampling Trajectory on Arterial tSNR
| Protocol | Radial | Cartesian | P-Value |
|---|---|---|---|
| 1-shot (2 min 21 s) | 22.2±3.6 | 10.2±3.1 | <0.001 |
| 2-shot (4 min 42 s) | 24.1±3.5 | 10.4±4.7 | <0.001 |
| 3-shot (7 min 3 s) | 25.0±4.1 | 13.8±4.1 | <0.001 |
| All Protocols | 23.8±3.7 | 11.5±4.2 | <0.001 |
Data are from the imaging volunteer group and are presented as mean ± standard deviation. Data obtained from the common carotid artery.
tSNR = temporal signal-to-noise ratio.
Deep Learning Image Processing: Comparison to BM3D in the Training Volunteer Group
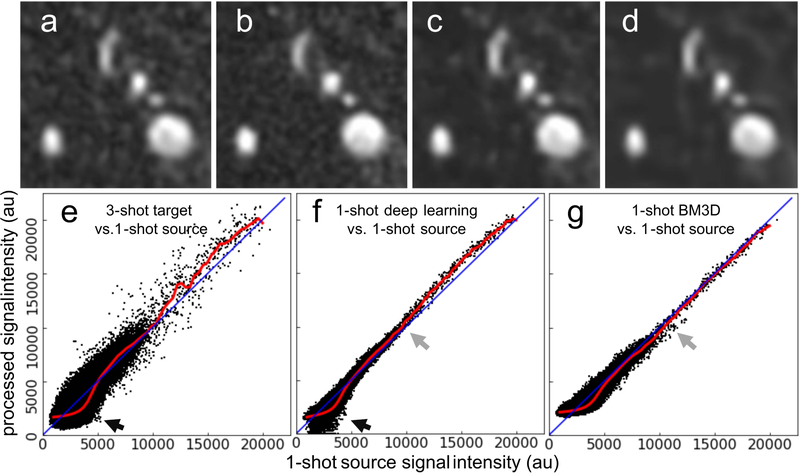
Images provided by deep learning image processing and BM3D denoising are shown in Figure 3. Quantitative impacts on arterial-to-background contrast, aCNR, and SSIM on source images are summarized in Table 3. Deep learning-based image processing provided 44% larger arterial-to-background contrast and 195% larger aCNR than the original input data, while retaining small and large arterial detail and providing the largest SSIM values with respect to the reference 3-shot images (P<0.05). In comparison, BM3D denoising provided lower arterial-to-background contrast (P<0.001) and a smoother image appearance that reduced the conspicuity of small arterial detail and structure. With reference to the input 1-shot images, scatterplots of voxel signal intensity for the 3-shot target, 1-shot deep learning processed, and 1-shot BM3D denoised images (Figure 3e–3g, respectively) revealed that the deep learning processing retained voxels with high signal intensities corresponding to arteries, and suppressed low signal bearing voxels corresponding to background which improved arterial-to-background contrast (Figure 3f). On the other hand, BM3D denoising demonstrated greater scatter than deep learning processing, especially in intermediate and high signal intensity voxels which correspond to arterial structures, and did not suppress low signal intensity background voxels to the same degree as deep learning processing (Figure 3g). Using the 3-shot results as the reference, the running median transformation line of input 1-shot to output data (red line shown in Figure 3e) was more similar to that provided by deep learning image processing than by BM3D denoising. Supporting Information Figure S1 shows plots of the 1-shot data in Figure 3e–g all with respect to the 3-shot target reconstruction, where it was seen that deep learning processing provided the best agreement with respect to the 3-shot target reconstruction. Processing times for deep learning image processing and BM3D denoising were approximately 0.2s per slice on a graphics processing unit and 11s per slice on a central processing unit, respectively.
Figure 3.
