Abstract
Purpose:
Transverse relaxation time (T2) mapping with MRI has a plethora of clinical and research applications. Current T2 mapping techniques are based primarily on spin-echo (SE) relaxometry strategies that rely on the signal magnitude, and often suffer from lengthy acquisition times. In this work we propose a phase-based T2 mapping technique where T2 information is encoded into the signal phase of rapid gradient echo (GRE) acquisitions.
Theory:
Bloch equation simulations demonstrate that the phase of GRE acquisitions obtained with a very small inter-repetition RF phase increment has a strong monotonic dependence on T2, resulting from coherent transverse magnetization. This T2-dependent phase behavior forms the basis of the proposed T2 mapping technique. To isolate T2-dependent phase from background phase, at least two datasets with different RF phase increments are acquired. The proposed method can also be combined with chemical shift encoded MRI to separate water and fat signals.
Methods:
The feasibility of the proposed technique was validated in a phantom experiment. In vivo feasibility was demonstrated in the brain, knee, abdomen and pelvis. Comparisons were made with SE-based T2 mapping, spectroscopy and T2 values from the literature.
Results:
The proposed method produced accurate T2 maps compared with SE-based T2 mapping in the phantom. Good qualitative agreement was observed in vivo between the proposed method and the reference. T2 measured in various anatomies agreed well with values reported in the literature.
Conclusion:
A phase-based T2 mapping technique was developed and its feasibility demonstrated in phantoms and in vivo.
Keywords: magnetic resonance imaging, T2 mapping, relaxometry, phase, gradient echo, RF spoiling, quantitative imaging biomarker
Introduction
The transverse relaxation time (T2) is associated with important microscopic tissue properties such as the concentration and cluster-size of paramagnetic particles and the mobility of hydrogen atoms. Importantly, T2 is well known to characterize a plethora of important disease processes such as iron deposition, fibrosis, edema, malignancy, and inflammation, among others. As a result, quantitative T2 mapping with MRI has many applications, including assessment of neuro-degenerative diseases and characterization of malignant lesions1, detection of myocardial edema2, detection of chronic rejection after heart transplant3,4, detection of early cartilage degeneration5, quantification of liver iron overload6 and even identification of myofascial trigger points7 .
Spin-echo (SE) based methods are commonly used to map T2. By varying the echo time and fitting the signals to a mono-exponential decay model (multi-exponential if a multi-component model is appropriate8,9), T2 can be estimated. Unfortunately, lengthy exams are needed due to the long repetition time (TR) to minimize T1 weighting. Acquisition times can be reduced by acquiring multiple echoes (multi-echo SE) in a single TR10,11, although the use of multi-echo methods may lead to different measurement of T212.
Magnetization prepared T2 contrast (“T2-prep”) is a method used to encode T2 relaxation into the longitudinal magnetization13. This technique is advantageous for imaging blood vessels and the heart13, and relies on modulation of the longitudinal magnetization prior to a readout acquisition. Although faster than SE-based acquisitions, T2-prep-based T2 quantification also suffers from relatively long acquisition times14,15.
Steady-state short TR methods based on spoiled gradient echo (SGRE), balanced-steady state free precession (bSSFP)16, and gradient-refocused acquisition in the steady-state (GRASS)17,18 are time efficient compared to spin-echo (SE) T2 mapping techniques. For example, two SGRE acquisitions with varying flip angle combined with bSSFP contain the necessary information for joint T1 and T2 estimation16. Due to the use of short TR acquisitions, these methods can deliver simultaneous T1 and T2 quantification of spatially resolved 3D volumes within clinically acceptable acquisition times16.
To further reduce acquisition time for T2 mapping, Welsch et al. proposed a multi-echo GRE acquisition known as double echo steady-state (DESS)17. This approach can also be extended for joint estimation of T1 and T2 using the triple echo steady-state (TESS) method proposed by Heule el al.18. In these methods, T2 information is encoded into the relative magnitude between echoes. In vivo feasibility of these methods has been demonstrated11,19. A variation of the DESS T2 mapping technique developed by Staroswiecki et al.20, has demonstrated high potential for accurate in vivo T2 mapping. Although only a single gradient echo (GRE) acquisition is required, these methods rely on water specific RF pulses for fat suppression, which may be unreliable in the setting of B0 inhomogeneities18. Differential T2* weighting in the various echoes can also confound T2 estimates.
We note that none of the above methods exploit signal phase to encode T2 relaxation. In this work, we propose a major modification of an RF phase scheme first proposed by Zur et al21 to achieve robust spoiling of transverse magnetization for GRE acquisitions. As we propose below, the use of very small RF phase increments, rather than large RF phase increments needed for RF spoiling, can create T2-dependent changes in both the phase and magnitude of the GRE signal. In this work we propose a novel quantitative T2-mapping technique that encodes T2 information into the phase of the GRE signal by manipulating the RF phase increment.
Theory
Complete spoiling of transverse magnetization is generally assumed when using spoiled gradient echo (SGRE) acquisitions. RF spoiling is a well-known approach used for spoiling transverse magnetization21,22. As first proposed by Zur et al.22 , RF spoiling methods use a pseudo-random sequence of phases of the RF excitation. The phase sequence is defined by the difference between the nth and the (n+1)th RF excitation, i.e.: ΦRF(n) = ΦRF(n-1) + Φ0 + n·ΔΦ (n=0, ….). If the RF phase increment (ΔΦ) is chosen carefully, transverse magnetization accumulates in an incoherent manner and is effectively spoiled.
The choice of RF phase increment is important for effective RF spoiling. Specific choices of RF phase increment (e.g. 117°) lead to excellent RF spoiling and the signal closely approximates the ideal SGRE signal magnitude21. Other choices of RF phase increment may lead to less effective RF spoiling21. Importantly, we note that the phase of gradient echo signals in the context of RF spoiling has not been well described.