Comparison of image quality obtained with deep learning-based processing and BM3D denoising. Montage showing cropped source slices through the proximal left carotid artery obtained (a) with a 1-shot acquisition, (b) the target corresponding 3-shot image, (c) the deep learning processed 1-shot image, and (d) the BM3D denoised 1-shot image. Note the improved arterial-to-background contrast obtained with (c) deep learning-based processing with respect to (a). Scatter plots showing original input 1-shot voxel signal intensities over the entire field-of-view versus (e) the 3-shot target signal intensities, and the 1-shot signal intensities obtained with (f) deep learning image processing and (g) BM3D denoising. Red lines show the moving median trendlines of output signal intensity levels across the input signal intensity spectrum; lines of unity are shown in blue. Compared to BM3D denoising, deep learning processing provided a median trendline that more closely mimicked that of the reference 1-shot to 3-shot transformation shown in (e), by selectively suppressing low signal intensity background voxels (black arrows) and exhibiting less scatter at intermediate signal intensities (gray arrows).
Table 3.
Comparison of Deep Learning Image Processing and BM3D Denoising
| Image Set | Contrast | aCNR | SSIM1 |
|---|---|---|---|
| 3-shot Target | 7.03±2.42 | 36.5±13.1 | - |
| 1-shot Source | 4.89±1.45 | 25.8±6.17 | 0.772±0.020 |
| 1-shot Deep Learning | 6.47±1.99a | 76.1±38.9b | 0.870±0.019c |
| 1-shot BM3D | 4.64±1.00 | 69.1±27.4 | 0.849±0.013 |
Data are from offline reconstructed radial images obtained in the training volunteer group and are presented as mean ± standard deviation. Data summarize measurements obtained in both the common carotid and internal carotid arteries. Deep learning processed images were generated using neural networks trained using a leave-one-out training strategy. aCNR = apparent contrast-to-noise ratio. SSIM = structural similarity index (SSIM). BM3D = block matching and 3D filtering.
SSIM computed using the 3-shot target data as the reference.
P<0.001, matched pairs t-test, versus all other 1-shot results for contrast. No difference between 1-shot deep learning processed and 3-shot target (P=NS).
P<0.01, matched pairs t-test, versus 1-shot source and 3-shot target for aCNR.
P<0.001, matched pairs t-test, versus 1-shot source and 1-shot BM3D images.
Deep Learning Image Processing of Prospectively Acquired QISS Data
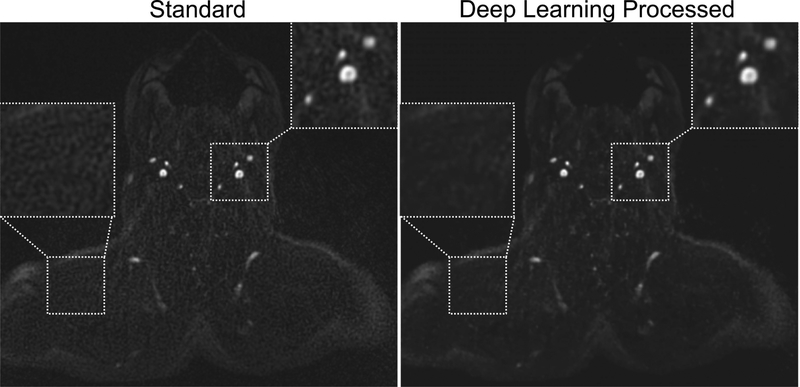
An example of the impact of deep learning-based image processing on prospectively acquired QISS data in the imaging volunteer group is shown in Figure 4. Quantitative measures of arterial-to-background contrast and aCNR are provided in Table 4. Compared to conventional images reconstructed by the MRI system, deep learning-based image processing improved arterial-to-muscle contrast by 14–29% (depending on protocol configuration; P<0.05 for all comparisons except 1-shot radial), and produced 167–224% increases in aCNR (P<0.05, all comparisons). Aggregating measurements made in the six protocols, deep learning processing increased arterial cross-sectional area by 1.7% (30.0±4.5 mm2 [standard] versus 30.5±4.6 mm2 [deep learning], P<0.001) and increased arterial sharpness by 2.8% (0.64±0.09 mm−1 [standard] versus 0.66±0.07 mm−1 [deep learning], P<0.001) with respect to standard image reconstruction. On the other hand, BM3D denoising reduced arterial cross-sectional area by 1.4% (29.6±4.7 mm2, P<0.01) and sharpness by 7.2% (0.59±0.08 mm−1, P<0.001).
Figure 4.
Impact of deep learning-based image processing on source image appearance. Ungated 1-shot radial QISS images obtained without (left) and with (right) U-net-based deep learning image processing. Note the reduced apparent noise levels as well as improved arterial-to-background contrast and image quality obtained with deep learning processing.
Table 4.
Impact of Deep Learning Image Processing on Arterial-Background Contrast and aCNR
| Contrast | aCNR | |||||
|---|---|---|---|---|---|---|
| Imaging Protocol | Standard | U-net | % Increase | Standard | U-net | % Increase |
| 1-shot Radial | 8.4±1.9 | 9.6±1.6 | 14 | 19.5±3.0 | 60.5±37.5a | 210 |
| 1-shot Cartesian | 5.8±1.2 | 7.4±1.4b | 28 | 14.2±4.4 | 39.9±22.2b | 181 |
| 2-shot Radial | 7.1±1.3 | 8.7±1.3c | 23 | 19.1±3.4 | 51.0±15.4c | 167 |
| 2-shot Cartesian | 5.8±0.7 | 7.3±1.0c | 26 | 17.4±2.9 | 48.5±20.1b | 179 |
| 3-shot Radial | 6.5±1.0 | 8.4±1.5c | 29 | 19.0±4.3 | 61.6±29.5b | 224 |
| 3-shot Cartesian | 6.1±0.6 | 7.7±1.1c | 26 | 18.8±3.3 | 57.2±32.1a | 198 |
| All Radial | 7.3±1.6 | 8.9±1.5c | 22 | 19.2±3.4 | 57.7±27.9c | 201 |
| All Cartesian | 5.9±0.8 | 7.5±1.1c | 27 | 16.8±3.9 | 48.5±25.1c | 189 |
| All Protocols | 6.6±1.5 | 8.2±1.5c | 24 | 18.0±3.8 | 53.1±26.6c | 195 |
Data are from inline reconstructed images obtained in the imaging volunteer group and are presented as mean ± standard deviation. Measurements made in the common carotid artery. aCNR = apparent contrast-to-noise ratio.
P<0.05, with respect to standard reconstruction.
P<0.01, with respect to standard reconstruction.
P<0.001, with respect to standard reconstruction.
Evaluation of source images by two radiologists revealed that deep learning processed images were preferred over standard images in 41 of 42 head-to-head comparisons irrespective of the k-space sampling trajectory (P<0.001, both reviewers), in 20 of 21 data set pairs obtained with radial k-space sampling (P<0.001, both reviewers), and in all 21 of 21 data set pairs obtained with Cartesian k-space sampling (P<0.001, both reviewers). Deep learning processed images were also consistently preferred over standard images across for all three spatial resolutions and acquisition times, with 14 of 14 instances preferred for rapid 1-shot imaging (P<0.001, both reviewers), 14 of 14 instances preferred for 2-shot imaging (P<0.001, both reviewers), and 13 of 14 instances preferred for 3-shot imaging (P<0.001, both reviewers).
Supporting Information Figure S2 shows MIP images corresponding to Figure 2 obtained without and with deep learning image processing. Qualitative scoring of rotating MIP images revealed that deep learning image processing provided image quality scores that were slightly improved but not significantly different than those obtained with standard image reconstruction (composite image quality scores of 3.5±0.4 versus 3.4±0.4 for deep learning image processing and standard reconstruction, respectively, P=NS).