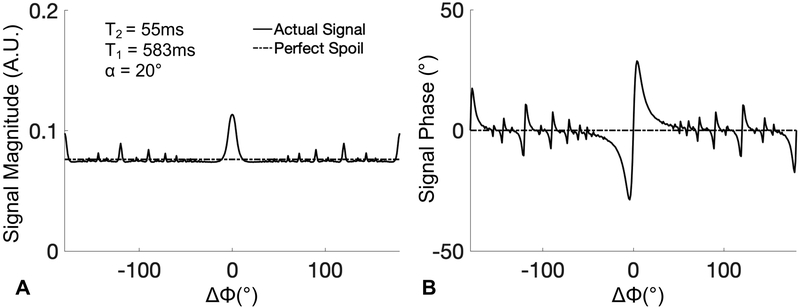
In this study, we investigate the effects of the RF phase increment on the phase of the complex-valued gradient echo signal. Figure 1 plots the results of a Bloch equation simulation showing both the signal magnitude (η) and phase (θ) of the gradient echo signal, using the RF phase increment method proposed by Zur et al.22. In this computer simulation, an ensemble of 1000 spins periodically experienced a sequence consisting of an RF pulse, T1 and T2 relaxation, and at the end of each repetition, a 2π phase dispersion across the isochromats due to an unbalanced readout gradient. Note that the acquisition reference frame matches the excitation phase.
Figure 1.
GRE signal magnitude (A) and phase (B) over the full range of RF phase increments (ΔΦ), according to the method of Zur et al.22 By varying the RF phase increment, large variations in the magnitude and phase of the GRE signal are observed. Signal shown in this plot was generated using a Bloch equation simulation assuming T1=583ms and T2=55ms to simulate normal liver tissue21 at 1.5T, with TR=10ms and flip angle=20°.
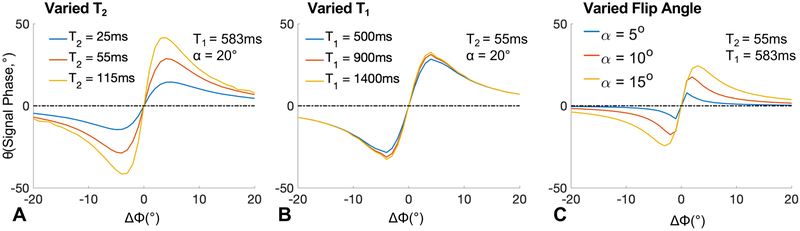
Figure 2 focuses on a narrow range of small RF phase increments, also with varying T1, T2 and flip angle. As can be seen in Figure 2, significant variations in the signal phase occur with changes in T2 and flip angle, and to a much lesser extent with T1. The largest signal phase was observed with small RF phase increments between 1° to 4°. Various combinations of T2 (25ms, 55ms, 115ms), T1 (500ms, 900ms, 1400ms) and flip angle (5°, 10°, 15°) are used in the simulation assuming a TR of 10ms and simulated TE of 0ms to ignore the effects of T2* decay, for simplicity.
Figure 2.
GRE signal phase is heavily influenced by T2 (A), but only minimally by T1 (B) for very small RF phase increments (ΔΦ), forming the basis for the proposed T2 mapping method. The phase of the GRE signal over the low range of RF phase increments were generated using Bloch equation simulations with physiological T1 and T2 values and TR=10ms. The dotted lines are the case where transverse magnetization is spoiled perfectly.
The effects of T2, T1 and flip angle are also plotted in Figure 3, demonstrating not only a strong dependence of the signal phase on T2 and flip angle, but also a relatively weak dependence on T1. We can express the steady-state gradient echo signal acquired with an RF phase increment as:
| (1) |
where η(ΔΦ,α,TR; T1, T2) is the signal magnitude relative to M0, θ(ΔΦ, α,TR; T1, T2) is the signal phase immediately after excitation and is dependent on T2, T1, flip angle (α) and θ′, which is the local background phase caused by complex coil sensitivity, eddy currents, magnetic field inhomogeneities, etc.
Figure 3.
GRE signal phase (A) increases monotonically with increasing T2 for small RF phase increments (ΔΦ), e.g. 1-4°. Using a small RF phase increment (e.g. 2°), the signal phase is sensitive to T2 over a wide range of T2 values, a property that is favorable for encoding T2 information. Note also that the signal phase is relatively insensitive to varying T1 (B) and flip angle (for midrange flip angles such as 18°) for phase increments of 1° and 2°. The phase and magnitude of the GRE signal over the low range of RF phase increments were generated using Bloch equation simulations with physiological T1 and T2 values and TR=10ms.
To the best of our knowledge, simple analytical forms of η(ΔΦ,α,TR;T1, T2) and θ(ΔΦ, α,TR;T1,T2) have not been derived. In this work, calculation of these two functions is based on the use of a lookup table. Lookup tables are constructed from Bloch equation simulations based on wide ranges of possible T1 and T2 values, and the known acquisition parameters used in the experiment: ΔΦ, α, TR. All lookup tables used in this work are constructed using the same Bloch equation simulation described above.
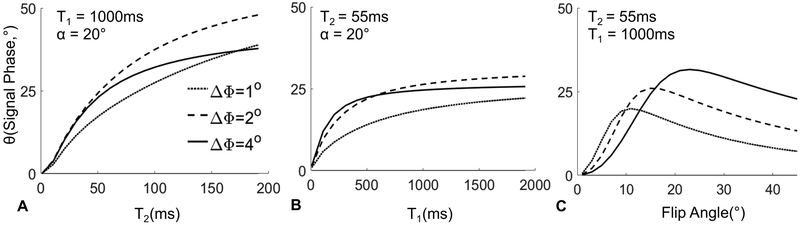
Figure 3 depicts in greater detail the dependence of the signal phase with respect to T2, T1 and flip angle over a few small RF phase increments. A pronounced monotonic increase in the observed phase with T2 is noted over a wide range of physiological T2 values23 with RF phase increments between 1° and 4°. For an RF phase increment of 2°, the signal phase is consistently sensitive to T2 over a wide range of T2 values (Figure 3, A). Unlike the strong dependence on T2, the signal phase changes minimally over a wide range of T1 values between 1000ms and 2000ms (Figure 3, B). Given these observations, a small RF phase increment such as 2° will encode the tissue T2 into the signal phase.
In actual MRI acquisitions the received signal phase contains an additional background phase term, i.e.: θ′(Eq. 1). Estimates of the signal phase resulting from T2 of the tissue must be isolated from the background phase. In this work we propose the following method to isolate θ from θ′, and subsequently estimate tissue T2.