Pilot Evaluation of Rapid Ungated Protocols in Patients with Carotid Stenosis
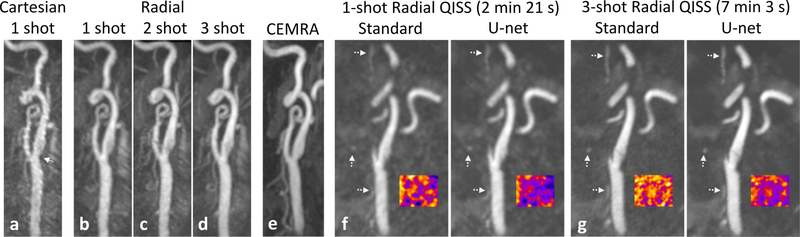
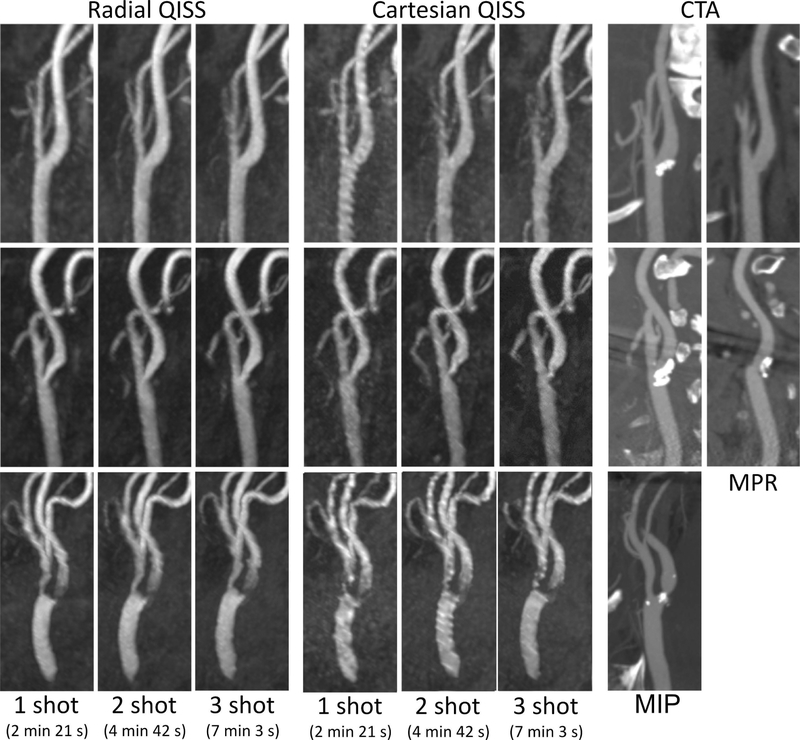
In our pilot feasibility study of patients with recent stroke, radial k-space sampling consistently produced better image quality than Cartesian k-space sampling and correlated well with contrast-enhanced CTA or MRA. Figure 5 shows the images obtained with ungated 1-shot and multi-shot QISS in a patient with mild carotid stenosis. In comparison with ungated 1-shot QISS MRA performed with Cartesian k-space sampling, radial k-space sampling provided better image quality for displaying the carotid bifurcation. Moreover, arterial-to-background contrast for 1-shot as well as multi-shot ungated radial QISS was improved with the use of deep learning processing. Figure 6 shows results obtained in three additional patients, in whom radial outperformed Cartesian QISS and rapid (2 min 21 s) 1-shot radial QISS was found feasible.
Figure 5.
Images obtained in a patient with mild carotid stenosis. Maximum intensity projection (MIP) images comparing ungated QISS MRA in a patient with: (a) 1-shot Cartesian k-space sampling (2 min 21 s); (b) 1-shot radial, (c) 2-shot radial and (d) 3-shot radial k-space sampling (scan times of 2 min 3 sec, 4 min 42 s, 7 min 3 s, respectively); and (e) contrast-enhanced MRA (CEMRA) obtained 9 weeks prior to QISS MRA. Arrow in (a) points to a mild stenosis of the left carotid bifurcation. Note the reduced pulsation artifact and improved image quality obtained with 1-shot radial sampling as compared with 1-shot Cartesian sampling, the similar appearances of the radial QISS acquisitions despite major differences in scan time, and the correlation of radial QISS with CEMRA. Panels (f) and (g): Sagittal 2-mm-thick multiplanar reformations through the proximal internal carotid artery showing comparisons of standard and deep learning processed QISS reconstructions obtained with (f) 1-shot and (g) 3-shot radial sampling. Deep learning processing reduced signal variability in background tissue (false color insets) without degrading small arterial detail (dashed arrows).
Figure 6.