Proposed phase-based T2 mapping:
In principle, two identical acquisitions with equal and opposite RF phase increments will generate equal but opposite phase responses (Figure 2), i.e.: θ(ΔΦ, α,TR ;T1, T2) = - θ(-ΔΦ, α,TR ;T1, T2). Using two such acquisitions, θ can be isolated from M0, η and θ′ in Eq.1 by taking the phase difference of two gradient echo signals acquired with opposite RF phase increments, and with all other acquisition parameters identical, i.e.:
| (2) |
Using a well-chosen RF phase increment (e.g. ΔΦ=2 °) and a relatively large flip angle (e.g. α=18 °), the estimated signal phase can be used to estimate T2. Based on the model presented above, it is expected that only a small error might occur in the T2 estimate related to T1 and unanticipated errors in flip angle (Figure 3 B,C). Note that the same figure shows a maximum signal phase of 50°, which will result in a phase difference of 100° between two signals. This phase difference is well below 2π, suggesting that even with higher T2 values, there should be no risk of phase wrap in the proposed method.
In this work, we propose to estimate T2 from through the use of a lookup table generated from a Bloch equation simulation that uses the known TR and flip angle of the acquisition. We note that the phase is weakly dependent on T1, and therefore T1 values measured using other methods or values reported in the literature for the anatomy of interest can be used to generate the lookup table.
Synthetic T2-weighted images:
In addition to the phase maps used to generate the T2 map, magnitude images are also acquired. By multiplying these magnitude images with the inferred T2 decay from the phase-based T2 map, synthetic T2-weighted images can be generated. The synthesis of the signal in each voxel can be expressed as:
| (3) |
where Ssyn is the synthesized T2-weighted signal, TEv is the virtual echo time, S denotes the signal acquired with one (or a combination) of the images acquired at the two phase increments. denotes the estimated phase-based T2 value.
Methods
Phantom experiments:
The accuracy of the proposed method was evaluated using a phantom constructed with varying concentrations of agarose and NiCl2 to modulate the T2 and T1 relatively independently24. The T1 was varied such that the confounding effect of T1 variation in the proposed method can be demonstrated. A 4×4 grid of cylindrical vial agarose gel phantom was constructed for this experiment. Each vial is approximately 3cm in diameter and 4.8cm in height. Each column was constructed with a varying concentration of agarose (0.5%, 1%, 2%, 4%) to modulate T2. Each row is doped with a varying concentration of NiCl2 (0mM, 0.5mM, 1mM, 2mM) to modulate T1.
All phantom experiments were performed on a clinical 3.0T MRI system (Signa Premier, GE Healthcare, Waukesha, WI) using a high channel density posterior and anterior receive array coil with up to 90 independent coil elements (Air coil, GE Healthcare, Waukesha, WI). Single-echo SE-based T2 mapping was performed to provide a reference standard. Echo times of 11ms, 50ms, 100ms, 150ms were acquired with TR of 6000ms. Other acquisition parameters include: axial acquisition; field of view (FOV)=18cm×18cm; matrix=128×128; slices=1; slice thickness=15mm; receiver bandwidth=±83.33kHz. Signals were fit to a mono-exponential decay signal model offline in Matlab (MathWorks, Natick, MA) to estimate T2 on a voxel by voxel basis. A circular ROI was drawn in each vial. The T2 measurements were averaged in each ROI for comparison with the proposed method.
T1 maps of the phantoms were generated using inversion recovered fast spin-echo (FSE-IR) MRI. Acquisition parameters were as follows: inversion time=50ms, 500ms, 1000ms, 1500ms, 2500ms, 3500ms, 4000ms; TR=6000ms; FOV=18cm×18cm; matrix=256×256; slices=1; slice thickness=15mm; receiver bandwidth=±25kHz. T1 estimation was performed on a voxel by voxel basis using the standard inversion recovery signal model25.
GRE images for phase-based T2 map were acquired using an axial acquisition; TR=5.0ms; FOV=18cm×18cm×24cm; matrix=128×128×24; receiver bandwidth=±50.1kHz; number of signal averages=4. Two complex GRE datasets with ΔΦ=2° and −2°, each with 18° flip angle were acquired for the proposed method. The sum of squares image was used as a virtual body coil image. The complex sensitivity map was generated using the source images with one RF phase schedule (the first echo if multiple echoes are acquired) and used to combine complex channel images for both sets of images with difference RF phase schedule, and all acquired echoes. This process was used to generate complex coil combined images. T2 maps were reconstructed as described in the theory section. For the reconstruction of phase-based T2 maps, a T1 of 1850ms was assumed (mid-point of the range of the T1 measured in the phantom, as reported in the Results section).
For each reconstruction by the proposed method, T2 measurements were averaged in a circular ROI in each vial, on the center slice. Linear regression was used to compare the T2 measurements obtained using the proposed phase-based method and SE-based T2 mapping.
In vivo experiments:
The proposed methods were also evaluated in healthy volunteers to demonstrate in vivo feasibility. All human imaging was performed after obtaining approval from our institutional review board (IRB) and informed written consent. All in vivo experiments were performed on 3.0T clinical MRI systems (abdomen, pelvis, brain experiments on Signa Premier; knee experiments on Discovery MR 750w, GE Healthcare, Waukesha, WI). Various phased array receive coils appropriate for the specific anatomy were used including: 8-channel head coil (brain), 8-channel knee coil (knee) and high channel density posterior and anterior receive array coils with up to 90 independent coil elements (Air coil, GE Healthcare, Waukesha, WI) for the abdomen and pelvis.
For the proposed method, the choice of RF phase increment and flip angle are as follows: ΔΦ = 2° and −2°, each with an 18° flip angle.
In the knee volunteer experiments, a total of 6 knees were scanned in 4 volunteers (4 males, ages 28-35). The acquisition parameters of the proposed method are as follows: 3D acquisition; sagittal plane; FOV=14cm×14cm×9.6cm; TR=5.9ms; matrix=256×256×32; bandwidth=±90.91kHz; signal averages=3; acquisition time=4:48 minutes. Due to the difficulty of limiting motion over long acquisition times, a commercial multi-echo SE T2 mapping was used as reference instead of single-echo SE. Acquisition parameters include: sagittal plane; FOV=14×14cm; slice thickness=2.7mm; gap=0.3mm; slices=28; TR=1.0s; acquisition matrix=256×256; echo times=8.6ms, 14.8ms, 22.2ms, 29.5ms, 36.9ms, 44.3ms, 51.7ms, 59.1ms; bandwidth=±31.25kHz; signal averages=1; exam time=12:56 minutes. The acquisition volumes of the two methods were precisely colocalized.
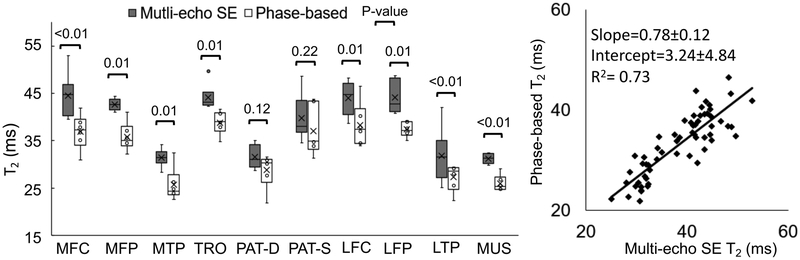
Phase-based T2 maps were reconstructed as described in the theory section. T1 of 1198ms was assumed (midpoint between the T1 of the medial femoral cartilage and patella)26. The reference T2 map from multi-echo SE images were calculated by fitting the signal to a single-exponential decay model to minimize least square error on a voxel by voxel basis. To compare the proposed method and the reference, T2 measurements were averaged inside ROIs drawn directly on the T2 maps in the following regions described by Fang Liu et, al27: medial femoral central (MFC) condyle, medial femoral posterior (MFP) condyle, medial tibial plateau (MTP), patella-deep (PAT-D), patella-superficial (PAT-S), lateral femoral central (LFC) condyle, lateral femoral posterior (LFP) condyle, lateral tibial plateau (LTP), as well as T2 measurements from the gastrocnemius muscle (MUS). For each individual region, box-whisker plots were created to demonstrate the distribution of T2 measurements by the two compared methods. A Student’s t-test was performed for paired samples. For measurements across all the regions, the Pearson coefficient was computed.
A brain study was performed on one volunteer (male, age 30). The acquisition parameters of the proposed method were as follows: 3D acquisition; axial plane; FOV=24cm×24cm×12.8cm; TR=5.6ms; acquisition matrix=256×256×32; bandwidth=±90.91kHz; signal averages=3; exam time=4:30 minutes. Single-echo SE T2 mapping was used as reference for T2 measurements, acquisition parameters include: axial plane; FOV=24cm×24cm; slice thickness=3.6mm; slice spacing=0.4mm; number of slices=22; TR=5s; acquisition matrix=256×256; echo times=11ms, 70ms; bandwidth=±31.25kHz; signal averages=1; acquisition time=24:00 minutes. The acquisition volumes of the two methods were precisely colocalized.
Phase-based T2 maps were reconstructed as described in the theory section. T1 of 915ms was assumed (midpoint between the T1 of white matter and the putamen at the age of 20) 28. The reference T2 map was reconstructed using least square error fitting to a single-exponential model. Synthetic T2-weighted images were also generated with virtual TE values of 70ms and 100ms as described in the theory section.
For imaging in the abdomen and pelvis, separation of water and fat signals was performed by combining the proposed method with a multi-echo 3D GRE chemical shift encoded (CSE) acquisition. Abdomen (male, age 54) and pelvis (male, age 47) experiments were conducted on one volunteer respectively. The acquisition parameters in the abdomen included: axial plane; FOV=40cm×32cm×26cm; TR=6.5ms; acquisition matrix=100×80×26; 5 echoes with echo times=0.9ms, 2.0ms, 3.0ms, 4.0ms, 5.1ms; bandwidth=±100kHz; signal averages=1; exam time=20 seconds in a single breath-hold. In the pelvis, the same acquisition parameters were used with the following exceptions: slice thickness=8mm; slices=32, TR=6.4ms; bandwidth=±90.91kHz; exam time of 25 seconds in a breath-hold.
For the image reconstruction, the proposed method was combined with CSE-MRI. Using complex fitting with single R2* least-squares fitting reconstruction29 from the ISMRM Fat-Water Toolbox30 (http://ismrm.org/workshops/FatWater12/data.htm), water and fat signals were separated. The magnitudes and phases of each chemical species were then used to reconstruct individual T2 maps for each chemical species.
Single voxel multi-TE stimulated echo acquisition mode (STEAM)-MR spectroscopy31 (MRS) was acquired in the liver and the spleen to provide reference values for phase-based T2 measurements. STEAM-MRS data was acquired with the following parameters: TR=3500ms; TE=10ms, 15ms, 20ms, 25ms, 30ms; number of points=2048; spectral width=5000Hz; 5ms mixing time. The voxel size was 15mm×15mm×20mm in the liver and 15mm×15mm×10mm in the spleen. Signal magnitude as well as T2 of water and fat signal was estimated jointly using non-linear least square fitting32.
Simulation experiment to evaluate the sensitivity of phase to motion:
Although no apparent effect of motion was observed in vivo (below), it is well known that GRE acquisitions with unbalanced gradients and no RF spoiling (i.e. unspoiled GRE) can be sensitive to motion33,34. In the presence of unbalanced gradients, moving spins will accrue a different phase during each TR. This phase accrual may impact the T2-dependent phase of the method proposed in the current work, potentially confounding T2 measurements.
For the proposed method, phase accrual resulting from the unbalanced readout gradient is a linear function of the voxel location. Assuming that the phase dispersion from the unbalanced gradient is 2π across the voxel in the readout direction, the additional phase accrual from the overall voxel can be written as: n×TR×Vx×2π/ΔX, for a voxel moving from the image isocenter, where Vx is the velocity of the voxel in the readout direction and ΔX is the voxel dimension in the readout direction. This effect potentially confounds quantification of the phase shift used to encode T2.
To assess the magnitude of velocity effects on T2 quantification, we performed a Bloch equation simulation experiment where the effects of the first order motion (velocity) in the readout direction were modeled. This simulation was performed using a modification of the simulation described in the Theory section. In addition to a 2π phase dispersion, a velocity dependent common phase was added to all isochromats in a voxel at the end of each repetition. This simulation experiment was conducted with velocity values ranging from −1mm/s to 1mm/s, with a 2mm voxel dimension. Other parameters used in the simulation included: flip angle = 18°, TR = 5ms, ΔΦ=±2°, T2=50ms, and T1=1000ms. Signal phase attributed to T2 was estimated from the phase difference between the two signals (i.e. with ΔΦ=±2°) divided by 2, and compared with a T2 lookup table for T2 estimation, using the proposed method described in the Theory section. The lookup table was generated using Bloch equation simulation without motion and the same acquisition parameters over a wide range of tissue relaxation parameters.”