Maximum intensity projection (MIP) images comparing ungated radial and Cartesian QISS obtained in three patients (top, middle, and bottom panels) with disease at the carotid bifurcation. Note the consistently improved arterial portrayal obtained with radial as opposed to Cartesian QISS, the feasibility of rapid ungated 1-shot radial QISS, and the correlation of ungated radial QISS with contrast-enhanced computed tomography angiography (CTA) obtained between 3 to 9 weeks prior. Multiplanar reformations (MPR) shown for bifurcations obscured by substantial calcification.
DISCUSSION
We found that ungated QISS MRA using a single-shot radial k-space trajectory in combination with deep learning-based image processing enabled depiction of the extracranial carotid arteries from thoracic inlet to skull base in less than three minutes. This is more than two-fold faster than prior QISS MRA studies of the neck, which relied on the use of multi-shot imaging to obtain adequate image quality. Comparing radial and Cartesian k-space sampling for single-shot QISS, the former provided higher tSNR (i.e. reduced variation in arterial signal) and better image quality. Deep learning-based image processing was helpful as it substantially increased arterial-to-background contrast and aCNR, and generated source images that were preferred for diagnostic use.
Prior reports of QISS MRA for evaluation of the extracranial carotid arteries (20,21) used multi-shot acquisitions that were two to three times less efficient than the single-shot acquisition used in the current study. We found that radial k-space sampling, in comparison with Cartesian k-space sampling, reduced the arterial signal variability (i.e. increased tSNR) across all imaging configurations (1-shot through 3-shot) and generally improved image quality. This was especially evident in the patient studies. We thus recommend the use of radial k-space sampling in lieu of Cartesian k-space sampling for ungated QISS MRA of the neck. Due to oversampling of central k-space and the effective signal averaging that occurs in this important region of k-space, radial sampling suppresses artifacts (e.g. from pulsation and motion) that are more apparent with Cartesian sampling (which only samples the center of k-space once), and therefore elevates tSNR. The tendency of radial k-space sampling to provide better image quality (in comparison with Cartesian k-space sampling) is consistent with prior work in the field of nonenhanced MRA (29–33).
This study also examined whether substantial reductions of acquisition time could be achieved through modest reductions of the imaging spatial resolution, without markedly impacting signal-to-noise and image quality for portraying the carotid arteries. Compared to the 3-shot QISS FLASH MRA protocol, we found that 2-shot and 1-shot QISS FLASH MRA was feasible and allowed for markedly reduced scan times with minimal impact on image quality (≈10% difference between 1-shot and 3-shot protocols), temporal signal-to-noise ratio, apparent contrast-to-noise ratio, and arterial detail. This was further corroborated in the patient studies where 1-shot radial QISS MRA displayed the carotid bifurcation similarly to the lengthier 3-shot protocol. If short scan times are not the highest priority and acquisition of higher spatial resolution is desired, our data suggest that one can apply a 2-shot radial protocol rather than the lengthier 3-shot radial protocol.
Deep learning-based image processing significantly improved the arterial-to-background contrast and apparent CNR on single-shot QISS source images, although it did not significantly improve the subjective image quality of rotating MIP images. Nonetheless, the application of deep learning processing produced diagnostically preferred source images, irrespective of the number of imaging shots and k-space trajectory utilized. While MIP images are often used to quickly identify locations of possible pathology, detailed evaluation and grading of disease is usually done using reformatted source images (12,34,35). Given that the deep neural network utilized is pre-computed and can rapidly process images with minimal latency (<0.2 s per image), we anticipate that it will prove useful for nonenhanced QISS MRA of the neck, especially when rapid imaging protocols are sought.
We found that deep learning-based image processing outperformed BM3D denoising in terms of SSIM, arterial-to-background contrast, and arterial sharpness. Further improvements in the deep learning image processing network may be possible with more training data and with refinements in the neural network architecture and training approach. In our experience, the deep learning processing strategy applied in this work appears to be of particular use when desired image details are present in the input image, but noise levels are higher than would be desirable, as is often the case with undersampled radial imaging. The impact of the small (≈1.7%) computer-measured differences in arterial cross-sectional area with deep learning-based image processing (which correspond to even smaller ≈0.8% changes in arterial diameter assuming a circular lumen) is unknown, but should be further examined and possibly accounted for in future patient studies.