Results
Phantom experiments:
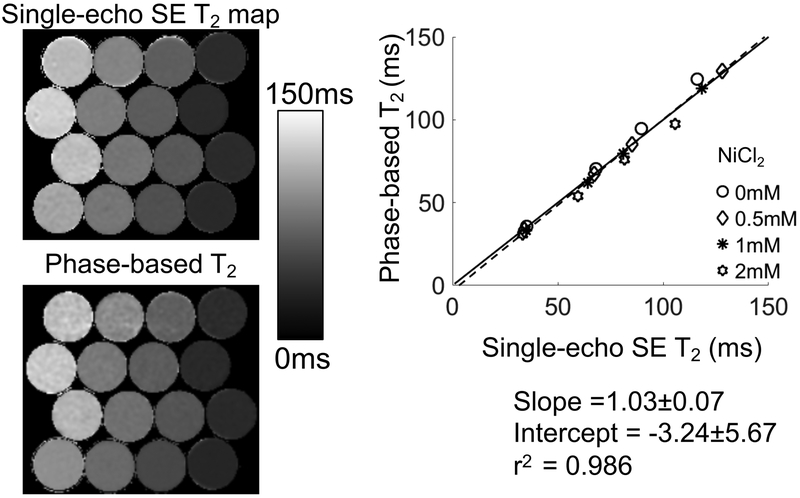
In the phantom experiment the T1 of the phantom vials were estimated by FSE-IR to be 873ms, 932ms, 829ms, 925ms, corresponding to agar concentrations of 0.5%, 1%, 2%, 4% in phantoms with 2mM NiCl2; 1390ms, 1315ms, 1279ms, 1332ms in phantoms with 1mM NiCl2; 1725ms, 1698ms, 2053ms, 1792ms in phantoms with 0.5mM NiCl2; 2848ms, 2902ms, 2788ms, 2888ms in phantoms with 0mM NiCl2. The proposed method (which did not correct for T1 effect in the signal phase) demonstrated close agreement with reference T2 estimates (Figure 4) (slope=1.03±0.07, intercept=−3.24±5.67). For vials of vastly different T1 measurements (2mM NiCl2 and 0.5mM NiCl2), the T2 measurements show slightly higher deviation from the reference at high T2 values.
Figure 4.
T2 maps generated using the proposed method provided accurate T2 measurements agreeing closely with spin-echo T2 mapping. Phantom T2 maps generated using single-echo SE MRI and the center slice of the phase-based T2 mapping are shown. The agreement between the phase-based T2 map and single-echo SE MRI was evidenced by linear regression between T2 values averaged in ROIs drawn in the center of the vials, with a slope and intercept statistically equal to one and zero, respectively.
In vivo experiments:
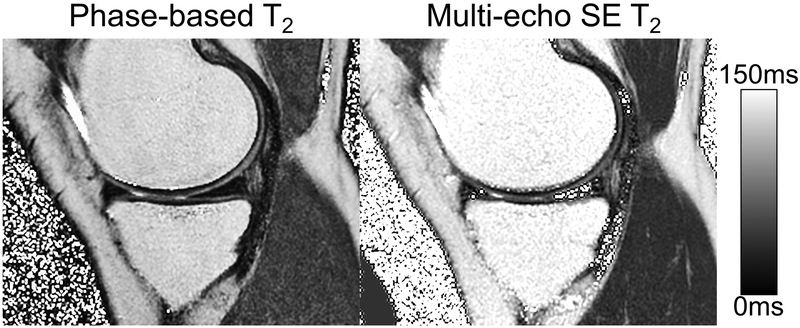
In the knee imaging experiments, the proposed method produced high quality T2 maps in all knees (Figure 5), including high apparent SNR and excellent depiction of anatomical detail. In areas where water signal is dominant (cartilage and muscle), similar T2 values were observed between the proposed method and the multi-echo SE T2 map used as a reference. In Figure 6, the box-whisker plot and scatter plot showed strong correlation between the phase-based T2 and the reference T2 measurements (Pearson correlation coefficient = 0.86), with slope=0.78±0.12 and intercept=3.24±4.84. Quantitative T2 values measured using the proposed method were very similar to the multi-echo SE based T2 measurements, although many of these measurements showed statistical differences.
Figure 5.
T2 map generated with the phase-based T2 mapping showed excellent image quality in all six knees. Similar intensities can be observed in regions with dominant water signal such as cartilage and the muscle. An example of T2 maps generated using multi-echo SE MRI and the proposed method are shown.
Figure 6.
The box and whisker plot and scatter plot showed strong correlation between the phase-based T2 and the multi-echo SE T2 with a high Pearson correlation coefficient (0.86). Measurements made in 10 different regions on each of six different knees. The regions measured were medial femoral central condyle (MFC), medial femoral posterior condyle (MFP), medial tibial plateau (MTP), patella-deep (PAT-D), patella-superficial (PAT-S), lateral femoral central condyle (LFC), lateral femoral posterior (LFP), lateral tibial plateau (LTP), gastrocnemius muscle (MUS).