This study has some possible limitations. First, this study was limited to a relatively small cohort of human subjects. Second, the deep learning reconstructive approach used may not have been optimal, which may understate the potential benefits of using such a strategy. Further optimization of the deep learning network architecture and comparison with other methods for image quality improvement is outside the scope of this pilot study and represents a topic of future investigation. Third, due to the focus on streamlined ungated imaging (which is the norm for neurological MRA), as well as practical constraints of limited acquisition time and unavoidable differences in experimental parameters to accommodate different heart rates (e.g. QISS shot TR, TI, FLASH TR, scan time), cardiac gating was not performed. Of note, arterial tSNR values obtained with ungated radial QISS sampling in the present study (≈24) are consistent with the SNR values obtained in the seminal QISS FLASH study (20) which was performed with cardiac gating and Cartesian k-space sampling (≈18). Moreover, the image quality scores of ≈3 obtained in the present study are consistent with those obtained in the prior report. Lastly, although good initial correlation with CTA or CEMRA was obtained at the carotid bifurcation in our pilot evaluation of patients with recent stroke, further evaluation of the rapid single-shot radial QISS protocol in a larger patient cohort presenting with more diverse arterial pathology is needed.
In conclusion, our study demonstrates that rapid, sub-three minute depiction of the extracranial carotid arteries is feasible with ungated single-shot radial QISS and that deep learning-based image processing can be applied to enhance source image quality.
Supplementary Material
Supporting Information Figure S1. Scatter plots of voxel signal intensities for the 1-shot reconstructions (source, deep learning, BM3D) of Figure 3 with respect to the 3-shot target reconstruction. Deep learning processing provided the best agreement with respect to the 3-shot reconstruction; intraclass correlation coefficient values for the three plots were 0.79 (source), 0.92 (deep learning), and 0.83 (BM3D). Red lines are moving median trendlines; lines of unity are shown in blue.
Supporting Information Figure S2. Comparison of maximum intensity projection images obtained with standard reconstruction (top panel) and deep learning image processing (bottom panel). Aside from the slightly improved image contrast obtained with deep learning image processing, note the similar overall image appearance of the two reconstructions, and the retention of both small and large arterial branches with deep learning image processing. Insets show magnified views of the right carotid bifurcation.
ACKNOWLEDGEMENT
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB027475. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
APPENDIX 1
Training and Reconstruction Methodology for the U-net Deep Convolutional Neural Network
Training Methodology:
Twelve 16×16 voxel image patches were obtained from each QISS slice for the purpose of training the U-net. So that the U-net was amply trained to reconstruct bright arterial structures for the intended application of MRA, 6 of these 12 patch locations were positioned corresponding to the 6 highest signal bearing locations as identified on frontal MIPs from the “3-shot” offline reconstructions; to ensure that these 6 “arterial” patches were largely unique, a maximum patch overlap of 4 pixels was enforced. So that the network was also trained to reconstruct non-arterial background tissue, an additional 6 patches from each slice were obtained from randomly selected locations within the field of view. To improve network generalizability, data augmentation of input and output patches during the training phase was performed by applying an additional 29 random rotations (range: ±180 degrees), in-plane shifts (range: ±4 pixels), spatial scaling (i.e. zoom) factors (range: 0.8 to 1.2), and signal intensity scaling factors (range: 0.4 to 1.0). The network was trained using a batch size of 1000, an adaptive moment estimation optimizer (learning rate of 0.001, β1=0.9, β2=0.999, ε=0.0), root mean squared error loss function, dropout of 50%, and early stopping based on validation loss. For example, when using training patch data from six subjects (276,480 total training patches separated into 221,184 training patches and 55,296 validation patches), network training time was ≈36 s per epoch on a commodity graphics processing unit (GTX 750Ti, Nvidia Corporation, Santa Clara, CA).
Reconstruction Methodology:
Images were constructed on a patch-by-patch basis with patches tiled together to generate the final reconstructed image. To eliminate block artifact at patch boundaries, patches were overlapped by 8 voxels in vertical and horizontal directions and a weighted average (with weights decreasing linearly from patch center) was used to merge overlapping patch data.