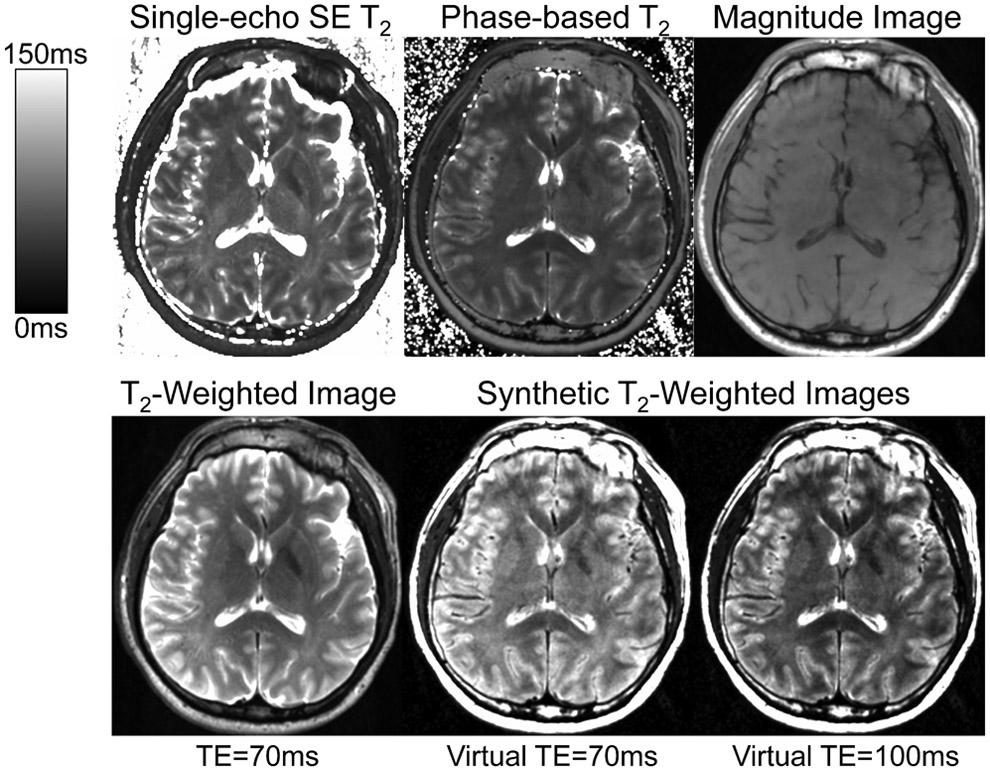
Similarly, high quality T2 maps were generated by the proposed method in the brain (Figure 7). However, some discrepancies in T2 values were observed between the two methods, especially in the grey matter. Average T2 values in regions of interest (ROIs) were 39ms (phase-based) and 53ms (SE) in the genu of corpus callosum; 40ms (phase-based) and 61ms (SE) in the splenium of corpus callosum; 47ms (phase-based) and 59ms (SE) in the white matter; 35ms (phase-based) and 40ms (SE) in the globus pallidus of basal ganglia; 45ms (phase-based) and 54ms(SE) in the putamen of basal ganglia.
Figure 7.
High quality T2 maps were generated in the brain using the proposed phase-based method. The phase-based T2 appeared lower than the reference T2 map especially in the grey matter. The magnitude image used in the proposed method is also shown. Synthesized T2-weighted images (virtual TE=70ms, TE=100ms) showed overall similar appearance although with less grey-white matter contrast than T2-weighted SE at the same TE (70ms).
Synthetic T2-weighted images generated from the phase-based T2 map and the simultaneously acquired magnitude images are also shown with two different virtual echo times (70ms, 100ms). Compared with a T2-weighted SE image with TE of 70ms, the synthesized T2-weighted image with virtual TE of 70ms showed overall similar appearance, although with slightly reduced apparent grey-white matter contrast..
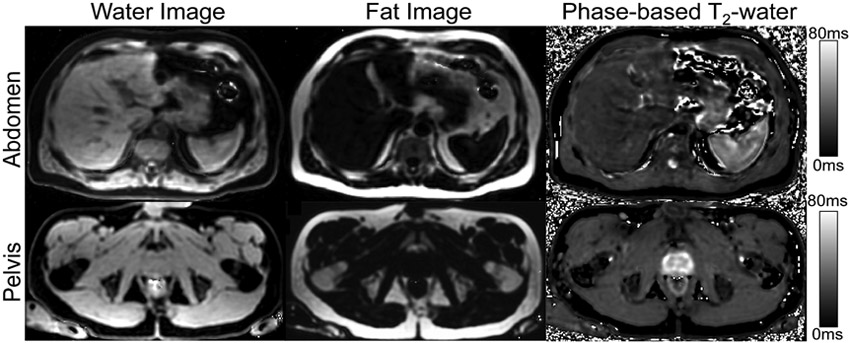
In the abdomen and pelvis, 3D spatially resolved T2 maps generated from separated water signal were successfully reconstructed after water-fat separation (Figure 8). Close agreement between T2 value of the water signal estimated by the proposed method and MRS was observed, with estimates of 20ms and 22ms respectively, using a co-localized voxel. Similarly, in the spleen, the proposed method measured a T2 value of 38ms compared to 34ms with MRS.
Figure 8.
The proposed phase-based 3D T2 mapping combined with CSE-MRI water-fat separation is feasible over the entire abdomen or the pelvis within a single breath-hold. Water and fat images were calculated from multi-echo gradient echo images with varied RF phase increments. A phase-based T2 map for water signal was also generated from the water phase of different RF phase increments. Simultaneous R2* and B0 field maps were also generated, but not included for brevity.
Finally, in the peripheral zone of the prostate, phase-based T2 measurements (72ms) were comparable to values reported in literature (74±9ms in the prostate)23.
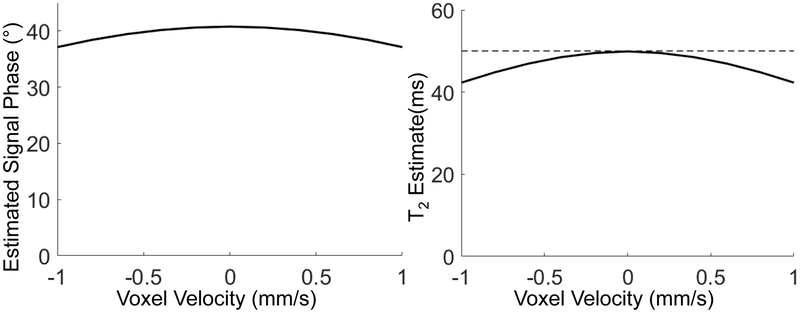
Simulation experiment to study the sensitivity of signal phase to motion:
As shown in Figure 9, the signal phase in the proposed method was sensitive to motion. For example, a velocity of 1mm/s lead to a 3.6° change in the signal phase, relative to no motion. Accordingly, the apparent T2 estimation was reduced from 50ms to 42ms.
Figure 9.
Sensitivity of the proposed method to motion. Signal phase generated using Bloch-equation simulation modeling constant velocity leads to small errors in the apparent T2-dependent phase and subsequent underestimation of T2.