REFERENCES
- 1.World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon BK, Demchuk AM. Computed Tomography Angiography in the Assessment of Patients With Stroke/TIA. Neurohospitalist 2011;1:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr JC, Ma J, Desphande V, Pereles S, Laub G, Finn JP. High-resolution breath-hold contrast-enhanced MR angiography of the entire carotid circulation. AJR Am J Roentgenol 2002;178:543–549. [DOI] [PubMed] [Google Scholar]
- 5.Yang CW, Carr JC, Futterer SF, et al. Contrast-enhanced MR angiography of the carotid and vertebrobasilar circulations. AJNR Am J Neuroradiol 2005;26:2095–2101. [PMC free article] [PubMed] [Google Scholar]
- 6.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 2006:S11–5. [DOI] [PubMed] [Google Scholar]
- 7.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 8.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 9.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007;242:647–649. [DOI] [PubMed] [Google Scholar]
- 10.Keller PJ, Drayer BP, Fram EK, Williams KD, Dumoulin CL, Souza SP. MR angiography with two-dimensional acquisition and three-dimensional display. Work in progress. Radiology 1989;173:527–532. [DOI] [PubMed] [Google Scholar]
- 11.Wagle WA, Dumoulin CL, Souza SP, Cline HE. 3DFT MR angiography of carotid and basilar arteries. AJNR Am J Neuroradiol 1989;10:911–919. [PMC free article] [PubMed] [Google Scholar]
- 12.De Marco JK, Nesbit GM, Wesbey GE, Richardson D. Prospective evaluation of extracranial carotid stenosis: MR angiography with maximum-intensity projections and multiplanar reformation compared with conventional angiography. AJR Am J Roentgenol 1994;163:1205–1212. [DOI] [PubMed] [Google Scholar]
- 13.Kramer H, Runge VM, Morelli JN, et al. Magnetic resonance angiography of the carotid arteries: comparison of unenhanced and contrast enhanced techniques. Eur Radiol 2011;21:1667–1676. [DOI] [PubMed] [Google Scholar]
- 14.Koktzoglou I, Gupta N, Edelman RR. Nonenhanced extracranial carotid MR angiography using arterial spin labeling: improved performance with pseudocontinuous tagging. J Magn Reson Imaging 2011;34:384–394. [DOI] [PubMed] [Google Scholar]
- 15.Takei N, Miyoshi M, Kabasawa H. Noncontrast MR angiography for supraaortic arteries using inflow enhanced inversion recovery fast spin echo imaging. J Magn Reson Imaging 2012;35:957–962. [DOI] [PubMed] [Google Scholar]
- 16.Raoult H, Gauvrit JY, Schmitt P, Le Couls V, Bannier E. Non-ECG-gated unenhanced MRA of the carotids: optimization and clinical feasibility. Eur Radiol 2013;23:3020–3028. [DOI] [PubMed] [Google Scholar]
- 17.Koktzoglou I, Meyer JR, Ankenbrandt WJ, et al. Nonenhanced arterial spin labeled carotid MR angiography using three-dimensional radial balanced steady-state free precession imaging. J Magn Reson Imaging 2015;41:1150–1156. [DOI] [PubMed] [Google Scholar]
- 18.Koktzoglou I, Walker MT, Meyer JR, Murphy IG, Edelman RR. Nonenhanced hybridized arterial spin labeled magnetic resonance angiography of the extracranial carotid arteries using a fast low angle shot readout at 3 Tesla. J Cardiovasc Magn Reson 2016;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelman RR, Sheehan JJ, Dunkle E, Schindler N, Carr J, Koktzoglou I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med 2010;63:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koktzoglou I, Murphy IG, Giri S, Edelman RR. Quiescent interval low angle shot magnetic resonance angiography of the extracranial carotid arteries. Magn Reson Med 2016;75:2072–2077. [DOI] [PubMed] [Google Scholar]