Discussion
We have proposed and successfully demonstrated preliminary feasibility of a phase-based T2 mapping technique based on GRE imaging. The theory and technique for encoding T2 information into the signal phase of a gradient echo acquisition were developed. We demonstrated that using a small RF phase increment in gradient echo acquisitions, a signal phase that increases monotonically with the transverse relaxation time can be generated. This behavior forms the basis of the proposed method for encoding information into the GRE signal phase. The feasibility of the proposed method was successfully demonstrated in phantoms and in vivo experiments, including in combination with chemical shift encoded water-fat separation. Further, the proposed approach can be used to generate high quality synthetic T2-weighted images that can be acquired in relatively short acquisition times.
Compared with traditional SE-based T2 mapping and T2-prep-based methods, the proposed method reduces acquisition time and would potentially render quantitative T2 mapping feasible for many clinical applications, including those that require short breath-holds. Compared with DESPOT2, the proposed method requires fewer GRE source images for parametric mapping and consequently shorter acquisition time. Further, the proposed method is also immune to signal voids caused by banding artifacts seen with bSSFP methods. Compared with the TESS T2 mapping technique, T2 weighting and T2* weighting in the signals of the proposed method are naturally separated, because the T2 information is contained in the signal phase, which is not affected by T2* effects. The proposed technique is also compatible with CSE-MRI, which is useful in many extra-cranial imaging applications, particularly in the abdomen and pelvis. This feature would enable simultaneous generation of T2 maps for both water and fat signals as well as the R2* and B0 field map.
In this work, the proposed GRE-based method was able to shorten the minimum acquisition time compared to spin-echo-based T2-mapping methods. Among the steady-state methods, DESPOT2 requires at least three of more acquisitions. The proposed phase-based T2 mapping technique requires two acquisitions, while DESS and TESS require only one. The number of acquisitions required would normally determine the minimum acquisition time needed to create a T2 map of a certain resolution and FOV. However, it is worth noting that the relative advantage of DESS and TESS in this comparison is offset by their generally longer TR (14ms, 20ms, 26ms, 21ms) 17-20 compared to DESPOT2 (e.g. 3.6ms) 16 and the phase-based T2 mapping (e.g. 5.9ms, 5.6ms and 6.5ms in this work). Further, depending on the application, multiple signal averages are often acquired, when there is sufficient acquisition time (e.g. in the knee and in the brain). In such applications, the SNR efficiency is a more important measure of acquisition speed. Rigorous evaluation of the SNR performance of the proposed method is beyond the scope of this work but will be an important component of future investigations.
Small discrepancies between the T2 measured with the proposed method and SE-based methods was observed, particularly in the brain. While the reasons for these discrepancies are unclear, possible reasons include multi-component T2 effects, magnetization transfer, B1 inhomogeneities, motion, or combinations of these factors. Further work will be needed to determine the cause of these discrepancies.
Previous studies have demonstrated that motion can lead to change in the signal magnitude in steady-state acquisitions using unbalanced gradient echo acquisitions and pseudo-random RF phase spoiling. In this work, we have performed preliminary Bloch equation simulations modeling constant linear motion, demonstrating that small changes in the signal phase may result from motion, leading to underestimation of T2. Although no definite effects of motion on artifacts or T2 estimation accuracy were observed in the experimental studies, some underestimation of T2, relative to references standard measurements was observed in cartilage and in the brain. It is uncertain whether this apparent underestimation in T2 was related to motion or not. Future rigorous evaluation of the potential effects of motion on T2 estimation is warranted, especially for applications where tissue motion may be an important factor, eg. heart, flowing blood
There are several limitations of this work. First, although the feasibility of this method has been successfully demonstrated, considerable technical optimization and substantial further clinical validation is needed. Further studies will be needed to evaluate the technical accuracy and noise performance of the proposed method, as well as to optimize acquisition parameters. In addition, the precise impact of B1 inhomogeneities, motion, variation in T1 of the tissues, magnetization transfer effects35 and multi-exponential relaxation27 requires further evaluation. These effects may explain the apparent discrepancies between the T2 measurements in the brain between the proposed phase-based method and conventional SE-based T2 mapping.
Another major limitation of the proposed method is that the T2 mapping algorithm requires knowledge or assumption of the T1 of tissue to map the signal phase into a T2 value. Although the signal phase is relatively independent of T1 for long T1 values, this is not the case for shorter T1 values. Thus, the overall accuracy of this method is unknown for T2 quantification, especially in tissues with short T1 values.
Further, the proposed method can be used to generate synthetic T2-weighted images. However, due to the relatively large flip angle in the proposed method, the source magnitude images are T1-weighted. For this reason, the T2-weighted images synthesized from the phased-based T2 maps and simultaneously acquired magnitude images will be T1-weighted as well, similar to short tau inversion recovery (STIR) based methods.
In addition, the proposed method currently requires the use of 3D acquisitions, due to the need for a uniform flip angle across the tissue of interest. Extension to 2D imaging should be feasible but will require more complex lookup table construction that accounts for acquisition slice profiles.
In conclusion, we have presented and successfully demonstrated the feasibility of a novel phase-based T2 mapping method based on gradient echo imaging. This approach has the potential for rapid, 3D mapping of T2 in tissue. Further technical development, optimization and clinical validation are needed.
Acknowledgements
The authors wish to thank David Harris, PhD for his assistance preparing and reviewing this manuscript. The authors also wish to thank Daiki Tamada, PhD for helpful discussions. We acknowledge the use of the ISMRM Fat-Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm) for some of the reconstruction methods described in this article. The authors also wish to acknowledge support from the NIH (R01 DK100651, R01 DK088925, R01 DK117354, K24 DK102595, and R41 EB025729), as well GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.