- 21.Koktzoglou I, Aherne EA, Walker MT, Meyer JR, Edelman RR. Ungated nonenhanced radial quiescent interval slice-selective (QISS) magnetic resonance angiography of the neck: Evaluation of image quality. J. Magn. Reson. Imaging 2019. doi: 10.1002/jmri.26781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters S, Huhndorf M, Jensen-Kondering U, et al. Non-Contrast-Enhanced Carotid MRA: Clinical Evaluation of a Novel Ungated Radial Quiescent-Interval Slice-Selective MRA at 1.5T. Am. J. Neuroradiol 2019;40:1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas A, Iliadis M, Molina R, Katsaggelos AK. Using Deep Neural Networks for Inverse Problems in Imaging: Beyond Analytical Methods. IEEE Signal Process. Mag 2018;35:20–36. [Google Scholar]
- 24.Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys 2019;29:102–127. [DOI] [PubMed] [Google Scholar]
- 25.Reeder SB, Wintersperger BJ, Dietrich O, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med 2005;54:748–754. [DOI] [PubMed] [Google Scholar]
- 26.Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics).; 2015. doi: 10.1007/978-3-319-24574-4_28. [DOI] [Google Scholar]
- 27.Dabov K, Foi A, Katkovnik V, Egiazarian K. Image Denoising by Sparse 3-D Transform-Domain Collaborative Filtering. IEEE Trans. Image Process. 2007;16:2080–2095. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Bovik AC, Sheikh HR, Simoncelli EP. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004. doi: 10.1109/TIP.2003.819861. [DOI] [PubMed] [Google Scholar]
- 29.Spuentrup E, Katoh M, Buecker A, et al. Free-breathing 3D Steady-State Free Precession Coronary MR Angiography with Radial k-Space Sampling: Comparison with Cartesian k-Space Sampling and Cartesian Gradient-Echo Coronary MR Angiography—Pilot Study. Radiology 2004;231:581–586. [DOI] [PubMed] [Google Scholar]
- 30.Edelman RR, Giri S, Murphy IG, Flanagan O, Speier P, Koktzoglou I. Ungated radial quiescent-inflow single-shot (UnQISS) magnetic resonance angiography using optimized azimuthal equidistant projections. Magn. Reson. Med 2014. doi: 10.1002/mrm.25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelman RR, Giri S, Pursnani A, Botelho MP, Li W, Koktzoglou I. Breath-hold imaging of the coronary arteries using Quiescent-Interval Slice-Selective (QISS) magnetic resonance angiography: pilot study at 1.5 Tesla and 3 Tesla. J Cardiovasc Magn Reson 2015;17:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koktzoglou I, Edelman RR. Super-resolution intracranial quiescent interval slice-selective magnetic resonance angiography. Magn Reson Med 2018;79:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koktzoglou I, Edelman RR. Radial fast interrupted steady-state (FISS) magnetic resonance imaging. Magn Reson Med 2018;79:2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lell M, Fellner C, Baum U, et al. Evaluation of carotid artery stenosis with multisection CT and MR imaging: Influence of imaging modality and postprocessing. Am. J. Neuroradiol 2007;28:104–110. [PMC free article] [PubMed] [Google Scholar]
- 35.Runck F, Steiner RP, Bautz WA, Lell MM. MR imaging: Influence of imaging technique and postprocessing on measurement of internal carotid artery stenosis. Am. J. Neuroradiol 2008;29:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. Scatter plots of voxel signal intensities for the 1-shot reconstructions (source, deep learning, BM3D) of Figure 3 with respect to the 3-shot target reconstruction. Deep learning processing provided the best agreement with respect to the 3-shot reconstruction; intraclass correlation coefficient values for the three plots were 0.79 (source), 0.92 (deep learning), and 0.83 (BM3D). Red lines are moving median trendlines; lines of unity are shown in blue.
Supporting Information Figure S2. Comparison of maximum intensity projection images obtained with standard reconstruction (top panel) and deep learning image processing (bottom panel). Aside from the slightly improved image contrast obtained with deep learning image processing, note the similar overall image appearance of the two reconstructions, and the retention of both small and large arterial branches with deep learning image processing. Insets show magnified views of the right carotid bifurcation.