References
- 1.Kosta P, Argyropoulou MI, Markoula S, Konitsiotis S. MRI evaluation of the basal ganglia size and iron content in patients with Parkinson’s disease. J Neurol. 2006;253(1):26–32. [DOI] [PubMed] [Google Scholar]
- 2.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: A hybrid method for T2-weighted imaging of edema in the heart. Magnetic Resonance in Medicine. 2008;59(2):229–235. [DOI] [PubMed] [Google Scholar]
- 3.Aherne T, Yee ES, Tscholakoff D, Gollin G, Higgins C, Ebert PA. Diagnosis of acute and chronic cardiac rejection by magnetic resonance imaging: a non-invasive in-vivo study. J Cardiovasc Surg (Torino). 1988;29(5):587–590. [PubMed] [Google Scholar]
- 4.Dolan RS, Rahsepar AA, Blaisdell J, et al. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection After Heart Transplantation. JACC: Cardiovascular Imaging. 2019;12(8, Part 2):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Benjamin Ma C, Link TM, et al. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthritis and Cartilage. 2007;15(7):789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sollmann N, Mathonia N, Weidlich D, et al. Quantitative magnetic resonance imaging of the upper trapezius muscles – assessment of myofascial trigger points in patients with migraine. The Journal of Headache and Pain. 2019;20(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter DA, Roque RA, Lin P-C, Doty SB, Pleshko N, Spencer RG. Improved specificity of cartilage matrix evaluation using multiexponential transverse relaxation analysis applied to pathomimetically degraded cartilage. NMR in Biomedicine. 2011;24(10):1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter DA, Lin P-C, Fishbein KW, Spencer RG. Multicomponent T2 relaxation analysis in cartilage. Magnetic Resonance in Medicine. 2009;61(4):803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaia E, Toffanin R, Guglielmi G, et al. Fast T2 mapping of the patellar articular cartilage with gradient and spin-echo magnetic resonance imaging at 1.5 T: validation and initial clinical experience in patients with osteoarthritis. Skeletal Radiol. 2008;37(6):511–517. [DOI] [PubMed] [Google Scholar]
- 11.Juras V, Bohndorf K, Heule R, et al. A comparison of multi-echo spin-echo and triple-echo steady-state T2 mapping for in vivo evaluation of articular cartilage. Eur Radiol. 2016;26(6):1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Eliezer N, Sodickson DK, Block KT. Rapid and accurate T2 mapping from multi–spin-echo data using Bloch-simulation-based reconstruction. Magnetic Resonance in Medicine. 2015;73(2):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary Angiography with Magnetization-Prepared T2 Contrast. Magnetic Resonance in Medicine. 1995;33(5):689–696. [DOI] [PubMed] [Google Scholar]
- 14.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: Relaxation Times and Image Contrast. American Journal of Roentgenology. 2004;183(2):343–351. [DOI] [PubMed] [Google Scholar]
- 15.Foltz WD, Al-Kwifi O, Sussman MS, Stainsby JA, Wright GA. Optimized spiral imaging for measurement of myocardial T2 relaxation. Magnetic Resonance in Medicine. 2003;49(6):1089–1097. [DOI] [PubMed] [Google Scholar]
- 16.Deoni SCL, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magnetic Resonance in Medicine. 2003;49(3):515–526. [DOI] [PubMed] [Google Scholar]
- 17.Welsch GH, Scheffler K, Mamisch TC, et al. Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magnetic Resonance in Medicine. 2009;62(2):544–549. [DOI] [PubMed] [Google Scholar]
- 18.Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magnetic Resonance in Medicine. 2014;71(1):230–237. [DOI] [PubMed] [Google Scholar]
- 19.Heule R, Ganter C, Bieri O. Rapid estimation of cartilage T2 with reduced T1 sensitivity using double echo steady state imaging. Magnetic Resonance in Medicine. 2014;71(3):1137–1143. [DOI] [PubMed] [Google Scholar]
- 20.Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magnetic Resonance in Medicine. 2012;67(4):1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawley AP, Wood ML, Henkelman RM. Elimination of transverse coherences in FLASH MRI. Magnetic Resonance in Medicine. 1988;8(3):248–260. [DOI] [PubMed] [Google Scholar]
- 22.Zur Y, Wood ML, Neuringer LJ. Spoiling of transverse magnetization in steady-state sequences. Magnetic Resonance in Medicine. 1991;21(2):251–263. [DOI] [PubMed] [Google Scholar]
- 23.de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR Imaging Relaxation Times of Abdominal and Pelvic Tissues Measured in Vivo at 3.0 T: Preliminary Results. Radiology. 2004;230(3):652–659. [DOI] [PubMed] [Google Scholar]
- 24.Christoffersson JO, Olsson LE, Sjöberg S. Nickel-doped agarose gel phantoms in MR imaging. Acta radiologica. 1991;32(5):426–431. [PubMed] [Google Scholar]
- 25.Pykett IL, Rosen BR, Buonanno FS, Brady TJ. Measurement of spin-lattice relaxation times in nuclear magnetic resonance imaging. Phys Med Biol. 1983;28(6):723–729. [DOI] [PubMed] [Google Scholar]
- 26.Wiener E, Pfirrmann CWA, Hodler J. Spatial variation in T1 of healthy human articular cartilage of the knee joint. BJR. 2010;83(990):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Chaudhary R, Hurley SA, et al. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3.0T. Journal of Magnetic Resonance Imaging. 2014;39(5):1191–1197. [DOI] [PubMed] [Google Scholar]
- 28.Eminian S, Hajdu SD, Meuli RA, Maeder P, Hagmann P. Rapid high resolution T1 mapping as a marker of brain development: Normative ranges in key regions of interest. PLOS ONE. 2018;13(6):e0198250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernando D, Liang Z-P, Kellman P. Chemical shift–based water/fat separation: A comparison of signal models. Magnetic Resonance in Medicine. 2010;64(3):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HH, Börnert P, Hernando D, et al. ISMRM workshop on fat–water separation: insights, applications and progress in MRI. Magnetic resonance in medicine. 2012;68(2):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton G, Middleton MS, Bydder M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. Journal of Magnetic Resonance Imaging. 2009;30(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernando D, Artz NS, Hamilton G, Roldan-Alzate A, Reeder S. Fully automated processing of multi-echo spectroscopy data for liver fat quantification. In: Proceedings of the 22nd Annual Meeting of ISMRM Milan, Italy Vol 2884 ; 2014. [Google Scholar]
- 33.Henkelman RM, McVeigh ER, Crawley AP, Kucharczyk W. Very slow in-plane flow with gradient echo imaging. Magnetic Resonance Imaging. 1989;7(4):383–393. [DOI] [PubMed] [Google Scholar]
- 34.Reeder S, McVeigh E. Velocity Spoiling of Transverse Magnetization: Implications for CINE MRI. In: Ney York, NY, U.S.A; 1993:1268. [Google Scholar]
- 35.Liu F, Block WF, Kijowski R, Samsonov A. Rapid multicomponent relaxometry in steady state with correction of magnetization transfer effects. Magnetic Resonance in Medicine. 2016;75(4):1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]